Abstract
Objectives
Coronavirus‐19 is a rapidly progressing disease that can result in mortality. We aimed to evaluate the efficacy of the delta neutrophil index in predicting mortality in intensive care patients diagnosed with Coronavirus‐19.
Materials and methods
Patients with a positive polymerase chain reaction test and/or computed tomography findings compatible with the disease were included in the study. The demographic characteristics of the patients, polymerase chain reaction test results, chest computed tomography findings, blood parameters at the time of presentation, 30‐day mortality, and the number of days in the intensive care unit were assessed.
Results
Of the 388 patients receiving intensive care, 220 (56.7%) were men and 168 (43.3%) were women. The mean age was 70 ± 15 years. The evaluation of mortality, 264 (68%) of the patients survived and 124 (32%) died. The delta neutrophil index, neutrophil lymphocyte ratio, lactate, interleukin‐6 and C‐reactive protein values were statistically significantly higher and the lymphocyte value was significantly lower in the mortality group (P = .003, .034, .000, .002, .000 and .024, respectively). In the receiver operating characteristic curve analysis, the area under the curve values of the delta neutrophil index, lymphocyte, neutrophil lymphocyte ratio, lactate, interleukin‐6 and C‐reactive protein levels in predicting mortality were 0.718, 0.416, 0.628, 0.585, 0.701 and 0.684, respectively.
Conclusion
We consider that the delta neutrophil index can be used as an effective prognostic parameter to show intensive care mortality in patients with Coronavirus‐19.
1. INTRODUCTION
Coronavirus‐19 (COVID‐19) is a disease that progresses rapidly and can lead to severe morbidity and mortality. According to the data of the World Health Organization, 62 363 527 COVID‐19 cases and 1 456 687 (2.3%) deaths occurred during the pandemic until November 30, 2020. 1 For the diagnosis of COVID‐19, physical examination findings, haematological tests, polymerase chain reaction (PCR) test and/or chest computed tomography (CT) are utilised. 2 , 3 Currently, some biochemical, haematological and immune markers, such as leukocyte, lymphocyte, neutrophil/lymphocyte ratio (NLR), C‐reactive protein (CRP), lactate, platelet, red cell distribution width (RDW), procalcitonin and interleukin‐6 are used in the evaluation of disease progression. 4 , 5 , 6 The delta neutrophil index (DNI), a marker obtained by measuring the fraction of circulating immature granulocytes and increases in infection and inflammation states, 7 has become a frequently used marker for the assessment of mortality in intensive care patients with sepsis. 8 , 9 In the current study, we aimed to evaluate the efficacy of DNI in predicting mortality in intensive care patients diagnosed with COVID‐19 based on PCR and/or chest CT.
2. MATERIAL AND METHODS
2.1. Patient data
Approval was obtained from the Turkish Ministry of Health and the ethics committee of Ankara City Hospital for this study (approval number: E1‐20‐1015). The study included patients aged 18 and over, who presented to the emergency service of Ankara City Hospital between April 2020 and July 2020 with the symptoms of fever, cough and shortness of breath. The sample consisted of patients who were suspected to have or were diagnosed with COVID‐19 based on examinations performed and thus admitted to the general intensive care unit, as well as those who were followed up with various diagnoses in other departments of the hospital and referred to the intensive care unit with the suspicion of COVID‐19 based on related symptoms and findings. The data of 602 cases in which COVID‐19 treatment was initiated in the general intensive care unit were retrospectively analysed. A total of 388 patients with a positive COVID‐19 PCR test and/or CT findings consistent with COVID‐19 were included in the study. Patients whose CT findings were not compatible with COVID‐19 and PCR test was negative, those with haematological malignancies and pregnant women were excluded. The demographic characteristics of the patients, COVID‐19 PCR test results, chest CT findings, blood parameters at the time of presentation to the emergency service or transfer from this service to the intensive care unit (procalcitonin, delta neutrophil index, leukocyte, platelet count, lymphocyte, neutrophil lymphocyte ratio, RDW, blood lactate level, interleukin‐6 and CRP values), 30‐day mortality and the number of days in the intensive care unit were evaluated. At least one positive value was considered significant in the PCR test. The patients were evaluated in two groups as those who died in intensive care (mortality group) and those who recovered (surviving group).
2.2. Statistical analysis
Data analysis was carried out using SPSS for Windows program v. 15. Normality was assessed using the Kolmogorov‐Smirnov test. The descriptive statistics of normally distributed continuous variables were expressed as mean ± standard deviation (SD) while those of non‐normally distributed continuous variables were given as median (interquartile range) values. The differences between the groups in terms of normally distributed variables were analysed with the Student's t‐test while non‐normally distributed variables were analysed using the Mann‐Whitney U test. The discriminative power of variables was measured as the area under the receiver operating characteristic (ROC) curve. The discriminative power of the prognostic variables was considered perfect if the area under the curve (AUC) was 1, good if >0.8, moderate if 0.6‐0.8 and poor if <0.6. Specificity, sensitivity, positive predictive value (PPV), negative predictive value (NPV), negative likelihood ratio and positive likelihood ratio were also calculated.
3. RESULTS
Of the 388 patients hospitalised in intensive care, 220 (56.7%) were men and 168 (43.3%) were women. The mean age of the patients was 70 ± 15 years. The PCR test was (‐) in 217 (55.9%) of the patients and (+) in 171 (44.1%). The chest CT findings were evaluated as negative for COVID‐19 in 27 (7%) of the patients and compatible with COVID‐19 in 361 (93%). The median value of the number of days in intensive care was 7 (4‐12), and concerning 30‐day mortality, 264 (68%) of the patients survived and 124 (32%) died in the intensive care unit.
Of the 264 patients in the surviving group, 141 (53.4%) were men and 123 (46.6%) were women, with a mean age of 69 ± 15 years. In 159 (60.2%) of the patients, PCR was (‐) for COVID‐19 in 159 (60.2%) and (+) in 105 (39.8%). The median values of the blood parameters were 14 µg/L (0.04‐0.52) for procalcitonin, 0.10 (0.10‐0.55) for DNI%, 8430 × 106/L (6015‐12 255) for leukocyte, 250 00D 109/L (179‐318) for platelet, 0.90 × 109/L (0.61‐1.41) for lymphocyte, 7.47 (3.92‐13.01) for NLR, 1.68 (1.25‐ 2.21) for lactate, 46.10 pg/mL (23.70‐92) for interleukin‐6 and 65 mg/L (26‐129) for CRP, and the mean RDW% value was 15.40 ± 2.49. The number of patients with suspected COVID‐19 based on chest CT was 242 (91.7%), and the number of those without COVID‐19 suspicion on chest CT was 22 (8.3%).
The median value of the number of hospitalised days in intensive care was 8 (5‐13), and 79 (63.7%) of the 124 patients who died were men while 45 (36.3%) were women. The mean age value of the mortality group was found to be 72 ± 15 years. PCR was (‐) for COVID‐19 in 58 (46.8%) of the patients and (+) in 66 (53.2%). In these patients, the median values of procalcitonin, DNI%, leukocyte, platelet, lymphocyte, NLR, lactate, interleukin‐6 and CRP were 0.35 µg/L (0.17‐1.36), 1.00 (0.10‐2.60), 9365 × 106/L (6755‐12 640), 235 × 109/L (141‐315), 0.77 × 109/L (0.50‐1.14), 9.36 (5.93‐15.90), 2.15 (1.52‐3.09), 94.20 pg/mL (46.20‐239) and 119 mg/L (60‐185), respectively, and the mean RDW% value was 15.87 ± 2.17. Furthermore, the number of patients with suspected COVID‐19 on a chest CT was determined as 119 (96%), and the number of those without COVID‐19 suspicion on a chest CT as 5 (4%). The median value for the number of days in the intensive care unit was 5 (3‐11).
When the blood parameters were compared between the mortality and surviving groups for the first 30 days of intensive care, it was observed that the mean age was higher in the mortality group, but there was no statistically significant difference (P = .182). The procalcitonin, RDW and leukocyte values were also found to be higher among the patients in the mortality group without a statistically significant difference (P = .243, .075 and .498 respectively). The platelet value was lower in the mortality group with no statistical significance (P = .058). The DNI, lactate, interleukin‐6, CRP and NLR values were significantly higher (P = .003, .000, .002, .000 and .034, respectively) and the lymphocyte value was significantly lower (P = .024) in the mortality group compared to the surviving group. It was observed that the percentage of patients with suspected COVID‐19 on a chest CT was higher in the mortality group, but there was no statistically significant difference (P = .081). However, the number of hospitalised days in the intensive care unit was significantly lower in the mortality group (P = .027) (Table 1).
TABLE 1.
Comparison of the mortality and surviving groups in terms of various parameters
| n = 388 | Surviving (n = 264) | Mortality (n = 124) | P value | |
|---|---|---|---|---|
| Gender F/M (%) | 123/141 (46.6/53.4) | 45/79 (36.3/63.7) | ||
| Age, years | Mean ± SD | 69 ± 15 | 72 ± 15 | .182 |
| Procalcitonin µg/L | Median (percentile 25‐75) | 0.14 (0.04‐0.52) | 0.35 (0.17‐1.36) | .243 |
| DNI% | Median (percentile 25‐75) | 0.10 (0.10‐0.55) | 1.00 (0.10‐2.60) | .003 |
| Leukocyte × 106/L | Median (percentile 25‐75) | 8430 (6015‐12 255) | 9365 (6755‐12 640) | .498 |
| Platelet × 109/L | Median (percentile 25‐75) | 250 (179‐318) | 235 (141‐315) | .058 |
| Lymphocyte × 109/L | Median (percentile 25‐75) | 0.90 (0.60‐1.41) | 0.77 (0.50‐1.14) | .024 |
| Neutrophil/lymphocyte ratio | Median (percentile 25‐75) | 7.47 (3.92‐13.01) | 9.36 (5.93‐15.90) | .034 |
| RDW% | Mean ± SD | 15.40 ± 2.49 | 15.87 ± 2.17 | .075 |
| Lactate | Median (percentile 25‐75) | 1.68 (1.25‐2.21) | 2.15 (1.52‐3.09) | .000 |
| Interleukin‐6 pg/mL | Median (percentile 25‐75) | 46.10 (23.70‐92.00) | 94.20 (46.20‐239.00) | .002 |
| CRP mg/L | Median (percentile 25‐75) | 65 (26‐129) | 119 (60‐185) | .000 |
| COVID‐19 PCR | Negative | 159 (60.2%) | 58 (46.8%) | .014 |
| Positive | 105 (39.8%) | 66 (53.2%) | ||
| Chest CT (COVID‐19 suspicion) | Negative | 22 (8.3%) | 5 (4%) | .081 |
| Positive | 242 (91.7%) | 119 (96%) | ||
| Number of hospitalised days | Median (percentile 25‐75) | 8 (5‐13) | 5 (3‐11) | .027 |
Abbreviations: COVID‐19, Coronavirus‐19; CRP, C‐reactive protein; CT, computed tomography; DNI, delta neutrophil index; PCR, polymerase chain reaction; SD, standard deviation.
P values written in bold show statistically significance.
In the ROC analysis (Figure 1) of 30‐day mortality prediction, the AUC values of DNI, lymphocyte, NLR, lactate, interleukin‐6 and CRP were determined as 0.718, 0.416, 0.628, 0.585, 0.701 and 0.684, respectively. At the cut‐off value of 0.15, DNI% had a sensitivity of 62.90%, specificity of 68.94%, PPV of 48.75% and NPV of 79.82%. At the cut‐off value of 0.80 × 109/L, lymphocyte had sensitivity, specificity, PPV and NPV values of 55.46%, 57.87%, 38.15% and 73.50% respectively. The cut‐off value of NLR was 8.72, at which the sensitivity, specificity, PPV and NPV values were 55.00%, 59.84%, 39.29% and 72.79% respectively. The cut‐off value of lactate was 1.80, at which the sensitivity, specificity, PPV and NPV values were 61.04%, 59.26%, 46.08% and 72.73% respectively. At the cut‐off value of 62.25 pg/mL, interleukin‐6 had sensitivity, specificity, PPV and NPV values of 63.64%, 65.42%, 48.61% and 77.78% respectively. Lastly, at the cut‐off value of 108.5 mg/L, the sensitivity, specificity, PPV and NPV of CRP were 54.78%, 65.08%, 41.72% and 75.93% respectively (Table 2).
FIGURE 1.
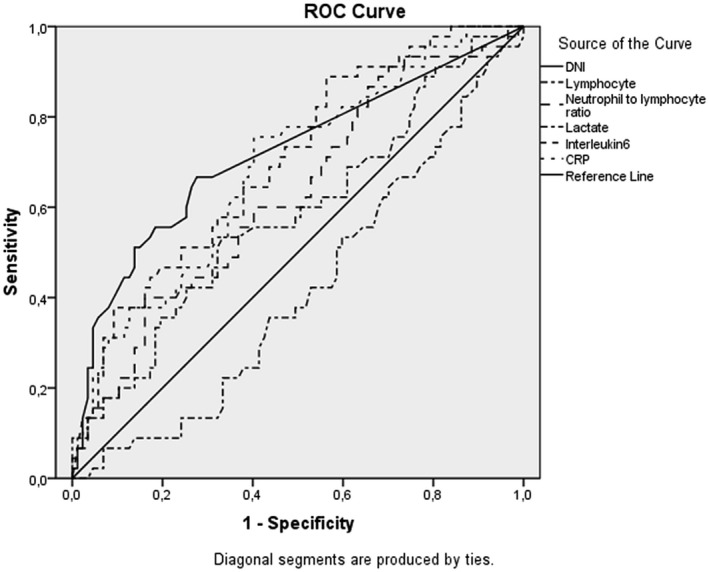
ROC analysis graph of the blood markers in the prediction of morality
TABLE 2.
ROC curve analysis of the blood markers in the prediction of mortality
| AUC | 95% CI | Cut‐off value | Sensitivity | Specificity | PPV | NPV | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| DNI | 0.718 | 0.620 | 0.815 | 0.15 | 62.90% | 68.94% | 48.75% | 79.82% |
| Lymphocyte | 0.416 | 0.315 | 0.516 | 0.80 | 55.46% | 57.87% | 38.15% | 73.50% |
| Neutrophil/lymphocyte ratio | 0.628 | 0.528 | 0.728 | 8.72 | 55.00% | 59.84% | 39.29% | 73.79% |
| Lactate | 0.585 | 0.481 | 0.690 | 1.80 | 61.04% | 59.26% | 46.08% | 72.73% |
| Interleukin‐6 | 0.701 | 0.609 | 0.793 | 62.25 | 63.64% | 65.42% | 48.61% | 77.78% |
| CRP | 0.684 | 0.589 | 0.779 | 108.5 | 54.78% | 65.08% | 41.72% | 75.93% |
Abbreviations: AUC, area under the curve; CI, confidence interval; CRP, C‐reactive protein; DNI, delta neutrophil index; NPV, negative predictive value; PPV, positive predictive value.
4. DISCUSSION
Simple laboratory parameters are more useful in predicting patient prognosis, so they are used more commonly in daily medical practice. 4 , 10 DNI is a frequently used parameter in the evaluation of mortality in patients with sepsis. Today, it is important to evaluate patients with a diagnosis of COVID‐19, especially those at risk of secondary bacterial sepsis. 6 To our knowledge, there is no study in the literature on the predictability of intensive care mortality in COVID‐19 patients based on DNI values. Therefore, we consider that our study can be useful in terms of contributing to the literature.
Frater et al stated that the procalcitonin value was mostly in the normal range in the early stages of COVID‐19, but increased in cases where the severity of infection increased. 6 In our study, although the procalcitonin value was elevated on the first day of intensive care, this was not statistically significant, suggesting that it is a parameter to be evaluated according to consecutive daily changes rather than as an early marker. Sharma et al reported that the RDW value increased in COVID‐19 patients, but did not create a statistically significant difference in terms of disease severity, in contrast to lymphopenia that provided significant results for the evaluation of disease severity. 5 Similarly, in our study, although the RDW value was higher in the mortality group, no statistically significant difference was observed compared to the surviving group. In addition, we determined that lymphocytes were lower in the mortality group, and they were statistically significant in predicting disease severity. Li et al stated that leukocytosis and high lactate levels were associated with mortality in severe COVID‐19 cases and reported the 32‐day mortality rate as 32.5%. 11 In our study, the median leukocyte values remained within the normal range in both groups and did not present a significant difference for the evaluation of disease severity; however, the lactate levels were significant in evaluating the severity of the disease. In addition, our intensive care mortality rate was similar to previously reported. Xia et al commented that an elevated NLR level was associated with the greater severity of COVID‐19. 12 Similarly, in our study, we found this parameter to be significantly higher in the mortality group. Lippi et al determined that thrombocytopenia was associated with the severity of COVID‐19 and mortality. 13 In contrast, in our study, although the platelet values were lower in the mortality group, no statistically significant difference was observed. Liu et al also noted that high IL‐6 and CRP values were independent prognostic factors for the evaluation of COVID‐19 severity. 14 Consistent with that research, we also detected a statistically significant increase in these parameters in the mortality group. Li et al emphasised the importance of chest tomography in diagnosing viral pneumonia and suggested that this modality might be useful in making a rapid diagnosis in the early period. 15 In a similar study, Fang et al argued that chest tomography was more successful than the PCR technique in the diagnosis of COVID‐19. 16 In a study conducted by Chan et al, the positivity rate of the PCR test reached 43% among COVID‐19 cases. In our study, although the chest CT findings were highly compatible with COVID‐19 in the mortality group, no statistically significant difference was observed. In addition, the rate of PCR positivity for COVID‐19 was higher in the mortality group. Polidoro et al stated that immature granulocytes caused acute respiratory distress syndrome (ARDS) in patients with COVID‐19. 17 In our study, DNI was found to be significantly higher in the mortality group. We consider that the association of high DNI with mortality may be related to ARDS. Predicting the mortality in Covid‐19 patients is important for regulating patients’ treatment. Scoring systems which are formed by using these parameters could be extremely useful for determining the high‐risk patient groups. 18
In conclusion, we consider that DNI can be used as an effective prognostic parameter to show intensive care mortality in COVID‐19 patients. There is a need for further research in this area, especially in terms of determining cases that will progress in intensive care.
DISCLOSURE
All authors declare that there is no conflict of interest.
Birben B, Birben OD, Akın T, et al. Efficacy of the delta neutrophil index in predicting 30‐day mortality in COVID‐19 patients requiring intensive care. Int J Clin Pract.2021;75:e13970. 10.1111/ijcp.13970
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.World Health Organization. WHO coronavirus disease (COVID‐19) Dashboard. Data last updated: 2020//11. 2020.
- 2. Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID‐19): a pictorial review. Eur Radiol. 2020;30:4381‐4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology. 2020;296:E32‐E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henry BM, De Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med (CCLM). 2020;58:1021‐1028. [DOI] [PubMed] [Google Scholar]
- 5. Sharma D, Dayama A, Banerjee S, Bhandhari S, Chatterjee A, Chatterjee D. To study the role of absolute lymphocyte count and RDW in COVID 19 patients and their association with appearance of symptoms and severity. J Assoc Physicians India. 2020;68:39‐42. [PubMed] [Google Scholar]
- 6. Frater JL, Zini G, d’Onofrio G, Rogers HJ. COVID‐19 and the clinical hematology laboratory. Int J Lab Hematol. 2020;42:11‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shin DH, Cho YS, Cho GC, et al. Delta neutrophil index as an early predictor of acute appendicitis and acute complicated appendicitis in adults. World J Emerg Surg. 2017;24:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahn C, Kim W, Lim TH, Cho Y, Choi K‐S, Jang B‐H. The delta neutrophil index (DNI) as a prognostic marker for mortality in adults with sepsis: a systematic review and meta‐analysis. Sci Rep. 2018;8:6621‐6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kong T, Park YS, Lee HS, et al. The delta neutrophil index predicts development of multiple organ dysfunction syndrome and 30‐day mortality in trauma patients admitted to an intensive care unit: a retrospective analysis. Sci Rep. 2018;8:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang L, Yan X, Fan Q, et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost. 2020;18:1324‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xia X, Wen M, Zhan S, He J, Chen W. An increased neutrophil/lymphocyte ratio is an early warning signal of severe COVID‐19. Nan fang yi ke da xue xue bao= J South Med Univ. 2020;40:333‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu F, Li L, Xu MD, et al. Prognostic value of interleukin‐6, C‐reactive protein, and procalcitonin in patients with COVID‐19. J Clin Virol. 2020;127:104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, Xia L. Coronavirus disease 2019 (COVID‐19): role of chest CT in diagnosis and management. Am J Roentgenol. 2020;214:1280‐1286. [DOI] [PubMed] [Google Scholar]
- 16. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID‐19: comparison to RT‐PCR. Radiology. 2020;296:E115‐E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polidoro RB, Hagan RS, de Santis SR, Schmidt NW. Overview: systemic inflammatory response derived from lung injury caused by SARS‐CoV‐2 infection explains severe outcomes in COVID‐19. Front Immunol. 2020;11:1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID‐19. JAMA Intern Med. 2020;180:1081‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


