Abstract
Human immunodeficiency virus (HIV) infection leads to the establishment of a long-lived latent cellular reservoir. One strategy to eliminate quiescent reservoir cells is to reactivate virus replication to induce HIV envelope glycoprotein (Env) expression on the cell surface exposing them to subsequent antibody targeting. Via the interactions between the antibody Fc domain and Fc-γ receptors (FcγRs) that are expressed on innate effector cells, such as natural killer cells, monocytes, and neutrophils, antibodies can mediate the elimination of infected cells. Over the last decade, a multitude of human monoclonal antibodies that are broadly neutralizing across many HIV-1 subtypes have been identified and are currently being explored for HIV eradication strategies. Antibody development also includes novel Fc engineering approaches to increase engagement of effector cells and optimize antireservoir efficacy. In this review, we discuss the usefulness of antibodies for HIV eradication approaches specifically focusing on antibody-mediated strategies to target latently infected cells and options to increase antibody efficacy.
Keywords: HIV reservoir, monoclonal antibodies, Infected cell recognition, Fc function, HIV cure
Although antiretroviral therapy (ART) has enabled pharmacological suppression of human immunodeficiency virus (HIV) type 1 replication, it has become evident that even lifelong therapy with ART will not eradicate the virus. HIV-1 infection remains incurable because it establishes a pool of long-lived memory CD4+ T cells in which replication-competent virus persists as integrated proviral DNA. This latent cellular reservoir is established within days of virus exposure, even before virus can be detected in peripheral blood [1]. Latently infected cells are invisible to the immune system as they lack active viral replication and therefore do not express viral proteins on the cell surface. However, once suppressive ART is stopped, these cells are capable of rapidly reigniting new rounds of infection. The latent reservoir therefore presents a significant hurdle for cure approaches. Novel concepts for viral eradication strategies combine pharmacological induction of the latently infected cells to produce virus together with immune enhancing interventions to enable the host to clear these cells. Over the last decade there has been significant progress in the identification and development of broadly neutralizing antibodies (bNAbs) against HIV.
The introduction of high-throughput single B-cell receptor sequencing technology using HIV-1 Env probes to identify HIV-1–specific B cells [2, 3], has generated a slew of extremely potent bNAbs [2, 4, 5]. Numerous antibodies have been reported to target various sites of vulnerability on the HIV-1 Env trimer, including the CD4-binding site (CD4bs) of glycoprotein (gp) 120, the V2 glycan site at the apex of the Env trimer, the V3 glycan site, the membrane-proximal external region of gp41, and more recently the interface region between gp120 and gp41 (reviewed in [6, 7]). Data from preclinical animal models but also from early-phase clinical trials suggest that bNAbs were not only capable of reducing plasma viremia, demonstrating their potent antiviral activity, but were also associated with a delay in viral rebound after analytical ART interruption, suggesting an effect of the bNAb therapy on the cellular viral reservoir [8–10]. Antibodies against HIV might be a promising new tool for viral eradication. Here, we will review the recent developments in the use of antibodies for HIV-1 therapy, specifically focusing on antibody-mediated strategies to target latently infected cells and options to increase antibody efficacy for HIV cure approaches.
THE TARGET: RECOGNITION OF THE CELLULAR HIV RESERVOIR
The process of HIV Env expression on the cell surface during viral replication is poorly understood, and several mechanisms have been proposed (reviewed in [11]). HIV buds from the plasma membrane, a process which exposes Env on the cell surface. The infected cell can be recognized and labeled by antibodies in this transient/vulnerable state, which can lead to recruitment of innate immune cell, such as natural killer (NK) cells, in an Fc-dependent fashion and elimination of the infected cell by direct effector cell functions. Figure 1 shows the surface of an HIV-infected lymphocyte decorated with Env as detected by the fluorescently labeled antibody 2G12. The kinetics of viral packaging on the surface are a battle between virus and host; host factors such as CD4 and tetherin proteins work to retain viral epitopes on the cell surface to allow sufficient exposure to antibody recognition or inhibit the release of viruses [12], respectively, and are antagonized by, for example, the HIV protein Vpu or a membrane-proximal endocytosis motif of gp41, which counter these defense mechanisms to promote efficient viral release [13–15]. Specifically, this process is unknown in the setting of viral reactivation from latency and Env synthesis and processing might differ significantly from productively infected cells.
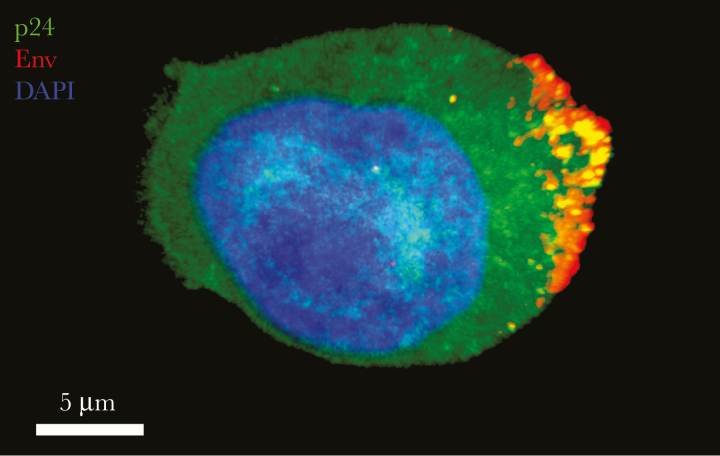
Figure 1.
Distribution of human immunodeficiency virus (HIV) envelope glycoprotein (Env) on the cell surface. Env is distributed (red) on the surface of an HIV NLAD8-infected (p24+; green) lymphocyte and is detected by the fluorescently labeled broadly neutralizing antibody 2G12 (red). Bright puncta of p24+ and Env+ suggest virions budding from the plasma membrane. The image is a projection of confocal sections through the entire thickness of the cell. Nuclear morphological characteristics are shown by 4′,6-diamidino-2-phenylindole (DAPI) staining (blue).
TOPOLOGY AND STOICHIOMETRY OF INFECTED CELL RECOGNITION
The topology of Env expression on the surface of infected cells is poorly understood. On the virion surface, electron cryomicroscopy reveals the presence of approximately 14 copies of Env trimers, the main target of neutralizing antibodies, distributed roughly isotropically [16]. Viral assembly and budding might bring these Env trimers in close proximity to each other on one hemisphere of the virion, increasing the density of bound antibodies and therefore potentially increasing the Fc accessibility by innate effector cells. Indeed, most FcγRs have a low affinity for immunoglobulin (Ig) G, and activation of effector cells and subsequent cytotoxicity is dependent on clustering of FcγRs on the cell surface. Antibody-binding stoichiometry to Env trimers seems to be important, because more available Fc domains will result in sufficient activating signals to augment, for example, antibody-dependent cellular cytotoxicity (ADCC) [17]. The binding stoichiometry of neutralizing antibodies vary from 1 antibody per Env spike (eg, the V2 glycan antibody PGDM1400 [18]), to the more typical 3 antibodies per spike (eg, the V3 glycan antibody PGT121 [19]), to the V3 antibody 2G12 antibody, which dimerizes by domain swapping [20], creating a potential occupancy of 6 antibodies per Env spike. It is reasonable to assume that effector cells may react differently to the single Fc domain presented by PGDM1400 than to 3 presented by a fully occupied PGT121 Env spike, or 6 potential Fcs presented by 2G12, because a threshold of sufficient FcγR interactions needs to be met to activate the effector cells.
OPEN QUESTIONS ABOUT THE EPITOPE LANDSCAPE ON REACTIVATED INFECTED CELLS
Although neutralizing antibodies specifically target functional Env trimers, the antibody response induced by natural HIV-1 infection consists largely of nonneutralizing antibodies (nNabs) that bind to gp120/gp41 monomers, gp120-depleted gp41 stumps or nonfunctional conformational variants of the trimer on the virion surface [21]. To what degree these nonfunctional Env structures are exposed on the surface of reactivated or actively infected cells is not known, but antibodies specific for such antigens could play a role in reservoir targeting strategies because they might increase the overall breadth of cell recognition. Furthermore, with latency at a cellular level generally defined as a state of infection without viral production, it is likely that immune activation is necessary to activate virus production so that Env may be exposed for targeting by antibodies.
Currently, latency reversal agents (LRAs) such as histone deacetylase (HDAC) inhibitors, protein kinase C agonists, Toll-like receptor (TLR) agonists, second mitochondrial-derived activator of caspase mimetics [22], and cytokine superagonists (ie, interleukin 15 [23, 24]) are being explored (reviewed in [25–29]). Although robust induction of viral replication in latently infected cells is the goal, it is critical that the LRA does not interfere with the immune response itself, that is, via off-site effects. It has been demonstrated for certain HDAC inhibitors, such as romidepsin, that NK cell and cytotoxic T-lymphocyte cytolytic activity is significantly decreased in the presence of the LRA, at least in vitro [30–32]. Conversely, some compounds, such as the protein kinase C agonist prostratin or the cytokine interleukin 15, have been demonstrated to increase NK antiviral activity [33, 34]. Conceptually, the ideal LRAs for antibody-based HIV cure approaches would lead to high levels of Env on the surface of reservoir cells under ART, thus exposing latently infected cells to antibody recognition and subsequent elimination while also stimulating innate effector cell activity.
PRECLINICAL AND CLINICAL EXPERIENCE WITH ANTIBODIES FOR HIV-1 THERAPY
Animal Models
The effects of bNAbs against plasma viremia, but also cell-associated virus, have been documented in several preclinical studies using mice and nonhuman primate models [35–41]. Intravenous infusion of PGT121 in rhesus macaques infected with simian-human immunodeficiency virus (SHIV) SF-162P3 resulted not only in rapid and profound suppression of plasma viral RNA, but also in substantial reductions of proviral DNA in peripheral blood, lymph nodes, and gastrointestinal mucosa [35]. In particular, the latter demonstrated that this bNAb had neutralizing activities but also seemed to clear infected cells, suggesting an Fc-mediated mechanism. Indeed, in the humanized mouse models, Bournazos and colleagues [40] demonstrated that enhanced antiviral potency of bNAbs was associated with preferential engagement of activating FcγRs by comparing the abilities of Fc-enhanced, knockout, and wild-type 3BNC117 to reduce viral load.
Furthermore, a rapid anti-infected cell effect by bNAbs was demonstrated by Lu and colleagues [41], in a model measuring cell-associated RNA reduction in response to wild-type or Fc function knockout mutants that ablated effector function, as well as direct FcγR blocking. Further bNAb-directed anti-infected cell effects were demonstrated in macaques; PGT121 protected 50% of macaques from infection that were challenged with SHIV SF162P3–infected splenocytes [42]. These animal data therefore confirmed the importance of Fc-mediated mechanisms of infected cell clearance. Additional studies focused on the direct antireservoir effects of bNAbs by performing analytical treatment interruptions. For example, the combination of the CD4bs antibody 3BNC117, the V3 glycan antibody 10–1074, and the V2 glycan antibody PG16 together with several LRAs, abrogated viral rebound after washout of the antibodies in 57% of HIV-infected humanized mice [43].
Borducchi et al [44] treated rhesus macaques infected with SHIV SF-162P3 with ART administered for 96 weeks, starting 7 days after infection. Under continued ART, macaques received a TLR7 agonist (Vesatolimod), PGT121, or both, followed by a washout period to allow PGT121 to be cleared before ART cessation. After ART discontinuation, a subset of the macaques (5 of 11) treated with PGT121 and TLR7 agonist did not rebound. In addition, bNAb administration to macaques acutely infected with SHIV AD8-EO led to controlled plasma viremia in a subset of animals, after washout of the bNAbs, in a CD8+ T-cell–dependent manner. Rapid reappearance of plasma viremia occurred in these animals once CD8+ T cells were depleted, suggesting that the bNAbs facilitated the emergence of potent CD8+ T-cell immunity [45]. It remains to be determined whether enhanced T-cell immunity will contribute to viral control during reservoir eradication strategies in humans,. Nevertheless, the accumulating evidence for therapeutic effects of bNAbs in animal models has led to a resurgence of interest in evaluating bNAbs for therapeutic indications in humans.
Clinical Data
Several first in-human studies demonstrated that single administrations of bNAbs, including 3BNC117, the CD4bs antibody VRC01, and 10–1074 or bNAb combinations, were able to reduce viral loads by several log10 in HIV-1–infected individuals not receiving ART. Furthermore, repeated dosing of single bNAbs (eg, 3BNC117 or VRC01) or a dual-bNAb combination (3BNC117 + 10–1074) in HIV -infected individuals receiving ART resulted in delayed viral rebound once ART was interrupted [8–10]. Specifically, when the latter bNAb combination was given to individuals with antibody-sensitive viral reservoirs, as 3 infusions of 30 mg/kg of each antibody every 3 weeks, viral suppression was maintained for a median of 21 weeks. In contrast, 2 individuals harboring 10–1074–resistant or 3BNC117-resistant viruses rebounded early.
The degree of viral control after ART interruption was in general variable between the studies, and antibody-resistant viruses emerged frequently. It therefore remains to be determined what effect bNAbs have on the size of the inducible viral reservoir. To improve antibody efficacy and reduce the risk for antibody therapeutic failure due to viral escape, triple-bNAb cocktails, combining antibodies with complementary epitope target regions (eg, targeting the CD4bs, V3, and V2) are currently being evaluated with the thought that this will elevate the threshold for selecting viral variants with preexistent escape mutations or the inducing novel resistance mutations (clinical trials NCT03205917 and NCT03721510). Following this concept, a trispecific antibody (SAR441236), which combines the antigen-binding fragments (Fabs) of the CD4bs antibody VRC01, the membrane-proximal external region antibody 10E8 and the V2 glycan antibody PGDM1400 is under clinical evaluation in HIV-infected viremic and ART-suppressed individuals (NCT03705169). Furthermore, multiple studies are underway or in development to evaluate the combination of bNAbs with LRAs and immunomodulators, such as TLR agonists and HDAC inhibitors (NCT03837756 and NCT03041012).
ANTIBODY FC FUNCTIONALITY IN HIV
Accumulating evidence demonstrates that antibody effector functions play a critical role in conferring therapeutic efficacy against HIV [40, 41, 46–48]. Indeed, the antibody Fc domain is able to mediate a wide range of effector functions through interaction with Fc receptors, complement proteins, and lectins. These functions include lysis by NK cells (ADCC), antibody-dependent complement deposition, trogocytosis (FcγR-dependent “nibbling” of the plasma membrane capable of killing the opsonized cell [49, 50]), and phagocytosis by monocytes/macrophages, neutrophils, and dendritic cells (DCs) [51, 52] (Figure 2). Numerous reports demonstrate that a humoral response to HIV with enhanced activity of Fc-dependent functions is critical in slowing HIV disease progression [53–57], and enhanced Fc functionality is even correlated with the development of bNAb responses [58, 59].
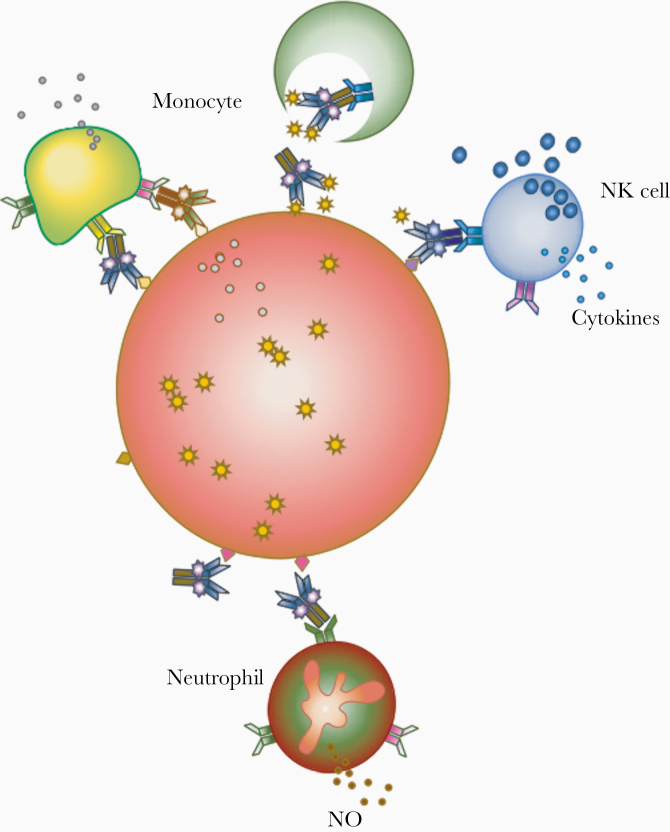
Figure 2.
Fc-mediated antibody effector functions, depicting innate effector recognition of immune complexes of antibodies and infected cells. Antibody-opsonized cells display the Fc in a conformation recognized by innate effectors, such as monocytes, natural killer (NK) cells, and neutrophils, which are capable of responses that vary by effector type. Abbreviation: NO, Nitric Oxide.
ANTIBODY-DEPENDENT CELLULAR CYTOTOXICITY
ADCC is a mechanism for clearance of infected cells (Figure 3), and an enrichment of potent ADCC-mediating antibodies has been described in serum samples from HIV elite controllers, rare individuals who are capable of spontaneous viremic control [54, 56, 60–64], but they also have been associated with antibody-mediated protection against infection in the RV144 HIV vaccine study [65]. ADCC is dependent on the ability of the antibody to recognize infected cells and present the Fc domain in a conformation accessible to FcγRIIIa (CD16a) on NK cells. Activation of NK degranulation depends on cross-linking of FcγRIIIa, leading to intracellular signaling events that led to NK cell activation and proliferation, and it can be inhibited by engagement of the inhibitory FcγRIIb (reviewed in [66]). Therefore, the balance of activating and inhibitory signals is critical, whereas the topological arrangement of target (infected cell) recognition and Fc presentation by antibodies is important. Indeed, recognition of infected cells by bNAbs and subsequent clearance by NK cells is highly heterologous [67–70].
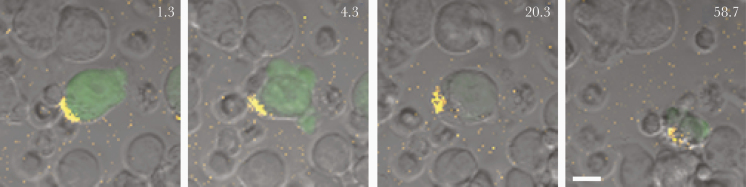
Figure 3.
Time-lapse confocal microscopy of a live human immunodeficiency virus (HIV)–infected (green) cell and antibody-dependent cellular cytotoxicity (ADCC) by natural killer (NK) cells. Purified primary NK cells (smaller cells) were coincubated with HIV-1 JR-CSF–infected CEM cells that express green fluorescent protein (GFP) when infected. Envelope glycoprotein recognition was mediated by a mix of AF647-conjugated 2G12 and unlabeled 2G12 (1:5 ratio). NK cells appear to be associated with the yellow envelope patch (first panel), the cell blebs (second panel), show diminished GFP expression (third panel), and are ultimately destroyed by NK cells (fourth panel). Numbers indicate time in minutes after coculture of effectors and targets; scale bar represents 10 μm.
Some groups report that infected primary cells seem to be more susceptible to elimination by opsonization of V1-V2–targeting bNabs PG9 and PG16, but not CD4bs antibodies [68], in contrast to target cells generated by infecting cell lines [67, 69]. Others, however show relatively high ADCC activity by CD4bs antibody 3BNC117 against reactivated primary cells from ART-treated individuals [71]. Reported differences are likely due to confounding variables such as virus strains used and origin (from infectious molecular clones or passaged primary virus), target cells (primary or cell line), antibody Fc glycosylation differences, and cellular and NK heterogeneity [72]. Furthermore, discordant results have been reported based on the design of the different ADCC assays [73].
In principle, neutralization is not required for ADCC-mediating antibodies, and, hypothetically, nNabs can bind to infected cells and mediate Fc functionality. A comparison of the ADCC activity for a panel of 9 nNabs targeting various epitopes, showed limited ability to recognize and eliminate infected peripheral blood mononuclear cells from ART-treated patients, compared with bNAbs [71]. One explanation might be that current bNAbs have been selected for high-affinity binding to the Env trimer, but the same efforts have not been applied to the identification of nNabs directed against nontrimer targets. Investing more in the identification of novel antibodies that bind nontrimer Env structures with highest affinity might result in a new class of therapeutics that can be used in cellular reservoir targeting strategies. Specifically, sequential antibody binding to different epitopes may act in concert to enhance ADCC [74–76]. For example, binding of certain CD4bs and glycan-targeting bNAbs to the Env trimer results in exposure of CD4-induced epitopes [77], creating a hypothetical scenario in which a bNAb could enhance ADCC by exposing epitopes to the patient’s own ADCC-capable antibody response or to passively infused antibody therapeutics.
PHAGOCYTOSIS
Antibody-dependent phagocytosis (ADCP) (reviewed in [78]) is another Fc function capable of clearance of HIV virions and HIV-infected cells. This function is principally carried out via the FcγRIIa on monocytes, macrophages, neutrophils, eosinophils, mast cells, and DCs. Phagocytic activity occurs in response to FcγRIIa stimulation and is inhibited by FcγRIIb. Similar to findings with ADCC, ADCP activity is higher in HIV elite controllers [57]. ADCP can directly phagocytose antibody-opsonized virions [79], and it can also result in antigen presentation, and interferon α signaling in DCs. Macrophages are capable of engulfing whole infected cells, but this can lead to productive infection in the macrophage [80]. ADCP activity has certain advantages over ADCC, for example, in lymph nodes, which are considered a sanctuary site of the viral reservoir [81], NK cells are infrequently found, whereas macrophages and neutrophils are more abundant [82]. In monoclonal antibody-based HIV cure approaches, Fc domains that stimulate ADCP add another dimension to the fight against the reservoir, through the recruitment of effectors with inflammatory and signaling potential.
FC-ENGINEERED ANTIBODIES
Using antibodies as monoclonal therapeutics presents an opportunity to optimize the Fc domain, resulting in enhanced Fc functionality over IgG1 [83]. Although in vivo data are limited in the HIV field, there are multiple Fc-engineered monoclonal antibodies approved for cancer [84, 85]. Fc enhancement can be accomplished in 3 principal ways: by altering the isotype, the amino acid sequence of the Fc domain, or the Fc glycans, all of which modulate affinity to FcγRs and complement protein C1q [85, 86]. Most antibodies in clinical use for treatment of autoimmune disorders or cancers are IgG1, but there is growing appreciation of the importance of other isotypes. Passive immunization of monoclonal IgM and IgA at mucosal surfaces has been demonstrated to offer superior protection against mucosal transmission of SHIV in macaques, compared with IgG1, potentially owing to the superior viral capture and avidity of IgM [87, 88]. IgG3 variants of the V2-directed bNAb CAP256-VRC26.25 exhibit increased neutralization potency and breadth, as well as increased ADCP and antibody-dependent complement deposition compared with IgG1 [89]. IgG1 and IgG3 are topologically similar in structure, with the exception of the extended hinge region of IgG3 separating the Fab and Fc domains. The length of this flexible domain modulates phagocytic activity [90] but may contribute to decreased antibody half-life of IgG3 relative to IgG1. However, the increased Fab and Fc functions of IgG3 could potentially complement IgG1 in the development of antireservoir monoclonal antibody combinations.
Amino acid sequence modifications of the IgG1 Fc domain focus on increasing or decreasing affinity for FcγRs. The most commonly used Fc mutation, M428L/N434S (commonly shortened to LS), increases antibody half-life in vivo by increasing affinity for the neonatal Fc receptor [91] while retaining binding and functional capacity to FcγRIIIA and C1q [92]. Currently, multiple antibodies with LS mutations are being explored clinically in HIV [93, 94]. Other Fc mutations can increase (or reduce) Fc functionality [86]. Examples include Fc mutations S298A/E333A/K334A (AAA) and G236A/S239D/A330L/I332E (GASDALIE), which increase ADCC activity by increasing affinity for FcγRIIIA [40, 95–97], (reviewed in [85]). In addition to increasing affinity for FcγRIIIA, the GASDALIE Fc mutations exhibit >10-fold increases in affinity for FcγRIIA as well and slightly decreases affinity for the inhibitory receptor FcγRIIB [40]. The use of function-enhanced antibodies in the fight against HIV is still in nascent stages, as demonstrated by protection studies [40, 96]; collectively, however, these data highlight the potential for modulation of antireservoir functional activity.
Similar to amino acid mutations, the glycosylation of the Fc domain modulates FcγR affinity and result in altered Fc functionality (reviewed in [98]). Deglycosylation has been demonstrated to abolish FcγR binding [99], and ADCC is significantly increased in antibodies lacking core fucose [85, 100–105]. Hypergalactosylation (G2 glycans) improves ADCP [106] and ADCC to a lesser relative extent than afucosylation, but it can be synergistically combined with afucosylation for increased FcγRIIIa affinity [105]. Approaches under development include glycoengineering antibodies (eg, by using specifically engineered production cell lines, tobacco plants, etc) and applying chemoenzymatic approaches to generate antibodies with specific glycan signatures (reviewed in [107]), and the first monoclonal antibodies (eg, with nonfucosylated glycan mixtures) have been approved for clinical use to eliminate blast cells during lymphoma [100, 108]. As the principles behind antibody-mediated elimination of cancer cells are similar to what is attempted with antibody-mediated clearance of HIV-infected cells, the potential for functional enhancement in vivo by glycoengineering of antireservoir antibodies should be explored.
VIRAL DIVERSITY IN THE LATENT RESERVOIR
Env sequence diversity in the HIV reservoir is a major obstacle for antibody-based cure approaches. ART cessation in HIV-infected patients leads to a rebound of diverse viruses from multiple tissue sources and compartments [109], with the consequence that reactivated viral populations are being dispersed via the blood and repopulate tissues throughout the body [110]. Although there is evidence for viral compartmentalization—for example, in the central nervous system (CNS) compared with blood [111]—a recent study performing rapid autopsies in deceased HIV-infected individuals combined with tissue reservoir analysis found evidence of identical intact full-length Env proviruses within and across tissues, but the differences across compartments varied between individuals [110]. Beside the fact that intravenously administered antibodies do not access the CNS owing to the blood-brain barrier, the CNS or potentially other tissue compartments could harbor a reservoir of bNAb-resistant viruses that could migrate to blood, therefore refueling systemic viremia.
To test the susceptibility of peripheral blood reservoir viruses to different bNAbs, Ren et al [70] characterized the ability of 14 anti-Env antibodies to neutralize virus outgrown from infected peripheral blood mononuclear cells from 36 ART-treated HIV-infected individuals but also assessed the ability of these antibodies to bind to cells infected with the same viruses ex vivo. They demonstrated that neutralization susceptibility and infected cell-binding efficacy was quite heterogenous, with single-antibody neutralization breadths ranging from 0% to 64% of viruses neutralized (80% inhibitory concentration, ≤10 μg/mL) and from 0% to 89% for binding of infected cells [70].
Identifying viral resistance in patients before antibody treatment is therefore critical to rational design of antibody combination regimens for HIV reservoir eradication strategies. Defining reservoir susceptibility using quantitative viral outgrowth assays and testing of neutralization sensitivity of these viruses in standard TZM-bl assays is time consuming and expensive (reviewed in [112]). Furthermore, this approach does not guarantee that all viral variants that could emerge are considered, because not every replication-competent provirus can and will be induced for outgrowth under in vitro conditions [113]. Several computational concepts have therefore been developed to use machine learning algorithms for predicting bNAb susceptibility based on patient-derived proviral Env sequences [114–117], and the models have achieved high prediction accuracy that might facilitate the selection of personalized antibody regimens for cure approaches. Nevertheless, we will need a better understanding of the landscape of neutralization resistance of the HIV reservoir across tissue compartments and methods to robustly assess these before antibody therapy. Until then, selection of bNabs/bNAb combinations with the broadest viral coverage possible may still be advantageous.
CONCLUSIONS
Antibodies have the potential to harness the innate immune system against the reactivated HIV reservoir and result in viral remission. The ideal antibody combination for reservoir targeting recognizes all reactivated cells and efficiently mediates destruction through the recruitment of innate effector cells. This will require exquisite breadth in viral coverage combined with the ability to bind to Env structures on reactivated cells while allowing easy accessibility of the Fc domain to FcγRs. The vast Env sequence diversity across the globe but also within each host’s reservoir, with potential archived antibody resistance mutations, inaccessibility of antibodies to (for example) the CNS, and limitations in innate effector cell availability within certain tissue compartments might represent obstacles that need to be overcome. Future studies in this field may benefit from advances in LRA development, resulting in products that are capable of robustly inducing viral activation and increasing infected cell visibility for the immune system, as well as from novel antibody Fc modifications that robustly boost effector functions.
Notes
Acknowledgments. We thank Hacheming Compere for proofreading the manuscript.
Financial support. This work was supported by the National Institute of Health (grant AI138790 to B. J.).
Supplement sponsorship. This supplement is sponsored by the Harvard University Center for AIDS Research (CFAR), an NIH funded program (P30 AI060354), and the Ragon Institute of MGH, MIT and Harvard. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Whitney JB, Hill AL, Sanisetty S, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 2014; 512:74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walker LM, Phogat SK, Chan-Hui PY, et al. Protocol G Principal Investigators Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 2009; 326:285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 2010; 329:856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walker LM, Huber M, Doores KJ, et al. ; Protocol G Principal Investigators Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 2011; 477:466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheid JF, Mouquet H, Feldhahn N, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 2009; 458:636–40. [DOI] [PubMed] [Google Scholar]
- 6. Burton DR, Mascola JR. Antibody responses to envelope glycoproteins in HIV-1 infection. Nat Immunol 2015; 16:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sok D, Burton DR. Recent progress in broadly neutralizing antibodies to HIV. Nat Immunol 2018; 19:1179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bar KJ, Sneller MC, Harrison LJ, et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med 2016; 375:2037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendoza P, Gruell H, Nogueira L, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 2018; 561:479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scheid JF, Horwitz JA, Bar-On Y, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 2016; 535:556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freed EO HIV-1 assembly, release and maturation. Nat Rev Microbiol 2015; 13:484–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008; 451:425–30. [DOI] [PubMed] [Google Scholar]
- 13. Arias JF, Heyer LN, von Bredow B, et al. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proc Natl Acad Sci U S A 2014; 111:6425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. von Bredow B, Arias JF, Heyer LN, et al. Envelope glycoprotein internalization protects human and simian immunodeficiency virus-infected cells from antibody-dependent cell-mediated cytotoxicity. J Virol 2015; 89:10648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pham TNQ, Lukhele S, Dallaire F, Perron G, Cohen ÉA. Enhancing virion tethering by BST2 sensitizes productively and latently HIV-infected T cells to ADCC mediated by broadly neutralizing antibodies. Sci Rep 2016; 6:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu P, Liu J, Bess J Jr, et al. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 2006; 441:847–52. [DOI] [PubMed] [Google Scholar]
- 17. Klein JS, Webster A, Gnanapragasam PN, Galimidi RP, Bjorkman PJ. A dimeric form of the HIV-1 antibody 2G12 elicits potent antibody-dependent cellular cytotoxicity. AIDS 2010; 24:1633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee JH, Andrabi R, Su CY, et al. A broadly neutralizing antibody targets the dynamic HIV envelope trimer apex via a long, rigidified, and anionic β-hairpin structure. Immunity 2017; 46:690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garces F, Lee JH, de Val N, et al. Affinity maturation of a potent family of HIV antibodies is primarily focused on accommodating or avoiding glycans. Immunity 2015; 43:1053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Y, West AP Jr, Kim HJ, Thornton ME, Ward AB, Bjorkman PJ. Structural basis for enhanced HIV-1 neutralization by a dimeric immunoglobulin G form of the glycan-recognizing antibody 2G12. Cell Rep 2013; 5:1443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moore PL, Crooks ET, Porter L, et al. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J Virol 2006; 80:2515–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nixon CC, Mavigner M, Sampey GC, et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 2019; 578:160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones RB, Mueller S, O’Connor R, et al. A subset of latency-reversing agents expose HIV-infected resting CD4+ T-cells to recognition by cytotoxic T-lymphocytes. PLoS Pathog 2016; 12:e1005545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mcbrien JB, Mavigner M, Franchitti L, et al. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8+ cells. Nature 2020; 578:154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sengupta S, Siliciano RF. Targeting the latent reservoir for HIV-1. Immunity 2018; 48:872–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sadowski I, Hashemi FB. Strategies to eradicate HIV from infected patients: elimination of latent provirus reservoirs. Cell Mol Life Sci 2019; 76:3583–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Campbell GR, Spector SA. DIABLO/SMAC mimetics selectively kill HIV-1-infected resting memory CD4+ T cells: a potential role in a cure strategy for HIV-1 infection. Autophagy 2019; 15:744–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Macedo AB, Novis CL, Bosque A. Targeting cellular and tissue HIV reservoirs with Toll-like receptor agonists. Front Immunol 2019; 10:2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bashiri K, Rezaei N, Nasi M, Cossarizza A. The role of latency reversal agents in the cure of HIV: a review of current data. Immunol Lett 2018; 196:135–9. [DOI] [PubMed] [Google Scholar]
- 30. Kelly-Sell MJ, Kim YH, Straus S, et al. The histone deacetylase inhibitor, romidepsin, suppresses cellular immune functions of cutaneous T-cell lymphoma patients. Am J Hematol 2012; 87:354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pace M, Williams J, Kurioka A, et al. Histone deacetylase inhibitors enhance CD4 T cell susceptibility to NK cell killing but reduce NK cell function. PLoS Pathog 2016; 12:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones RB, O’Connor R, Mueller S, et al. Histone deacetylase inhibitors impair the elimination of HIV-infected cells by cytotoxic T-lymphocytes. PLoS Pathog 2014; 10:e1004287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garrido C, Spivak AM, Soriano-Sarabia N, et al. HIV latency-reversing agents have diverse effects on natural killer cell function. Front Immunol 2016; 7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garrido C, Abad-Fernandez M, Tuyishime M, et al. Interleukin-15-stimulated natural killer cells clear HIV-1-infected cells following latency reversal ex vivo. J Virol 2018; 92:e00235–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barouch DH, Whitney JB, Moldt B, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 2013; 503:224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Horwitz JA, Halper-Stromberg A, Mouquet H, et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A 2013; 110:16538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Julg B, Pegu A, Abbink P, et al. Virological control by the CD4-binding site antibody N6 in simian-human immunodeficiency virus-infected rhesus monkeys. j Virol 2017; 91:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shingai M, Nishimura Y, Klein F, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 2013; 503:277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klein F, Halper-Stromberg A, Horwitz JA, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 2012; 492:118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 2014; 158:1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lu CL, Murakowski DK, Bournazos S, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science 2016; 352:1001–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parsons MS, Lloyd SB, Lee WS, et al. Partial efficacy of a broadly neutralizing antibody against cell-associated SHIV infection. Sci Transl Med 2017; 9: eaaf1483. [DOI] [PubMed] [Google Scholar]
- 43. Halper-Stromberg A, Lu CL, Klein F, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 2014; 158:989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Borducchi EN, Liu J, Nkolola JP, et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 2018; 563:360–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nishimura Y, Gautam R, Chun TW, et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 2017; 543:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hessell AJ, Hangartner L, Hunter M, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 2007; 449:101–4. [DOI] [PubMed] [Google Scholar]
- 47. Hessell AJ, Poignard P, Hunter M, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 2009; 15:951–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Flerin NC, Bardhi A, Zheng JH, et al. Establishment of a novel humanized mouse model to investigate in vivo activation and depletion of patient-derived HIV latent reservoirs. J Virol 2019; 93:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taylor RP, Lindorfer MA. Fcγ-receptor-mediated trogocytosis impacts mAb-based therapies: historical precedence and recent developments. Blood 2015; 125:762–6. [DOI] [PubMed] [Google Scholar]
- 50. Richardson SI, Crowther C, Mkhize NN, Morris L. Measuring the ability of HIV-specific antibodies to mediate trogocytosis. J Immunol Methods 2018; 463:71–83. [DOI] [PubMed] [Google Scholar]
- 51. Bournazos S, Ravetch JV. Diversification of IgG effector functions. Int Immunol 2017; 29:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Parsons MS, Chung AW, Kent SJ. Importance of Fc-mediated functions of anti-HIV-1 broadly neutralizing antibodies. Retrovirology 2018; 15:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baum LL, Cassutt KJ, Knigge K, et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol 1996; 157:2168–73. [PubMed] [Google Scholar]
- 54. Lambotte O, Ferrari G, Moog C, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 2009; 23:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang J, Kang BH, Ishida E, et al. Identification of a CD4-binding-site antibody to HIV that evolved near-pan neutralization breadth. Immunity 2016; 45:1108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ackerman ME, Mikhailova A, Brown EP, et al. Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog 2016; 12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ackerman ME, Dugast AS, McAndrew EG, et al. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcγR2a and FcγR2b. J Virol 2013; 87:5468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lofano G, Gorman MJ, Yousif AS, et al. Antigen-specific antibody Fc glycosylation enhances humoral immunity via the recruitment of complement. Sci Immunol 2018; 3:eaat7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Richardson SI, Chung AW, Natarajan H, et al. HIV-specific Fc effector function early in infection predicts the development of broadly neutralizing antibodies. PLoS Pathog 2018; 14:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Madhavi V, Wren LH, Center RJ, et al. Breadth of HIV-1 Env-specific antibody-dependent cellular cytotoxicity: relevance to global HIV vaccine design. AIDS 2014; 28:1859–70. [DOI] [PubMed] [Google Scholar]
- 61. Madhavi V, Wines BD, Amin J, et al. HIV-1 Env- and Vpu-specific antibody-dependent cellular cytotoxicity responses associated with elite control of HIV. J Virol 2017; 91:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lambotte O, Pollara J, Boufassa F, et al. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PLoS ONE 2013; 8:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alsahafi N, Ding S, Richard J, et al. Nef proteins from HIV-1 elite controllers are inefficient at preventing antibody-dependent cellular cytotoxicity. J Virol 2016; 90:2993–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ackerman ME, Crispin M, Yu X, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest 2013; 123:2183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012; 366:1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol 2008; 26:513–33. [DOI] [PubMed] [Google Scholar]
- 67. Bruel T, Guivel-Benhassine F, Amraoui S, et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun 2016; 7:10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mujib S, Liu J, Rahman AKMN, et al. Comprehensive cross-clade characterization of antibody-mediated recognition, complement-mediated lysis, and cell-mediated cytotoxicity of HIV-1 envelope-specific antibodies toward eradication of the HIV-1 reservoir. J Virol 2017; 91:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. von Bredow B, Arias JF, Heyer LN, et al. Comparison of antibody-dependent cell-mediated cytotoxicity and virus neutralization by HIV-1 Env-specific monoclonal antibodies. J Virol 2016; 90:6127–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ren Y, Korom M, Truong R, et al. Susceptibility to neutralization by broadly neutralizing antibodies generally correlates with infected cell binding for a panel of clade B HIV reactivated from latent reservoirs. J Virol 2018; 92:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bruel T, Guivel-Benhassine F, Lorin V, et al. Lack of ADCC breadth of human nonneutralizing anti-HIV-1 antibodies. J Virol 2017; 91:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bernard NF, Kiani Z, Tremblay-McLean A, Kant SA, Leeks CE, Dupuy FP. Natural killer (NK) cell education differentially influences HIV antibody-dependent NK cell activation and antibody-dependent cellular cytotoxicity. Front Immunol 2017; 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lewis GK, Ackerman ME, Scarlatti G, et al. Knowns and unknowns of assaying antibody-dependent cell-mediated cytotoxicity against HIV-1. Front Immunol 2019; 10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Anand SP, Prévost J, Baril S, et al. Two families of Env antibodies efficiently engage Fc-gamma receptors and eliminate HIV-1-infected cells. J Virol 2019; 93:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pollara J, Bonsignori M, Moody MA, et al. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J Virol 2014; 88:7715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Davis-Gardner ME, Gardner MR, Alfant B, Farzan M. eCD4-Ig promotes ADCC activity of sera from HIV-1-infected patients. PLoS Pathog 2017; 13:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Derking R, Ozorowski G, Sliepen K, et al. Comprehensive antigenic map of a cleaved soluble HIV-1 envelope trimer. PLoS Pathog 2015; 11:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tay MZ, Wiehe K, Pollara J. Antibody dependent cellular phagocytosis in antiviral immune responses. Front Immunol 2019; 10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tay MZ, Liu P, Williams LTD, et al. Antibody-mediated internalization of infectious HIV-1 virions differs among antibody isotypes and subclasses. PLoS Pathog 2016; 12:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Baxter AE, Russell RA, Duncan CJ, et al. Macrophage infection via selective capture of HIV-1-infected CD4+ T cells. Cell Host Microbe 2014; 16:711–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fukazawa Y, Lum R, Okoye AA, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 2015; 21:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sips M, Krykbaeva M, Diefenbach TJ, et al. Fc receptor-mediated phagocytosis in tissues as a potent mechanism for preventive and therapeutic HIV vaccine strategies. Mucosal Immunol 2016; 9:1584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Huang Y, Ferrari G, Alter G, et al. Diversity of antiviral IgG effector activities observed in HIV-infected and vaccinated subjects. J Immunol 2016; 197:4603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kellner C, Otte A, Cappuzzello E, Klausz K, Peipp M. Modulating cytotoxic effector functions by Fc engineering to improve cancer therapy. Transfus Med Hemother 2017; 44:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Saunders KO Conceptual approaches to modulating antibody effector functions and circulation half-life. Front Immunol 2019; 10:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang X, Mathieu M, Brezski RJ. IgG Fc engineering to modulate antibody effector functions. Protein Cell 2018; 9:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gong S, Tomusange K, Kulkarni V, et al. Anti-HIV IgM protects against mucosal SHIV transmission. AIDS 2018; 32:F5–F13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Watkins JD, Sholukh AM, Mukhtar MM, et al. CAVD Project Group Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. AIDS 2013; 27:F13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Richardson SI, Lambson BE, Crowley AR, et al. IgG3 enhances neutralization potency and Fc effector function of an HIV V2-specific broadly neutralizing antibody. PLoS Pathog 2019; 15:e1008064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chu TH, Crowley AR, Backes I, et al. Hinge length contributes to the phagocytic activity of HIV-specific IgG1 and IgG3 antibodies. PLoS Pathog 2020; 16:e1008083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zalevsky J, Chamberlain AK, Horton HM, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol 2010; 28:157–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Booth BJ, Ramakrishnan B, Narayan K, et al. Extending human IgG half-life using structure-guided design. MAbs 2018; 10:1098–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Caskey M Broadly neutralizing antibodies for the treatment and prevention of HIV infection. Curr Opin HIV AIDS 2020; 15:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gaudinski MR, Coates EE, Houser KV, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med 2018; 15:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shields RL, Namenuk AK, Hong K, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem 2001; 276:6591–604. [DOI] [PubMed] [Google Scholar]
- 96. Santra S, Tomaras GD, Warrier R, et al. Human non-neutralizing HIV-1 envelope monoclonal antibodies limit the number of founder viruses during SHIV mucosal infection in rhesus macaques. PLoS Pathog 2015; 11:1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ahmed AA, Keremane SR, Vielmetter J, Bjorkman PJ. Structural characterization of GASDALIE Fc bound to the activating Fc receptor FcγRIIIa. J Struct Biol 2016; 194:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li W, Zhu Z, Chen W, Feng Y, Dimitrov DS. Crystallizable fragment glycoengineering for therapeutic antibodies development. Front Immunol 2017; 8:1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lund J, Tanaka T, Takahashi N, Sarmay G, Arata Y, Jefferis R. A protein structural change in aglycosylated IgG3 correlates with loss of huFc gamma R1 and huFc gamma R111 binding and/or activation. Mol Immunol 1990; 27:1145–53. [DOI] [PubMed] [Google Scholar]
- 100. Beck A, Reichert JM. Marketing approval of mogamulizumab: a triumph for glyco-engineering. MAbs 2012; 4:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Boesch AW, Kappel JH, Mahan AE, et al. Enrichment of high affinity subclasses and glycoforms from serum-derived IgG using FcγRs as affinity ligands. Biotechnol Bioeng 2018; 115:1265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV, Wang LX. Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci U S A 2017; 114:3485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pereira NA, Chan KF, Lin PC, Song Z. The “less-is-more” in therapeutic antibodies: afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs 2018; 10:693–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Houde D, Peng Y, Berkowitz SA, Engen JR. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol Cell Proteomics 2010; 9:1716–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Thomann M, Reckermann K, Reusch D, Prasser J, Tejada ML. Fc-galactosylation modulates antibody-dependent cellular cytotoxicity of therapeutic antibodies. Mol Immunol 2016; 73:69–75. [DOI] [PubMed] [Google Scholar]
- 106. Chung AW, Crispin M, Pritchard L, et al. Identification of antibody glycosylation structures that predict monoclonal antibody Fc-effector function. AIDS 2014; 28:2523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mastrangeli R, Palinsky W, Bierau H. Glycoengineered antibodies: towards the next-generation of immunotherapeutics. Glycobiol 2018; 29:199–210. [DOI] [PubMed] [Google Scholar]
- 108. Gagez AL, Cartron G. Obinutuzumab: a new class of anti-CD20 monoclonal antibody. Curr Opin Oncol 2014; 26:484–91. [DOI] [PubMed] [Google Scholar]
- 109. Rothenberger MK, Keele BF, Wietgrefe SW, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci U S A 2015; 112:E1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chaillon A, Gianella S, Dellicour S, et al. HIV persists throughout deep tissues with repopulation from multiple anatomical sources. J Clin Invest 2020; 130:1699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Stefic K, Chaillon A, Bouvin-Pley M, et al. Probing the compartmentalization of HIV-1 in the central nervous system through its neutralization properties. PLoS One 2017; 12:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang Z, Simonetti FR, Siliciano RF, Laird GM. Measuring replication competent HIV-1: advances and challenges in defining the latent reservoir. Retrovirol 2018; 15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ho YC, Shan L, Hosmane NN, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013; 155:540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hake A, Pfeifer N. Prediction of HIV-1 sensitivity to broadly neutralizing antibodies shows a trend towards resistance over time. PLoS Comput Biol 2017; 13:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Magaret CA, Benkeser DC, Williamson BD, et al. Prediction of VRC01 neutralization sensitivity by HIV-1 gp160 sequence features. PLoS Comput Biol 2019; 15:e1006952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yu W-H, Su D, Torabi J, et al. Predicting the broadly neutralizing antibody susceptibility of the HIV reservoir. JCI Insight 2019; 4: e130153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rawi R, Mall R, Shen CH, et al. Accurate prediction for antibody resistance of clinical HIV-1 isolates. Sci Rep 2019; 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]