Abstract
Background
The preliminary report of the RECOVERY large randomised controlled trial indicated a promising survival effect for dexamethasone therapy of coronavirus disease 2019 (COVID‐19). This study aimed to investigate the anti‐hypoxic activities of dexamethasone to understand a possible mechanism of its action in hypoxia‐induced lethality through experimental models of hypoxia.
Methods
In this investigation, 84 Male BALB/c mice were randomly divided into groups of seven (12 groups). Treatment groups received 10 days of dexamethasone intraperitoneal injection at both human dose (~0.1 mg/kg) and the animal does (~1 mg/kg). Control negative and positive groups were treated with 10 ml/kg of normal saline and 30 mg/kg of propranolol, respectively. Three experimental models of hypoxia, asphyctic, circulatory, and hemic were applied in this study.
Results
The findings showed that dexamethasone significantly prolonged the latency for death in the asphyctic model concerning the control group in both humans (P < .0001) and animal dose (P < .0001). The results were also highly significant for both doses in the hemic model (P < .001). In the circulatory model, although a small increase was observed in death prolongation, results were not statistically significant for both doses in this model (P > .05).
Conclusions
This experimental in vivo investigation demonstrated an excellent protective effect for 10 days of dexamethasone treatment against hypoxia, especially in asphyctic and hemic models. In addition to promising dexamethasone outcomes, using propranolol as the positive control illustrated a very substantial anti‐hypoxic effect even much better than dexamethasone in all models. It seems that propranolol would be a safe, potential, and prudent choice to invest in treating COVID‐19 patients.
What’s known
The RECOVERY large randomised controlled trial indicated a promising survival effect for dexamethasone therapy of coronavirus disease 2019.
Corticosteroids (eg, methylprednisolone and dexamethasone) have anti‐inflammatory, antifibrotic, and vasoconstrictive activities.
What’s new
Dexamethasone has an excellent anti‐hypoxia effect, which may reveal one of the primary mechanisms for dexamethasone's clinical benefits against COVID‐19.
Propranolol illustrated a very significant anti‐hypoxic effect even much better than dexamethasone.
1. INTRODUCTION
A novel coronavirus appeared in Wuhan, China, in December 2019 and has been named severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), and it induces coronavirus disease 2019 (COVID‐19). The World Health Organization (WHO) declared the novel coronavirus outbreak as a pandemic on March 11, 2020. 1 At the time of writing, SARS‐CoV‐2 has caused >40 000 000 infections, and >1 000 000 deaths worldwide. Despite intensive efforts, much remains unknown of many aspects of SARS‐CoV‐2 infection. Many treatment approaches have been proposed with many rapid preprints and publications with conflicting results. Hence, there is much to learn about the pathogenesis and effective treatment approaches for SARS‐CoV‐2 and COVID‐19.
Corticosteroids (eg, methylprednisolone and dexamethasone) have anti‐inflammatory, antifibrotic, and vasoconstrictive activities. Such medications have been widely used to improve outcomes in individuals with acute respiratory distress syndrome (ARDS), pneumonia, and septic shock. 2 , 3 In recent years, different well‐designed clinical trials have demonstrated promising clinical benefit for corticosteroids in ARDS and septic shock. 4 , 5 , 6
In this case, one of the conflicting treatment choices since the emergence of the COVID‐19 was dexamethasone. The preliminary report of the RECOVERY large randomised controlled trial 7 indicated a promising survival effect for dexamethasone therapy of coronavirus disease 2019 (COVID‐19) patients at a dose of 6 mg q.d. for up to 10 days. The majority of the patients infected with SARS‐CoV‐2 are asymptomatic or only manifest a mild disease. However, the infection can lead to critical stages and cause acute hypoxemic respiratory failure requiring supplemental oxygen. 7 , 8 Remarkably, the RECOVERY study indicated that the treatment approach was significant amongst patients with hypoxemia under the invasive/non‐invasive respiratory support, but not in mild patients without hypoxemia and breathing support.
As a corticosteroid, dexamethasone widely affects innate and adaptive immunity, especially as an anti‐inflammatory agent. Regardless of dexamethasone's studied activities, following the RECOVERY study results, we aimed to simulate this study's treatment approach and investigate the anti‐hypoxic activities of dexamethasone to understand a possible mechanism of its action in hypoxia‐induced lethality through experimental models of hypoxia.
2. METHODS
2.1. Ethics statement and animal treatment protocols
All the experimental procedures were performed based on the US National Institutes of Health guidelines of the Laboratory Animal Care and Use. 9 The Animal Ethical Committee of Mazandaran University of Medical Sciences has approved the experimental protocol (IR.MAZUMS.REC.1399.546). Male BALB/c mice were purchased at 10‐12 weeks of age from Royan Institute (Tehran, Iran).
2.2. Animals
In this investigation, 84 Male BALB/c mice (32.98 ± 1.04 g) were randomly divided into groups of seven (12 groups) in polypropylene cages at ambient temperature, 25 ± 1°C and 45%‐55% relative humidity, with a 12 hours light: 12 hours dark cycle (lights on at 7 am). The animals had free access to standard water, pellet fuel by ad libitum access. Experiments were performed between 8:00 and 14:00 hours.
2.3. Treatments
According to Table 1, treatment groups received 10 days of dexamethasone intraperitoneal (ip) injection at both human dose (6 mg once daily based on body surface area = ~0.1 mg/kg) and the animal does (~1 mg/kg for mice). 10 Control negative groups were treated with 10 ml/kg of normal saline and control positive groups with 30 mg/kg of propranolol at the same time plan.
TABLE 1.
Treatment regimens
| Experiment model | Group | n | DEXH a | DEXA b | NS c | PRO d | Treatment period (d) |
|---|---|---|---|---|---|---|---|
| Asphyctic | GP1 | 7 | — | — | Yes | — | 10 |
| GP2 | 7 | Yes | — | — | — | 10 | |
| GP3 | 7 | — | Yes | — | — | 10 | |
| GP4 | 7 | — | — | — | Yes | 10 | |
| Circulatory | GP5 | 7 | — | — | Yes | — | 10 |
| GP6 | 7 | Yes | — | — | — | 10 | |
| GP7 | 7 | — | Yes | — | — | 10 | |
| GP8 | 7 | — | — | Yes | 10 | ||
| Hemic | GP9 | 7 | — | — | Yes | — | 10 |
| GP10 | 7 | Yes | — | — | — | 10 | |
| GP11 | 7 | — | Yes | — | — | 10 | |
| GP12 | 7 | — | — | — | Yes | 10 |
Abbreviations: DEX, dexamethasone; NS, normal saline; PRO, propranolol.
Dexamethasone human dose ~ 0.1 mg/kg.
Dexamethasone human dose ~ 1 mg/kg.
10 mL/kg.
30 mg/kg.
2.4. Experimental models
Three experimental models of hypoxia; asphyctic, circulatory, and hemic were applied in this study. Asphyctic hypoxia model induced to the animals by putting them individually in a closed 300 mL glass container coated tightly with parafilm 30 minutes after the final ip injection of dexamethasone on day 11. The animals died following convulsions because of hypoxia. The latencies for death were recorded. Circulatory and Hemic hypoxia models were applied to the animals through ip injection of NaF (150 mg/kg) and NaNO2 (360 mg/kg), respectively, on day 11, 30 minutes after the final dexamethasone treatment. Finally, the death latencies were recorded.
2.5. Statistical analysis
The GraphPad Prism v.8 was used for Statistical Analysis. Data were presented as mean ± SD. Tukey's multiple comparison test was used to determine the mean differences. All P‐value less than 0.05 is considered statistically significant.
3. RESULTS
According to Figure 1, the findings indicated that dexamethasone significantly prolonged the latency for death in the asphyctic model concerning the control group in both humans (15.05 ± 0.83 vs 21.43 ± 1.29 minutes, P < .0001) and animal dose (15.05 ± 0.83 vs 20.38 ± 1.73 minutes, P < .0001). The results were also highly significant for both doses in the hemic model (Human dose; 10.56 ± 1.20 vs 12.49 ± 0.96 minutes, P < .001, Animal dose; 10.56 ± 1.20 vs 12.50 ± 0.71 minutes, P < .001). In the circulatory model, although a small increase was observed in death prolongation with 0.1 mg/kg of dexamethasone (10.60 ± 1.46 vs 11.09 ± 1.24 minutes, P > .05), results were not statistically significant for both doses in this model (P > .05).
FIGURE 1.
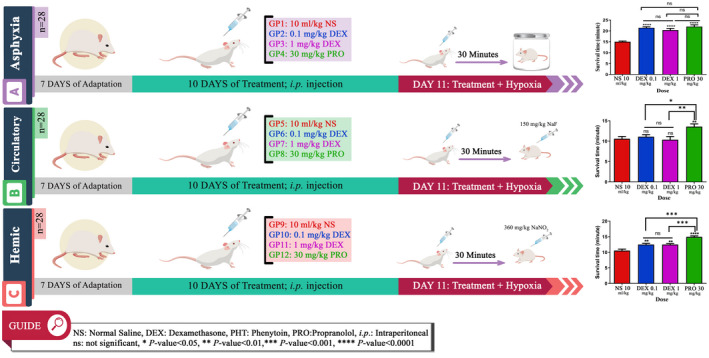
The experiment process and results
4. DISCUSSION
After the RECOVERY press release, several multicentre RCTs have investigated the effects of corticosteroids COVID‐19 patients. These studies and the WHO Rapid Evidence Appraisal for COVID‐19 Therapies (REACT) meta‐analysis indicated a promising clinical benefit for corticosteroids against COVID‐19 patients, especially in severe condition. 11 , 12 , 13 , 14 In this regard, some physicians may ask why corticosteroids are beneficial in COVID‐19‐related ARDS patients after many heterogeneous findings of corticosteroid therapy in ARDS patients in recent decades. To find an answer to one aspect of this question, we performed an experimental study investigating dexamethasone's protective effects against hypoxia‐induced lethality in mice.
Hypoxia condition leads to intense physiological stress and induces a wide range of toxic effects at the cellular level. Oxygen as a source of ROS formation is a vital component of organisms and normal redox reactions in the cell. 15 It has been indicated that both hypoxia and hyperoxia can promote oxidative damage and increase the risk of morbidity and mortality. 16 Studies demonstrated that hypoxia interrupts mitochondrial function and increases ROS production and oxidative stress, which could trigger apoptosis signalling. 17 , 18
The brain uses a large amount of oxygen, extremely susceptible to low oxygen levels. 19 Free radicals play a critical role in signalling species in many regular physiological processes, but such radicals’ excessive production leads to damage biological material. The high levels of ROS in hypoxia caused by the accumulation of reducing equivalents in the mitochondrial electron transport system. 20 ROS’s effects can be observed mainly in specific tissues, such as the brain because it uses approximately 1/5 of the basal oxygen.
Hemoglobin is an oxygen carrier in red blood cells. Any interferences in hemoglobin's performance with oxygen transport lead to hypoxia conditions. Then, lack of oxygen in the environment, followed by mitochondrial low oxygen partial pressure, causes cell death because of inadequate energy. Available research studies illustrate that using NaF induces circulatory hypoxia, increasing the blood histamine content and decreasing the oxygen‐carrying capacity. 21 In the hemic hypoxia model induced to the mice, NaNO2 reduces the blood's oxygen‐carrying capacity by converting hemoglobin to methemoglobin and breaking the respiratory chain, preventing the cell from using oxygen to produce energy. 22 , 23
Herein, this experimental in vivo investigation, which was designed and simulated based on the RECOVERY study, demonstrated an excellent protective effect for 10 days of dexamethasone treatment against hypoxia, especially in asphyctic and hemic models. These findings may reveal one of the primary mechanisms for dexamethasone's clinical benefits against COVID‐19.
More interesting, in addition to promising outcomes of dexamethasone, using 30 mg/kg of propranolol as the positive control drug in this study illustrated a very considerable anti‐hypoxic effect even much better than dexamethasone in all models (Figure 1).
The propranolol is a nonselective β‐blocker agent that has indicated fantastic activities such as an increase of oxygen utilisation during hypoxia, 24 regulation of inflammatory cytokines, 25 cell membrane stabilisation, 26 anti‐thrombotic properties, 27 etc in different studies. Hence, this medication, which is available as 10, 20, 40, 60, and 80 mg tablets for oral administration with the maximum recommended human oral daily dose of 640 mg (FDA Reference ID: 2919389), would be a potential and prudent choice to invest for the treatment of COVID‐19 patients with hypoxemia through randomised clinical trials, especially in the continuation of the RECOVERY Collaborative Group investigation, who are highly appreciated by our team and world medical communities in these challenging times.
5. CONCLUSION
This experimental in vivo investigation demonstrated an excellent protective effect for 10 days of dexamethasone treatment against hypoxia, especially in asphyctic and hemic models. In addition to promising dexamethasone outcomes, using 30 mg/kg of propranolol as the positive control drug in this study illustrated a very considerable anti‐hypoxic effect even much better than dexamethasone in all models. It seems that propranolol would be a safe, potential, and prudent choice to invest in treating COVID‐19 patients with hypoxemia through a randomised clinical trial, especially in the continuation of the RECOVERY study.
DISCLOSURE
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
All of the authors (MA.E, MH.H, and A.Sh) were contributed to study design, performing experiments, and manuscript preparation.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Animal Ethical Committee of Mazandaran University of Medical Sciences has approved the experimental protocol (IR.MAZUMS.REC.1399.546).
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
We express our gratitude to the Vice‐Chancellor for Research at Mazandaran University of Medical Sciences for supporting this study.
Hosseinzadeh MH, Shamshirian A, Ebrahimzadeh MA. Dexamethasone vs COVID‐19: An experimental study in line with the preliminary findings of a large trial. Int J Clin Pract.2021;75:e13943. 10.1111/ijcp.13943
Funding information
Funded by Vice‐Chancellor for Research at Mazandaran University of Medical Sciences.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Organization WH . Coronavirus disease (COVID‐2019) situation reports. Geneva, Switzerland: WHO; 2020. [Google Scholar]
- 2. Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788‐800. [DOI] [PubMed] [Google Scholar]
- 3. Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet (London, England). 1967;2:319‐323. [DOI] [PubMed] [Google Scholar]
- 4. Villar J, Ferrando C, Martínez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267‐276. [DOI] [PubMed] [Google Scholar]
- 5. Venkatesh B, Finfer S, Cohen J, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378:797‐808. [DOI] [PubMed] [Google Scholar]
- 6. Annane D, Renault A, Brun‐Buisson C, et al. Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med. 2018;378:809‐818. [DOI] [PubMed] [Google Scholar]
- 7. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid‐19 ‐ preliminary report. N Engl J Med. 2020. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infect Dis. 2020;20:669‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Institute of Laboratory Animal Resources. Committee on C, Use of Laboratory A . Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 10. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator‐free in patients with moderate or severe acute respiratory distress syndrome and COVID‐19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID‐19: a meta‐analysis. JAMA. 2020;324:1330‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dequin P‐F, Heming N, Meziani F, et al. Effect of hydrocortisone on 21‐day mortality or respiratory support among critically ill patients with COVID‐19: a randomized clinical trial. JAMA. 2020;324:1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Angus DC, Derde L, Al‐Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID‐19: the REMAP‐CAP COVID‐19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Omrani H, Alipour MR, Mohaddes G. Ghrelin improves antioxidant defense in blood and brain in normobaric hypoxia in adult male rats. Adv Pharm Bull. 2015;5:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao HW, Haddad GG. Review: Hypoxic and oxidative stress resistance in Drosophila melanogaster. Placenta. 2011;32:S104‐S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guzy RD, Mack MM, Schumacker PT. Mitochondrial complex III is required for hypoxia‐induced ROS production and gene transcription in yeast. Antioxid Redox Signal. 2007;9:1317‐1328. [DOI] [PubMed] [Google Scholar]
- 18. Satoh T, Enokido Y, Aoshima H, Uchiyama Y, Hatanaka H. Changes in mitochondrial membrane potential during oxidative stress‐induced apoptosis in PC12 cells. J Neurosci Res. 1997;50:413‐420. [DOI] [PubMed] [Google Scholar]
- 19. Warner DS, Sheng H, Batinić‐Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221‐3231. [DOI] [PubMed] [Google Scholar]
- 20. Tibor B, Zsolt R. High altitude and free radicals. J Sports Sci Med. 2004;3:64‐69. [PMC free article] [PubMed] [Google Scholar]
- 21. Eslami B, Nabavi SF, Nabavi SM, Ebrahimzadeh MA, Mahmoudi M. Pharmacological activities of Hypericum scabrum L. Eur Rev Med Pharmacol Sci. 2011;15:532‐537. [PubMed] [Google Scholar]
- 22. Prabhakaran K, Li L, Borowitz JL, Isom GE. Caspase inhibition switches the mode of cell death induced by cyanide by enhancing reactive oxygen species generation and PARP‐1 activation. Toxicol Appl Pharmacol. 2004;195:194‐202. [DOI] [PubMed] [Google Scholar]
- 23. Bingquan C, Weiqiao H, Yuanzao L. Experimental study of the anti‐fatigue and anti‐hypoxia function of Phyllanthus emblica L. in mice. Mod Chin Med. 2008:11. [Google Scholar]
- 24. Khambatta HJ, Stone JG, Askanazi J, Khan E. Propranolol increases oxygen utilization during hypoxia. Br J Anaesth. 1987;59:1171‐1176. [DOI] [PubMed] [Google Scholar]
- 25. Hajighasemi F, Mirshafiey A. Regulation of inflammatory cytokines in human immunocompetent cells by propranolol in vitro. Eur Respir J. 2016;48:PA1083. [Google Scholar]
- 26. Montazeri M, Ebrahimzadeh MA, Ahmadpour E, Sharif M, Sarvi S, Daryani A. Evaluation of propranolol effect on experimental acute and chronic toxoplasmosis using quantitative PCR. Antimicrob Agents Chemother. 2016;60:7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bonten TN, Plaizier CE, Snoep JJ, Stijnen T, Dekkers OM, van der Bom JG. Effect of β‐blockers on platelet aggregation: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2014;78:940‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


