Dear Editor,
Different cutaneous manifestations of coronavirus disease 2019 (COVID‐19) has been reported, some of which are due to the adverse effects of the medications. Favipiravir, a broad spectrum antiviral, effective against RNA viruses including influenza and Ebola, is one of the current medications approved for the treatment of Covid‐19. 1
Favipiravir selectively inhibits the RNA‐dependent RNA polymerase by acting as a guanine analog. It transforms into active phosphoribosylated form in cells, causing chain termination, slowing viral RNA synthesis and lethal mutagenesis. 2 Despite the lack of double‐blind randomized controlled studies regarding its effects on severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), favipiravir has been employed in patients in our country among with Russia, China and India. 3 It is prescribed to all patients with a positive Reverse Transcriptase Polymerase Chain Reaction (PCR) test. Favipiravir is effective on COVID‐19 only at high doses. 4 Current treatment scheme consists of 1600 mg bid on the first day followed by 600 mg bid po reaching a total dose of 8000 mg in Turkey. Lungs, kidneys, spleen, and brain are main targets for Favipiravir. 5 No cutaneous side effect of favipiravir has been reported. 6
The main purpose of the Wood's lamp examination is to enhance the visibility of some substances by using their fluorescence properties. Fluorescence may occur due to certain substances like elastin, collagen, melanin precursors found naturally in the skin or external factors like drugs. Drug metabolites or excipients may accumulate in the skin and cause fluorescence. Yellow fluorescence is observed in both lunula and nails after oral tetracycline and yellow‐green fluorescence on the nails is seen due to quinacrine hydrochloride. 7
We aimed to present the fluorescence of the nails and hair of our five patients, four of whom received only oral favipiravir treatment and paracetamol for COVID‐19 (Table 1). It was observed that the fluorescent parts of the nails were proportionally elongated with the drug initiation time. While fluorescence was seen in the middle portion of nail plates in Case 1, it was seen half portion of the nail plates near the proximal nail folds in Case 2 and 3. Greenish fluorescence was seen in the lunula and nail plate portion near proximal nail fold in Case 4 who used the drug recently. Green fluorescence was also observed on the hair of the four patients. No fluorescence was observed on the nails and hair of Case 5 who did not have favipiravir but had paracetamol (Figures 1, 2, 3, 4). This suggests that the fluorescence formation in the nails is due to favipiravir use.
TABLE 1.
The demographic properties and clinical findings of the patients
| Case | Age | Gender | BMI kg/m2 | Symptoms | Days passed a | Total dose of favipiravir | Fluorescence on nails | Fluorescence on hair |
|---|---|---|---|---|---|---|---|---|
| 1 | 27 | Female | 19.8 | None | 54 | 8000 mg | The middle portion of the nail plate and no fluorescence on the proximal and distal part of the nail plate. | Yes |
| 2 | 42 | Female | 32.8 | Loss of taste and smellHeadacheNausea vomitingDiarrhea | 36 | 8000 mg | One half of the nail plate beginning from the proximal nail fold | Yes |
| 3 | 51 | Female | 27.9 | CoughBack painWeaknessLoss of taste and smell | 30 | 8000 mg | One half of the nail plate beginning from the proximal nail fold | Yes |
| 4 | 30 | Female | 26.6 | Nausea vomitingHeadache | 17 | 5600 mg b | The portion of the nail plate near to the proximal nail fold and lunula. | Yes |
| 5 | 29 | Male | 34.7 | None | 17 | None | No | No |
Days passed after PCR positivity on Wood's examination day.
Quitted the drug because of nausea.
FIGURE 1.
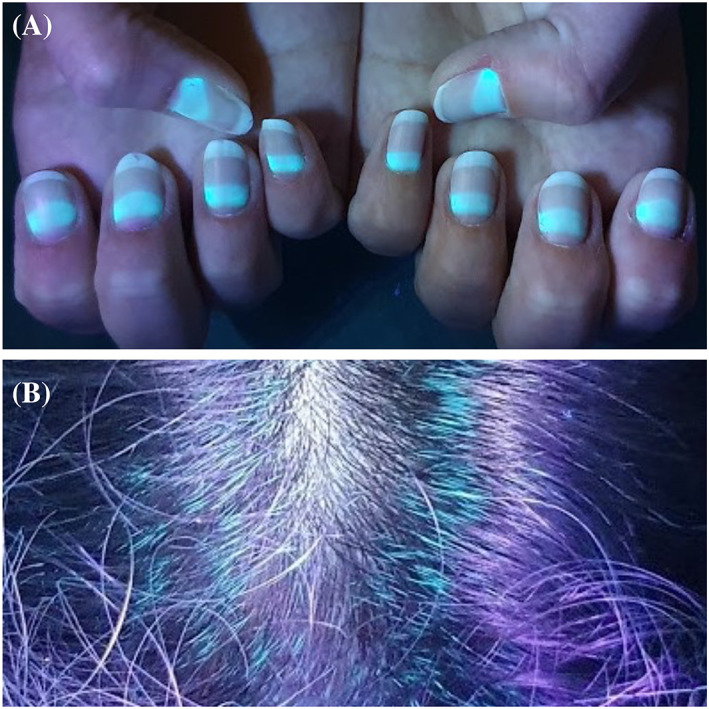
Fluorescence on the nail and hair on Case 1. Fluorescence is seen on the middle portion of the nail plates and no fluorescence on the proximal and distal part of the nail plates
FIGURE 2.

A,B, Fluorescence on the nail and hair on Case 2. C,D, Fluorescence on the nail and hair on Case 3. Fluorescence is seen on one half of the nail plate beginning from the proximal nail fold
FIGURE 3.
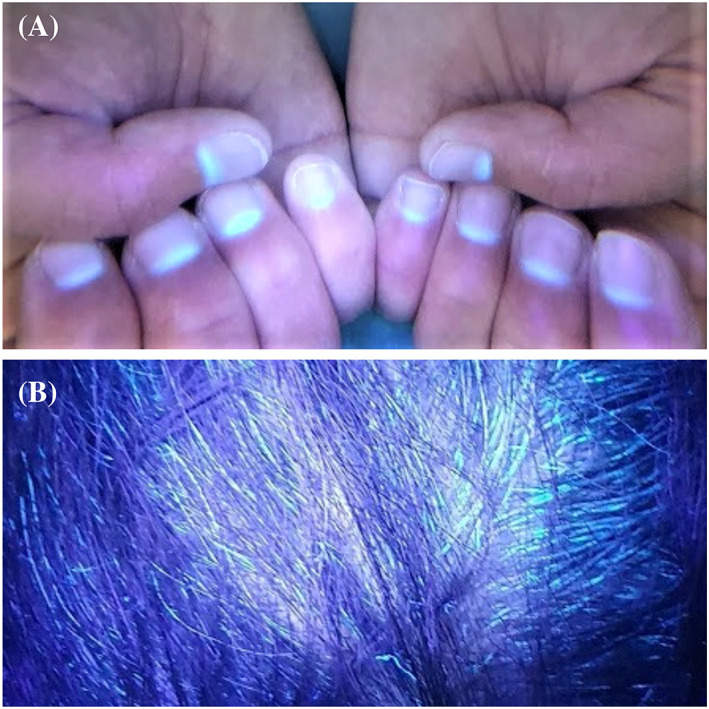
Fluorescence on the nail and hair on Case 4. Fluorescence is seen on the portion of the nail plate near to the proximal nail fold and lunula
FIGURE 4.
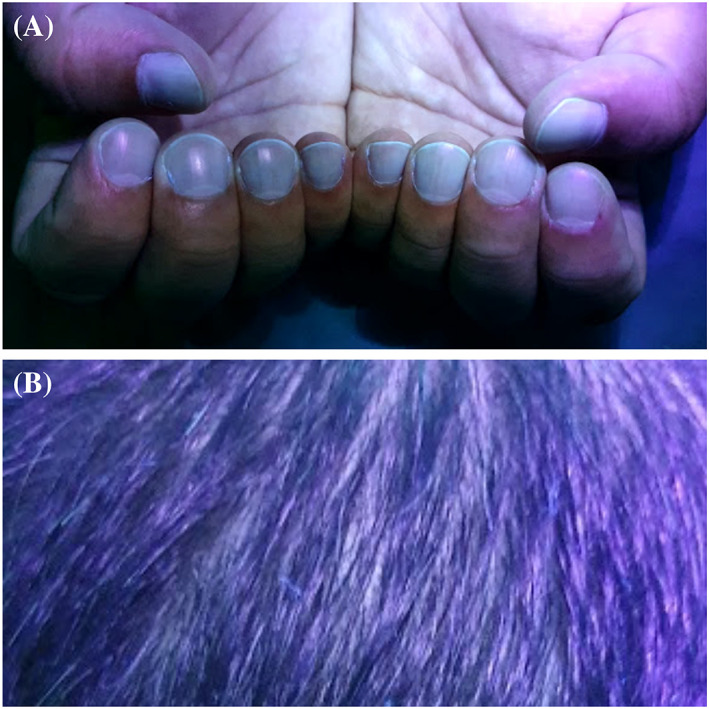
The nail and hair of Case 5. No fluorescence is seen
The fluorescence appearance seen in human hair and nails due to favipiravir use has not been reported. In fact, it is a mystery whether the fluorescent substance is mainly caused by the drug metabolites or by the ingredients such as titanium dioxide, yellow ferric oxide in the tablet. The drug's active phosphorylated metabolite was shown in human plasma and its concentration and fluorescence intensity had shown a linear relationship. 8 Systemically taken metals like titanium create temporary concentrations in blood/body fluids, but have continuity in the the skin appendages like nails and hair which could show the past exposure. 9 , 10 The phosphorylated metabolite or the ingredients may explain the fluorescence being present only in the proximal part of the nail plate in patients with recent drug use and the fluorescence retention of the ½ portion of the nail plate in the patients who used the drug a month ago.
Considering that the substance accumulated in the tissues like hair and nails with a high dose of favipiravir, it may be necessary to be careful in accumulation in the other tissues. Since it is known that the drug distributes in the kidney, lungs, spleen and brain, and titanium dioxide in organs such as the liver and spleen more, caution should be taken in patients with comorbidities such as liver and kidney dysfunction.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank our patients, all of whom are health care workers.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Yaghoubi A, Amel Jamehdar S, Movaqar A, Milani N, Soleimanpour S. An effective drug against COVID‐19: reality or dream? Expert Rev Respir Med. 2020;1‐14. 10.1080/17476348.2021.1854092. [DOI] [PubMed] [Google Scholar]
- 2. Shannon A, Selisko B, Le N, et al. Favipiravir strikes the SARS‐CoV‐2 at its achilles heel, the RNA polymerase. bioRxiv. 2020. 10.1101/2020.05.15.098731. [DOI] [Google Scholar]
- 3. Coomes EA, Haghbayan H. Favipiravir, an antiviral for COVID‐19? J Antimicrob Chemother. 2020;75(7):2013‐2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaptein SJF, Jacobs S, Langendries L, et al. Favipiravir at high doses has potent antiviral activity in SARS‐CoV‐2‐infected hamsters, whereas hydroxychloroquine lacks activity. Proc Natl Acad Sci U S A. 2020;117(43):26955‐26965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Y, Chen L. Tissue distributions of antiviral drugs affect their capabilities of reducing viral loads in COVID‐19 treatment. Eur J Pharmacol. 2020;889:173634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez‐Lopez A, Cuenca‐Barrales C, Montero‐Vilchez T, Molina‐Leyva A, Arias‐Santiago S. Review of adverse cutaneous reactions of pharmacologic interventions for COVID‐19: a guide for the dermatologist. J Am Acad Dermatol. 2020;83(6):1738‐1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hendricks AA. Yellow lunulae with fluorescence after tetracycline therapy. Arch Dermatol. 1980;116(4):438‐440. [PubMed] [Google Scholar]
- 8. Megahed SM, Habib AA, Hammad SF, Kamal AH. Experimental design approach for development of spectrofluorimetric method for determination of favipiravir; a potential therapeutic agent against COVID‐19 virus: application to spiked human plasma. Spectrochim Acta A Mol Biomol Spectrosc. 2020;249:119241 10.1016/j.saa.2020.119241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jani PU, McCarthy DE, Florence AT. Titanium dioxide (rutile) particle uptake from the rat GI tract and translocation to systemic organs after oral administration. Int J Pharm. 1994;105(2):157‐168. [Google Scholar]
- 10. Abdulrahman FI, Akan JC, Chellube ZM, Waziri M. Levels of heavy metals in human hair and nail samples from Maiduguri metropolis, Borno state, Nigeria. World Environ. 2012;2(4):81‐89. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


