Abstract
Objective
The repurposing of nitazoxanide, doxycycline and azithromycin may be effective to improve the symptoms in mild and moderate COVID‐19 subjects. This study aimed to detect and explain the efficacy of reusing of these drugs in treating COVID‐19.
Methods
The study was divided into two parts: clinical and computational parts. In the clinical part, 80 (30 females) subjects with reverse transcription‐polymerase chain reaction‐confirmed COVID‐19 with mild and moderate symptoms were enrolled in the study. Subjects were treated with azithromycin or doxycycline, and nitazoxanide was added to the treatment if the subject had diarrhoea. Subjects were divided into four groups: Group 1: subjects treated with azithromycin (20 subjects); Group 2: subjects treated with doxycycline (20 subjects); Group 3: subjects treated with a combination of nitazoxanide and doxycycline (20 subjects); and Group 4: subjects treated with a combination of nitazoxanide and azithromycin (20 subjects).
In the computational part, we docked the three drugs against all currently available COVID‐19‐related protein targets (viral and non‐viral). Subsequently, top hits were subjected to molecular dynamic simulations (MDSs) (50 ns) and binding free energy calculations to further validate the docking experiments and to investigate the binding modes of the potential inhibitors.
Results
The symptomatic improvement of mild to moderate subjects was seen on the fifth day after starting treatment in Group 3 and Group 4 and on the seventh day in Group 2. However, for Group 1, the symptomatic improvement of mild to moderate subjects was not seen on the fifth day and required replacement by doxycycline to get the symptomatic improvement. None of the subjects needed intensive care admission and no deaths were reported.
In silico, results were in good accordance with the clinical outcomes, where both nitazoxanide and doxycycline achieved the best docking scores against the viral ADP‐ribose phosphatase (ADPRP) and the human Adaptor‐Associated Kinase 1 (AAK1). MDSs revealed that both drugs were stable in their bindings indicating that they can be considered as lead molecules for targeting ADPRP and AAK1.
Conclusion
The clinical and computational studies applied on three FDA‐approved antimicrobials together with their recent clinical findings revealed that both nitazoxanide and doxycycline have great therapeutic potential against COVID‐19. The future in vitro mechanistic investigation may confirm our primary computational outcomes, and in turn, these classes of compounds provide a promising starting point for further anti‐COVID‐19 therapeutics.
What’s known
The repurposing of nitazoxanide, doxycycline and azithromycin may be effective to improve the symptoms in mild and moderate COVID‐19 subjects.
This study aimed to detect and explain the efficacy of reusing of these drugs in treating COVID‐19.
What’s new
The clinical and computational studies applied on three FDA‐approved antimicrobials together with their recent clinical findings revealed that both nitazoxanide and doxycycline have great therapeutic potential against COVID‐19.
The future in vitro mechanistic investigation may confirm our primary computational outcomes, and in turn, these classes of compounds provide a promising starting point for further anti‐COVID‐19 therapeutics.
1. INTRODUCTION
Until the development of safe, specific and effective therapy against COVID‐19, repurposing of the already available drugs to control the disease's complications remains the primary management protocol. Recently, many FDA‐approved drugs have been repurposed for COVID‐19. Remdesivir has suggested being the most promising anti‐COVID‐19 agent in the ongoing randomised trials. Additionally, nitazoxanide, doxycycline and azithromycin have also been proposed as potential treatment options (Figure 1). 1
FIGURE 1.
Structures of the studied drugs in the present investigation
Nitazoxanide is an FDA‐approved drug for the treatment of a wide range of parasite‐related infectious diarrhoea and enteritis with a considerable safety profile. 2 Besides, this thiazolidine anti‐infective agent has shown broad‐spectrum antiviral activity including the previously spread coronavirus associated with the Middle East respiratory syndrome (MERS‐CoV). 3 Previous reports have suggested that nitazoxanide may mediate its antiviral activity by down‐regulation of protein kinase R (PKR) phosphorylation and in turn impair the cellular translation machinery, viral reproduction. 3 Another study has revealed that nitazoxanide can suppress the production of interleukin‐6 (IL‐6) in mice. 4
Doxycycline and azithromycin are well‐defined antibiotics with good safety profiles that their use has also been suggested in the management of COVID‐19. 5 Both antibiotics have been revealed to have antiviral properties against several RNA viruses. 5 , 6 Moreover, the anti‐inflammatory activity of doxycycline has been well‐established through the suppression of multiple interleukin production, notably IL‐6. 7 Consequently, it has been proposed as a promising agent in controlling the cytokine storm associated with SARS‐CoV‐2. 8
Hence, nitazoxanide, doxycycline and azithromycin hypothetically can be considered as a potential therapeutics against SARS‐CoV‐2.
Computation‐based (ie, in silico) drug screening has emerged during this outbreak as a rapid and efficient tool in repurposing the already available therapy, so that the health systems around the globe can handle the rapidly spreading outbreak. Thus, we aimed in this study to highlight the efficacy of the three aforementioned antimicrobial drugs in the management of COVID‐19 and proposing their possible mode of action via a comprehensive in silico investigation.
2. MATERIAL AND METHODS
2.1. Subjects methods
2.1.1. Design and subjects
This was a randomised, open‐label, drug interventional study including 80 (30 females) subjects with reverse transcription‐polymerase chain reaction (RT‐PCR)‐confirmed COVID‐19 with mild and moderate symptoms, who met the inclusion criteria listed below. The study protocol was approved by the Research Ethical Committee of the Faculty of Pharmacy, Beni‐Suef University (REC‐H‐PhBSU‐19004), and by the Declaration of Helsinki. Participants provided written informed consent.
2.1.2. Inclusion criteria
Subjects within the age group 18 years and more
With either sex, male or female
Positive cases of COVID‐19 by RT‐PCR test
Mild and moderate cases with typical symptoms
Subjects who are not already treated with any other antiviral drugs.
2.1.3. Exclusion criteria
Subjects who are critically ill
Subjects with chronic liver diseases
Pregnant and lactating women
Subjects less than 18 years of age.
2.1.4. Procedure
The subjects were given a treatment of azithromycin or doxycycline and added nitazoxanide to the treatment if the subject had diarrhoea. Subjects were divided into four groups: Group 1: subjects treated with azithromycin (20 subjects); Group 2: subjects treated with doxycycline (20 subjects); Group 3: subjects treated with a combination of nitazoxanide and doxycycline (20 subjects); and Group 4: subjects treated with a combination of nitazoxanide and azithromycin (20 subjects). The dose of azithromycin was 500 mg once daily for 5 days; doxycycline 100 mg daily for 10 days; nitazoxanide was 600 mg twice daily for 5 days. The subjects were followed up daily for symptomatic improvement.
2.2. Molecular docking analysis
All available crystal structures (Figure 2) of SARS‐CoV‐2 proteins (6 proteins) in the Protein Data Bank (PDB) along with the other four human proteins involved in the entry and possessing of the virus were used for the docking analysis using Autodock Vina docking machine. 9 The applied docking protocol dealt with the protein as a rigid structure and the tested compound as a flexible structure during its computations. The co‐crystallised ligands (ADP and staurosporine) were used to determine the binding sites. The ligand‐to‐binding‐site shape matching root mean square deviation (RMSD) threshold was set to 2.0 Å. The interaction energies were determined using the Charmm Force Field (v.1.02) with 10.0 Å as a non‐bonded cut‐off distance and distance‐dependent dielectric. Then, 5.0 Å was set as an energy grid extending from the binding site. The tested compounds were energy‐minimised inside the selected binding pocket. The editing and visualisation of the generated binding poses were performed using Pymol software. 10
FIGURE 2.
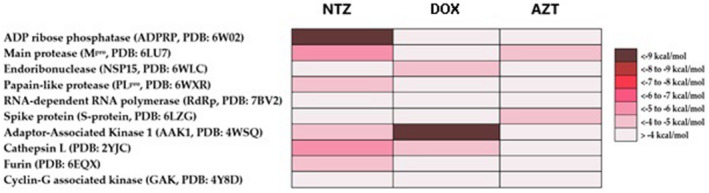
Docking scores of nitazoxanide, doxycycline and azithromycin against all viral‐based and human‐based protein targets hosted in the protein data bank
2.3. Molecular dynamic simulation
Molecular dynamic simulations (MDSs) for ligand‐enzyme complexes were performed using the Nanoscale Molecular Dynamics (NAMD) 2.6 software, 11 by applying the CHARMM27 force field. 12 Hydrogen atoms were added to the protein structures using the psfgen plugin included Antibiotics 2020, 9, 562 14 of 16 in the Visual Molecular Dynamic (VMD) 1.9 software. 13
Afterwards, the whole systems were solvated using TIP3P water particles and 0.15 mol/L NaCl. The energy of the generated systems was firstly minimised and gradually heated to 300 K and equilibrated for 200 pseconds. Subsequently, the MDS was continued for 20 ns, and the trajectory was stored every 0.1 ns and further analysed with the VMD 1.9 software. The MDS output was sampled every 0.1 ns to evaluate the conformational changes of the entire system to analyse the RMSD and root mean square fluctuation (RMSF). The topologies and parameters of the tested compounds were prepared using the VMD Force Field Toolkit (ffTK) 13 and the online software Ligand Reader & Modeler (http://www.charmm‐gui.org/?doc=input/ligandrm). 14 Binding free energies were calculated using the free energy perturbation (FEP) method through the web‐based software Absolute Ligand Binder. 15
3. RESULTS
3.1. Subjects’ results
In this study, 80 (30 females) subjects with RT‐PCR‐confirmed COVID‐19 with mild and moderate symptoms from Beni‐Suef University hospital clinic were included from June to July 2020. The oldest subject was 70 years and the youngest one was 18 years. Most subjects were within the age group of 25 to 45 years. Subjects were divided into four groups: Group 1: subjects treated with azithromycin (20 subjects; six females); Group 2: subjects treated with doxycycline (20 subjects; eight females); Group 3: subjects treated with a combination of nitazoxanide and doxycycline (20 subjects; seven females); and Group 4: subjects treated with a combination of nitazoxanide and azithromycin (20 subjects; nine females).
The symptomatic improvement (especially if there was diarrhoea) of mild to moderate subjects was seen on the fifth day after starting treatment in Group 3 and Group 4 and was seen on seventh day after starting treatment in Group 2; however, for Group 1, the symptomatic improvement of mild to moderate subjects was not seen at fifth day after starting the treatment. So, the subjects in this group needed replacing azithromycin with doxycycline to get the symptomatic improvement. None of the subjects needed intensive care admission and no deaths were reported.
3.2. Molecular docking study and molecular dynamic refinement
Docking of the three proposed drugs against all available viral‐based and human‐based targets (Figure 2) revealed that both nitazoxanide and doxycycline have the potential to modulate the viral ADP‐ribose phosphatase (ADPRP) and the human Adaptor‐Associated Kinase 1 (AAK1), respectively. ADPRP is a phosphatase that removes the terminal 1″‐phosphate group of ADP‐ribose‐1″‐phosphate (Appr 1″‐p). Such a process can breakdown the cellular innate immunity, and hence, facilitate the completion of viral replication and release without a host immune response. 16 Recently, the activity of ADPRP has been linked to the cytokine storm syndrome that is commonly observed in COVID‐19 severe cases. 17
Herein, nitazoxanide showed a high binding affinity towards ADPRP (Docking score = −10.1 kcal/mol) and comparable with that of the co‐crystallised ligand ADP‐ribose (PDB entry 6W02, Docking score = −9.5 kcal/mol). Nitazoxanide was able to interact with most of the reported amino acid residues (ALA‐38, VAL‐49, SER‐128, GLY, 130, ILE‐131, and PHE‐132) through strong H‐bonds (<2 Å). Additionally, its phenyl moiety was perfectly sandwiched between ILE‐131 and PHE‐132 (Figure 3).
FIGURE 3.
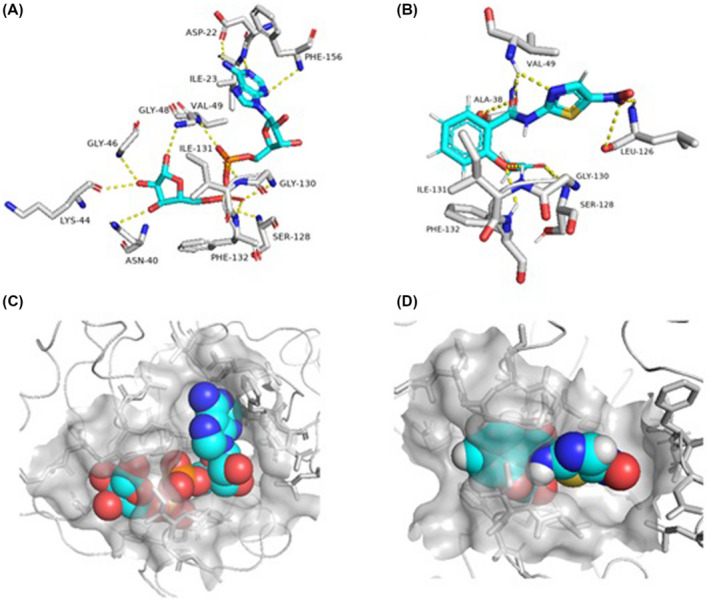
Binding mode of nitazoxanide inside the active site of ADPRP (B and D) together with its co‐crystallised ligand, ADP (A and C)
On the other hand, doxycycline was able to strongly bind with AAK1 active site (Docking score = −9.4 kcal/mol) in comparison with the co‐crystallised inhibitor (Docking score = −8.6 kcal/mol). AAK1 plays a crucial role in the endocytosis, assembly and release of multiple unrelated RNA viruses including SARS‐CoV‐2. 18 , 19 , 20 Hence, inhibiting this serine‐threonine kinase can disrupt the intracellular viral trafficking and processing.
Doxycycline was able to construct a network of strong H‐bonds (8 H‐bonds) with several amino acid residues inside the enzyme's active site (eg, GLU‐54, GLY‐55, GLN‐133, LYS‐178, and ASP‐194). Additionally, GLU‐180 was able to form strong electrostatic interaction with the protonated tertiary amine of doxycycline. Moreover, ALA‐53 and VAL‐60 formed hydrophobic interactions with the body of doxycycline (Figure 4).
FIGURE 4.
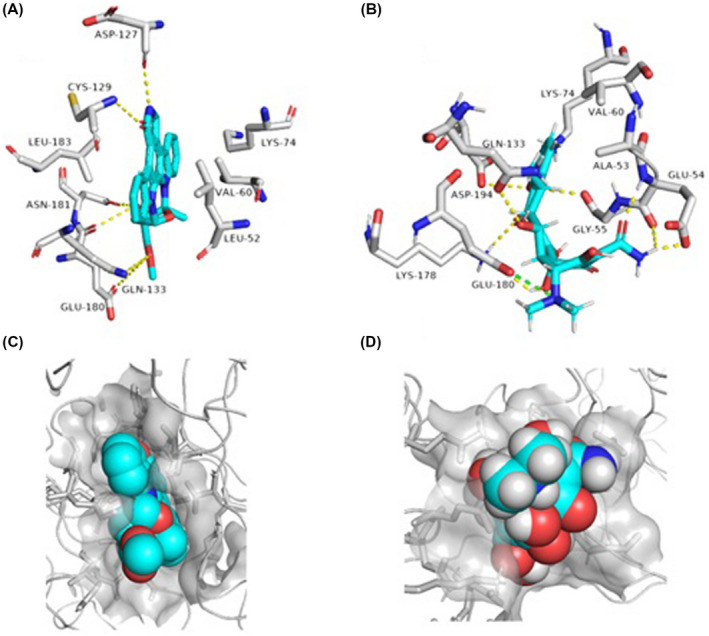
Binding mode of doxycycline inside the active site of AAK1 (B and D) together with its co‐crystallised ligand, staurosporine (A and C)
To further validate the docking results, both ADPRP‐nitazoxanide and AAK1‐doxycycline complexes were subjected to 50 ns MDSs.
Both nitazoxanide and doxycycline were able to stabilise ADPRP and AAK1, respectively, throughout the course of MDS (RMSD ~ 2.51 Å and 3.16 Å, respectively, Figure 6A,B). Additionally, the MDS‐derived binding free energy of both complexes (calculated by the FEP method) was comparable or even better than the co‐crystallised ligands (Figure 5).
FIGURE 6.
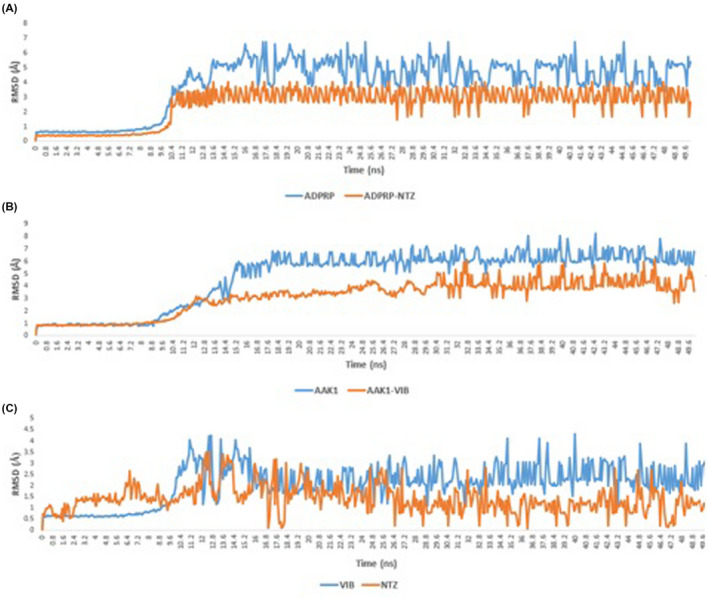
A and B, RMSD of ADPRP and AAK1 in their free and complex forms with both nitazoxanide and doxycycline. RMSD of nitazoxanide and doxycycline inside the active sites of both ADPRP and AAK1
FIGURE 5.
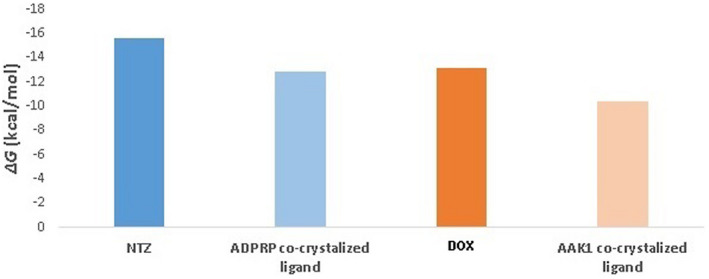
Binding free energy of both nitazoxanide and doxycycline with ADPRP and AAK1, respectively, calculated by FEP, a molecular dynamics‐based method
Furthermore, both drugs were rapidly stabilised inside the active site of both protein targets (RMSD ~ 1.37 Å and 2.0 Å, respectively; Figure 6C).
Regarding ligand‐protein interaction during the course of MDS, nitazoxanide was stabilised inside the ADPRP active site by several H‐bonds, water bridges and hydrophobic interactions. As shown in Figure 7A, ALA‐38, VAL‐49, LEU‐126, SER‐128, GLY‐130, ILE‐131 and PHE‐132 amino acid residues were the major contributors in H‐bonding with nitazoxanide during the MDS, where ALA‐38, VAL‐49, SER‐128 and GLY‐130 were the most important interacting residues (ie, having 61% to 75% interaction time). Moreover, VAL‐49, ILE‐131 and PHE‐132 were also highly involved in hydrophobic interactions with the aromatic moieties of nitazoxanide (72% to 79% interaction time). In addition to direct H‐bonding, water molecules‐mediated H‐bonding (ie, water bridges) also played a significant role in stabilising nitazoxanide, particularly, those with GLY‐46 and VAL‐49.
FIGURE 7.
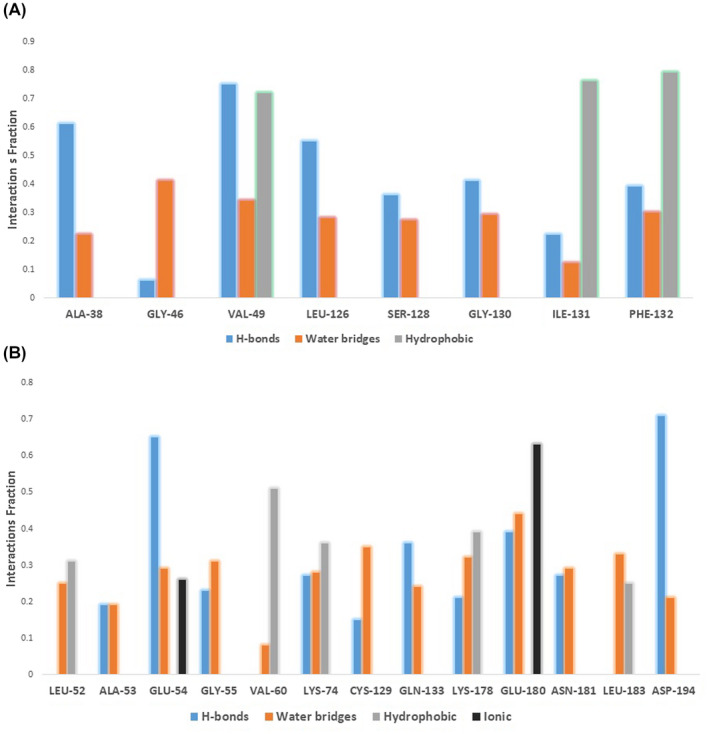
Interacting amino acid residues with both nitazoxanide (A) and doxycycline (B) during the course of MDS
Regarding doxycycline (Figure 7B), H‐bonding with ALA‐53, GLU‐54, GLY‐55, LYS‐74, GLN‐133, LYS‐178, GLU‐180 and ASP‐194 amino acids was the major stabiliser inside the AAK1 active site, particularly, GLU‐54 and ASP‐194 (65% and 71% interaction time, respectively). All of these H‐bonds‐interacting residues were also involved in water bridges that further stabilised doxycycline during the MDS. Besides the aforementioned H‐bond‐based interactions, doxycycline's aromatic moiety interacted significantly with VAL‐60 and LYS‐74 via hydrophobic contacts.
Unlike nitazoxanide, the positively charged tertiary amine of doxycycline was involved in strong ionic interaction with the negatively charged carboxylate side chain of GLU‐180 (69% interaction time).
4. DISCUSSION
COVID‐19 pandemic is a serious public threat, where it led to more around 40 million reported cases and over 1 million deaths. Despite the enormous global efforts to develop therapeutics specific for SARS‐CoV‐2, this process is time‐consuming with limited progress to date. Therefore, repurposing of the currently available drugs can serve as a transient option to modulate this rapidly spreading disease. Several drugs have been suggested to be used as treatment protocols around the world, and some of them have shown acceptable clinical efficacy (eg, dexamethasone). 21 Later on, nitazoxanide, doxycycline and azithromycin have shown significant efficacy in controlling mild to moderate cases.
Consequently, we conducted the present clinical study to detect and explain the efficacy of reusing of anti‐infective drugs in treating COVID‐19; in addition to a computational study to investigate the possible molecular basis that can explain the efficacy of these antimicrobial agents in the management of COVID‐19.
From the clinical results, we noticed that nitazoxanide, doxycycline and azithromycin in combination gave good results in treating COVID‐19. The Egyptian protocol for treating COVID‐19 includes hydroxychloroquine. However, the hydroxychloroquine and azithromycin combination has some safety problems, as drug–drug interactions and cardio‐toxicity, specifically in old subjects. 22
The results of this study demonstrate that the treatment with azithromycin alone was associated with a non‐significant symptomatic improvement of mild to moderate subjects on the fifth day after starting treatment and required replacement by doxycycline. Previously, subjects who received azithromycin alone were shown to have the strongest cumulative hazard. 23
The high evidence of effectiveness for azithromycin is because of its role as an antibacterial drug. Although there is no direct proven effect of azithromycin in COVID‐19, some scientists have suggested that the antibacterial properties of azithromycin are still clinically useful as empirical treatment of COVID‐19 infection. 24 Because of the risks of using azithromycin especially in combination with hydroxychloroquine, we suggest the use of doxycycline as an alternative to azithromycin. Doxycycline has anti‐inflammatory effects with in vitro antiviral efficacy against many RNA viruses. The use of this drug in our study was associated with clinical improvement and decreased cytokine production in some infections with RNA viruses. 5
Doxycycline is safe to use in the treatment of acute respiratory distress syndrome. It could be a better option for COVID‐19 treatment. 25
Nitazoxanide is an orally broad‐spectrum anti‐parasitic and antiviral drug and unlike metronidazole, its metabolites are safer and free of mutagenic factors. 26 Nitazoxanide also potentiates the production of interferon alfa and interferon beta and it has been previously shown to exhibit an in vitro activity against MERS‐CoV and other coronaviruses. 27 Moreover, when nitazoxanide was given in a dose of 600 mg twice daily for 5 days, it was noticed that it reduces the duration of symptoms in subjects with acute uncomplicated influenza with little adverse effects. 28 So, this dose regimen can be used in combination with azithromycin or doxycycline to decrease SARS‐CoV‐2 morbidity and mortality.
The nitazoxanide when used in combination with azithromycin or doxycycline appeared to be a safe and more effective regimen that might replace hydroxychloroquine/azithromycin combination as standard care for COVID‐19, especially if the preliminary data regarding the low efficacy of azithromycin alone have been detected. 29
In the computational study, we were able to virtually screen these drug molecules against several viral protein targets, thanks to the rapid characterisation of SARS‐CoV‐2 vital proteins. Additionally, previous reports on other viral diseases including SARS‐CoV‐1 revealed that other host‐based target proteins can enhance viral virulence. Hence, these targets were also included in our primary screening step. As a result, two enzymes, ADPRP and AAK1, appeared to be the possible targets of both nitazoxanide and doxycycline.
ADPRP is a key enzyme that enables the virus to bypass the host immunity, while AAK1 is a host‐based kinase that controls the viral entry and processing inside the host cell. Consequently, targeting both of these enzymes can provide effective anti‐COVID‐19 therapeutics.
Subsequent MDSs and binding free energy calculations tentatively illustrated the mode of interactions of nitazoxanide and doxycycline inside the active sites of both proteins. Surprisingly, each tested drug molecule achieved binding free energies higher than the reported co‐crystallised inhibitors, and thus, they possess the interesting potential to be considered as drug candidates for further in vitro evaluation.
Besides the observed therapeutic efficacy of these antimicrobial agents in treating COVID‐19, they can alleviate the secondary bacterial infection associated with the disease, particularly in immunocompromised subjects, since 15% prevalence of secondary bacterial infection were found among hospitalised subjects. 30
5. CONCLUSION
The repurposing nitazoxanide, doxycycline and azithromycin are highly effective for the symptomatic improvement of mild and moderate COVID‐19 subjects. Dual therapy by combining nitazoxanide with azithromycin or doxycycline is considered to be better than monotherapy. The use of azithromycin alone is not recommended, as it was not effective against symptoms compared with the other regimens used.
The clinical and computational studies applied on three FDA‐approved antimicrobials together with their recent clinical findings revealed that both nitazoxanide and doxycycline have great therapeutic potential against COVID‐19. The future in vitro mechanistic investigation may confirm our primary computational outcomes, and in turn, these classes of compounds provide a promising starting point for further anti‐COVID‐19 therapeutics.
DISCLOSURE
No conflict to declare.
AUTHOR CONTRIBUTIONS
(1) Conception and design: Marwa O. Elgendy. (2) Administrative support: All authors. (3) Provision of study materials or subjects: Ahmed M Khalaf, Marwa O. Elgendy. (4) Collection and assembly of data: All authors. (5) Data analysis and interpretation: Ahmed M. Sayed. (6) Manuscript writing: All authors. (7) Final approval of manuscript: All authors.
Sayed AM, Khalaf AM, Abdelrahim MEA, Elgendy MO. Repurposing of some anti‐infective drugs for COVID‐19 treatment: A surveillance study supported by an in silico investigation. Int J Clin Pract.2021;75:e13877. 10.1111/ijcp.13877
DATA AVAILABILITY STATEMENT
The datasets analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;323:1824‐1836. [DOI] [PubMed] [Google Scholar]
- 2. Martins‐Filho PR, Barreto‐Alves JA, Fakhouri R. Potential role for nitazoxanide in treating SARS‐CoV‐2 infection. Am J Physiol‐Lung Cell Mol Physiol. 2020;319:L35‐L36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashiru O, Howe JD, Butters TD. Nitazoxanide, an antiviral thiazolide, depletes ATP‐sensitive intracellular Ca2+ stores. Virology. 2014;462:135‐148. [DOI] [PubMed] [Google Scholar]
- 4. Hong SK, Kim HJ, Song CS, Choi IS, Lee JB, Park SY. Nitazoxanide suppresses IL‐6 production in LPS‐stimulated mouse macrophages and TG‐injected mice. Int Immunopharmacol. 2012;13:23‐27. [DOI] [PubMed] [Google Scholar]
- 5. Malek AE, Granwehr B, Kontoyiannis DP. Doxycycline as a Potential Partner of COVID‐19 Therapies. Elsevier; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schögler A, Kopf BS, Edwards MR, et al. Novel antiviral properties of azithromycin in cystic fibrosis airway epithelial cells. Eur Respir J. 2015;45:428‐439. [DOI] [PubMed] [Google Scholar]
- 7. Conforti C, Giuffrida R, Zalaudek I, Di Meo N. Doxycycline, a widely used antibiotic in dermatology with a possible anti‐inflammatory action against IL‐6 in COVID‐19 outbreak. Dermatol Ther. 2020;33:e13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sodhi M, Etminan M. Therapeutic potential for tetracyclines in the treatment of COVID‐19. Pharmacotherapy. 2020;40:487‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lill MA, Danielson ML. Computer‐aided drug design platform using PyMOL. J Comput Aided Mol Des. 2011;25:13‐19. [DOI] [PubMed] [Google Scholar]
- 11. Phillips JC, Braun R, Wang W, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781‐1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. MacKerell AD Jr, Bashford D, Bellott ML, et al. All‐atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B. 1998;102:3586‐3616. [DOI] [PubMed] [Google Scholar]
- 13. Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33‐38. [DOI] [PubMed] [Google Scholar]
- 14. Jo S, Kim T, Iyer VG, Im W. CHARMM‐GUI: a web‐based graphical user interface for CHARMM. J Comput Chem. 2008;29:1859‐1865. [DOI] [PubMed] [Google Scholar]
- 15. Jo S, Jiang W, Lee HS, Roux B, Im W. CHARMM‐GUI Ligand Binder for Absolute Binding Free Energy Calculations and Its Application. Chicago, IL: ACS Publications; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michalska K, Kim Y, Jedrzejczak R, et al. Crystal structures of SARS‐CoV‐2 ADP‐ribose phosphatase: from the apo form to ligand complexes. IUCrJ. 2020;7:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Claverie J‐M. A putative role of de‐Mono‐ADP‐ribosylation of STAT1 by the SARS‐CoV‐2 nsp3 protein in the cytokine storm syndrome of COVID‐19. Viruses. 2020;12:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gil C, Ginex T, Maestro I, et al. COVID‐19: Drug targets and potential treatments. J Med Chem. 2020;63:12359–12386. [DOI] [PubMed] [Google Scholar]
- 19. Ceribelli A, Motta F, De Santis M, et al. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J Autoimmun. 2020;102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019‐nCoV acute respiratory disease. Lancet (London, England). 2020;395:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lammers T, Sofias AM, van der Meel R, et al. Dexamethasone nanomedicines for COVID‐19. Nat Nanotechnol. 2020;15:622‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osama El‐Gendy A, Saeed H, Ali AMA, et al. Bacillus Calmette‐Guérin vaccine, antimalarial, age and gender relation to COVID‐19 spread and mortality. Vaccine. 2020;38:5564‐5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arshad S, Kilgore P, Chaudhry ZS, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID‐19. Int J Infect Dis. 2020;97:396‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sultana J, Cutroneo PM, Crisafulli S, Puglisi G, Caramori G, Trifirò G. Azithromycin in COVID‐19 patients: pharmacological mechanism, clinical evidence and prescribing guidelines. Drug Saf. 2020;43:691‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alam MT, Murshed R, Bhiuyan E, Saber S, Alam RF, Robin RC. A case series of 100 COVID‐19 positive patients treated with combination of Ivermectin and doxycycline. J Bangladesh Coll Phys Surg. 2020;38:10‐15. [Google Scholar]
- 26. Wasuna A. Repositioning Fusidic Acid for Tuberculosis: Semi‐Synthesis of Analogues and Impact of Mycobacterial Biotransformation on Antibiotic Activity. Cape Town: University of Cape Town; 2018. [Google Scholar]
- 27. Rossignol J‐F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health. 2016;9:227‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haffizulla J, Hartman A, Hoppers M, et al. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double‐blind, randomised, placebo‐controlled, phase 2b/3 trial. Lancet Infect Dis. 2014;14:609‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelleni M.Nitazoxanide/Azithromycin combination for COVID‐19: A suggested new protocol for COVID‐19 early management; 2020. [DOI] [PMC free article] [PubMed]
- 30. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.


