Abstract
This retrospective matched cohort study describes 30 solid organ transplant (SOT) patients with Coronavirus Disease 2019 (COVID‐19) matched 1:2 to 60 non‐SOT patients (control group) based on age, body mass index (BMI), and comorbidities (hypertension and diabetes mellitus with hemoglobin A1c > 8.0%). The SOT group had a higher proportion of cardiovascular disease (P < .05). During the index hospitalization, there were no significant differences with regard to disease severity or critical care needs (mechanical intubation, vasopressors, and renal replacement therapy). At 28 days, 4 (13%) patients died in the SOT group and 8 (13%) patients died in the control group (P = 1.0). Nineteen patients received tocilizumab in the SOT group compared to 29 patients in the control group. Among these patients, interleukin‐6 (IL‐6) and soluble interleukin‐2 receptor (sIL2R) levels increased after tocilizumab and interleukin‐10 (IL‐10) levels decreased after tocilizumab. Overall, SOT patients had comparable mortality to non‐SOT patients, although numerically more SOT patients received tocilizumab (63% vs 48%) and steroids (37% vs 20%). Larger, multi‐center studies are needed to ascertain these findings. Lastly, the complex cytokine release syndrome in COVID‐19 remains an area of intense research and the analysis of key interleukin levels (IL‐6, IL‐10, and sIL2R) in this study contributes to the understanding of this process.
Keywords: COVID‐19, cytokines, outcomes, solid organ transplant, tocilizumab
1. INTRODUCTION
Owing to their immunosuppressed state, solid organ transplant (SOT) recipients are feared to be at increased risk for worse outcomes with Coronavirus Disease 2019 (COVID‐19). Such concern was raised due to prior experience with other respiratory viruses such as influenza which was associated with increased mortality among immunosuppressed hosts. 1 In the early stages of the pandemic, several studies suggested mortality rate was higher among SOT patients compared to the general population. 2 , 4 , 5 However, more recent studies, including a large multicenter SOT cohort study reported a mortality of 20.5%, similar to that of the general population. 6 SOT recipients are more likely to have comorbidities associated with poor outcomes in COVID‐19 including hypertension, chronic kidney disease, and heart disease. In addition, correlates of increased immunosuppression, such as lower absolute lymphocyte counts, have been predictive of mortality. 6 , 7 However, it was also speculated that their immunosuppression could be protective resulting in a blunted immune response and subsequently impeding progression of cytokine release syndrome (CRS) that can occur in COVID‐19. 8
The CRS in severe COVID‐19 is clinically characterized by acute respiratory distress syndrome (ARDS), shock, and multisystem organ failure. 9 In addition to antivirals, management of this disease has focused on immunomodulators to temper CRS. Interleukin‐6 (IL‐6), secreted by monocytes and macrophages, was identified as a key driver of this immunologic response. 9 Therefore, IL‐6 inhibition is theorized to be a preferred mode of therapy over steroids because it spares the T‐cell response while preserving the adaptive immune response triggered by inflammation. 10 In the early stages of the pandemic, several immunomodulators, including IL‐6 inhibitors, were used with varying success in treatment of severe COVID‐19. 11 , 12 A recent randomized controlled trial found that tocilizumab administered to hospitalized patients with partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio of 200‐300 did not reduce the risk of requiring Intensive Care Unit (ICU) admission, mechanical ventilation, death, or progression to ARDS. 13 On the other hand, interim results from a clinical trial using tocilizumab showed that patients on tocilizumab were 44% less likely to progress to mechanical ventilation or death compared to placebo. 14 It remains to be seen if this therapy has further impact on disease progression in different stages of COVID‐19. It is worth noting that in a randomized controlled trial, dexamethasone demonstrated a survival benefit in severe COVID‐19. 15 To date, there is no consensus regarding the adjustment of immunosuppressive agents for SOT patients with COVID‐19, but observational studies have suggested that reducing immunosuppression did not lead to worse outcomes. 6 In our center, the antimetabolite, mycophenolate mofetil (MMF), was preferentially discontinued upon diagnosis of COVID‐19.
In this study, we evaluate the mortality of SOT patients in contrast to non‐SOT patients with COVID‐19 through a retrospective, matched cohort study. We also attempt to characterize cytokine signatures based on disease severity and exposure to the IL‐6 inhibitor, tocilizumab, in order to gain a better understanding of how immunosuppression impacts CRS.
2. METHODS
2.1. Study Design
We conducted a retrospective medical chart review of adult patients diagnosed with COVID‐19 between March 10, 2020 and May 11, 2020 at Yale New Haven Health. This study was approved by the Yale University Human Investigation Committee (#2000027876).
We included hospitalized adult patients age ≥18 years with laboratory‐confirmed COVID‐19 infection by polymerase chain reaction of nasopharyngeal swab or other respiratory samples, COVID‐19 diagnosis within 72 hours of hospital admission, and a cytokine panel drawn within 72 hours of admission. SOT patient had to be on active immunosuppression at time of COVID‐19 diagnosis; hence, excluding those with failed graft and those off immunosuppression. SOT patients were matched to a control group of non‐SOT patients by age (±10 years), body mass index (BMI) ≥ 30, hypertension, and diabetes mellitus with hemoglobin A1c > 8.0%. The control group was selected from a large database of hospitalized non‐SOT patients in a 1:2 ratio. Two patients were randomly chosen if they fit the match criteria. Immunosuppression was an exclusion criterion for the control group. The last three variables were selected because of correlation with COVID‐19 disease severity. 16 Several patients were included in a previously reported multicenter SOT cohort, 6 but without the case‐control and cytokine‐specific analyses described here.
The data extracted from electronic medical chart were as follows: demographics, co‐morbidities, calculated body mass index, symptom onset of COVID‐19, C‐reactive protein (CRP), D‐dimer, absolute lymphocyte count (ALC), procalcitonin, lactate dehydrogenase (LDH), ferritin, critical care need (ie, required mechanical ventilation, vasopressor, renal replacement therapy), co‐infections or superinfection, receipt of COVID‐19‐related therapeutic agents (tocilizumab, corticosteroid, ie, prednisone equivalent of ≥20 mg/d, hydroxychloroquine, azithromycin, atazanavir, and convalescent plasma), maintenance of immunosuppression, changes to immunosuppression in response to COVID‐19, cytokine panels [including IL‐6, interleukin‐10 (IL‐10), and soluble interleukin‐2 receptor (sIL2R)] within 28 days from admission, duration of hospitalization, discharge disposition, and status (alive/dead) on last follow‐up date.
2.2. Ascertainment of Co‐infection and Superinfection
We also evaluated for any co‐infection or superinfection with COVID‐19. All infection events were reviewed by infectious disease physicians. Of note, no preemptive monitoring or screening for infections in the cohort was implemented during the study period. Infections were categorized per consensus definitions of the Centers for Disease Control and Prevention's National Healthcare Safety Network (NHSN) 17 with the exception of pneumonia. Because of a similar clinical presentation of COVID‐19 and bacterial pneumonia, a modified composite score for pneumonia based on NHSN definitions was calculated, which also included worsening respiratory parameters (increased oxygen requirements, worsening chest radiograph, worsening secretions), inflammatory parameters (fever, white blood cell (WBC) count, procalcitonin), microbiologic parameters (positive sputum culture or positive bacterial antigen test) within 48 hours of initiating antibiotic therapy for a suspected infection, and procalcitonin ≥2.5 ng/mL (with the exception of patients with chronic kidney disease or requiring renal replacement therapy; Supporting Information S1). Timing of onset of infection relative to tocilizumab was determined and designated as either pre‐tocilizumab if it occurred prior to or within 48 hours of tocilizumab administration, or post‐tocilizumab if it occurred ≥48 hours after tocilizumab administration.
2.3. Therapeutic Approach
At Yale New Haven Health, a treatment algorithm was implemented to standardize the treatment approach to hospitalized patients with COVID‐19. Tocilizumab was used off‐label for patients who met criteria for severe disease. This evolved to include patients with either a minimum of 3 L/min oxygen supplementation or CRP > 70 mg/L and requiring minimum of 2 L/min oxygen supplementation. Patients admitted with moderate disease which evolved into severe or critical also qualified for tocilizumab. Tocilizumab was administered 8 mg/kg, not to exceed 800 mg, intravenously in a single dose. We defined steroid use as COVID‐19 treatment if they received ≥20 mg prednisone equivalent per day. Of note, the treatment algorithm was frequently updated and antiviral therapy recommendations were modified since the onset of the pandemic. Hydroxychloroquine and protease inhibitors were potential treatment options in the early months of the pandemic but were subsequently removed because of published randomized controlled trials (Supporting Information S2). As previously mentioned, MMF was preferentially discontinued upon diagnosis of COVID‐19 in any solid organ transplant recipient.
2.4. Analytic Approach
Clinical characteristics were compared between SOT and non‐SOT control groups. The primary outcome measured was mortality as of June 26, 2020, which was determined per last documented contact with patient in the chart. Secondary outcomes included requirement of critical care (ie, mechanical ventilation, vasopressors, and renal replacement therapy) and disease severity. Disease severity was defined as maximum oxygen requirement during their hospitalization. The disease severity classification was based on the treatment algorithm used at our institution. Moderate disease was defined as the need for <3 L/min of supplemental oxygen by nasal cannula. Severe disease was defined as the need for 4‐6 L/min of supplemental oxygen by nasal cannula or use of a non‐rebreather mask. Critical disease was defined as the need for high‐flow nasal cannula, non‐invasive positive pressure ventilation, and/or mechanical ventilation.
Patient characteristics between cases and controls were compared using sample t‐test for continuous variables and Chi‐square test for categorical variables. Survival analysis was done with log‐rank P‐value reported between SOT and non‐SOT group using R's survival package (version 3.1‐8).
For cytokine‐level comparisons across stratified groups, to address the longitudinal nature of the data, we regarded multiple observations from each individual as separate data points. All entries with value of ‘<5’ were replaced with a value of 2.5 for analysis purposes. Cytokine levels between groups were compared using Welch's t‐test.
Given the data were recorded using 0.5‐day intervals, to standardize time, we assumed each patient was admitted at 0:00am on the day of admission, the cytokine test was conducted at 6:00 AM for the first half day, and 6:00 PM for the second half day. All of the above data cleaning, processing, and date standardization were done using R's Tidyverse packages collection (version 1.3.0). For the longitudinal cytokine level analysis, all data points were further realigned using date of symptom onset as day 0, and group mean was obtained as the average of all samples with non‐missing data at a certain time.
3. RESULTS
A total of 33 SOT patients were hospitalized with COVID‐19 during the study period. Three patients were excluded: one was diagnosed with COVID‐19 >72 hours after admission, one did not have a cytokine panel collected, and one was a kidney transplant patient with failed graft and who was off immunosuppression for >10 years. Ultimately, 30 SOT were included in the analysis. A 1:2 case‐control match was undertaken, yielding 60 control patients.
The clinical characteristics of 30 SOT patients and the 60 control patients are described in Table 1. Males represented the majority in both groups (53% and 60% in SOT and control groups, respectively). African Americans comprised 43% of the SOT group and 47% of the control group. Chronic cardiac disease (P ≤ .05) was more prevalent in the SOT group than the control group. CRP, ferritin, D‐dimer, ALC, procalcitonin, and LDH were not different between groups. Rates of co‐infections were not different between groups. With regard to treatment, there was a similar frequency of tocilizumab (63% vs 48%) and steroid use (37% vs 20%) between groups, although immunomodulatory therapy was numerically more common in the SOT group.
TABLE 1.
Patient characteristics in solid organ transplant (SOT) patients and control (non‐SOT) patients
| Patient characteristics | SOT (n = 30) | Control (n = 60) | P‐value |
|---|---|---|---|
| Age—median (range) | 60 (29‐78) | 60.5 (23‐83) | .882 |
| Gender—no (%) | .546 | ||
| Male | 16 (53%) | 36 (60%) | |
| Female | 14 (47%) | 24 (40%) | |
| Race—no (%) | |||
| African American | 13 (43%) | 28 (47%) | .765 |
| White | 10 (33%) | 25 (42%) | .445 |
| Asian | 0 | 1 (2%) | .477 |
| American Indian or Alaska Native | 1 (3%) | 0 | .155 |
| Unknown | 6 (20%) | 6 (10%) | .188 |
| Ethnicity—no (%) | |||
| Non‐Hispanic | 24 (80%) | 47 (78%) | .855 |
| Hispanic | 6 (20%) | 12 (20%) | 1.0 |
| Unknown | 0 | 1 (2%) | .477 |
| BMI—median (range) | 28.0 (21.3‐40.9) | 29.1 (17.9‐51.4) | .686 |
| Length from symptom onset to admission in days—median (range) | 3 (0‐16) | 3 (0‐14) | .086 |
| Comorbidities—no (%) | |||
| Chronic heart disease | 18 (60%) | 21 (35%) | .024 |
| Chronic lung disease | 10 (33%) | 21 (35%) | .875 |
| Diabetes Mellitus with A1c > 8.0% | 12 (40%) | 24(40%) | 1.0 |
| Hypertension | 28 (93%) | 56 (93%) | 1.0 |
| Human Immunodeficiency Virus (HIV) | 1 (3%) | 2 (3%) | 1.0 |
| Laboratory data—median (range) | |||
| Highest CRP (mg/L) | 140.8(3.1‐300) | 108.3 (1.7‐300) 1 | .125 |
| Highest D‐dimer (mg/L) | 2.4 (0.29‐33.89) | 1.6 (0.33‐33.89) 2 | .191 |
| Lowest ALC (1000 cells/μL) | 0.5 (0.1‐1.6) | 0.9 (0.3‐2.2) | 2.46 |
| Highest Procalcitonin (ng/mL) | 0.5 (0.1‐90.5) | 0.3 (0‐54.2) 3 | .390 |
| Highest LDH (U/L) | 413.5 (189‐9,350) 4 | 365 (165‐1,531) 5 | .269 |
| Highest Ferritin (ng/mL) | 1,816 (168‐13,127) | 870.5 (17‐24,118) 6 | .241 |
| Treatment—no (%) | |||
| Tocilizumab | 19 (63%) | 29 (48%) | .179 |
| Steroids b | 11 (37%) | 12 (20%) | .088 |
| Hydroxychloroquine | 28 (93%) | 47 (78%) | .072 |
| Azithromycin | 3 (10%) | 6 (10%) | 1.0 |
| Atazanavir | 7 (23%) | 20 (33%) | .329 |
| Remdesivir | 0 | 3 (5%) | .213 |
| Convalescent Plasma | 1 (3%) | 0 | .155 |
| Severity—no (%) a | |||
| Moderate | 14 (47%) | 33 (55%) | .456 |
| Severe | 6 (20%) | 13 (22%) | .855 |
| Critical | 10 (33%) | 14 (23%) | .312 |
| Critical Care Needs—no (%) | 9 (30%) | 13 (22%) | .386 |
| Mechanical ventilation | 8 (27%) | 12 (20%) | .473 |
| Vasopressors | 8 (27%) | 13 (22%) | .597 |
| New renal replacement therapy | 2 (7%) | 3 (5%) | .745 |
| Co‐infection or superinfection—no (%) | 6 (20%) | 14 (23%) | .720 |
| No tocilizumab | 0 | 4 (7%) | .148 |
| Tocilizumab | 5 (17%) | 10 (17%) | 1.0 |
| Pre‐tocilizumab | 2 (7%) | 7 (12%) | .456 |
| Post‐tocilizumab | 3 (10%) | 5 (8%) | .793 |
| Duration of hospital stay in days—median (range) | 12 (3‐75) | 9 (2‐50) | .047 |
| Location at 28 d—no (%) | |||
| Deceased | 4 (13%) | 8 (13%) | 1.0 |
| Home | 16 (53%) | 42 (70%) | .119 |
| Facility (extended care facility or subacute rehabilitation facility) | 4 (13%) | 6 (10%) | .635 |
| Re‐admission | 2 (7%) | 1 (2%) | .213 |
| Remains Inpatient | 4 (13%) | 3 (5%) | .164 |
| Total follow‐up days after COVID‐19 diagnosis—median (range) | 66 (7‐100) | 48 (2‐101) | .078 |
| Death at last known follow up—no (%) | 4 (13%) | 8 (13%) | 1.0 |
| Death during index hospitalization related to COVID‐19 infection | 4 (100%) | 8 (100%) | |
Severity: Outcome defined as maximum oxygen requirement during hospitalization. Moderate disease was defined as the need for 0‐3 L/min of supplemental oxygen by nasal cannula. Severe disease was defined as the need for 4‐6 L/min of supplemental oxygen by nasal cannula or use of a non‐rebreather mask. Critical disease was defined as the need for high‐flow nasal cannula, non‐invasive positive pressure ventilation, and/or mechanical ventilation.
Steroids: ≥20 mg prednisone equivalent dose. This does not include maintenance immunosuppression.
Available in 56 patients.
Available in 58 patients.
Available in 58 patients.
Available in 24 patients.
Available in 45 patients.
Available in 58 patients.
Bold value indicates clinical significance (P value < .05).
There was no difference in critical care needs. In the SOT group, 8 patients required mechanical ventilation, compared to 12 in the non‐SOT group (P = .473). The median length of stay was 12 days in the SOT group and 9 days in the non‐SOT group (P = .047). At 28‐day follow‐up, 4 patients (13%) died in the SOT group versus 8 patients (13%) in the non‐SOT group (P = 1.0). Survival analyses of the 2 groups at the conclusion of this study on June 26, 2020 are illustrated in Figure 1.
FIGURE 1.
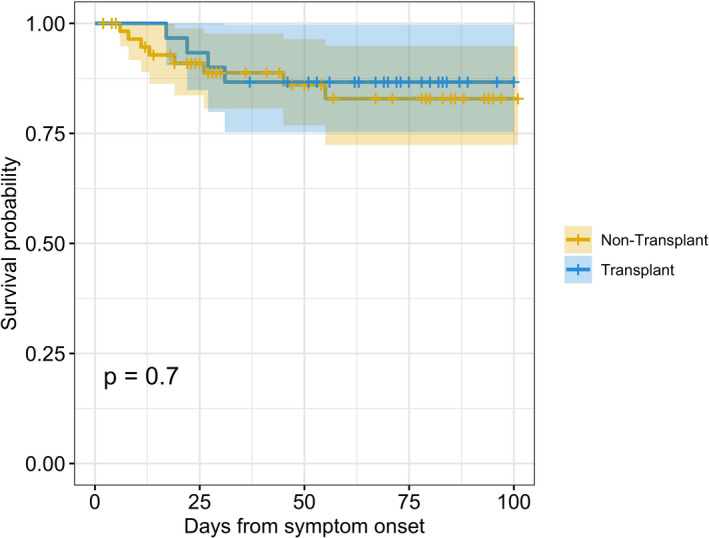
Kaplan Meier Survival Curve comparing SOT versus non‐SOT
Transplant‐specific characteristics are described in Table 2. Of the 30 SOT patients, 87% had received kidney transplant. The median time from transplant to COVID‐19 diagnosis was 4.2 years (range: 0.1‐24.3 years). With regard to maintenance immunosuppression, 25 (83%) patients were on prednisone <5 mg daily, 21 (70%) were on tacrolimus daily, and 19 (63%) were on MMF. With the exception of 2 SOT patients, MMF was held on admission (n = 17/19, 89%). Prednisone and tacrolimus were continued throughout admission for all patients.
TABLE 2.
Characteristics of 30 solid organ transplant recipients
| Transplant characteristics | Total SOT recipients (N = 30) |
|---|---|
| Type of SOT, no. (%) | |
| Kidney | 26 (87%) |
| Liver | 3 (10%) |
| Heart | 1 (3%) |
| Time from transplantation to COVID‐19 diagnosis in years—median (range) | 4.2 (0.1‐24.3) |
| Maintenance Immunosuppression—no. (%) | |
| Prednisone < 5 mg daily | 25 (83%) |
| Tacrolimus | 21 (70%) |
| Mycophenolate mofetil (MMF) | 19 (63%) |
| Belatacept | 6 (20%) |
| Azathioprine | 2 (7%) |
| Cyclosporine | 1 (3%) |
| Change to Immunosuppression on admission—no. (%) | |
| Discontinued MMF among SOT on MMF | 17 (89%) |
| Continued immunosuppression | 14 (45%) |
3.1. Cytokines in SOT
The IL‐6, IL‐10, and sIL2R levels were examined in the SOT group. Figure 2 describes mean cytokine levels over time from day of symptom onset stratified by severity of disease. IL‐6 and sIL2R remained elevated throughout hospitalization. IL‐10 remained low throughout the hospitalization.
FIGURE 2.
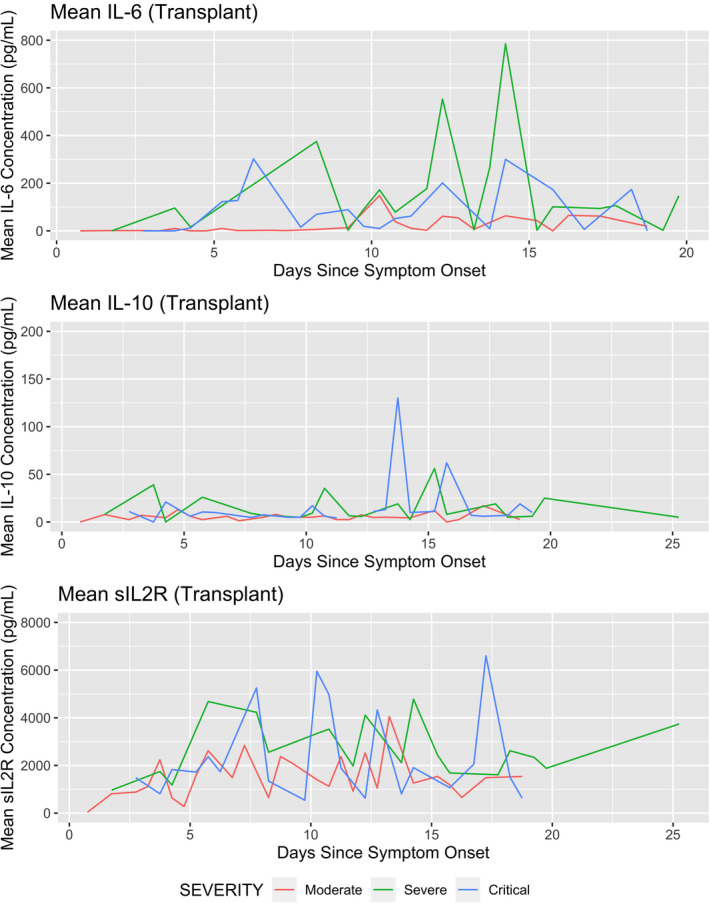
The trends of mean IL‐6, IL‐10, and sIL2R during hospitalization stratified according to the severity of COVID‐19 disease
Figure 3 and Table 3 present cytokine values in SOT patients who received tocilizumab stratified by pre‐ and post‐tocilizumab as well as disease severity. IL‐6 increased significantly in all severity groups after tocilizumab. Median IL‐10 decreased after tocilizumab in both severe and critical groups. This decrease, however, was statistically significant only in the severe group (P = .044). The IL‐10 in the moderate group significantly increased post tocilizumab (P = .002). sIL2R increased significantly after tocilizumab in the moderate and critical group (P < .001 and P = .025, respectively). In contrast, the sIL2R in severe group decreased after tocilizumab but change from pre‐ to post‐tocilizumab was not statistically significant (P = .052).
FIGURE 3.
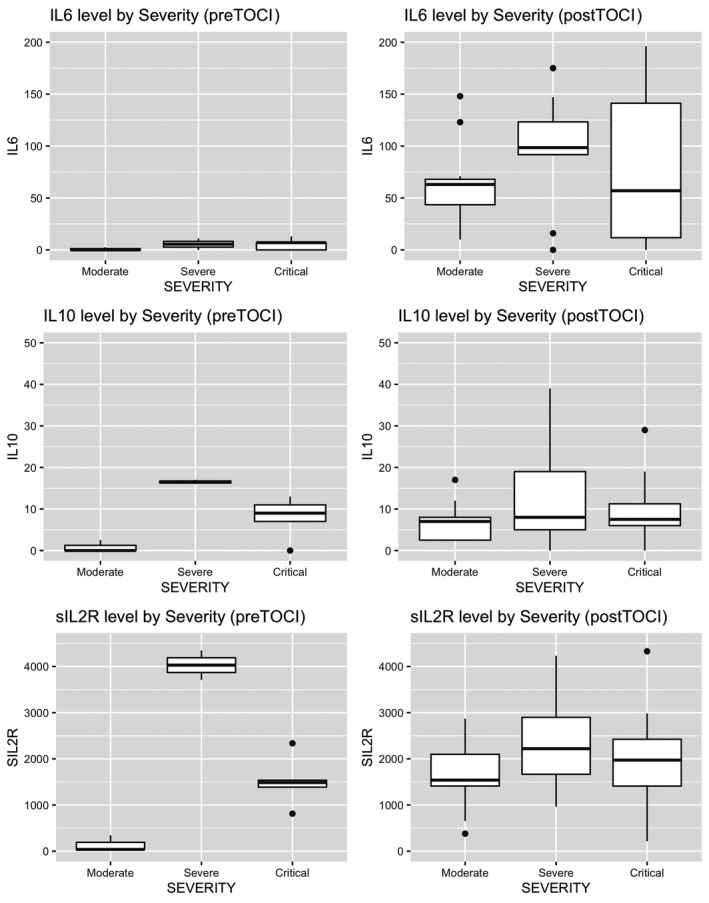
Box plots of IL‐6, IL‐10 and sIL2R pre and post tocilizumab in SOT recipients stratified by COVID‐19 severity. (TOCI, tocilizumab)
TABLE 3.
Cytokine analysis pre‐ and post‐tocilizumab in SOT patients stratified by severity of disease (median, IQR)
| CYTOKINES | All (N = 19) | Moderate (n = 5) | Severe (n = 5) | Critical (n = 9) | |
|---|---|---|---|---|---|
| IL‐6 (pg/mL) | Pre | 2.5 (0, 8.5) | 0 (0, 1.25) | 0 ( 0, 5.5) | 7 (7, 10) |
| Post | 96 (53, 186) | 63 (43.5, 68) | 161 ( 99.8, 286) | 133 (24, 196) | |
| P‐value | <.001 | <.001 | .002 | <.001 | |
| IL‐10 (pg/mL) | Pre | 9 (4.75, 14.5) | 0 (0, 1.25) | 16 (12, 16.5) | 11 (9, 13) |
| Post | 7 (5, 11.2) | 7 (2.5, 8) | 7.5 (5, 19) | 7 (6, 11) | |
| P‐value | .659 | .002 | .044 | .591 | |
| sIL2R (pg/mL) | Pre | 1486 (654, 3024) | 38 (36.5, 192) | 3710 (2336, 4030) | 1537 (1486, 2337) |
| Post | 2117 (1537, 2982) | 1631 (1427, 2270) | 2586 (1777, 3928) | 2116 (1692, 3656) | |
| P‐value | .103 | <.001 | .052 | .025 | |
4. DISCUSSION
We describe a case series of 30 SOT patients diagnosed with COVID‐19 in which mortality was similar to that of 60 matched non‐SOT controls. These data are consistent with previously published literature suggesting that transplant status is not associated with increased mortality and poor clinical outcomes in the setting of COVID‐19. 6 , 18 , 19 , 20 Further corroborating these findings, a recent multicenter study by Molnar, et al matched 98 SOT patients to 288 non‐SOT patients in which mortality and critical care requirements were similar between groups. 19 Similarly, Chaudhry, et al found that despite more co‐morbidities, SOT patients had similar mortality compared to non‐SOT controls. 21 Given the changing dynamics of COVID‐19 management, the varying standards of care and patient demographics at different medical centers, comparing results from different studies is fraught with difficulties. In order to better assess for the differences between SOT and non‐SOT populations in this single‐center study, we employed a case‐control design based on parameters including age, BMI, hypertension, and diabetes mellitus (A1c > 8.0%), which are associated with increased mortality. 7
In comparison with non‐SOT patients in our study, SOT patients had an increased prevalence of chronic cardiac disease, a finding that is not unexpected in SOT recipients and which may have resulted from their other pre‐existing comorbidities. SOT patients also had longer length of hospital stay. One possible explanation is the higher degree of medical care provided to SOT patients as seen in other non–COVID‐related hospitalizations. 22 , 23 Another possibility is that the SOT patients in our study had a higher proportion of critical disease and ICU needs (although not found statistically significant).
Despite the immunosuppression, the survival rate among SOT was not different from non‐SOT patients. Although reasons for this are unclear, one possibility is a protective effect of immunosuppression from the deleterious effects of CRS, the development of which can be monitored using specific biomarkers such as CRP and IL‐6, as previously reported in general population. 24 Similarly, CRP has been found to be a useful marker of disease severity in SOT recipients with COVID‐19. 25 Interestingly, in our study, SOT patients had a higher, although not statistically significant, median CRP level compared with the non‐SOT group despite the presence of immunosuppression. Whether this indicates that iatrogenic immunosuppression stymied the progression of the inflammatory cascade into full‐blown CRS is unclear, but preliminary data do not suggest that increased CRP in SOT patients is associated with worse outcomes.
In addition to CRP, other markers may be useful to gauge CRS. IL‐6, IL‐10, and sIL2R were previously described as key cytokines that drive COVID‐19 inflammation. 26 , 27 IL‐6, a pro‐inflammatory cytokine with a pleiotropic effect on the immune system, was discovered early on to be elevated in affected patients, which heralded the trial of tocilizumab as potential therapy. 26 , 28 A recent randomized controlled trial in the general population found that tocilizumab in select patients with PaO2/FiO2 of 200‐300 did not decrease the risk of clinical progression. 13 In a recent matched case‐control focused on only SOT patients by Pereira, et al, 29 tocilizumab did not have an impact on mortality, hospital discharge, or secondary infections in SOT patients with severe COVID‐19. However, there was trend toward lower number of deaths in the non‐intubated versus intubated SOT patients who received tocilizumab, although not statistically significant. Given the increased number of patients who received tocilizumab and steroids in the SOT group, it is possible that this contributed to favorable outcomes. Further studies by means of randomized control trials are needed to verify therapeutic role of tocilizumab and steroids in SOT with COVID‐19. We speculate that timing of immunomodulatory therapy in the disease course is critical and influences clinical outcomes.
Pre‐tocilizumab, SOT patients with critical disease have higher IL‐6 compared to those with moderate and severe disease. An increase after IL‐6 tocilizumab administration was observed, an occurrence which has been previously described in the literature; upon encountering tocilizumab, IL‐6 receptors become saturated, leading to an increase in free IL‐6. 30 Given this finding, our study suggests that using post‐tocilizumab IL‐6 as a marker of severity of disease in SOT patients might not be useful, but further studies are needed.
IL‐10 has not been previously described as a marker in SOT with COVID‐19; however, it has been evaluated in SOT patients with influenza. A study demonstrated that SOT patients had lower baseline levels of IL‐10 and less robust increases compared to non‐SOT in response to influenza. 31 Our findings are similar in that SOT patients had overall low levels of IL‐10. One possible explanation is that SOT patients have less overall inflammation because of their immunosuppression, and therefore do not require robust anti‐inflammatory action via upregulation of IL‐10. The increase in IL‐10 levels in SOT patients with moderate disease post tocilizumab may be a signal addressing the balance between hyperactive immune response and immunosuppression that merits further investigation.
sIL2R, a marker for T‐cell activation and proliferation, is seldom seen outside of hematologic malignancies or macrophage activation syndrome (MAS)/hemophagocytic lymphohistiocytosis (HLH), making the elevated levels seen in COVID‐19 hyperinflammation worth further investigation. 32 In SOT patients, sIL2R has been most closely studied in the monitoring of organ rejection and infection. In a study by Mehta, et al, kidney transplant patients were found to have baseline sIL2R levels that were almost twice that of healthy volunteers (698 U/mL vs 349 U/mL). 33 This finding is reproducible in our population, as overall sIL2R levels were elevated. Additionally, we see in our cohort that sIL2R levels continue to rise after administration of tocilizumab. 34 Based on our findings, it seems that tocilizumab has little effect on reducing T‐cell activation.
Given the uncertainty of how immunosuppression impacts outcomes, it remains the standard of care to decrease immunosuppression in the setting of acute infection. In this study, despite reduction in immunosuppression in most SOT patients, we did not observe a negative impact on mortality and clinical outcomes. This is consistent with other studies that favor decreasing immunosuppression at time of diagnosis. 6 Appropriate immunosuppression management for SOT patients with COVID‐19 will require further analysis in prospective studies.
An interesting finding from our study is that tocilizumab, an immunosuppressive agent, in SOT patients was not associated with an increased risk of infection. This is in contrast to a single‐center cohort study from Michigan in which patients who received tocilizumab had higher rates of superinfection despite improved overall survival. 35 In our study, co‐infections after tocilizumab exposure occurred at comparable rates in both SOT and non‐SOT groups. It is unclear whether these infections were directly caused by tocilizumab or associated with prolonged hospitalization and critical illness.
Our study has several limitations, primarily stemming from its retrospective design. Despite the healthcare system's protocol on therapeutics and diagnostic testing, certain laboratory parameters were not present at specific time points for certain patients. Another limitation is that 10 of 19 patients who received tocilizumab also received steroids, which likely impact the cytokine analysis. Additionally, SOT patients are generally viewed as higher risk hosts, which potentially may lead to provider bias, with earlier infectious disease and transplant specialist consultation and more aggressive treatment. We also only focused on hospitalized patients in a single center, so these results might not be generalizable to outpatients and to patient populations that differ in their demographics.
In conclusion, this study suggested that SOT patients had comparable mortality rates to non‐SOT patients but larger and randomized controlled trials are needed to ascertain these findings. Lastly, the CRS remains an area of intense research and the analysis of key interleukin levels (IL‐6, IL‐10, and sIL2R) in this study contributes to the understanding of this process and discovery of future potential therapeutics.
CONFLICT OF INTEREST
The authors have no financial disclosures related to this study.
AUTHOR CONTRIBUTION
MR—Review of Literature, Manuscript Draft, Data Collection. VA and KK—Data Collection, Critical Review of Manuscript. DT—Statistical Analysis and Creation of Figures. HR—Data Collection. MA and CP—Conceptualization, Critical Review of Manuscript. MM—Conceptualization, Critical Review, and Edit of Manuscript.
Supporting information
Supplementary Material
Ringer M, Azmy V, Kaman K, et al. A retrospective matched cohort single‐center study evaluating outcomes of COVID‐19 and the impact of immunomodulation on COVID‐19‐related cytokine release syndrome in solid organ transplant recipients. Transpl Infect Dis. 2021;23:e13556. 10.1111/tid.13556
Veronica Azmy, Kelsey Kaman and Daiwei Tang contributed equally as second co‐authors.
Funding information
No funding for this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.
REFERENCES
- 1. Martin ST, Torabi MJ, Gabardi S. Influenza in solid organ transplant recipients. Ann Pharmacother. 2012;46(2):255‐264. [DOI] [PubMed] [Google Scholar]
- 2. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nacif LS, Zanini LY, Waisberg DR, et al. COVID‐19 in solid organ transplantation patients: a systematic review. Clinics. 2020;75:e1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fung M, Babik JM. COVID‐19 in immunocompromised hosts: what we know so far. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kates OS, Haydel BM, Florman SS, et al. COVID‐19 in solid organ transplant: a multi‐center cohort study. Clin Infect Dis. 2020;ciaa1097. [Google Scholar]
- 7. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID‐19. The Journal of infection. 2020;80(6):607‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fishman JA, Roberts MB, Zhang EW, Kumar D, Hirsch HH, Maggiore U. Case 29–2020: a 66‐year‐old man with fever and shortness of breath after liver transplantation. N Engl J Med. 2020;383(12):1168‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rojas‐Marte G, Khalid M, Mukhtar O, et al. Outcomes in patients with severe COVID‐19 disease treated with tocilizumab: a case–controlled study. QJM: Int J Med. 2020;113(8):546‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID‐19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID‐19 Pneumonia: a randomized clinical trial. JAMA Intern Med. 2020;181(1):24‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roche’s phase III EMPACTA study showed Actrema/RoActemra reduced the likelihood of needing mechanical ventilation in hospitalised patients with COVID‐19 associated pneumonia. https://www.roche.com/media/releases/med‐cor‐2020‐09‐18.htm.
- 15. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid‐19 ‐ preliminary report. N Engl J Med. 2021;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. CDC . People with Certain Medical Conditions. https://www.cdc.gov/coronavirus/2019‐ncov/need‐extra‐precautions/people‐with‐medical‐conditions.html
- 17. CDC/NHSN Surveillance Definitions for Specific Types of Infections. https://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf
- 18. Di Maira T, Berenguer M. COVID‐19 and liver transplantation. Nat Rev Gastroenterol Hepatol. 2020;17(9):526‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Molnar MZ, Bhalla A, Azhar A, et al. Outcomes of critically ill solid organ transplant patients with COVID‐19 in the United States. Am J Transplant. 2020;20(11):3061‐3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singhvi A, Barghash M, Lala A, et al. Challenges in heart transplantation during COVID‐19: a single center experience. J Heart Lung Transplant. 2020;39(9):894‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chaudhry ZS, Williams JD, Vahia A, et al. Clinical characteristics and outcomes of COVID‐19 in solid organ transplant recipients: a cohort study. Am J Transplant. 2020;20(11):3051–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Navale SM, Szubski CR, Klika AK, Schiltz NK, Desai PP, Barsoum WK. The impact of solid organ transplant history on inpatient complications, mortality, length of stay, and cost for primary total hip arthroplasty admissions in the United States. J Arthroplasty. 2017;32(4):1107‐1116.e1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malcolm TL, Chatha K, Breceda AP, et al. The impact of solid organ transplant history on inpatient complications, mortality, length of stay, and cost for primary total shoulder arthroplasty admissions in the United States. J Shoulder Elbow Surg. 2018;27(8):1429‐1436. [DOI] [PubMed] [Google Scholar]
- 24. Moore JB, June CH. Cytokine release syndrome in severe COVID‐19. Science. 2020;368(6490):473. [DOI] [PubMed] [Google Scholar]
- 25. Roberts MB, Izzy S, Tahir Z, Al Jarrah A, Fishman JA, El Khoury J. COVID‐19 in solid organ transplant recipients: dynamics of disease progression and inflammatory markers in ICU and non‐ICU admitted patients. Transpl Infect Dis. e13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Del Valle DM, Kim‐Schulze S, Huang H‐H, et al. An inflammatory cytokine signature predicts COVID‐19 severity and survival. Nat Med. 2020;26(10):1636‐1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Wang X, Li X, et al. Potential contribution of increased soluble IL‐2R to lymphopenia in COVID‐19 patients. Cell Mol Immunol. 2020;17(8):878‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pereira MR, Aversa MM, Farr MA, et al. Tocilizumab for severe COVID‐19 in solid organ transplant recipients: a matched case‐control study. Am J Transplant. 2020;20(11):3198‐3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin‐6 (IL‐6) and soluble IL‐6 receptor after administration of an anti–IL‐6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112(10):3959‐3964. [DOI] [PubMed] [Google Scholar]
- 31. L’Huillier AG, Ferreira VH, Hirzel C, et al. Cytokine profiles and severity of influenza infection in transplant recipients. J Infect Dis. 2019;219(4):535‐539. [DOI] [PubMed] [Google Scholar]
- 32. Kleynberg RL, Schiller GJ. Secondary hemophagocytic lymphohistiocytosis in adults: an update on diagnosis and therapy. Clin Adv Hematol Oncol. 2012;10(11):726‐732. [PubMed] [Google Scholar]
- 33. Mehta R, Shah G, Adler W, Kittur D. Soluble interleukin 2 receptor (sIL‐2R) levels in renal transplant recipients. Clin Transplant. 2004;18(Suppl 12):67‐71. [DOI] [PubMed] [Google Scholar]
- 34. Price CC, Altice FL, Shyr Y, et al. Tocilizumab treatment for cytokine release syndrome in hospitalized COVID‐19 patients: survival and clinical outcomes. CHEST. 2020;158(4):1397‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID‐19. medRxiv. 2005:2020.2005.2029.20117358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.


