Abstract
Purpose
Pediatric oncology patients undergoing active chemotherapy are suspected to be at a high risk for severe disease secondary to severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) infection; however, data to support this are lacking. We aim to describe the characteristics of coronavirus disease 2019 (COVID‐19) in this population and also its impact on pediatric cancer care in the New York region during the peak of the pandemic.
Patients and Methods
This multicenter, retrospective study included 13 institutions. Clinical and laboratory information on 98 patients ≤21 years of age receiving active anticancer therapy, who tested positive for SARS‐CoV‐2 by nasopharyngeal swab polymerase chain reaction (PCR), was collected.
Results
Of the 578 pediatric oncology patients tested for COVID‐19, 98 were positive, of whom 73 were symptomatic. Most experienced mild disease, 28 required inpatient management, 25 needed oxygen support, and seven required mechanical ventilation. There is a slightly higher risk of severe disease in males and obese patients, though not statistically significant. Persistent lymphopenia was noted in severe cases. Delays in cancer therapy occurred in 67% of SARS‐CoV‐2‐positive patients. Of four deaths, none were solely attributable to COVID‐19. The impact of the pandemic on pediatric oncology care was significant, with 54% of institutions reporting delays in chemotherapy, 46% delays in surgery, and 30% delays in transplant.
Conclusion
In this large multi‐institutional cohort, we observed that mortality and morbidity from COVID‐19 amongst pediatric oncology patients were low overall, but higher than reported in general pediatrics. Certain subgroups might be at higher risk of severe disease. Delays in cancer care due to SARS‐CoV‐2 remain a concern.
Keywords: chemotherapy, COVID‐19, immunocompromised, immunotherapy, pediatric oncology, SARS‐CoV‐2
Abbreviations
- ALC
absolute lymphocyte count
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- HSCT
hematopoietic stem cell transplant
- ICU
intensive care unit
- LOS
length of stay
- PCR
polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
1. INTRODUCTION
The novel severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) virus, which causes coronavirus disease 2019 (COVID‐19), has led to a global pandemic, with significant morbidity and mortality in high‐risk populations. Due to their immunocompromised state, children undergoing cancer‐directed therapy have been suspected to be at a higher risk for complications of COVID‐19; however, data to support this are lacking. Early reports from China on adult oncology patients suggested a higher risk of severe disease in these patients, lending support to this theory. 1 Children undergoing cancer‐directed therapy have been similarly suspected to be at higher risk for complications of COVID‐19.
Early data on pediatric patients in China suggested that malignancy and chemotherapy may be a risk factor for severe disease. Of 171 children who were positive for SARS‐CoV‐2, only three required mechanical ventilation, one of whom was receiving therapy for acute lymphoblastic leukemia (ALL). 2 A report published by the French Society of Pediatric Oncology described severe disease in five out of 33 patients who tested positive for the SARS‐CoV‐2 virus. 3 Additionally, two cases of severe COVID‐19 in children with cancer were reported. 4 These early results, however, were not replicated in the initial reports from New York City, which reported lower rates of infection, lower morbidity, and no mortality. 5 , 6 In contrast, the American Society of Hematology (ASH) registry suggested more severe outcomes with a pediatric mortality rate of 5.3% as of September 16, 2020. 7 Two pediatric‐specific registries, the Pediatric Oncology COVID Case Report (POCC Report) and the St. Jude Children's Research Hospital global registry also reported higher mortality rates (2.7% in POCC, 4.6% in St. Jude) and high rates of admission to the intensive care unit (ICU). 8 , 9
In the United States, the New York region was an early epicenter of the pandemic, providing the opportunity to study the behavior of the disease even within a niche population like pediatric oncology. Investigators formed a rapid data collection collaborative of 13 institutions with the overarching goal to describe the clinical characteristics and natural history of the disease to help devise surveillance and treatment guidelines.
2. METHODS
This was a multicenter retrospective cohort study. All pediatric oncology patients ≤21 years receiving anticancer therapy who were diagnosed with SARS‐CoV‐2 by polymerase chain reaction (PCR) nasopharyngeal swab, regardless of symptomatology, between the dates of January 15, 2020 and April 27, 2020 were eligible. The primary coordinating site for this study was the Icahn School of Medicine at Mount Sinai in New York, NY. The 13 participating institutions are listed in Table S1. In total, participating sites cared for approximately 1200 pediatric oncology patients on active treatment during this time period. The primary aim was to describe the severity and clinical course of COVID‐19 disease in these children, while secondary aims included identifying clinical risk factors for severe disease, profiling the changes in laboratory parameters and their correlation to illness severity, as well as study the impact of COVID‐19 on the course of treatment. Additionally, we aimed to characterize the impact of the pandemic on staffing, testing policies, and delays in care delivery. The protocol was reviewed by each site's local institutional review board (IRB) and was granted exemption from informed consent due to the deidentified nature of the data collected.
2.1. Data collection
Data were collected utilizing two REDCap questionnaires. The first questionnaire evaluated the impact of the pandemic on institutional practice guidelines, while the second assessed demographics, clinical characteristics, disease course, and laboratory parameters of individual cases. Laboratory parameters were collected at three time points: (a) at diagnosis; (b) during course of illness: most clinically significant value during course of illness (eg, nadirs for blood counts, peak values for inflammatory markers like C‐reactive protein (CRP), ferritin, D‐dimer, fibrinogen); (c) at resolution, where SARS‐CoV‐2 infection resolution was defined by resolution of clinical symptoms in symptomatic patients or 2 weeks from initial diagnosis in asymptomatic patients. All the data were deidentified with an institutional case ID number. After data collection was complete, each entry was reviewed by the lead authors and clarifications were sought from primary sites where applicable in an effort to minimize confounding factors. Individual charts were also reviewed for age‐adjusted body mass index (BMI), and patients were classified as underweight, healthy, overweight, or obese as per Center for Disease Control (CDC) guidelines. 10 Chemotherapeutic regimens were graded as mildly, moderately, or severely immunosuppressive based upon relative risk of fever and neutropenia. In cases of nonstandard or experimental therapy, the level of immunosuppression was assigned by the treating institution. Details can be found in the Supporting Information.
Patients were assigned a “disease severity score” (DSS) by categorizing them into one off our groups, asymptomatic, mild, moderate, or severe disease as detailed in Table 1.
TABLE 1.
COVID‐19 disease severity score
| Disease severity | Definition |
|---|---|
| Asymptomatic | No symptoms of COVID‐19 at any time point |
| Mild disease | Illness that did not require hospitalization or if hospitalization was required, it was for indications other than management of COVID‐19‐associated signs or symptoms |
| Moderate disease | Requiring inpatient management for COVID‐19‐associated symptoms, without the need for ICU‐level care |
| Severe | Requiring ICU‐level care for COVID‐19‐related symptoms |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2.2. Statistical analysis
The cross‐sectional information was presented using standard descriptive statistics. Frequency, percentage, and Fisher's exact test were provided for categorical variables (Table S2). We estimated median and interquartile range (IQR) for continuous variables, which were tested using Kruskal‐Wallis test as appropriate (Table S3). All the tests were two‐sided at a significance level of .05. We assessed normality of the continuous variables, and log‐transformation of the data was made to render it appropriate for parametric testing. Several laboratory variables were collected from three time points in this study: diagnosis, peak or nadir during course of illness, and resolution. Cross‐sectional analyses at each time point and longitudinal analyses were performed. A linear mixed‐model approach, accounting for correlation of the data from the same source (patient) across time points, was used for the longitudinal data. 11 All analyses were conducted using SAS Institute 9.4 software (Cary, NC). 12
3. RESULTS
3.1. Institutional data
Nearly all institutions (12 out of 13) were testing asymptomatic patients for COVID‐19 for multiple indications, commonest being prior to anesthesia for procedures (92%) or surgery (75%). Others included positive household contacts, radiation therapy, initiation of chemotherapy, or start of a new cycle of chemotherapy. Postexposure prophylaxis with hydroxychloroquine was only initiated at one institution briefly at the beginning of the pandemic. A majority (78%) did not routinely screen staff for SARS‐CoV‐2. Pediatric oncology staff was commonly deployed to other areas; however, only two institutions felt that redeployment impacted patient care. Clinic visits were delayed or converted to telehealth visits at 12 institutions. Eleven institutions reported that chemotherapy normally delivered inpatient was administered in the outpatient clinic. Importantly, half (54%) of the institutions reported delays in chemotherapy. At six institutions, cancer‐directed surgeries were delayed due to concerns about sedation or resource utilization, but delays in radiation therapy were only reported by two sites. Where directed surgery or radiation was delayed, 67% reported neoadjuvant chemotherapy was administered as a bridge to local control. Only three institutions reported delays in lumbar punctures for patients with ALL in premaintenance phases of therapy (33%), but six reported delays during maintenance. Steroid pulses for children with ALL in maintenance were delayed or modified in two institutions. Of the participating sites, five performed hematopoietic stem cell transplants (HSCTs) with three reporting delays in HSCT due to concerns of myeloablative therapy amidst a pandemic.
3.2. Patient data
3.2.1. Patient demographics and clinical characteristics
Patient demographics and details of patients’ cancer diagnosis and therapy (Table 2) are provided for the 98 SARS‐CoV‐2‐positive patients. Patients ranged from 2 to 21 years old, with a median of 12.8 years. Sixteen children were ≤5 years of age; 20 were of ages 5‐9 years; 25 were of ages 10‐15; and 37 were >15. There was a strong male predominance (70%). A majority of the patients were of Hispanic/Latino ethnicity (55%). The majority of patients had no reported comorbidities. However, the most common morbidity was obesity, reported in 22% of patients. As expected, the commonest underlying diagnosis was ALL (53%). The majority of patients had newly diagnosed disease with a minority having relapsed/refractory cancer (75% vs 25%). Approximately half (46%) of patients were receiving mildly immunosuppressive chemotherapy, whereas 21% were receiving moderately and 33% severely immunosuppressive therapy. Ten patients received immunotherapy including blinatumomab, rituximab, basiliximab, and other investigational agents. Two patients received radiation therapy within 30 days of diagnosis and three had undergone surgical therapy within 28 days prior to COVID‐19 diagnosis. Eight patients had a distant (>1 year) history of bone marrow transplant, three of which were allogeneic and five were autologous transplants. There were no patients actively undergoing allogeneic HSCT or cellular therapy in this cohort.
TABLE 2.
Demographic data and details of cancer diagnosis and therapy
| N (98) | % | |
|---|---|---|
| Age group, years | ||
| <5 | 16 | 16.3 |
| 5.1‐10 | 20 | 20.4 |
| 10.1‐15 | 25 | 25.5 |
| 15 or older | 37 | 37.8 |
| Race/ethnicity | ||
| White | 25 | 25.5 |
| Hispanic or Latino | 54 | 55.1 |
| Black or African American | 13 | 13.3 |
| Asian or Pacific Islander | 6 | 6.1 |
| Other | 7 | 7.1 |
| Gender | ||
| Male | 69 | 70.4 |
| Female | 29 | 29.6 |
| Age‐adjusted BMI | ||
| Underweight | 10 | 10.2 |
| Healthy | 51 | 52.0 |
| Overweight | 15 | 15.3 |
| Obese | 22 | 22.4 |
| Comorbidities | ||
| Diabetes | 2 | 2.0 |
| Hypertension | 7 | 7.1 |
| Smoking/vaping | 0 | 0 |
| Asthma | 7 | 7.1 |
| Obesity | 22 | 22.4 |
| None | 51 | 52.0 |
| Other* | 22 | 22.4 |
| Cancer diagnosis | ||
| Leukemia‐ALL | 52 | 53.0 |
| Leukemia‐AML | 9 | 9.2 |
| Lymphoma | 3 | 3.1 |
| CNS tumor | 9 | 9.2 |
| Neuroblastoma | 5 | 5.1 |
| Solid tumor | 16 | 16.3 |
| Other | 4 | 4.1 |
| Diagnosis etiology | ||
| Initial diagnosis | 73 | 74.5 |
| Relapse/refractory | 25 | 25.5 |
| Immunosuppression score # | ||
| Mild | 45 | 45.9 |
| Moderate | 21 | 21.4 |
| Severe | 32 | 32.7 |
| Additional cancer therapy | ||
| Immunotherapy | 10 | 10.2 |
| Autologous HSCT | 5 | 5.1 |
| Allogeneic HSCT | 3 | 3.1 |
| Radiation therapy | 2 | 2.0 |
| Cancer‐directed surgery | 3 | 3.1 |
Patient demographic and diagnosis information is included above. For race/ethnicity and comorbidities, sites were allowed to select all that applied. Age‐adjusted BMI group was assigned by reviewing the patients' biological gender, BMI and age and categorized based on CDC guidelines.
*Other comorbidities included pulmonary metastases, GVHD, renal insufficiency, trisomy 21, hydrocephalus, Soto syndrome, adrenal insufficiency, DVT, bronchiolitis obliterans, history of MAS (Perforin A91V mutation).
#Immunosuppressive score was assigned based on degree of immunosuppression for each patients' course of chemotherapy as outlined in supplemental methods.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2.2. Diagnosis and clinical course of COVID‐19
Of the 98 SARS‐CoV‐2‐positive patients among 578 tested, 32 were asymptomatic at the time, of whom seven ultimately developed symptoms, while 66 (65.7%) were tested due to symptoms of COVID‐19 (Figure 1). The median time for asymptomatic patients to develop symptoms was 7 days (range 2‐11 days). Presenting symptoms included fever (82.2%), cough (61.6%), or respiratory distress (26%) with fatigue, myalgias, vomiting/diarrhea, anosmia, ageusia, and sore throat being reported less frequently. Other rare symptoms that prompted testing included rhinorrhea, increased oxygen requirement, headache, anorexia, and irritability. Patients’ clinical details are outlined in Table 3. Reasons for testing asymptomatic patients are detailed in the institutional data section above and were not collected on an individual basis.
FIGURE 1.
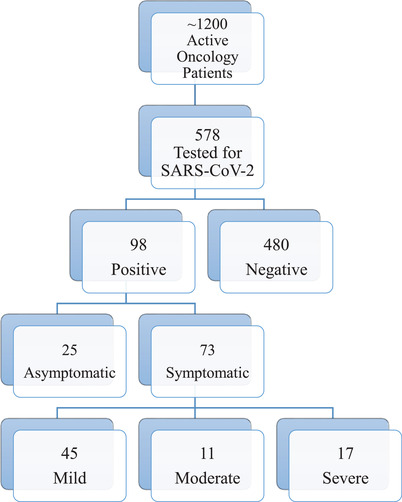
Patient population flow diagram
TABLE 3.
Clinical characteristics of SARS‐CoV‐2‐positive patients
| N (98) | % | |
|---|---|---|
| Rationale for initial testing | ||
| Asymptomatic | 32 | 32.7 |
| Initially asymptomatic, who developed symptoms | 7 | 7.1 |
| Symptomatic | 66 | 67.3 |
| Symptoms | 73 | 100 |
| Fever | 60 | 82.2 |
| Cough | 45 | 61.6 |
| Respiratory distress | 19 | 26.0 |
| Vomiting or diarrhea | 8 | 11.0 |
| Anosmia | 4 | 5.5 |
| Ageusia | 3 | 4.1 |
| Myalgias | 11 | 15.1 |
| Fatigue | 18 | 24.7 |
| Sore throat | 5 | 6.8 |
| Other* | 17 | 23.3 |
| COVID‐19 disease severity (DSS) | 98 | 100 |
| Asymptomatic | 25 | 25.5 |
| Mild | 45 | 45.9 |
| Moderate | 11 | 11.2 |
| Severe | 17 | 17.3 |
| Respiratory support | 98 | 100 |
| Supplemental oxygen | 25 | 25.5% |
| Mechanical ventilation | 7 | 7.1% |
| Complications attributable to COVID‐19 | 98 | 100 |
| Bacterial superinfection | 7 | 7.1% |
| Acute respiratory distress syndrome | 12 | 12.2% |
| Acute kidney injury | 4 | 4.1% |
| Other ± | 9 | 9.2% |
| COVID‐19‐directed treatments | 98 | 100 |
| Hydroxychloroquine | 15 | 15.3 |
| Azithromycin | 15 | 15.3 |
| Tocilizumab | 5 | 5.1 |
| Remdesivir | 4 | 4.1 |
| Other | 7 | 7.1 |
| Anakinra, prednisolone | 1 | 2.0 |
| Convalescent plasma | 2 | 2.0 |
| Additional therapies | 98 | 100 |
| Hematopoietic growth factors | 18 | 18.4 |
| Anticoagulation therapies | 19 | 19.4 |
| Prophylactic dosing | 13 | 13.3 |
| Therapeutic dosing, no VTE | 3 | 3.1 |
| Therapeutic dosing, + VTE | 3 | 3.1 |
| Death # | 4 | 4.1% |
Clinical characteristics of pediatric oncology patients undergoing active anti‐cancer therapy with SARS‐CoV‐2 positivity by nasopharyngeal swab PCR are outlined above.
*Other COVID‐19 symptoms included (N) increased supplemental O2 support from baseline (2), congestion (4), headache (5), rhinorrhea (3), irritability (1) and anorexia (1).
±Other complications of COVID‐19 included fungemia, aspiration pneumonia, seizures, thromboembolism and one case of suspected “hyperinflammatory syndrome” (as reported by one institution).
#No death was solely attributed to COVID‐19.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Of the total 73 symptomatic patients, the majority (61.6% [45/73]) had mild disease. While 75% (55/73) of these patients required hospitalization, only 38.4% (28/73) required inpatient care specifically for COVID‐19‐related symptoms. The remaining were admitted for other indications, most commonly febrile neutropenia (10) or scheduled chemotherapy (12). Amongst those requiring inpatient care for COVID‐19, 15% (11/73) were classified as having moderate disease, while 23.2% (17/73) had severe disease requiring ICU admission, of which 8.2% (6/73) required mechanical ventilation. Reported complications from COVID‐19 included acute respiratory distress syndrome in 16.4%, bacterial superinfection in 9.6%, and acute kidney injury in 5.5%. The median duration of symptoms was 10 days (range 1‐52 days). Patients requiring inpatient care for management of COVID‐19 had a median length of stay (LOS) of 12 days (range 2‐65 days). Supplemental oxygen was required for a median of 8.5 days (range 1‐52 days), whereas mechanical ventilation, when required, had a median duration of 8 days (range 1‐32 days). Of the 64 patients on whom this data were available, SARS‐CoV‐2 PCR remained positive for a median of 27.5 days, with a minimum of 1 day and a maximum of 119 days.
Twenty‐three patients received COVID‐19‐specific therapy. Hydroxychloroquine and azithromycin were used frequently (15/23). Tocilizumab, remdesivir, and convalescent plasma were used in a small minority (Table 3). Anticoagulation was started in 19 hospitalized patients, either at prophylactic or therapeutic dosing. Indications for anticoagulation varied by site, however the majority of patients on anticoagulation (15/19) had moderate to severe disease. Three patients had a thrombus identified, though it was difficult to ascertain if this was related to COVID‐19. Eighteen patients received hematopoietic growth factors.
There was a greater frequency of severe disease in patients who were older (>15 years); however, this was not statistically significant (Figure 2A). Male patients had 1.74 times the risk and obese patients had 1.19 times the risk of developing severe disease, again without statistical significance (95% CI: 0.69‐4.29, P = .19; 95% CI: 0.57‐2.51, P = .56, respectively). However, when comparing obese patients to nonobese, there was a statistically significant difference in distribution across disease severity groups (P = .0088) (Figure 2B).
FIGURE 2.
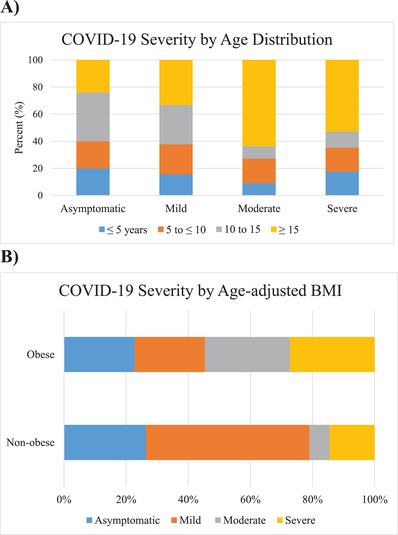
Relationship of age and obesity to disease severity. A, Distribution of age groups within disease severity groups, showing a predominance of older patients in the moderate and severe disease groups. B, Distribution of disease severity within obese patients as compared to nonobese patients showing a trend toward more severe disease in obese patients
Overall, four deaths were reported in the cohort. Of these three had relapsed/refractory disease and one had newly diagnosed acute myeloid leukemia (AML) with hyperleukocytosis, none of whom were in remission. Thus, while the all‐cause mortality in this cohort was 4.08% (4/98), no deaths were solely attributed to COVID‐19 disease. Additional details of each patient mortality can be found in the Supplemental Results.
There were interruptions in chemotherapy in 67% (66/98) of patients who tested positive for SARS‐CoV‐2, with delays ranging from 2 to 78 days. This included both symptomatic and asymptomatic patients, but interruptions were more common in patients with severe disease (14 out of 17). Delays in cancer‐directed surgery or radiation were reported in 6.1% (6/98) of SARS‐CoV‐2 patients regardless of disease severity. Reasons for delays in therapy are again as detailed in the institutional data section and are not elicited specifically for each individual patient.
3.2.3. Laboratory values
Laboratory parameters were collected at three time points: diagnosis, peak or nadir during course of illness, and resolution. None significantly correlated with a more severe course of illness. While previous reports found a correlation of absolute neutrophil count (ANC) to absolute lymphocyte count (ALC) ratio with disease severity, 13 we did not observe this in our cohort (P = .12). However, while testing mixed model effects, we observed a statistically significant difference in lymphocyte counts between COVID‐19 disease severity groups (P = .02). This difference is also impacted by time point (P = .03). The ALC counts at diagnosis were variable and did not correlate to clinical severity, but patients with severe disease had 2.5 times the risk of lymphopenia at recovery which was statistically significant (95% CI: 1.35‐4.9, P = .01) (Figure 3). Alanine transaminase levels were significantly higher among obese patients at all time points (diagnosis P = .006; peak P = .02; resolution P = .001). Among the small number of patients for whom this data were available, inflammatory markers such as CRP, D‐dimer, and interleukin‐6 did not correlate with disease severity (Table S4).
FIGURE 3.
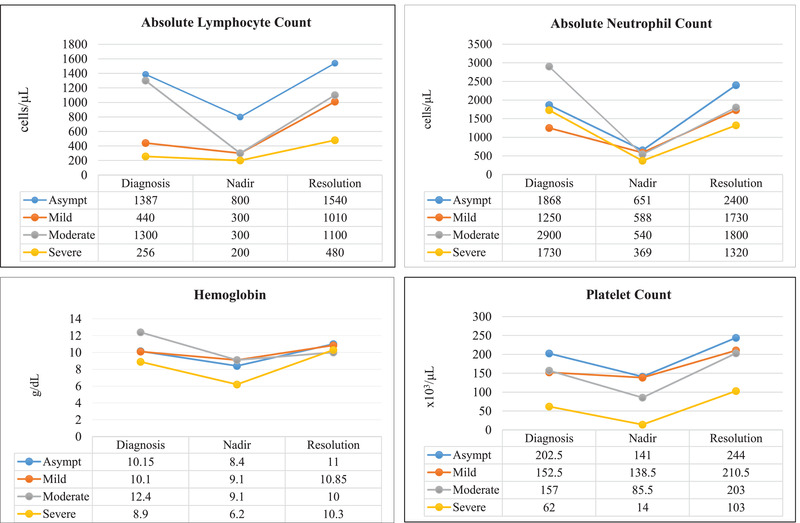
Median blood counts amongst disease severity groups at time of COVID‐19 diagnosis, peak or nadir during course of COVID‐19 illness, and at COVID‐19 resolution
4. DISCUSSION
Pediatric oncology patients straddle an interesting clinical space in the SARS‐CoV‐2 world, one in which their age would predict a mild course but their underlying malignancy and treatment may increase the risk of severe disease. The observations captured in this study shed considerable light on this debate and suggest that while overall risk of severe disease is less than predicted, the risk may be higher than healthy children and with certain demographic and disease features.
Patients of Hispanic/Latino ethnicity and male gender were a majority in this cohort, but it is difficult to comment on whether this is truly reflective of disease impact or is purely a reflection of institutions’ general population, because data on the overall demographics of the patient population at these institutions were not collected. Published literature on COVID‐19 supports that these patients are at a higher risk. 14 , 15 , 16 The majority of children were between 10 and 21 years of age, and a significant proportion of older children developed moderate to severe disease as compared to their younger counterparts. Male patients and obese patients had a higher relative risk of severe disease, although this did not reach statistical significance. Underlying diagnosis did not show any statistically significant correlation with severity of COVID‐19, though a relatively larger number of patients with AML (55%, 5/9) required ICU‐level care. There appeared to be no correlation of COVID‐19 severity with the degree of chemotherapy‐induced immunosuppression, which is similar to the findings of the United Kingdom Coronavirus Cancer Monitoring Project. 17 Two patients in our cohort had trisomy 21 both requiring ICU care, suggesting susceptibility to severe disease, which has been reported. 18
In our cohort, 16.95% of tested pediatric oncology patients were positive for SARS‐CoV‐2, which is higher than the positivity rates amongst general pediatric patients and rates reported by the initial pediatric oncology reports (∼11%) from New York City. 5 , 6 , 19 These differences may be a result of heightened screening among immunocompromised patients, local testing availability/policies, and the phase of the pandemic. Only a minority of initially asymptomatic patients eventually developed symptoms and amongst the symptomatic patients, most had a mild illness. It is notable though, that of those requiring inpatient care for COVID‐19, 75% required oxygen support and 60% required ICU admission. This is significantly higher than what has been reported in hospitalized pediatric patients without cancer. Early studies from China reported very low rates of severe disease in patients who tested positive for SARS‐CoV‐2 (2.8%). 20 The COVID‐NET group showed a 33.2% rate of ICU admissions, whereas Kainth et al reported a 35% rate of ICU admissions, both among hospitalized children. 14 , 21 The median for measures such as LOS (12), days on oxygen support (8.5), days on mechanical ventilation (8) also appear to be markedly longer in our patients as compared to their general pediatric counterparts. Kainth et al reported a median of 3.2 days for LOS, 3 days on supplemental oxygen, and 5 days for mechanical ventilation. Similarly, in the COVID‐NET study, median LOS was 2.5 days with a median ICU stay of 2 days. Early reports from New York of pediatric oncology patients were conflicting, with one institution reporting only one hospitalization and no deaths among 20 patients, while another reported four of 14 patients (28.5%) required ICU‐level care. 5 , 6 In contrast, real‐time databases like the POCC reported hospitalization in 42% of leukemia patients and 36% of solid tumor patients, with 8% of leukemia patients requiring ICU‐level care and a mortality rate of 2.7%. The St. Jude global registry has reported 10.2% patients requiring ICU‐level care and a 4.6% death rate, further suggesting a more severe course. Interpreting these data, however, could be challenging without further details due to the medically complex nature of these patients.
While the all‐cause mortality was 4.1%, no deaths were attributable exclusively to COVID‐19. However in two cases, COVID‐19 significantly contributed to accelerating demise. Thus in our cohort, COVID‐19 disease does not seem to be associated with increased mortality, except in children who are at high risk of death from their malignancy.
Correlating laboratory values with prognosis in this group has been challenging, given the impact of their malignant diagnosis and its therapy upon these values. Despite this, the persistent lymphopenia noted in the severe disease group, is noteworthy. Lymphopenia has recurrently been discussed as a risk factor for severe disease in COVID‐19 in adults, 22 however, not in pediatrics. Kainth et al found a tendency for elevated total white blood cell counts in more severe pediatric cases, but not lymphopenia. 21 Additionally, in a systematic review of 7780 children with COVID‐19, Hoang et al described an overall normal white blood cell count, with a mild neutropenia and an elevated lymphocyte count. 23 Development of lymphopenia in pediatric oncology patients may have implications for subsequent therapy, particularly immunotherapy. None of the inflammatory markers were associated with disease severity, though we did not have consistent data for all patients. Lastly, another notable feature was the duration of persistent SARS‐CoV‐2 PCR positivity, with a median of nearly 4 weeks. Prolonged positivity might not be a true indicator of continued infectivity, but rather of nonreplicative virus. 24
While this study provides important information on SARS‐CoV‐2 infection in these patients, there are several limitations. First, the study was conceived early in the pandemic when hospital resources were strained due to explosive growth of the pandemic in the New York/New Jersey area and prior to the availability of antibody testing or the description of the multisystem inflammatory syndrome. Screening for SARS‐CoV‐2 was not population based, and therefore could have selection biases for the patients who were tested. The PCR testing for SARS‐CoV‐2 was also not centralized and is subject to variability in technique between institutions. Additionally, due to differences between management among institutions, lab values were not consistently available for all patients, further limiting analysis.
In conclusion, while pediatric oncology patients on active therapy appear to have higher risk of severe disease and need for critical care support as compared to the general pediatric population, this risk may be lower than initially perceived and is far lower than observed in their adult oncology counterparts. However, certain subgroups might be at higher risk for severe disease (males, older age, and obesity). Delays in therapy were common in our cohort due to uncertainties about the impact of the virus and the strain on hospital resources, but our data indicate that this approach may be indicated only in select symptomatic cases. The ultimate impact of the unprecedented COVID‐19 pandemic on pediatric oncology patients will be assessed through prospective studies that are underway.
FUNDING INFORMATION
National Cancer Institute, Grant Number: R01 CA140729‐05; Hyundai Hope on Wheels; Alex's Lemonade Stand; Perlmutter Cancer Center Airline and Norman M. Feinberg Pilot Grant for Lymphoid Malignancies and the Perlmutter Cancer Center, Grant Number: P30 CA016087; NCI, Grant Number: CA98543
CONFLICT OF INTEREST
Elizabeth A. Raetz receives research funding from Pfizer and serves on the DSMB for Celgene. Prakash Satwani is a consultant for Mesoblast, Takeda, and Sobi Pharmaceuticals.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors would like to acknowledge the contributions of the following individuals who helped facilitate this collaboration and/or participated in data collection and entry at their respective sites: Maria‐Luisa Sulis (Memorial Sloan Kettering Cancer Center, New York, NY), Peter Cole (Rutgers Cancer Institute of New Jersey, Robert Wood Johnson Medical School, Newark, NJ), Mitchell Cairo and Jessica Hochberg (Maria Fareri Children's Hospital, New York Medical College, Westchester, NY), Ludovico Guarini (Maimonides Medical Center, Brooklyn, NY), Leya Schwartz (The Children's Hospital at Montefiore, Albert Einstein College of Medicine, Bronx, NY), Elana Smilow (Joseph M. Sanzari Children's Hospital, Hackensack University Medical Center, Hackensack, NJ), Celia Grace Murnock (St. Joseph's Children's Hospital, Patterson, NJ) and Aurora Lewis (Mount Sinai Kravis Children's Hospital). We would also like to acknowledge Jennifer Levine (New York‐Presbyterian, Weill Cornell Medicine, New York, NY) and Prakash Satwani (Columbia University Medical Center) for their contribution toward helping us recruit collaborating institutions and structuring a regional research group on COVID‐19 disease in pediatric hematology‐oncology patients.
Madhusoodhan PP, Pierro J, Musante J, et al. Characterization of COVID‐19 disease in pediatric oncology patients: The New York‐New Jersey regional experience. Pediatr Blood Cancer. 2021;68:e28843 10.1002/pbc.28843
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andre N, Rouger‐Gaudichon J, Brethon B, et al. COVID‐19 in pediatric oncology from French pediatric oncology and hematology centers: high risk of severe forms? Pediatr Blood Cancer. 2020;67(7):e28392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stokes CL, Patel PA, Sabnis HS, Mitchell SG, Yildirim IB, Pauly MG. Severe COVID‐19 disease in two pediatric oncology patients. Pediatr Blood Cancer. 2020;67(9):e28432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boulad F, Kamboj M, Bouvier N, Mauguen A, Kung AL. COVID‐19 in children with cancer in New York City. JAMA Oncol. 2020;6(9):1459‐1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gampel B, Troullioud Lucas AG, Broglie L, et al. COVID‐19 disease in New York City pediatric hematology and oncology patients. Pediatr Blood Cancer. 2020;67(9):e28420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Society of Hematology Research Collaborative (ASH RC) . COVID‐19 Registry in Hematology . ASH Research Collaborative. https://www.ashresearchcollaborative.org/s/covid-19-registry-data-summaries.
- 8. UAB . The Pediatric COVID‐19 Cancer Case Report. 2020. https://www.uab.edu/medicine/icos/icos‐research/the‐pocc‐report.
- 9. St. Jude Global . Global Registry of COVID‐19 in Pediatric Cancer. 2020. https://global.stjude.org/en‐us/global‐covid‐19‐observatory‐and‐resource‐center‐for‐childhood‐cancer/registry.html.
- 10. CDC . Healthy Weight, Nutrition and Physical Activity . 2020. https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html.
- 11. Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Wiley; 2011. [Google Scholar]
- 12. SAS Institute Inc . SAS/ACCESS® 9.4 Interface to ADABAS: Reference . Cary, NC: SAS Institute Inc; 2013.
- 13. Liu J, Liu Y, Xiang P, et al. Neutrophil‐to‐lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim L, Whitaker M, O'Halloran A, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory‐confirmed COVID‐19 ‐ COVID‐NET, 14 States, March 1‐July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1081‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alkhouli M, Nanjundappa A, Annie F, Bates MC, Bhatt DL. Sex differences in case fatality rate of COVID‐19: insights from a multinational registry. Mayo Clin Proc. 2020;95(8):1613‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tirupathi R, Muradova V, Shekhar R, Salim SA, Al‐Tawfiq JA, Palabindala V. COVID‐19 disparity among racial and ethnic minorities in the US: a cross sectional analysis. Travel Med Infect Dis. 2020;38:101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. UK Coronavirus Cancer Monitoring Project team . The UK Coronavirus Cancer Monitoring Project: protecting patients with cancer in the era of COVID‐19. Lancet Oncol. 2020;21(5):622‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clift AK, Coupland CAC, Keogh RH, Hemingway H, Hippisley‐Cox J. COVID‐19 mortality risk in Down syndrome: results from a cohort study of 8 million adults. Ann Intern Med. 2020. 10.7326/M20-4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID‐19) in children in Madrid, Spain. JAMA Pediatr. 2020. 10.1001/jamapediatrics.2020.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145(6):e20200702. [DOI] [PubMed] [Google Scholar]
- 21. Kainth MK, Goenka PK, Williamson KA, et al. Early experience of COVID‐19 in a US children's hospital. Pediatrics. 2020;146(4):e2020003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao Q, Meng M, Kumar R, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a systemic review and meta‐analysis. Int J Infect Dis. 2020;96:131‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoang A, Chorath K, Moreira A, et al. COVID‐19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24:100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Korea Centers for Disease Control and Prevention . Findings from Investigation and Analysis of Re‐Positive Cases . KDCA; May 19, 2020. https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030&act=view&list_no=367267&nPage=1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


