Abstract
This study was performed to determine whether Autologous Matrix-Induced Chondrogenesis (AMIC) is an effective and safe treatment option for patients with symptomatic Osteochondral defects of the Talus (OCTs) and to identify factors that influence the clinical outcome. A systematic review of the literature was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Three reviewers independently conducted the literature search using the MEDLINE/PubMed database and the Cochrane Database of Systematic Reviews. The databases were queried using the terms “autologous” AND “matrix” AND “induced” AND “chondrogenesis.” Thirteen studies were eligible for review. All studies that compared the preoperative and postoperative mean values of different clinical/functional scores showed significant clinical improvement. The final postoperative mean Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score ranged from 50.9 to 74.5. The included studies indicated that age and body mass index may have a detrimental impact on the postoperative outcome. A higher re-intervention rate is expected with the open technique, mainly because of hardware removal after malleolar osteotomy. This data analysis demonstrated that both arthroscopic and open AMIC procedures are effective and safe for the treatment of OCTs. Level IV, systematic review of therapeutic studies
Key words: Autologous matrix-induced chondrogenesis, osteochondral lesion, talus, ankle, cartilage repair
Introduction
Since the late 1980s, the diagnosis of osteochondral talar lesions has dramatically increased because of the development of new diagnostic modalities.1 Many of these lesions are associated with traumatic events and are prevalent within active populations.2,3 According to some authors, osteochondral defects of the talus (OCTs) can occur in up to 70% of acute ankle sprains and fractures.4 Non-operative treatment has shown good results for selected indications in children and adolescents, especially in the early stages of osteochondritis dissecans. However, a recent study showed that half of patients with OCTs fail to experience resolution of their symptoms by conservative means.5 The other treatment option comprises surgical modalities, which are usually indicated for symptomatic osteochondritis dissecans in adolescents and adults depending on the size and location of the lesion.6
The surgical treatment options for cartilage defects have substantially increased during the past several years. Debridement, drilling, microfracture, and abrasion chondroplasty for osteochondral lesions of the knee stimulate the release of mesenchymal stem cells and growth factors that result in fibrocartilaginous tissue, which may degenerate with time.7 The use of microfractures for the treatment of OCTs has also been described.8 Similarly, treatment options such as Autologous Chondrocyte Implantation (ACI), matrix-induced ACI, and osteochondral transplantation procedures are also available for the talus. ACIrelated techniques are performed in two stages. During the first stage, a region of healthy articular cartilage, usually in the ipsilateral knee, is biopsied, and the harvested chondrocytes are isolated and cultured. A second procedure is necessary for chondrocyte reimplantation.9 The osteochondral transplantation technique involves harvesting of native cylindrical osteochondral grafts followed by transplantation of the grafts into the site of the talar defect.10 The concept of this technique is to replace the damaged cartilage with an autologous graft that has biological and mechanical properties similar to those of the ambient hyaline tissue. Despite many reports on the effectiveness and safety of all available treatment options and techniques, the lack of a homogenous methodology and standardized outcome evaluation has prevented the establishment of a gold standard treatment for this challenging clinical entity.5
A relatively new option for the treatment of OCTs is Autologous Matrix- Induced Chondrogenesis (AMIC). This method seems to provide a cost-effective alternative to cell-based therapies for articular cartilage repair, and it is highly autologous in nature.11 AMIC was first described by Benthien and Behrens12 for the treatment of cartilage defects of the knee. They used an awl to perform perforations in the subchondral bone and commercially available fibrin glue to adhere a collagen membrane (Chondro-Gide®; Geistlich Biomaterials, Wolhusen, Switzerland) to the lesion. The goal was to improve the quality of the repair tissue by capturing more mesenchymal cells and growth factors while additionally offering a scaffold on which the cells can proliferate. The Transforming Growth Factor beta (TGFβ) component of the fibrin glue used in this technique may contribute to the chondrogenic differentiation of mesenchymal stem cells.13
Several clinical trials have been published regarding the use of AMIC for ankle osteochondral lesions.14-26 The present systematic review was performed to determine whether the AMIC procedure is an effective and safe treatment for patients with symptomatic OCTs and whether differences exist between all-arthroscopic AMIC and open AMIC in the ankle. Additionally, we investigated whether any influencing factors consistently affect the postoperative outcome. Our hypothesis was that the AMIC procedure is safe and effective in the treatment of OCTs regardless of the way in which it is applied.
Materials and Methods
A systematic review of the literature was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Three reviewers (MAM, LK, and PDM) independently conducted the search using the MEDLINE/PubMed database and the Cochrane Database of Systematic Reviews. These databases were queried with the terms “autologous” AND “matrix” AND “induced” AND “chondrogenesis.” Backward chaining of the reference lists from the retrieved papers was performed to maximize the search range.
The inclusion criteria were full-text articles referring to clinical studies of patients with OCTs that were treated with AMIC. The clinical trials that were chosen contained a clinical follow-up evaluation (with tests and/or scores), and only articles in English were used. Furthermore, all articles were published until 15 March 2020 (end of our search).
The exclusion criteria were preclinical, cadaveric, or biomechanical studies; trials with no clinical and/or Magnetic Resonance Imaging (MRI) outcomes; studies with less than 2 years of follow-up; studies assessing the outcome of the AMIC procedure in other joints (knee, hip, shoulder); and studies focusing on other types of biological treatments such as ACI, matrix-induced ACI, osteochondral autograft transfer system, microfractures, bone marrow aspirate concentrate, and other types of surgical or conservative treatments. Studies involving patients with lesions other than OCTs were also excluded from our survey. Finally, we excluded abstracts, editorial comments, technical notes, expert opinions, case reports, and literature reviews or metaanalyses.
Differences among the reviewers (MAM, LK, and DC) were discussed until agreement was achieved. We independently extracted data from each study and assessed variable reporting of the outcome data. Descriptive statistics were calculated for each study and parameters were analyzed. The primary outcome variables were functional scores and radiological scores, as reported in the final follow-up of each study. The secondary outcomes were the reoperation and complication rates per study. We also searched for factors that may influence the postoperative outcome.
The level of evidence was categorized according to the definition established by the Oxford Centre for Evidence-Based Medicine.27 The modified Coleman Methodology Score was determined for each study to assess the methodological quality of the collected data.28,29 The studies were graded by assigning a score for each of 10 criteria (range, 0–100). Excellent studies had a score of 85 to 100, good studies had a score of 70 to 84, fair studies had a score of 55 to 69, and poor studies had a score of less than 55.30,31 The methodological quality of each study was independently assessed by each reviewer, and a consensus was then reached.
Results
Among the 145 initial studies, we finally chose and assessed 13 clinical studies that were eligible according to our inclusion and exclusion criteria.14-26A summary of our literature search is depicted in the PRISMA flow chart shown in Figure 1.
Level of evidence and quality of studies
The level of evidence was III in two studies15,23 and IV in the remaining studies. 14,16-22,24-26 The mean Coleman Methodology Score was 62.8 (range, 49– 68).
General characteristics
The 13 studies involved a total of 348 patients (mean age, 34.3 years; 200 males, 148 females). Seven studies focused on the open AMIC technique and comprised 196 patients (118 males, 78 females).14,19-21,24-26 The mean patient age ranged from 33 to 42.6 years, and the mean follow-up ranged from 30 to 60 months. The other six studies focused on the arthroscopic AMIC technique and comprised 152 patients (82 males, 70 females).15-18,22,23 The mean age of the patients in these studies ranged from 17.9 to 36.1 years, and the mean follow-up ranged from 24 to 68.4 months. D’Ambrosi et al.17 reported a case series of young patients (mean age, 17.9 years) with juvenile OCTs (Table 1).
Figure 1.
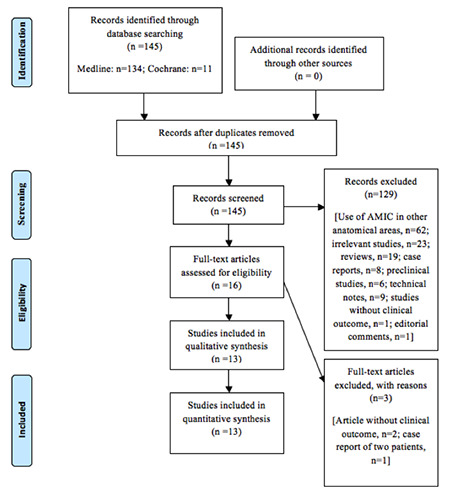
PRISMA flow chart.
Two of the 13 studies involved specific population groups.16,23 Usuelli et al.23 compared the outcomes of an obese group of patients with a mean body mass index (BMI) of 27.41 kg/m2 and a healthy-weight group of patients with a mean BMI of 21.90 kg/m2. Another study by the same team reported the results of two age groups: ≤33 and >33 years (Table 1).16
Autologous bone grafting was performed in 12 studies.14,16-26 In nine studies, all included patients underwent AMIC combined with autologous bone grafting16-19,21-24,26 while in three studies, only some patients underwent additional grafting.14,20,25 The use of a malleolar osteotomy to approach the talar lesion was reported in five studies.14,21,24-26 Concomitant surgical procedures were also identified: calcaneal osteotomy for hindfoot malalignment was performed in one study,26 and ligamentous repair to address ankle instability was performed in two studies.24,26 Posterior ankle arthroscopy and flexor hallucis longus debridement was additionally performed in another study.19 These concomitant procedures had no adverse effects on the final outcome and no associated complications.
Clinical outcomes
Seven different scores were used for the functional assessment. The American Orthopaedic Foot and Ankle Society (AOFAS) score was the most common; it was recorded in 10 studies.14,16-18,21-26 The difference between the preoperative and postoperative score was assessed, and the score was found to have improved (mean, 82.5 points) in 8 of the 10 studies.14,16-18,22-24,26 Twelve studies estimated the Visual Analog Scale (VAS) score for pain,14-19,21-26 and all of these studies demonstrated that the postoperative score was better than the preoperative score. The Foot Function Index (FFI) score was used in three studies, all of which focused on the open AMIC technique.14,20,21 In one of these studies, a significant improvement was shown between the preoperative and postoperative measurements.20 The 12-Item Short-Form Survey (SF-12) score was calculated in five studies, all of which focused on the arthroscopic AMIC technique.16-18,22,23 The mean final postoperative SF-12 score was improved in all of these studies in both the mental and physical subscales.16-18,22,23 Improvement between the preoperative and postoperative values was also found in one study that used the Hannover Scoring System.15 Some authors recorded improvement between the preoperative and postoperative Tegner scores,25 while others found no difference (Table 2).26
Imaging and lesion size assessment
MRI was used in most of the studies of the present review.14-25 The well-established Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) scoring system, or a modified form of it,32 was used in 8 of the 13 studies.14,15,19-22,24,25 This scale consists of 9 major parameters and 25 items, and the score ranges from 0 to 100 points (best possible result, 100 points). The final postoperative MOCART score of the identified studies ranged from 50.9 to 74.5 points. Becher et al.15 did not use all the variables of the scoring system; thus, no MOCART score was calculated. Two studies that compared the 1- and 2-year MOCART scores showed a significant improvement;14,22 however, a third study revealed no differences in this score during the same follow-up period.19
Ten of the 13 studies assessed the OCT lesion size.15-18,20-25 The most common imaging modality used to evaluate the lesion size was MRI, which was used in nine studies.15-18,20-23,25 Five studies used Computed Tomography (CT) scans to measure the lesion, 16-18,22,23 while one study utilized single- photon emission CT–CT.26 Six studies compared the mean preoperative lesion size with the mean final postoperative size.16-18,20,22,23 All of these studies showed a significant decrease in the lesion size at the final follow-up (Table 3).16-18,20,22,23
Usuelli et al.23 reported that the patients in the overweight group had a larger mean preoperative lesion size than the patients in the healthy-weight group; however, they found no significant difference in the final postoperative lesion size between the groups. In one study, D’Ambrosi et al.16 found no significant difference in the postoperative lesion size between younger and older patients. In another study, however, the same group reported that patients with underlying bone edema had larger defects than those without edema on follow-up MRI and CT scans.18
Age and BMI
Five of the 13 papers examined the impact of age on the clinical parameters. 16,17,20,21,25 According to Gottschalk et al.,20 younger patients exhibited better outcomes on the pain and function subscales of the preoperative FFI. One study showed that the postoperative AOFAS score was better in young patients.16 Another study demonstrated insignificant correlations between the age at surgery and the preoperative VAS pain score or intraoperative lesion size.17 The preoperative mental component summary of the SF-12 was higher in older than younger patients.17 There were insignificant differences in the lesion size on CT and MRI scans between older and younger patients at each control point.17 Kubosch et al.21 showed that patients younger than 45 years had increased values for postoperative pain and lower values for overall contentment; however, Weigelt et al.25 found no correlation between age and clinical outcomes.
Table 1.
Demographics of included studies.
| Author(s) | Publication year | Patients (n) | Gender | Mean age (years) | Mean follow-up (months) |
|---|---|---|---|---|---|
| Valderrabano et al.24 | 2013 | 26 | 18M/8F | 33 | 31.0 |
| Kubosch et al.21 | 2015 | 17 | 9M/8F | 38.8 | 39.5 |
| D’Ambrosi et al.16 | 2016 | 31 (G1: 17, G2: 14) | 18M/13F (G1: 8M/9F, G2: 10M/4F) | 34.9 (G1: 25, G2: 47) | 27.0 |
| Wiewiorski et al.26 | 2016 | 60 | 36M/24F | 34.9 | 46.9 |
| Albano et al.14 | 2017 | 16 | 8M/8F | 42.6 | 30.0 |
| D’Ambrosi et al.17 | 2017 | 11 | 2M/9F | 17.9 | 24.0 |
| Galla et al.19 | 2017 | 23 | 15M/8F | 35.6 | 33.5 |
| Gottschalk et al.20 | 2017 | 21 | 13M/8F | 37.0 | 60.0 |
| Usuelli et al.23 | 2017 | 37 (HG: 21, OG: 16) | 22M/15F (HG: 10M/11F, OG: 12M/4F) | 33.4 (HG: 33, OG: 34) | 24.0 |
| Becher et al.15 | 2018 | 16 | 7M/9F | 32.4 | 68.4 |
| D’Ambrosi et al.18 | 2018 | 37 (GNE: 24, GE: 13) | 22M/15F (GNE: 14M/10F, GE: 8M/5F) | 34.0 (GNE: 31, GE: 38) | 24.0 |
| Usuelli et al.22 | 2018 | 20 | 11M/9F | 36.1 | 24.0 |
| Weigelt et al.25 | 2019 | 33 | 19M/14F | 35.1 | 55.4 |
| Total | 348 | 200M/148F | 34.3 | 37.5 |
M: males, F: females; HG: healthy-weight group, OG: overweight group; G1: patients <33 years, G2: patients >33 years; GNE: non-edema group, GE: edema group.
Five papers assessed the influence of the BMI on OCTs. 17,20,21,23,25 D’Ambrosi et al.17 noticed a positive correlation between the BMI and the size of the intraoperative lesion. Usuelli et al.23 found that the BMI was positively correlated with the lesion size on preoperative MRI. Gottschalk et al.20 reported that an increased BMI was correlated with worse outcomes on the FFID and both of its subscales (pain and function). According to Kubosch et al.,21 a high BMI was negatively correlated with the AOFAS score and was accompanied by higher postoperative VAS pain scores. As such, patients with a BMI of >30 kg/m2 had a lower AOFAS score and a higher VAS pain score postoperatively than did patients with a BMI of <30 kg/m2.21 However, Usuelli et al.23 reported no significant difference between overweight and healthyweight patients and found that both groups exhibited clinical improvement after surgery. Similarly, another study showed a tendency of poorer AOFAS scores in patients with higher BMIs; however, this finding was not statistically significant (Table 4).25
Table 2.
Clinical scores.
| Author(s) | AOFAS | HSS | VAS pain | SF-12 PCS | SF-12 MCS | FFI pain | FFI function | Tegner |
|---|---|---|---|---|---|---|---|---|
| Valderrabano et al.24 | Pre: 60 Post: 89 | N/A | Pre: 5 Post: 1.6 | N/A | N/A | N/A | N/A | N/A |
| Kubosch et al.21 | N/A Post: 82.6 | N/A | Pre: 7.8 Post: 3.2 | N/A | N/A | N/A Post: 31.0 | N/A Post: 35.9 | N/A |
| D’Ambrosi et al.16 | Pre: 53.0 Post: 89.0 | N/A | Pre: 7.8 Post: 1.8 | Pre: 30.4 Post: 50.6 | Pre: 42.5 Post: 53.5 | N/A | N/A | N/A |
| Wiewiorski et al.26 | Pre: 43 Post: 76 | N/A | Pre: 6.9 Post: 2.3 | N/A | N/A | N/A | N/A | Pre: 3.3 Post: 3.4 |
| Albano et al.14 | Pre: 60.2 Post: 77.4 | N/A | Pre: 6.3 Post: 2.9 | N/A | N/A | N/A Post: 31.1 | N/A Post: 38.2 | N/A |
| D’Ambrosi et al.17 | Pre: 55.2 Post: 95.1 | N/A | Pre: 7.5 Post: 0.7 | Pre: 33.7 Post: 56.0 | Pre: 42.8 Post: 57.9 | N/A | N/A | N/A |
| Galla et al.19 | N/A | N/A | Pre: 7.6 Post: 1.4 | N/A | N/A | N/A | Pre: 46.8 Post: 15.9 | N/A |
| Gottschalk et al.20 | N/A | N/A | N/A | N/A | N/A | Pre: 53 Post: 21 | Pre: 58 Post: 27 | N/A |
| Usuelli et al.23 | Pre: 52 Post: 88.3 | N/A | Pre: 7.9 Post: 1.8 | Pre: 30.9 Post: 51.2 | Pre: 42.5 Post: 53.9 | N/A | N/A | N/A |
| Becher et al.15 | N/A | Pre:59 Post:88 | Pre:7.3 Post:1.0 | N/A | N/A | N/A | N/A | N/A |
| D’Ambrosi et al.18 | Pre: 51.97 Post: 89.32 | N/A | Pre: 7.89 Post: 1.84 | Pre: 30.88 Post: 51.19 | Pre: 42.50 Post: 53.86 | N/A | N/A | N/A |
| Usuelli et al.22 | Pre: 57.1 Post: 86.6 | N/A | Pre: 8.1 Post: 2.5 | Pre: 29.9 Post: 48.5 | Pre: 43.8 Post: 53.1 | N/A | N/A | N/A |
| Weigelt et al.25 | Pre: N/A Post: 93.0 | N/A | Pre: 6.4 Post: 1.4 | N/A | N/A | N/A | N/A | Pre: 3.5 Post: 5.2 |
Table 3.
MOCART score and difference between preoperative and postoperative lesion size.
| Author(s) | Mean MOCART score | Preoperative mean lesion size | Postoperative mean lesion size | Significant difference |
|---|---|---|---|---|
| Valderrabano et al.24 | Final follow-up: 62 | SPECT-CT:1680 mm3 | N/A | N/A |
| Kubosch et al.21 | Final follow-up: 52.7 | MRI: 2400 mm3 | N/A | N/A |
| D’Ambrosi et al.16 | N/A | CT: 118.3 mm2 MRI: 153.7 mm2 | CT: 79.2 mm2 MRI: 87.4 mm2 | CT: Yes MRI: Yes |
| Albano et al.14 | 1-year follow-up: 41.9 2-year follow-up: 51.9 | N/A | N/A | N/A |
| D’Ambrosi et al.17 | N/A | CT: 119.1 mm2 MRI: 132 mm2 | CT: 77.9 mm2 MRI: 85.3 mm2 | CT: Yes MRI: Yes |
| Galla et al.19 | 1-year follow-up: 74.1 2-year follow-up: 74.5 | N/A | N/A | N/A |
| Gottschalk et al.20 | Final follow-up: 54 | MRI: 140 mm2 | N/A | N/A |
| Usuelli et al.23 | N/A | CT: 121 mm2 MRI:153.6 mm2 | CT: 80.5 mm2 MRI: 88.2 mm2 | CT: Yes MRI: Yes |
| Becher et al.15 | N/A | N/A | MRI: 106 mm2 | N/A |
| D’Ambrosi et al.18 | N/A | CT: 121.0 mm2 MRI: 153.6 mm2 | CT: 80.5 MRI: 88.2 | CT: Yes MRI: Yes |
| Usuelli et al.22 | 1-year follow-up: 42.8 2-year follow-up: 50.9 | CT: 111.1 mm2 MRI: 154.1 mm2 | CT: 76.9 mm2 MRI: 94.3 mm2 | CT: Yes MRI: Yes |
| Weigelt et al.25 | Fnal follow-up: 60.6 | MRI: 90 mm2 | N/A | N/A |
Complications
Overall, complications were identified in 31 of the 348 patients (8.9%) treated with AMIC for OCTs. In the open AMIC technique, 28 of the 196 patients developed adverse events. The most frequent was tissue irritation that required hardware removal (14/196). Including cases of hardware removal, the re-intervention rate in the open technique group was 13.2% (26/196). Among these patients, five required arthroscopic re-intervention19,25 and five were treated with a metal implant (HemiCAP®; Arthrosurface, Franklin, MA, USA) because of progressive degenerative changes.14,25 Conversely, a 2.0% re-intervention rate was observed with the arthroscopic technique; additional arthroscopic debridement was needed in 3 of 152 patients because of impingement (Table 5).16,18,23
Discussion
The most important finding of this systematic review is that improvement of both clinical and radiological scores was achieved after the treatment of OCTs with AMIC. There is a significant lack of highquality evidence concerning the application of AMIC to the talus. Our hypothesis that AMIC is safe and effective in the treatment of OCTs cannot yet be fully supported. As such, AMIC is a promising treatment option for OCTs that merits further high-quality studies to prove its safety and efficacy.
AMIC is a single-stage procedure with no need for cartilage harvesting and no potential donor site morbidity. Furthermore, it is cost-effective with no need for in vitro cell processing.32,33 AMIC can be applied using either an open or arthroscopic technique. Both methods provide satisfactory clinical and radiological outcomes and are associated with low complication rates. We tend to consider arthroscopic treatment as advantageous because it is associated with significantly less surgical trauma than the open technique, and it avoids the need for a malleolar osteotomy and subsequent hardware removal.24,25 Possible complications of medial malleolus osteotomy include direct morbidity by injury to adjacent structures (posterior tibial tendon, posterior tibial artery, tibial nerve, or healthy tibial cartilage), mid-term morbidity by malunion or nonunion of the osteotomy, and long-term morbidity by the development of local cartilage degeneration and the need for hardware, which may become symptomatic in an area with a limited soft tissue envelope. 25,34 Most reoperations in patients who had undergone the open AMIC technique were attributed to tissue irritation and the need for hardware removal.25 Nevertheless, most of the studies involving the use of an open technique performed a malleolar osteotomy; the exceptions were the studies by alk et al.20 and Galla et al.19 It seems that surgeons who use the open procedure prefer to perform a malleolar osteotomy for better access and visualization of the lesion. A higher reoperation rate was observed with open AMIC, mostly because of the need for hardware removal. However, open surgery without malleolar osteotomy is also feasible. 19
Table 4.
Parameters significantly correlated with age and BMI.
| Author(s) | Parameter correlated with age | Parameter correlated with BMI |
|---|---|---|
| Kubosch et al.21 | More postoperative pain and lower values of overall contentment in young patients. | Negatively: AOFAS score Positively: VAS score (higher VAS score with higher BMI) |
| D’Ambrosi et al.16 | Postoperative AOFAS score better in young patients. Preoperative SF-12 MCS higher in older patients. | N/A |
| D’Ambrosi et al.17 | The preoperative SF-12 MCS was significantly higher in the older patients in comparison with the younger patients. | Positively: intraoperative lesion size (larger lesion with higher BMI) |
| Gottschalk et al.20 | Better preoperative FFI-D (both pain and function subscales) | Negatively: Preoperative FFI-D and both subscale scores (for pain and function) |
| Usuelli et al.23 | N/A | Negatively: mental component of SF-12 Positively: size of the lesions in MRI preoperatively (larger lesion with higher BMI) |
| Weigelt et al.25 | No correlation | No correlation (tendency of poorer AOFAS in higher BMI) |
AOFAS: American Orthopaedic Foot and Ankle Society; FFI: Foot Function Index; SF: Short Form; MCS: Mental Component Summary; VAS: Visual Analogue Scale; BMI: Body Mass Index.
Table 5.
Type of surgical approach and complications.
| Author(s) | Type of surgery | Complications |
|---|---|---|
| Valderrabano et al.24 | Open | None |
| Kubosch et al.21 | Open | None |
| D’Ambrosi et al.16 | Arthroscopic | None |
| Wiewiorski et al.26 | Open | None |
| Albano et al.14 | Open | Four patients needed revision with a HemiCAP® One patient needed AMIC revision |
| D’Ambrosi et al.17 | Arthroscopic | None |
| Galla et al.19 | Open | One transient deep peroneal nerve irritation (conservative treatment) One arthrofibrosis that needed arthroscopic arthrolysis One patient needed revision with a HemiCAP® |
| Gottschalk et al.20 | Open | Not reported |
| Usuelli et al.23 | Arthroscopic | One case of impingement needed new arthroscopy |
| Becher et al.15 | Arthroscopic | Not reported |
| D’Ambrosi et al.18 | Arthroscopic | One case of impingement needed new arthroscopy. |
| Usuelli et al.22 | Arthroscopic | One case of impingement needed new arthroscopy |
| Weigelt et al.25 | Open | One delayed union (conservative treatment) 19 re-interventions (14 hardware removal for irritation plus arthroscopy, 4 arthroscopies without hardware removal, one gastrocnemius recession) |
Most of the studies included in this review evaluated relatively young patients (age of <39 years in 12 of the 13 studies), and most patients were male (57.5%). The clinical and radiological outcomes were generally evaluated in the short to medium term, whereas two studies followed their patients for a minimum of 5 years.15,20 In the same setting, a general systematic review on AMIC that was not restricted to the ankle joint demonstrated favorable outcomes of the technique in the short to medium term. The authors found limited evidence to support better results in younger patients, and they suggested that high-level studies should be carried out to assess the mediumand long-term results.11
Various subjective clinical scores were deployed to evaluate the effectiveness of AMIC, namely the AOFAS score, VAS score, FFI score, Hannover Scoring System for the ankle, SF-12 score, and Tegner score. The validity of the AOFAS system has been shown to be limited by its overemphasis on evaluating pain,35 while the use of inhomogeneous outcome parameters does not allow quantification of the reported improvement. However, because the reported complication rates were low and no adverse events or complications were specific to the procedure, both the open and the arthroscopic technique can be considered safe treatment options. Impingement might be observed after the arthroscopic procedure but can be resolved with arthroscopic debridement. MRI was the most commonly used imaging modality. The MOCART score is the MRI-quantified score and was used to evaluate the cartilage repair in most of the studies.14,18-22,24,25 Although some authors reported that the mean MOCART score improved from the first to second year after surgery,14,22 some others found no significant change in the score,19 and its reliability and reproducibility in the morphological evaluation of OLTs was also questioned. 36 Another quantifiable method to evaluate the efficacy of AMIC was to document postoperatively the lesion size by means of CT or MRI. D’Ambrosi et al.18 stated that patients with additional bone edema had larger lesions at each follow-up, probably because bone edema is closely associated with the lesion area.3 However, all studies that measured the lesion found a decreased size after surgery.16-18,20-23
Predictive factors that might have influenced the outcome of AMIC were also assessed. The most widely discussed factors identified in this review were age and the BMI. In general, the data showed that younger patients would greatly benefit from AMIC and would exhibit better clinical scores than older patients.16,17,20 Only Kubosch et al.21 reported the opposite; they illustrated that patients aged <45 years presented worse outcomes than patients aged >45 years. Four studies documented significant correlations between the BMI and the clinical scores as well as the size of the index lesion.17,20,21,23 An increased BMI was correlated with a larger preoperative lesion size on MRI.25,43 However, the postoperative outcomes were conflicting.20,21,23 Two studies postulated that patients with an increased BMI are likely to present a deteriorated final outcome.20,24 However, a trial that directly compared the outcomes of overweight and normal-weight patients showed no significant differences between the two groups.23 Some authors have stated that tobacco use is a predictive factor for less favorable postoperative outcomes and increased pain,19 while others showed no difference between smokers and nonsmokers. 26
It is not easy to compare our results with those of studies examining other surgical options for OLTs because of wide heterogeneity in study designs and reporting of outcomes. Bone marrow stimulation, or microfracturing, is a well-established method for the management of OLTs. In a retrospective study comparing AMIC with microfractures, Becher et al.15 failed to show any superiority of one technique over the other in terms of clinical scores and MRI parameters. Chuckpaiwong et al.37 reported favorable results with microfractures at the 32-month follow-up in lesions <15 mm in diameter. Choi et al.38 stated that microfractures should be used for the treatment of lesions with a maximum size of 150 mm2. Although whether AMIC can potentially restore such lesions remains unclear, the studies included in this systematic review reported outcomes in larger preoperative defects. Nevertheless, in the 2018 International Consensus Group on Cartilage Repair of the Ankle, it was stated (92% consensus) that a scaffold can be added to a bone marrow stimulation procedure in primary and revision cases involving lesions of >1 cm2.39
The presence of subchondral bone cysts has been a controversial factor related to the outcome of OCT treatment with microfractures. In two studies, the implementation of microfractures in patients with subchondral bone cysts resulted in poor clinical outcomes. 40,41 However, a study with combined lesion–subchondral cyst defects of <1.5 cm2 showed no difference in functional or radiographic outcomes after 2 years between patients with and without subchondral cysts.42 In the present systematic review, 12 of 13 studies14,16-26 performed reconstruction of subchondral bone lesions by impacting cancellous bone, regardless of the technique used (arthroscopic or open). The bone graft was sealed with the collagen matrix, offering satisfactory results. The International Consensus Group stated (87% consensus) that bone grafting may be considered in cases with >3 mm of bone loss as measured intraoperatively after debridement.39
Cellular-based techniques such as ACI require a two-stage procedure. These techniques are indicated for larger cartilage defects, and their efficacy and safety in the knee have been established.43 However, their role in the ankle joint still lacks strong scientific evidence. A meta-analysis by Niemeyer et al.44 showed a high success rate of ACI for the treatment of isolated cartilage lesions and OCTs with a mean size of 2.3 cm2 after 32 months. However, the variety of different techniques applied and the limited numbers of published studies make it impossible to draw safe conclusions regarding the use of cellular-based techniques in the ankle joint.
Osteochondral autograft techniques aim to replace OCTs with native hyaline cartilage by harvesting osteochondral plugs from the talus, calcaneus, and ipsilateral knee.45 In a study by Scranton et al.,46 90% of patients with an OCT (range, 8–20 mm diameter) had a good to excellent outcome at a mean follow-up of 36 months. Valderrabano et al.47 described 12 patients who were treated with osteochondral grafts harvested from the ipsilateral knee. The authors reported significant pain relief and improvement in functional scores after a mean follow-up of 72 months; however, half of the patients reported knee pain, and 10 patients developed recurrent ankle lesions.47 This highlights the risk of donor site morbidity and the difference in the biochemical and biomechanical properties of the knee cartilage.
The studies included in this review had some limitations. First, the number of patients included was small, and no randomization or blinding process was utilized. Second, the design of the studies was relatively poor because most of them were case series. However, it must be acknowledged that this clinical entity is uncommon and there are objective difficulties in conducting larger, single-center trials. Third, an important concern was the possible overlapping of the reported cohorts by the same authors in different studies. Fourth, the quality of the included studies was low; no controlled clinical trial of with a level of evidence of I or II was identified in the literature. Finally, given the lack of randomized controlled trials, no comparisons with other techniques such as microfractures, ACI, or osteochondral transplantation could be performed. Performance of further higher-quality trials should be encouraged to validate the efficacy and safety of this technique.
Conclusions
This systematic data analysis showed that both arthroscopic and open AMIC are promising surgical techniques for the treatment of OCTs. Age and BMI may have a detrimental impact on the clinical outcome. A higher re-intervention rate is expected with the open AMIC technique. Further high-quality clinical trials are mandatory to validate these preliminary results.
Funding Statement
Funding: None.
References
- 1.Loomer R, Fisher C, Lloyd-Smith R, et al. Osteochondral lesions of the talus. Am J Sports Med 1993;21:13-9. [DOI] [PubMed] [Google Scholar]
- 2.O'Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med 2010;38:392-404. [DOI] [PubMed] [Google Scholar]
- 3.van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ. Osteochondral defects in the ankle: why painful? Knee Surg Sports Traumatol Arthrosc 2010;18:570-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hintermann B, Regazzoni P, Lampert C, et al. Arthroscopic findings in acute fractures of the ankle. J Bone Joint Surg Br 2000;82:345-51. [DOI] [PubMed] [Google Scholar]
- 5.Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc 2010;18:238-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aurich M, Albrecht D, Angele P, et al. Treatment of osteochondral lesions in the ankle: a guideline from the group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Traumatology (DGOU). Z Orthop Unfall 2017;155:92-9. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa T, Eyre DR, Koide S, Glimcher MJ. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am 1980;62:79-89. [PubMed] [Google Scholar]
- 8.Thermann H, Becher C. Microfracture technique for treatment of osteochondral and degenerative chondral lesions of the talus. 2-year results of a prospective study. Unfallchirurg 2004;107:27-32. [DOI] [PubMed] [Google Scholar]
- 9.Petersen L, Brittberg M, Lindahl A. Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin 2003;8:291-303. [DOI] [PubMed] [Google Scholar]
- 10.Flynn S, Ross KA, Hannon CP, et al. Autologous osteochondral transplantation for osteochondral lesions of the talus. Foot Ankle Int 2016;37:363-72. [DOI] [PubMed] [Google Scholar]
- 11.Shaikh N, Seah MKT, Khan WS. Systematic review on the use of autologous matrix-induced chondrogenesis for the repair of articular cartilage defects in patients. World J Orthop 2017;8:588-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benthien JP, Behrens P. The treatment of chondral and osteochondral defects of the knee with autologous matrixinduced chondrogenesis (AMIC): method description and recent developments. Knee Surg Sports Traumatol Arthrosc 2011;19:1316-9. [DOI] [PubMed] [Google Scholar]
- 13.Gille J, Meisner U, Ehlers EM, et al. Migration pattern, morphology and viability of cells suspended in or sealed with fibrin glue: a histomorphologic study. Tissue Cell 2005;37:339-48. [DOI] [PubMed] [Google Scholar]
- 14.Albano D, Martinelli N, Bianchi A, et al. Clinical and imaging outcome of osteochondral lesions of the talus treated using autologous matrix-induced chondrogenesis technique with a biomimetic scaffold. BMC Musculoskelet Disord 2017;18:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becher C, Malahias MA, Ali MM, et al. Arthroscopic microfracture vs. arthroscopic autologous matrix-induced chondrogenesis for the treatment of articular cartilage defects of the talus. Knee Surg Sports Traumatol Arthrosc 2019;27:2731-6. [DOI] [PubMed] [Google Scholar]
- 16.D'Ambrosi R, Maccario C, Serra N, et al. Osteochondral lesions of the talus and autologous matrix-induced chondrogenesis: is age a negative predictor outcome? Arthroscopy 2017;33:428-35. [DOI] [PubMed] [Google Scholar]
- 17.D'Ambrosi R, Maccario C, Ursino C, et al. Combining microfractures, autologous bone graft, and autologous matrixinduced chondrogenesis for the treatment of juvenile osteochondral talar lesions. Foot Ankle Int 2017;38:485-95. [DOI] [PubMed] [Google Scholar]
- 18.D'Ambrosi R, Maccario C, Ursino C, et al. The role of bone marrow edema on osteochondral lesions of the talus. Foot Ankle Surg 2018;24:229-35. [DOI] [PubMed] [Google Scholar]
- 19.Galla M, Duensing I, Kahn TL, Barg A. Open reconstruction with autologous spongiosa grafts and matrix-induced chondrogenesis for osteochondral lesions of the talus can be performed without medial malleolar osteotomy. Knee Surg Sports Traumatol Arthrosc 2019;27:2789-95. [DOI] [PubMed] [Google Scholar]
- 20.Gottschalk O, Altenberger S, Baumbach S, et al. Functional medium-term results after autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: a 5-year prospective cohort study. J Foot Ankle Surg 2017;56:930-6. [DOI] [PubMed] [Google Scholar]
- 21.Kubosch EJ, Erdle B, Izadpanah K, et al. Clinical outcome and T2 assessment following autologous matrix-induced chondrogenesis in osteochondral lesions of the talus. Int Orthop 2016;40:65-71. [DOI] [PubMed] [Google Scholar]
- 22.Usuelli FG, D'Ambrosi R, Maccario C, et al. All-arthroscopic AMIC((R)) (ATAMIC( (R))) technique with autologous bone graft for talar osteochondral defects: clinical and radiological results. Knee Surg Sports Traumatol Arthrosc 2018;26:875-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Usuelli FG, Maccario C, Ursino C, et al. The impact of weight on arthroscopic osteochondral talar reconstruction. Foot Ankle Int 2017;38:612-20. [DOI] [PubMed] [Google Scholar]
- 24.Valderrabano V, Miska M, Leumann A, Wiewiorski M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med 2013;41:519-27. [DOI] [PubMed] [Google Scholar]
- 25.Weigelt L, Hartmann R, Pfirrmann C, et al. Autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: a clinical and radiological 2- to 8- year follow-up study. Am J Sports Med 2019;47:1679-86. [DOI] [PubMed] [Google Scholar]
- 26.Wiewiorski M, Werner L, Paul J, et al. Sports activity after reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med 2016;44:2651-8. [DOI] [PubMed] [Google Scholar]
- 27.Hanzlik S, Mahabir RC, Baynosa RC, Khiabani KT. Levels of evidence in research published in The Journal of Bone and Joint Surgery (American Volume) over the last thirty years. J Bone Joint Surg Am 2009;91:425-8. [DOI] [PubMed] [Google Scholar]
- 28.Coleman BD, Khan KM, Maffulli N, et al. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports 2000;10:2-11. [DOI] [PubMed] [Google Scholar]
- 29.Mithoefer K, McAdams T, Williams RJ, et al. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med 2009;37:2053-63. [DOI] [PubMed] [Google Scholar]
- 30.Jakobsen RB, Engebretsen L, Slauterbeck JR. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am 2005;87:2232-9. [DOI] [PubMed] [Google Scholar]
- 31.Sambandam SN, Gul A, Priyanka P. Analysis of methodological deficiencies of studies reporting surgical outcome following cemented total-joint arthroplasty of trapezio-metacarpal joint of the thumb. Int Orthop 2007;31:639-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marlovits S, Singer P, Zeller P, et al. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol 2006;57:16-23. [DOI] [PubMed] [Google Scholar]
- 33.Valderrabano V, Barg A, Alattar A, Wiewiorski M. Osteochondral lesions of the ankle joint in professional soccer players: treatment with autologous matrix-induced chondrogenesis. Foot Ankle Spec 2014;7:522-8. [DOI] [PubMed] [Google Scholar]
- 34.Kreuz PC, Steinwachs M, Edlich M, et al. The anterior approach for the treatment of posterior osteochondral lesions of the talus: comparison of different surgical techniques. Arch Orthop Trauma Surg 2006;126:241-6. [DOI] [PubMed] [Google Scholar]
- 35.SooHoo NF, Shuler M, Fleming LL. American Orthopaedic Foot and Ankle Society Evaluation of the validity of the AOFAS Clinical Rating Systems by correlation to the SF-36. Foot Ankle Int 2003;24:50-5. [DOI] [PubMed] [Google Scholar]
- 36.Albano D, Martinelli N, Bianchi A, et al. Evaluation of reproducibility of the MOCART score in patients with osteochondral lesions of the talus repaired using the autologous matrix-induced chondrogenesis technique. Radiol Med 2017;122:909-17. [DOI] [PubMed] [Google Scholar]
- 37.Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy 2008;24:106-12. [DOI] [PubMed] [Google Scholar]
- 38.Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med 2009;37:1974-80. [DOI] [PubMed] [Google Scholar]
- 39.Rothrauff BB, Murawski CD, Angthong C, et al. International consensus group on cartilage repair of the ankle scaffoldbased therapies: proceedings of the International Consensus Meeting on Cartilage Repair of the Ankle. Foot Ankle Int 2018;39:41S-7S. [DOI] [PubMed] [Google Scholar]
- 40.Kumai T, Takakura Y, Higashiyama I, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am 1999;81:1229-35. [DOI] [PubMed] [Google Scholar]
- 41.Robinson DE, Winson IG, Harries WJ, Kelly AJ. Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Br 2003;85:989-93. [DOI] [PubMed] [Google Scholar]
- 42.Han SH, Lee JW, Lee DY, Kang ES. Radiographic changes and clinical results of osteochondral defects of the talus with and without subchondral cysts. Foot Ankle Int 2006;27:1109-14. [DOI] [PubMed] [Google Scholar]
- 43.Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am 2009;91:1778-90. [PubMed] [Google Scholar]
- 44.Niemeyer P, Salzmann G, Schmal H, et al. Autologous chondrocyte implantation for the treatment of chondral and osteochondral defects of the talus: a meta-analysis of available evidence. Knee Surg Sports Traumatol Arthrosc 2012;20:1696-703. [DOI] [PubMed] [Google Scholar]
- 45.Calder JD, Ballal MS, Deol RS, et al. Histological evaluation of calcaneal tuberosity cartilage--A proposed donor site for osteochondral autologous transplant for talar dome osteochondral lesions. Foot Ankle Surg 2015;21:193-7. [DOI] [PubMed] [Google Scholar]
- 46.Scranton PE Jr, Frey CC, Feder KS. Outcome of osteochondral autograft transplantation for type-V cystic osteochondral lesions of the talus. J Bone Joint Surg Br 2006;88:614-9. [DOI] [PubMed] [Google Scholar]
- 47.Valderrabano V, Leumann A, Rasch H, et al. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med 2009;37:105S-11S. [DOI] [PubMed] [Google Scholar]


