AUTHOR CONTRIBUTIONS
All authors have contributed to the work. Marta Drake‐Monfort, Susana Armesto, Marcos Antonio González‐López, and Pablo Munguía‐Calzada reported the four cases. Pablo Munguía‐Calzada and Marcos Antonio González‐López wrote the article. Leandra Reguero‐del Cura and Ana Elisabet López‐Sundh made the photographs, reviewed the bibliography, and performed the critical revision of the letter.
Dear Editor,
Psoriasis is a chronic inflammatory disease affecting 1% to 3% of the world's population 1 , 2 and results from the interaction between genetics and environmental factors such as stress, infections, and drugs, causing a T‐cell‐mediated response. 1 , 2 , 3 Vaccination is an uncommon triggering factor for the flare‐ups of several skin diseases, 2 , 4 , 5 , 6 and a potential association between vaccination and the onset or exacerbation of psoriasis has been previously documented. 2 , 5 , 6 , 7 In this letter, we report four new cases of psoriasis flare‐ups after an influenza vaccination (Table 1).
TABLE 1.
Summary of psoriasis flares following influenza vaccination
| Case | Age | Gender | Treatment before psoriasis flare | Vaccine type | Time from vaccination to psoriasis flare | Psoriasis type after vaccination | Treatment for flare |
|---|---|---|---|---|---|---|---|
| 1 | 41 | Male | Adalimumab | Chiroflu (anti‐H1N1, H3N2 and B) | 24 h | Plaque | Guselkumab Topical corticosteroids |
| 2 | 70 | Female |
Topical corticosteroids Vitamin D analogs |
Chiroflu (anti‐H1N1, H3N2 and B) | 7 d | Plaque |
Oral acitretin Oral prednisone in slow de‐escalation Topical corticosteroids |
| 3 | 55 | Female | Secukinumab | Chiroflu (anti‐H1N1, H3N2 and B) | 24 h | Plaque | Topical fluticasone |
| 4 | 67 | Female | Guselkumab | Chiroflu (anti‐H1N1, H3N2 and B) | 30 d | Guttate | Brodalumab |
The first patient was a 41‐year‐old man with chronic plaque psoriasis undergoing adalimumab therapy who developed a severe flare‐up that required hospital admission 24 hours after an intramuscular Chiroflu influenza vaccination (Trivalent A/Victoria/2452/2019 H1N1, A/Hong Kong/2671/2019 H3N2, and B/Victoria/705/2018) (Figure 1). The patient clinically improved after treatment with subcutaneous guselkumab, topical corticosteroids, and emollients.
FIGURE 1.
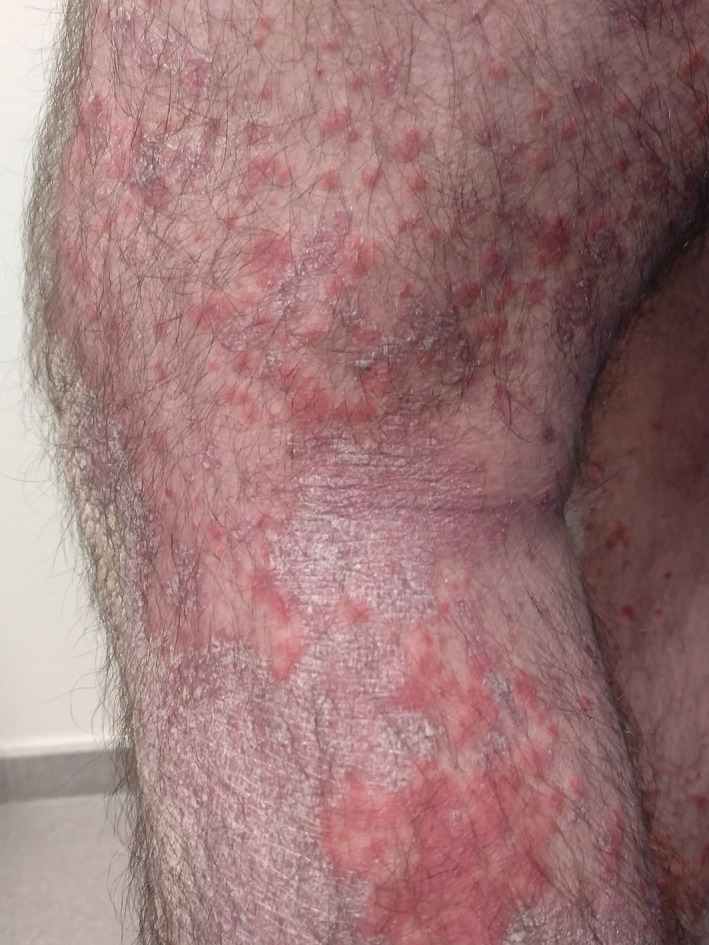
Chronic hyperkeratotic plaques on the legs with surrounding erythematous macules
The second patient was a 70‐year‐old woman with chronic psoriasis who was undergoing treatment with topical corticosteroids and vitamin D analogs. The patient was referred to our department from the emergency room because she had started to develop diffuse erythema and numerous plaques following an intramuscular Chiroflu influenza vaccination 7 days earlier (Figure 2). The patient was started on treatment with oral acitretin, oral prednisone in slow de‐escalation, and topical methylprednisolone aceponate, showing marked improvement after 3 weeks.
FIGURE 2.
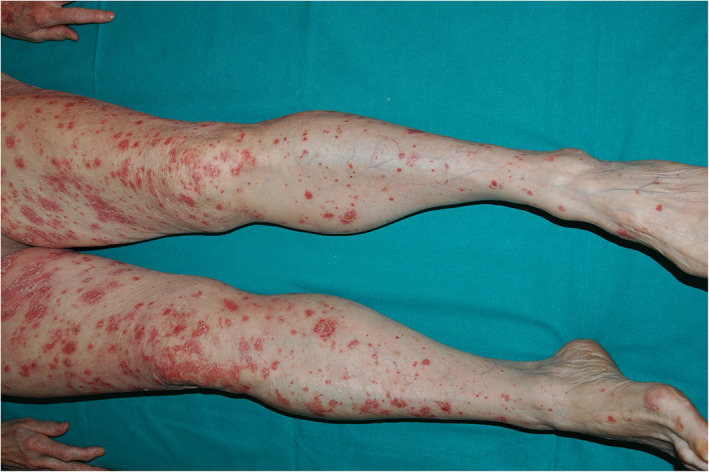
Diffuse erythematous scaly plaques on the legs
The third patient was a 55‐year‐old woman with severe chronic psoriasis treated with subcutaneous secukinumab (previously with etanercept, adalimumab, and ustekinumab) who developed a facial psoriasis plaque 24 hours after a subcutaneous Chiroflu influenza vaccination. The patient was treated with topical fluticasone, with complete resolution of the skin lesion in 2 weeks.
The fourth patient was a 67‐year‐old woman with severe chronic psoriasis undergoing guselkumab therapy who developed a guttate psoriasis flare‐up following a Chiroflu influenza vaccination 1 month earlier. The patient's biological therapy was changed to brodalumab, with improvement in the cutaneous lesions.
New‐onset or severe exacerbations of psoriasis following influenza vaccination are uncommon. Most reported vaccination‐related psoriasis flare‐ups have been classified as guttate and guttate/plaque variants. 2 , 4 , 5 , 6 , 7 We report four cases of psoriasis exacerbation following influenza vaccination with H1N1, H3N2, and B influenza strains. In our four patients, the close temporal relationship between the vaccination and the onset of the psoriasis flare‐ups suggests a possible causal association. 5 Although the etiological relationship between psoriasis and vaccination remains uncertain, it is known that the influenza vaccine generates T‐helper (Th)1 and Th17 immunologic responses, which could represent a possible mechanism for vaccination‐induced psoriasis. 2 The immunologic reaction to the influenza vaccination might rely on the generation of interleukin (IL)‐6 and IL‐22, producing Th17 cells that play a key role in the development of the characteristic epidermal changes of psoriasis. 5 , 6 , 8 In patients treated with IL‐17 inhibitors, Th1 cells might be involved in the development of psoriasis flare‐ups instead of Th17 cells. However, we found no differences in the clinical outcomes between the patient treated with secukinumab and the other patients. To date, “psoriasis vaccinalis” has also been described with Bacillus Calmette‐Guerin, tetanus‐diphtheria, and pneumococcal polysaccharide vaccines, including psoriasis‐like eruptions and psoriatic arthropathy. 8 , 9 , 10 , 11 The very low incidence of this condition and the favorable cost‐effectiveness of the influenza vaccine should not change the immunization practice, especially for patients with psoriasis undergoing immunosuppressive and/or biological therapy. 5 , 6 Nevertheless, it is important to acknowledge vaccination as a triggering factor of psoriasis flare‐ups, particularly in the COVID‐19 era, given that we do not yet know whether COVID‐19 vaccines will be a triggering factor for preexisting or new dermatological conditions, although it appears possible. Patients should therefore be carefully monitored once the COVID‐19 immunization process begins.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author.
REFERENCES
- 1. Shoenfeld Y, Aron‐Maor A. Vaccination and autoimmunity—'vaccinosis': a dangerous liaison? J Autoimmun. 2000;14(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 2. Gunes AT, Fetil E, Akarsu S, Ozbagcivan O, Babayeva L. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valdimarsson H, Thorleifsdottir RH, Sigurdardottir SL, Gudjonsson JE, Johnston A. Psoriasis—as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30(10):494‐501. [DOI] [PubMed] [Google Scholar]
- 4. Raaschou‐Nielsen W. Psoriasis vaccinalis; report of two cases, one following B.C.G. vaccination and one following vaccination against influenza. Acta Derm Venereol. 1955;35(1):37‐42. [PubMed] [Google Scholar]
- 5. Sbidian E, Eftekahri P, Viguier M, et al. National survey of psoriasis flares after 2009 monovalent H1N1/seasonal vaccines. Dermatology. 2014;229(2):130‐135. [DOI] [PubMed] [Google Scholar]
- 6. Shin MS, Kim SJ, Kim SH, Kwak YG, Park HJ. New onset guttate psoriasis following pandemic H1N1 influenza vaccination. Ann Dermatol. 2013;25(4):489‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi CR, Nambudiri VE. Widespread psoriasis flare following influenza vaccination. Vaccine. 2017;35(36):4785‐4786. [DOI] [PubMed] [Google Scholar]
- 8. Dudelzak J, Curtis AR, Sheehan DJ, Lesher JL Jr. New‐onset psoriasis and psoriatic arthritis in a patient treated with bacillus Calmette‐Guerin (BCG) immunotherapy. J Drugs Dermatol. 2008;7(7):684. [PubMed] [Google Scholar]
- 9. Macias VC, Cunha D. Psoriasis triggered by tetanusdiphtheria vaccination. Cutan Ocul Toxicol. 2013;32(2):164‐165. [DOI] [PubMed] [Google Scholar]
- 10. Yoneyama S, Kamiya K, Kishimoto M, Komine M, Ohtsuki M. Generalized exacerbation of psoriasis vulgaris induced by pneumococcal polysaccharide vaccine. J Dermatol. 2019;46(11):442‐443. [DOI] [PubMed] [Google Scholar]
- 11. Grafanaki K, Vryzaki E, Georgiou S, Liga M. Double trouble: influenza and pneumococcal vaccine exacerbation of psoriasis in a new‐onset polycythemia vera patient. J Dermatol. 2020;47(7):e263‐e264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.


