Abstract
Objective
In this study, we aim to investigate the thoughts and attitudes of individuals towards the future COVID‐19 vaccine.
Methods
This descriptive study was carried out on the web between 10/06/2020 and 10/07/2020. The sample constitutes all individuals above 18 years of age using social media and smartphone. The e‐survey form was shared by the researchers via the web for a month, and those who completed the survey were included in the study and formed the sample of the research.
Results
Seven‐hundred and fifty‐nine people participated. 49.7% of the participants stated to be vaccinated; 38.4% of them stated to be vaccinated their children against COVID‐19; if the vaccine for COVID‐19 is developed. The request for the COVID‐19 vaccine had relationship with gender, occupation, health insurance, anxiety level, having children and willing to get vaccinated for their children. “Afraid of the side effects of vaccine”, “don't think it can be reliable as it will be a new vaccine” and “COVID‐19 infection is a biological weapon and the vaccine will serve those who produce this virus” were the most common reasons for rejection of vaccine.
Conclusion
In our study, afraid of the side effects of vaccine and not thinking it can be reliable as it will be a new vaccine are the most reasons of indecision and rejection about COVID 19 vaccine. In order for the future COVID 19 vaccination campaign to not fail, media, politicians and healthcare professionals should closely follow the vaccination development processes, inform the public transparently and consider public's concerns.
What’s known
Vaccination researches for SARS‐CoV‐2 are also ongoing in the large centres.
The vaccines are known to be effective in creating a long‐lasting immune memory to control infectious diseases.
Vaccines currently prevent 2‐3 million deaths per year.
There is an urgent and important need to manufacture and distribute enough safe and effective vaccine to immunise individuals in order to protect the entire global community from the threat of morbidity and mortality from SARS‐CoV‐2.
What’s new
Forty‐nine point seven per cent of the participants stated that if the vaccine for COVID‐19 infection is developed, they will be vaccinated against COVID‐19.
The most important reason for willingness to be vaccinated is to be thinking that vaccination will be important not only for him/herself or his/her children but also for protecting the health of people around him/her or his/her children.
This shows that people are aware that vaccines provide not only individual but also social protection.
Five point eight per cent of the participants stated that they will be vaccinated if the vaccine is free.
Economic reasons are important too, also our study support this issue.
1. INTRODUCTION
The COVID‐19 infection, which the World Health Organization has declared as a “pandemic” because it has spread to more than 114 countries, has caused more than 43.140.173 confirmed cases and more than 1.155.235 deaths as of 25 October 2020. 1 , 2 , 3
Developing drugs for SARS COV‐2 is an important issue for the scientists, and currently there are no officially approved drugs to treat COVID‐19 infection. 1 , 2 , 3 In addition to the clinical research of old and new drugs, vaccination researches for SARS‐CoV‐2 are also ongoing in the large centres. 4 , 5 The vaccines are known to be effective in creating a long‐lasting immune memory to control infectious diseases. Vaccines currently prevent 2‐3 million deaths per year. 4 Various vaccines have been developed in pandemics such as the 1957, 1968, 1976 and 1977 outbreaks and the H5N1 outbreak (1997‐1998), and the 2009 H1N1 outbreak. 5 In the studies conducted in the H1N1 pandemic, it has been stated that the senior citizens, men, those from an ethnic minority and those who are doctors among healthcare workers are more moderate and willing to vaccinate. 6
It is believed that with the availability of a safe and effective vaccine for COVID‐19, a great progress will be made in controlling the pandemic. On April 2, 2020, “ Vaccine and Drug Development virtual conference" was held with coordination of Turkish COVID‐19 platform in Turkey. In the opening speech of the conference, Turkey's Minister of Industry and Technology said, "Under the COVID‐19 Platform, there are seven different vaccine development projects and seven different drug development projects that both chemical and biotechnological methods will be applied". 7 More than a hundred companies or academic institutions around the world are working on COVID‐19 vaccines with strategies that include recombinant vectors, mRNA, DNA, inactivated virus, live attenuated virus, virus‐like particles and protein subunits in lipid nanoparticles. 8 As of 19 October 2020, there are 44 candidate vaccines in the clinical evaluation. 9
While studies on COVID‐19 vaccine are ongoing, the vaccine hesitancy or refusal for vaccine‐preventable disease reverses the progress made in the fight for these diseases. Therefore, it is of great importance to evaluate the perspective of the society in this regard. In this study, we aim to investigate the thoughts and attitudes of individuals towards the future COVID‐19 vaccine.
2. METHODS
This descriptive study was carried out on the web between 10/06/2020 and 10/07/2020. The research population constitutes all individuals above 18 years of age using social media and smartphone in Turkey. The e‐survey form was shared by the researchers, and those who completed the survey were included in the study and formed the sample of the research. The survey form was shared on whatsapp, facebook and instagram. The target number of people was reached by the snowball method. Sampling calculation has not been made and people answered the questionnaire within the specified period were included in the study. It is aimed to reach at least 500 adults.
The questionnaire to be used in the research was prepared by the researchers and there are 24 questions in total. The questionnaire contains 7 questions for sociodemographic characteristics (gender, age, occupation, etc) and 2 questions for health conditions. There are 3 questions about COVID‐19 infection, 12 questions about participants' opinions about vaccines and future COVID‐19 vaccine. Participants were asked to rate their anxiety levels against COVID‐19 between 0 and 10. After the approval of the ethics committee, data were collected via the web for 1 month. The e‐survey form developed by the researchers was shared 10 times in 3 days’ intervals on the web. Voluntary consent form was added to the questionnaire, participants who responded positively to the voluntary consent form completed the questionnaire. The people who answered all the questions were included in the study.
2.1. Data analysis
Mean ± standard deviation for the variables that were continuous from the demographic information of the participants, and frequency tables for the qualitative data were created. The consistency of continuous variables to normal distribution was examined using visual (histogram and possible graphics) and analytical methods (Kolmogorov‐Smirnov / Shapiro‐Wilk). Correlation coefficients and statistical significance were calculated by Pearson test when both variables were normally distributed in correlation analyses, or by Spearman test for at least one normal distribution or ordinal variables. Chi‐Square test was used to investigate the relationships between qualitative data. Differences between‐group values of continuous variables were investigated with t test, ANOVA test or tests with their non‐parametric equivalents. The value of α = 0.05 was chosen as the level of error and probability values obtained from statistical analysis were interpreted accordingly. Statistical analysis was done with SPSS 23 package program.
2.2. Permission and approval of the ethics committee
The study was conducted in accordance with the principles of the Helsinki Declaration related to conducting clinical trials on humans, and the research proposal was approved by the Ethics Committee of the XXX University with the number of GO 20/556 at June 2020.
3. RESULTS
Seven‐hundred and fifty‐nine participants participated. The mean age of the participants is 32.41 ± 9.92 (min = 18; max = 81) and 62.8% were women. Detailed sociodemographic characteristics of participants were at Table 1.
TABLE 1.
Sociodemographic characteristics of participants
| TOTAL | ||
|---|---|---|
| Number (n) | Percentage (%) | |
| Gender: | ||
| Female | 477 | 62.8 |
| Male | 282 | 37.2 |
| Educational status: | ||
| Not Literate | 2 | 0.3 |
| Literate | 3 | 0.4 |
| Primary school | 21 | 2.8 |
| High school | 83 | 10.9 |
| University | 483 | 63.6 |
| Master/PhD | 167 | 22 |
| Marital status | ||
| Married | 354 | 46.6 |
| Single | 405 | 53.4 |
| Profession | ||
| Not working | 96 | 12.6 |
| Student | 104 | 13.7 |
| Health employee | 308 | 40.6 |
| Other | 251 | 33.1 |
| Monthly income | ||
| <1000 | 103 | 13.6 |
| 1000‐2000 | 71 | 9.4 |
| 2000‐3000 | 82 | 10.8 |
| 3000‐4000 | 72 | 9.5 |
| >4000 | 431 | 56.8 |
| Health insurance type | ||
| SSI | 623 | 82.1 |
| Special | 49 | 6.5 |
| Other | 50 | 6.6 |
| Family type | ||
| Nuclear family | 690 | 90.9 |
| Extended family | 69 | 9.1 |
| Chronic disease state | ||
| Yes | 139 | 18.3 |
| No | 620 | 81.7 |
Forty‐nine point seven per cent of the participants stated that if the vaccine for COVID‐19 infection is developed, they will be vaccinated against COVID‐19. Vaccination requests of participants against COVID‐19 were at Figure 1.
FIGURE 1.
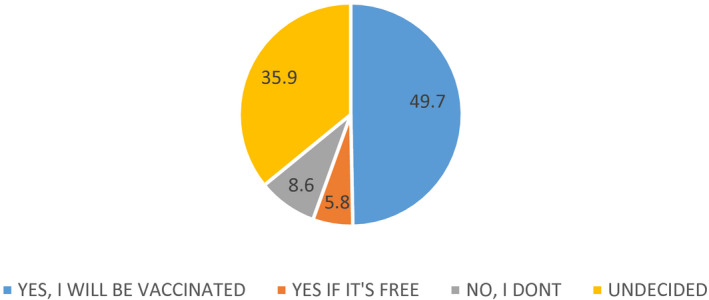
Vaccination requests of participants against COVID‐19
Of the participants, 0.8% (n = 6) had COVID‐19 infections, 17.1% (n = 130) of their relatives had COVID‐19 infections. The average level of anxiety for COVID 19 infection was 6.04 ± 2.30 (min = 0; max = 10). COVID‐19 infection measure practices of the participants are presented in Table 2.
TABLE 2.
COVID 19 infection measure practices of the participants
| Total | ||
|---|---|---|
| Number (n) | Per cent (%) | |
| Using a mask | 745 | 98.2 |
| Washing hands | 723 | 95.3 |
| Taking care not to touch eyes, mouth and nose with hands. | 645 | 85 |
| Avoiding to go out, going into crowded places | 609 | 80.2 |
| Ventilation of environment at work, at home | 559 | 73.6 |
| Avoiding to close contact with people such as handshaking, hugging, keep a distance of 1.5 m | 684 | 90.1 |
| Improving diet and sleep, started to consume healthy foods and drink more water. | 209 | 27.5 |
| None of them | 1 | 0.1 |
Thirty point six per cent (n = 232) of participants had children between the ages of 0‐18, 6.2% (n = 14) of those rejected any of the vaccines within the National Vaccination Program for their children and 58.5% (n = 134) of them were getting their children vaccines which were not at National Vaccination Program and had paid (meningococcal vaccine, influenza vaccine, HPV vaccine, etc). Of the participants, 38.4% stated that if the vaccine for COVID‐19 infection is developed, they will be vaccinating their children against COVID‐19. Vaccination requests of participants for their children against COVID‐19 were at Figure 2.
FIGURE 2.
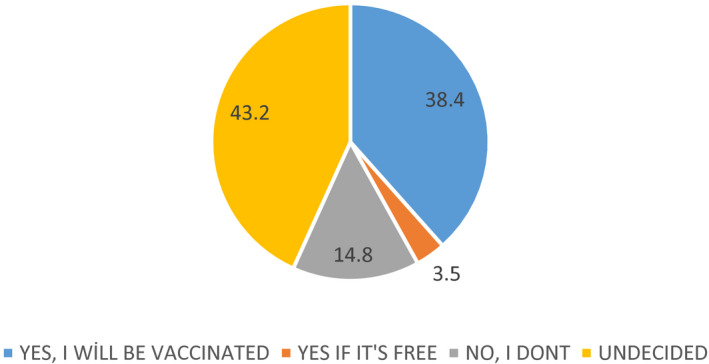
Vaccination requests of participants for their children against COVID‐19
The relationship of some factors and vaccination requests of participants for themselves and for their children against COVID‐19 are presented at Table 3. It was seen that women had more negative opinions (don't get vaccinated/ undecided) about getting vaccinated than men (P = .001). While students and health professionals were more willing to vaccinate, those who did not work stated that they were more undecided than other occupational groups (P = .026). Those who have SSI or private health insurance (P = .004), who got seasonal flu vaccine (P < .001), who had children (P = .048) and those who were thinking about getting their child COVID‐19 vaccine (P < .001) stated that they were more willing to get vaccinated than others. As the level of anxiety increases, the willingness to get vaccinated increases (P = .010). With the increasing level of education, the participants' thoughts about getting vaccinated for their children were increased (P < .001). Those who have SSI or private health insurance (P = .006), who got seasonal flu vaccine (P = .023) stated that they were more willing to get vaccinated than others.
TABLE 3.
The relationship of some factors and vaccination requests of participants against COVID 19
| Vaccination request against covid 19 | Vaccination request for their children against covid 19 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes, get vaccinated | If it is free, get it done | No, don't get vaccinated | Undecided | P | Yes, get vaccinated | If it is free, get it done | No, don't get vaccinated | Undecided | P | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |||
| Age | ||||||||||||||||||
| <25 | 105 | 27.9 | 14 | 31.8 | 13 | 20 | 69 | 25.2 | .78 | 1 | 1.1 | 0 | 0 | 2 | 5.9 | 1 | 1 | .58 |
| 26‐30 | 125 | 33.2 | 14 | 31.8 | 13 | 20 | 84 | 30.8 | 13 | 14.8 | 0 | 0 | 5 | 14.7 | 12 | 12.1 | ||
| 31‐35 | 42 | 11.1 | 3 | 6.8 | 17 | 26.2 | 43 | 15.8 | 20 | 22.7 | 2 | 25 | 10 | 29.4 | 35 | 35.4 | ||
| 36‐40 | 30 | 8 | 6 | 13.6 | 8 | 12.3 | 22 | 8.1 | 19 | 21.6 | 3 | 37.5 | 7 | 20.6 | 21 | 21.2 | ||
| >40 | 75 | 19.9 | 7 | 15.9 | 14 | 21.5 | 55 | 20.1 | 35 | 39.8 | 3 | 37.5 | 10 | 29.4 | 30 | 30.3 | ||
| Gender | ||||||||||||||||||
| Female | 214 | 56.8 | 24 | 54.5 | 47 | 72.3 | 192 | 70.3 | .001 | 47 | 53.4 | 3 | 37.5 | 21 | 61.8 | 67 | 67.7 | .12 |
| Male | 163 | 43.2 | 20 | 45.5 | 18 | 27.7 | 81 | 28.7 | 41 | 46.6 | 5 | 62.5 | 13 | 38.2 | 32 | 32.3 | ||
| Educational status | ||||||||||||||||||
| Not Literate | 0 | 0 | 0 | 0 | 1 | 1.5 | 1 | 0.4 | .26 | 0 | 0 | 1 | 12.5 | 0 | 0 | 0 | 0 | <.001 |
| Literate | 2 | 0.5 | 0 | 0 | 0 | 0 | 1 | 0.4 | 1 | 1.1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Primary school | 14 | 3.7 | 1 | 2.3 | 0 | 0 | 6 | 2.2 | 7 | 8 | 1 | 12.5 | 0 | 0 | 2 | 2 | ||
| High school | 32 | 8.5 | 7 | 15.9 | 11 | 16.9 | 33 | 12.1 | 15 | 17 | 0 | 0 | 9 | 26.5 | 19 | 19.2 | ||
| University | 240 | 63.7 | 31 | 70.5 | 40 | 61.5 | 172 | 63 | 47 | 53.4 | 6 | 75.0 | 19 | 55.9 | 58 | 58.6 | ||
| Master/PhD | 89 | 23.6 | 5 | 11.4 | 13 | 20 | 60 | 22 | 18 | 20.5 | 0 | 0 | 6 | 17.6 | 20 | 20.2 | ||
| Marital Status | ||||||||||||||||||
| Married | 160 | 42.4 | 19 | 43.2 | 36 | 55.4 | 139 | 50.9 | .07 | 84 | 95.5 | 8 | 100 | 30 | 88.2 | 95 | 96 | .29 |
| Single | 217 | 57.6 | 25 | 56.8 | 29 | 44.6 | 134 | 49.1 | 4 | 4.5 | 0 | 0 | 4 | 11.8 | 4 | 4 | ||
| Profession | .42 | |||||||||||||||||
| Not working | 38 | 10.1 | 8 | 18.2 | 9 | 13.8 | 41 | 15 | .026 | 14 | 15.9 | 1 | 12.5 | 7 | 20.6 | 15 | 15.2 | |
| Student | 63 | 16.7 | 8 | 18.2 | 5 | 7.7 | 28 | 10.3 | 0 | 0 | 0 | 0 | 1 | 2.9 | 0 | 0 | ||
| Health employee | 167 | 44.3 | 14 | 31.8 | 26 | 40.0 | 101 | 37.0 | 34 | 38.6 | 2 | 25 | 11 | 32.4 | 29 | 29.3 | ||
| Other | 109 | 28.9 | 14 | 31.8 | 25 | 38.5 | 103 | 37.7 | 40 | 45.5 | 5 | 62.5 | 15 | 44.1 | 55 | 55.6 | ||
| Monthly income | ||||||||||||||||||
| <1000 | 46 | 12.2 | 10 | 22.7 | 7 | 10.8 | 40 | 14.7 | .09 | 2 | 2.3 | 0 | 0 | 0 | 0 | 2 | 2 | .43 |
| 1000‐2000 | 40 | 10.6 | 4 | 9.1 | 3 | 4.6 | 24 | 8.8 | 7 | 8 | 1 | 12.5 | 2 | 5.9 | 8 | 8.1 | ||
| 2000‐3000 | 38 | 10.1 | 4 | 9.1 | 8 | 12.3 | 32 | 11.7 | 17 | 19.3 | 0 | 0 | 6 | 17.6 | 14 | 14.1 | ||
| 3000‐4000 | 23 | 6.1 | 5 | 11.4 | 9 | 13.8 | 35 | 12.8 | 8 | 9.1 | 1 | 12.5 | 10 | 29.4 | 13 | 13.1 | ||
| >4000 | 230 | 61 | 21 | 47.7 | 38 | 58.5 | 142 | 52 | 54 | 61.4 | 6 | 75 | 16 | 47.1 | 62 | 62.6 | ||
| Health insurance type | .006 | |||||||||||||||||
| SSI | 323 | 85.7 | 30 | 68.2 | 50 | 76.9 | 220 | 80.6 | .004 | 78 | 88.6 | 4 | 50 | 25 | 73.5 | 80 | 80.8 | |
| Special | 26 | 6.9 | 3 | 6.8 | 2 | 3.1 | 18 | 6.6 | 5 | 5.7 | 1 | 12.5 | 1 | 2.9 | 7 | 7.1 | ||
| Other | 15 | 4 | 6 | 13.6 | 10 | 15.4 | 19 | 7 | 1 | 1.1 | 3 | 37.5 | 4 | 11.8 | 9 | 9.1 | ||
| Family type | ||||||||||||||||||
| Nuclear family | 343 | 91 | 38 | 86.4 | 60 | 92.3 | 249 | 91.2 | .73 | 76 | 86.4 | 5 | 62.5 | 28 | 82.4 | 91 | 91.9 | |
| Extended family | 34 | 9 | 6 | 13.6 | 5 | 7.7 | 24 | 8.8 | 12 | 13.6 | 3 | 37.5 | 6 | 17.6 | 8 | 8.1 | .06 | |
| Chronic disease state | .88 | |||||||||||||||||
| Yes | 67 | 17.8 | 6 | 13.6 | 11 | 16.9 | 55 | 20.1 | .70 | 21 | 23.9 | 1 | 12.5 | 8 | 23.5 | 21 | 21.2 | |
| No | 310 | 82.2 | 38 | 86.4 | 54 | 83.1 | 218 | 79.9 | 67 | 76.1 | 7 | 87.5 | 26 | 76.5 | 178 | 78.8 | ||
| Vaccination against seasonal flu in the last 1 y | .023 | |||||||||||||||||
| Yes | 54 | 14.3 | 5 | 11.4 | 2 | 3.1 | 14 | 5.1 | <.001 | 14 | 15.9 | 1 | 12.5 | 0 | 0 | 6 | 6.1 | |
| No | 323 | 85.7 | 39 | 88.6 | 63 | 96.9 | 259 | 94.9 | 74 | 84.1 | 7 | 87.5 | 34 | 100 | 93 | 93.9 | ||
| Infection Status with COVID‐19 | ||||||||||||||||||
| Yes | 3 | 0.8 | 1 | 2.3 | 1 | 1.5 | 1 | 0.4 | .50 | 0 | 0 | 1 | 12.5 | 0 | 0 | 1 | 1.0 | .004 |
| No | 374 | 99.2 | 43 | 97.7 | 64 | 98.5 | 272 | 99.6 | 88 | 100.0 | 7 | 87.5 | 34 | 100 | 98 | 99.0 | ||
| Infection Status of relatives with COVID‐19 | ||||||||||||||||||
| Evet | 74 | 19.6 | 6 | 13.6 | 9 | 13.8 | 41 | 15 | .33 | 15 | 17 | 2 | 25 | 3 | 8.8 | 12 | 12.1 | .46 |
| Hayır | 303 | 80.4 | 38 | 86.4 | 56 | 86.2 | 232 | 85 | 73 | 83 | 6 | 75 | 31 | 91.2 | 87 | 87.9 | ||
| Anxiety level for COVID‐19 infection | ||||||||||||||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1.1 | .010 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | .23 |
| 1 | 13 | 3.4 | 6 | 13.6 | 6 | 9.2 | 12 | 4.4 | 6 | 6.8 | 1 | 12.5 | 4 | 11.8 | 2 | 2 | ||
| 2 | 7 | 1.9 | 2 | 4.5 | 2 | 3.1 | 11 | 4 | 1 | 1.1 | 0 | 0 | 1 | 2.9 | 4 | 4 | ||
| 3 | 24 | 6.4 | 2 | 4.5 | 5 | 7.7 | 18 | 6.6 | 1 | 1.1 | 1 | 12.5 | 3 | 8.8 | 8 | 8.1 | ||
| 4 | 21 | 5.6 | 0 | 0 | 5 | 7.7 | 18 | 6.6 | 4 | 4.5 | 0 | 0 | 3 | 8.8 | 6 | 6.1 | ||
| 5 | 71 | 18.8 | 10 | 22.7 | 23 | 35.4 | 48 | 17.6 | 21 | 23.9 | 2 | 25 | 7 | 20.6 | 21 | 21.2 | ||
| 6 | 60 | 15.9 | 6 | 13.6 | 5 | 7.7 | 35 | 12.8 | 5 | 5.7 | 1 | 12.5 | 6 | 17.6 | 6 | 6.1 | ||
| 7 | 66 | 17.5 | 5 | 11.4 | 7 | 10.8 | 66 | 24.2 | 11 | 12.5 | 2 | 25 | 3 | 8.8 | 23 | 23.2 | ||
| 8 | 54 | 14.3 | 8 | 18.2 | 6 | 9.2 | 30 | 11 | 20 | 22.7 | 1 | 12.5 | 2 | 5.9 | 11 | 11.1 | ||
| 9 | 27 | 7.2 | 3 | 6.8 | 2 | 3.1 | 12 | 4.4 | 7 | 8 | 0 | 0 | 2 | 5.9 | 4 | 4 | ||
| 10 | 34 | 9 | 2 | 4.5 | 4 | 6.2 | 20 | 7.3 | 12 | 13.6 | 0 | 0 | 3 | 8.8 | 13 | 13.1 | ||
| The condition of having paid vaccinations for the child | .07 | |||||||||||||||||
| Yes | 68 | 68.7 | 5 | 41.7 | 11 | 45.8 | 50 | 53.2 | .07 | 61 | 69.3 | 3 | 37.5 | 16 | 47.1 | 53 | 54.1 | |
| No | 29 | 29.3 | 7 | 58.3 | 12 | 50 | 37 | 39.4 | 25 | 28.4 | 5 | 62.5 | 17 | 50 | 38 | 38.8 | ||
| I have no idea/remember | 2 | 2 | 0 | 0 | 1 | 4.2 | 7 | 7.4 | 2 | 2.3 | 0 | 0 | 1 | 2.9 | 7 | 7.1 | ||
| Refusal of vaccinations to the child | ||||||||||||||||||
| Yes | 5 | 5.1 | 0 | 0 | 3 | 12.5 | 6 | 6.5 | .20 | 4 | 4.5 | 1 | 12.5 | 3 | 9.1 | 6 | 6.2 | .08 |
| No | 92 | 93.9 | 11 | 100 | 21 | 87.5 | 81 | 87.1 | 84 | 95.5 | 7 | 87.5 | 30 | 90.9 | 84 | 86.6 | ||
| I have no idea/remember | 1 | 1 | 0 | 0 | 0 | 0 | 6 | 6.5 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 7.2 | ||
| Presence of children between the ages of 0 and 18 | ||||||||||||||||||
| Yes | 99 | 26.3 | 12 | 27.3 | 24 | 36.9 | 97 | 35.5 | .048 | |||||||||
| No | 278 | 73.7 | 32 | 72.7 | 41 | 63.1 | 176 | 64.5 | ||||||||||
| The thought of getting COVID‐19 vaccine to child | ||||||||||||||||||
| Yes | 81 | 81.8 | 4 | 36.4 | 0 | 0 | 3 | 3.2 | <.001 | |||||||||
| If it is free, get it | 1 | 1 | 6 | 54.5 | 1 | 4.2 | 011 | 0 | ||||||||||
| No | 0 | 0 | 1 | 9.1 | 22 | 91.7 | 11.6 | |||||||||||
| Undecided | 17 | 17.2 | 0 | 0 | 1 | 4.2 | 81 | 85.3 | ||||||||||
“Afraid of the side effects of vaccine”, “don't think it can be reliable as it will be a new vaccine” and “COVID‐19 infection is a biological weapon and the vaccine will serve those who produce this virus” were the most common reasons for rejection of vaccine both for themselves and for their children. The reasons of indecision and rejection about COVID‐19 vaccine are presented at Table 4.
TABLE 4.
The reasons of indecision and rejection about COVID 19 vaccine
| The reasons of indecision and rejection about COVID 19 vaccine | ||||
|---|---|---|---|---|
| For themselves | For their children | |||
| n | % | n | % | |
| Vaccines to be produced for the COVID 19 virus can cause COVID 19 infection. | 55 | 7.2 | 30 | 4 |
| Afraid from the side effects of vaccine. | 210 | 27.7 | 90 | 11.9 |
| COVID 19 infection is a biological weapon and I think that the vaccine will serve those who produce this virus. | 104 | 13.7 | 42 | 5.5 |
| Don't think it can be reliable as it will be a new vaccine. | 208 | 27.4 | 78 | 10.3 |
| Don't think that the vaccines produced for COVID 19 virus can be effective. | 62 | 8.2 | 23 | 3 |
| Don't think to have enough information about vaccines. | 66 | 8.7 | 30 | 4 |
| COVID 19 infection is exaggerated, it is not a risky disease, so no vaccine is needed. | 11 | 1.4 | 7 | 0.9 |
| Preferring other ways of protection. | 55 | 7.2 | 29 | 3.8 |
| Do not intend to be vaccinated because of having COVID 19 infection. | 1 | 0.1 | — | — |
| Waiting for vaccination until child grows a little older. | — | — | 13 | 1.7 |
| In general, having doubts about vaccinations. | — | — | 21 | 2.8 |
| Other | 24 | 3.2 | 9 | 1.2 |
“Vaccination will be important to protect against COVID‐19 disease” and “vaccination will be important not only for myself/my children but also for protecting the health of people around me/my children” were the most common reasons for request of vaccine both for themselves and for their children. The reasons of request for COVID‐19 vaccine are presented at Table 5.
TABLE 5.
The reasons of request for COVID 19 vaccine
| The reasons of request for COVID 19 vaccine | ||||
|---|---|---|---|---|
| For themselves | For their children | |||
| n | % | n | % | |
| COVID 19 vaccine will end the outbreak. | 170 | 22.4 | 43 | 5.7 |
| Vaccination will be important to protect against COVID19 disease. | 287 | 37.8 | 85 | 11.2 |
| Vaccination will be important not only for myself/my children but also for protecting the health of people around me/my children. | 350 | 46.1 | 74 | 9.7 |
| COVID 19 vaccine will reduce the duration and severity of this disease. | 279 | 36.8 | 61 | 8 |
| Other | 10 | 1.3 | 3 | 0.4 |
4. DISCUSSION
The current estimated mortality rate of SARS‐CoV‐2 is around 6.9% but varies among different countries. 10 There is an urgent and important need to manufacture and distribute enough safe and effective vaccine to immunise individuals in order to protect the entire global community from the threat of morbidity and mortality from SARS‐CoV‐2. 11 Also in our study 49.7% of the participants stated that if the vaccine for COVID‐19 infection is developed, they will be vaccinated against COVID‐19. The most important reason for willingness to be vaccinated is to be thinking that vaccination will be important not only for him/herself or his/her children but also for protecting the health of people around him/her or his/her children. This shows that people are aware that vaccines provide not only individual but also social protection. The second reason for being vaccinated is to be thinking that vaccination will be important to protect against COVID‐19 disease.
However, there is not any vaccine currently licensed for any of the other coronaviruses affecting humans. These are as follows: SARS‐CoV‐1, MERS‐CoV and minor cold viruses. Economic reasons are of course a major factor for the absence of these vaccines. But despite economic challenges, vaccine design is also a challenge. Immune responses to natural coronavirus infections can be short lived, and some trial vaccines for SARS‐CoV‐1 raised safety concerns in animal models. 12
In our study, we investigated the thoughts and attitudes of individuals towards the future COVID‐19 vaccine. Eight point six per cent of the participants stated that if the vaccine for COVID‐19 infection is developed, they will not be vaccinated against COVID‐19 and 35.9% of them are undecided. Fourteen point eight per cent of the participants stated that if the vaccine for COVID‐19 infection is developed, they will not be vaccinating their children against COVID‐19 and 43.2% of the patents are undecided. In addition, people who had children and who were thinking about getting COVID‐19 vaccine to their children were more willing to get vaccinated than others. With the increasing level of education, the participants' thoughts about getting vaccinated for their children were increased. People are more reluctant to vaccinate their children than their own vaccination. This may be caused by different reasons related to vaccines and immunisation policies, which are being discussed and questioned with increasing momentum.
The current pandemic entails three important challenges for public confidence in and uptake of a future, licensed vaccine. First, evidence shows that if the vaccine is new, the level of hesitancy will be high. 13 Second, one reason; people trust vaccines is the slow and methodical process it takes to develop them, which may take up to several years before final approval. While evidence‐based medicine is essential, the marketing‐based pharmaceutical industry is the reality of our time. Pharmaceutical companies have captured science to make more profit. 14 A third challenge relates to misinformation by anti‐vaccination campaigners. Vaccines are also one of the topics that are increasingly being questioned and discussed.
Recent studies have shown that COVID‐19 vaccine hesitancy level varies from low to high, with some 29% of New York residents claiming they will refuse a vaccine, compared to 20% of those in Canada and 6% of those in the United Kingdom. 15 , 16 We currently know little about what constitutes a protective immune response against COVID‐19.
In our study, afraid of the side effects of vaccine and not thinking it can be reliable as it will be a new vaccine are the most reasons of indecision and rejection about COVID‐19 vaccine for themselves and for their children. There are many studies in the scientific literature on the content and side effects of the vaccines. 17 , 18 , 19 , 20 , 21 There are also publications in the literature about the autoimmune diseases reported after the vaccinations. 22
In our study, 5.8% of the participants stated that they will be vaccinated if the vaccine is free. Economic reasons are important too, also our study support this issue; not working people were more undecided and those who have social insurance (SSI) or private health insurance were more willing to get vaccinated. Studies in the literature, lower price barrier and higher ability to pay were associated with higher willingness to pay. 23 , 24 But the decision to add a new vaccine to the national immunisation program requires a very extensive and comprehensive assessment. Decision‐making process also influenced by social values, perceptions and political approaches. 25
Another important result in our study was that participants who got seasonal flu vaccine were more willing to get vaccinated and also to get vaccinated their children against COVID‐19 than others. Seasonal flu vaccine is salaried in Turkey. Also people could have paid for seasonal flu vaccine and those who care about vaccination were more willingness for vaccination against COVID‐19.
Heath anxiety occurs when perceived bodily sensations or changes, are interpreted as symptoms of being ill and play a vital role in the success of public health strategies used to manage epidemics and pandemics; that is, risk communication, vaccination and antiviral therapy, hygiene practices and social distancing. 26 As the level of anxiety increases, the willingness to get .
vaccinated increases in our study. In accordance with our study, anxiety level and vaccine history were the main affecting factors for the willingness of future A/H7N9 influenza vaccine uptake. 27
Health professionals were more willing to vaccinate in our study. During 2009 H1N1 Pandemic, because of the high‐risk perception of healthcare workers, vaccination rates have increased. 28 , 29 , 30 , 31 In one study from Italy (n = 2557) vaccination in pandemics 17%, while vaccination rate decreased to 7.8% in 2012‐2013 season after pandemic. 32
Limitations included the methodological limitation of web‐based survey. For our sample population, they had higher education level, higher rates of health care professional and cannot be generalised to society.
5. CONCLUSION
Health professionals should act with a sense of responsibility in the recommendations on immunisation policies and vaccinations and should know that each vaccine has different characteristics (content, side effects, efficacy, safety, importance and necessity). Recommendations from peers and healthcare providers increase willingness to vaccination. 24 Adverse effects on people's confidence in healthcare professionals should be taken into account when recommending or rejecting vaccinations. In order for the future COVID‐19 vaccination campaign to not fail, media, politicians and healthcare professionals should closely follow the vaccination development processes, inform the public transparently and consider public's concerns.
DISCLOSURE
The authors declared no conflict of interest concerning the research, authorship or publication of this article.
RESEARCH INVOLVING HUMAN PARTICIPANTS AND/OR ANIMALS
This research involved human participants’ data and authors include a statement that confirms that the study was approved by Hacettepe University research ethics committee and certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Akarsu B, Canbay Özdemir D, Ayhan Baser D, Aksoy H, Fidancı İ, Cankurtaran M. While studies on COVID‐19 vaccine is ongoing, the public’s thoughts and attitudes to the future COVID‐19 vaccine. Int J Clin Pract.2021;75:e13891. 10.1111/ijcp.13891
DATA AVAILABILITY STATEMENT
The authors declare that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non‐commercial purposes, without breaching participant confidentiality. Moreover, the authors ensure that their datasets are presented in the main manuscript.
REFERENCES
- 1. T. C. Ministry of Health General Directorate of Public Health . COVID‐19 (SARS‐CoV2 Infection) Guideline; 2020. https://covid19bilgi.saglik.gov.tr/tr/covid‐19‐rehberi.html. Accessed July 27, 2020.
- 2. Xu K, Cai H, Shen Y, et al. The Zhejiang experience 2020. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49: PMID: 32096367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO Coronavirus Disease (COVID‐19) Dashboard . https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed July 27, 2020.
- 4. WHO Coronavirus disease (COVID‐19) pandemic . https://www.who.int/news‐room/feature‐stories/ten‐threats‐to‐global‐health‐in‐2019. Accessed July 27, 2020.
- 5. Wood JM. Developing vaccines against pandemic influenza. Philos Trans R Soc Lond B Biol Sci. 2001;356:1951‐1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bish A, Yardley L, Nicoll C, Michie S. Factors associated with uptake of vaccination against pandemic influenza: a systematic review. Vaccine. 2011;29:6472‐6484. [DOI] [PubMed] [Google Scholar]
- 7. T. C. Ministry of Health General Directorate of Public Health . https://covid19.tubitak.gov.tr/duyurular/covid‐19‐turkiye‐platformu‐asi‐ve‐ilac‐gelistirme‐sanal‐konferansi‐duzenledi; 2020. Accessed July 27, 2020.
- 8. Le Thanh T, Andreadakis Z, Kumar A, et al. The COVID‐19 vaccine development landscape. Nat Rev Drug Discovery. 2020;19:305‐306. [DOI] [PubMed] [Google Scholar]
- 9. WHO Coronavirus Disease (COVID‐19) Dashboard . https://www.who.int/who‐documents‐detail/draft‐landscape‐of‐covid‐19‐candidate‐vaccines. Accessed July 27, 2020.
- 10. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corey L, Mascola JR, Fauci AS, Collins FS. A strategic approach to COVID‐19 vaccine R&D. Science. 2020;368:948‐950. [DOI] [PubMed] [Google Scholar]
- 12. Amanat F, Krammer F. SARS‐CoV‐2 vaccines: status report. Immunity. 2020;52:583‐589.PMID:32259480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger JA. Vaccine hesitancy: an overview. Hum Vaccines Immunother. 2013;9(8):1763‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spielmans GI, Parry PI. From evidence‐based medicine to marketing‐based medicine: evidence from internal industry documents. J Bioeth Inq. 2010;7:13‐29. [Google Scholar]
- 15. Latimer K.About 20% of people in recent survey said they wouldn’t take COVID‐19 vaccine; 2020. https://www.cbc.ca/news/canada/saskatchewan/covid‐survey‐first‐round‐results‐1.5541053. Accessed July 27, 2020.
- 16. Henley J; Guardian correspondents . Coronavirus causing some anti‐vaxxers to waver, experts say; 2020. https://www.theguardian.com/world/2020/apr/21/anti‐vaccination‐community‐divided‐how‐respond‐to‐coronavirus‐pandemic. Accessed July 27, 2020.
- 17. Ball LK, Ball R, Pratt RD. An assessment of thimerosal use in childhood vaccines. Pediatrics. 2001;107:1147‐1154. [DOI] [PubMed] [Google Scholar]
- 18. Drasch G, Aigner S, Roider G, Staiger E, Lipowsky G. Mercury in human colostrum and early breast milk, its dependence on dental amalgam and other factors. J Trace Elem Med Biol. 1998;12:23‐27. [DOI] [PubMed] [Google Scholar]
- 19. Bose‐O’Reilly S, Mccarty K, Steckling N, Lettmeier B. Mercury exposure and children’s health. Curr Probl Pediatr Adolesc. Health Care. 2010;40:186‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hessel L. Mercury in vaccines. Bull Acad Natl Med. 2003;187:1501‐1510. http://www.ncbi.nlm.nih.gov/pubmed/15146581 [PubMed] [Google Scholar]
- 21. Shaw C, Tomljenovic L. Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res. 2013;56:304‐316. [DOI] [PubMed] [Google Scholar]
- 22. Orbach H, Agmon‐Levin N, Zandman‐Goddard G. Vaccines and autoimmune diseases of the adult. Discov Med. 2010;9:90‐97. [PubMed] [Google Scholar]
- 23. Hou Z, Chang J, Yue D, Fang H, Meng Q, Zhang Y. Determinants of willingness to pay for self‐paid vaccines in China. Vaccine. 2014;32:4471‐4477. [DOI] [PubMed] [Google Scholar]
- 24. Bish AL, Yardley A, Nicoll S, Michie S. Factors associated with uptake of vaccination against pandemic influenza: a systematic review. Vaccine. 2011;29:6472‐6484. [DOI] [PubMed] [Google Scholar]
- 25. Kartal M, Güldal D. Yeni Aşıların Ulusal Programda Yer Alma Süreci. Türkiye Klin J Fam Med‐Special To. 2011;2:5‐10. [Google Scholar]
- 26. Asmundson GJG, Taylor S. How health anxiety influences responses to viral outbreaks like COVID‐19: what all decision‐makers, health authorities, and health care professionals need to know. J Anxiety Disord. 2020;71:102211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chan EY, Cheng CK, Tam GC, Huang Z, Lee PY. Willingness of future A/H7N9 influenza vaccine uptake: a cross‐sectional study of Hong Kong community. Vaccine. 2015;33:4737‐4740. PMCID: PMC7271220. [DOI] [PubMed] [Google Scholar]
- 28. Gürbüz Y, Tütüncü EE, Şencan İ, et al. İnfluenza A (H1N1) 2009 pandemisinde hastane çalışanlarının grip aşısına yaklaşımlarının araştırılması. Pamukkale Tıp Dergisi. 2013;6:12‐17. 10. [Google Scholar]
- 29. Sevencan F, Ertem M, Özçullu N, Dorman V, Kubat NK. The evaluation of the opinions and attitudes of healthcare personnel of the province Diyarbakir against influenza A (H1N1) and the vaccination. Hum Vaccin. 2011;7:945‐951. [DOI] [PubMed] [Google Scholar]
- 30. Örmen B, Türker N, Vardar İ, et al. Hastane personeline pandemik influenza A (H1N1) aşı uygulamasının ardından aşılama hakkındaki görüşler ve gözlenen yan etkiler. Mikrobiyol Bül. 2012;46:57‐64. [PubMed] [Google Scholar]
- 31. Arda B, Durusoy R, Yamazhan T, et al. Did the pandemic have an impact on influenza vaccination attitude? A survey among health care workers. BMC Infect Dis. 2011;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giannattasio A, Mariano M, Romano R, et al. Sustained low influenza vaccination in health care workers after H1N1 pandemic: a cross sectional study in an Italian health care setting for at‐risk patients. BMC Infect Dis. 2015;15:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non‐commercial purposes, without breaching participant confidentiality. Moreover, the authors ensure that their datasets are presented in the main manuscript.


