Abstract
This study aimed to assess changes in sleep pattern and their influence on people's daily life and emotion during the COVID‐19 pandemic. Self‐developed questionnaires were used to measure changes in nocturnal sleep, daytime napping, lifestyles and negative emotions in individuals before and after the COVID‐19 pandemic. Nine hundred and thirty effective questionnaires were collected in this study. Repeated measures analysis of variance and hierarchical regression analysis were applied. We found that individuals' sleep rhythms were delayed, and sleep duration and sleep latency were increased during the stay‐at‐home orders. Meanwhile, their exercise levels and learning/working efficiency were decreased, and electronic device use time, annoyance levels and anxiety levels were increased. Delayed sleep patterns affected lifestyles and emotions. Moreover, sleep quality positively predicted learning/working efficiency and exercise levels, and negatively predicted use of electronic devices and negative emotions. Sleep patterns became delayed on weekdays during stay‐at‐home orders in all four daytime napping groups (no daytime napping, daytime napping as before, more daytime napping and less daytime napping), and the group taking daytime naps as before had a minimal variation, and their lifestyles and emotions were significantly better than those of the other groups. This study demonstrated that under the influence of stress caused by the pandemic, maintaining regular daytime napping was an effective way to stabilize sleep patterns and biological rhythms, keep good lifestyles and alleviate the effect of acute psychological stress, and to prevent and control mental disorders during the pandemic.
Keywords: COVID‐19, mental health, napping, sleep, stress
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a public health emergency of international concern, causing global crises with regard to life, health, safety, economies and societies (World Health Organization, 2020). To control the spread of COVID‐19, many governments have adopted quarantine and 'stay‐at‐home' measures and temporarily closed educational institutions, workplaces and entertainment venues. During the COVID‐19 pandemic, concerns over infection and economic issues, as well as media broadcasts regarding the pandemic, became sources of psychological stress (Dong & Zheng, 2020; Léger et al., 2020), possibly resulting in stress responses that affected people's sleep, lifestyles and emotions. Stress is a state of threatened homeostasis (physical or perceived threat to homeostasis). In this state, adaptive compensatory responses by the organism might be activated to sustain homeostasis (Pacák & Palkovits, 2001). Psychological stress occurs when individuals perceive that environmental demands tax or exceed their adaptive capacity (Cohen et al., 1995, 2007), which can trigger stress responses in many areas, such as emotions (e.g., fear, anger, helplessness and hopelessness), cognitions (e.g., confusion, dissociation, difficulty concentrating, etc.), physiological responses (e.g., insomnia, increased heart rate, anxiety, fatigue, etc.) and interpersonal relationship issues (e.g., suspicion, isolation, sense of abandonment, etc.) (Friedman, 2015). Studies have shown that public health emergencies, such as the severe acute respiratory syndrome (SARS) and the 2009 H1N1/influenza A epidemics, caused acute stress responses in people, which generated negative emotions (e.g., fear, anxiety and anger) (Bai et al., 2004; Carmona et al., 2016; Tam et al., 2004; Yu et al., 2005). Stress also affects sleep, including sleep–wake patterns, wake after sleep onset (WASO) and sleep architecture, by affecting the activation of the hypothalamic‐pituitary‐adrenal (HPA) axis (Lo Martire et al., in press; Sanford et al., 2014).
Recent findings demonstrated that during COVID‐19, individuals experienced changes in sleep patterns, such as delayed sleep phases, increased sleep duration and poor sleep quality when staying at home (Cellini et al., 2020; Wright et al., 2020). In contrast to nocturnal sleep, daytime napping usually lasts from 15 min to 2 h (Dinges, 1989). An appropriate nap can not only reduce the level of subjective and objective sleepiness but also improve cognitive function and behavioural performance, such as short‐term memory and emotional control (Brooks & Lack, 2006; Evans et al., 1977; Jones, 2009; Tietzel & Lack, 2001). Although daytime napping might be beneficial, long naps lead to sleep inertia, which affects subsequent cognitive and emotional functions. In addition, long naps negatively affect the duration and pattern of nocturnal sleep (ÅKerstedt et al., 1989). People's schedules would be more flexible at home during the COVID‐19 pandemic, providing conditions for daytime napping or alterations in daytime napping time. Previous studies on daytime napping mainly focused on the effects of napping and factors that influence it, but paid less attention to individual differences in daytime napping and daily performance and nocturnal sleep.
Young adulthood (or early adulthood) is when individuals have the greatest energy and strong contradiction and stress (Levinson, 1986). Young adults, especially people aged 18–25 years, are in a maturing stage of their physiological and psychological development. They are in the process of stabilizing their career and life. However, they have to face social, emotional and life challenges and change, and their strengths and vulnerabilities continue to emerge (Wood et al., 2018). However, COVID‐19 has suddenly posed a threat to young adults' stability and they may exhibit strong stress responses to emergencies and emergency‐caused changes, thus causing significant changes to their sleep, lifestyles and emotions.
The present study focused on alterations in young adults' nocturnal sleep, daytime napping, lifestyle and negative emotions before and after the COVID‐19 pandemic. This study proposed the following hypotheses. (a) Compared to before the COVID‐19 pandemic, individuals' exercise and learning/working efficiency might be worse. Electronic device use and negative emotions might increase. Sleep patterns might be changed, such as delayed sleep rhythm and increased sleep duration and sleep latency, during the stay‐at‐home orders. (b) Changes in sleep patterns after the pandemic might negatively predict learning/working efficiency and exercise and positively predict electronic device use and negative emotions. (c) After the pandemic, changes in daytime napping have different effects on changes in nocturnal sleep patterns, lifestyles and negative emotions. Stabilized habitual daytime napping might have positive effects on nocturnal sleep, lifestyle and alleviating negative emotion.
2. METHODS
2.1. Participants
A total of 1,136 questionnaires were collected. After excluding unqualified questionnaires (67 out of the age range and 139 incomplete), 930 questionnaires were included in the final analysis. The valid response rate was 81.9%. The survey was internet based and investigated sleep, lifestyle and negative emotion among healthy Chinese young adults (18–35 years old) (see Table 1). The study was approved by the Ethics Committee of South China Normal University and was conducted from late March to April 2020.
Table 1.
Demographic statistics (n = 930)
| Demographic variables | Frequency | Percentage (%) | Minimum | Maximum | Mean (SD) |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 229 | 24.6 | |||
| Female | 701 | 75.4 | |||
| Age | 18 | 35 | 22.43 (3.77) | ||
| BMI | 14.69 | 30.67 | 20.33 (2.52) | ||
| Occupation | |||||
| Undergraduate | 564 | 60.6 | |||
| Graduate | 195 | 21.0 | |||
| Working | 171 | 18.4 | |||
2.2. Measures
This questionnaire was developed to assess people's physical and mental conditions under stay‐at‐home orders during the COVID‐19 pandemic. There were 25 items about sleep, lifestyle and negative emotion.
2.2.1. Sleep
Time to wake‐up and fall asleep
There were eight items about wake‐up time and time of falling asleep on weekdays and weekends before and after the pandemic. Participants needed to indicate the actual wake‐up time and time of falling asleep, not the time for getting up and going to bed. To facilitate statistical analysis, for the item "Before the pandemic, what time did you usually fall asleep on weekdays?", if the time of falling asleep exceeded midnight (24:00 PM), 24 h was added to indicate that the time was the next day. The participants' original data were converted into an hour unit (e.g., 24.25 h represents 00:15). Sleep duration on weekdays and weekends before and after the pandemic was calculated based on wake‐up times and times of falling asleep.
Sleep latency
There were four items about sleep duration on weekdays and weekends before and after the pandemic. Participants were asked to provide the time they needed from going to bed or putting down their phones to actually fall asleep.
Sleep quality
Sleep quality was assessed with a single item that asked respondents for the degree of change in their sleep quality during the stay‐at‐home orders (compared with that before the pandemic). A 5‐point Likert‐type scale was used: 1 = substantially worsened, 2 = slightly worsened, 3 = basically unchanged, 4 = slightly improved and 5 = substantially improved.
Daytime napping
Changes in daytime napping between before and after the pandemic were assessed with a single item evaluated on a 4‐point scale: 1 = no daytime napping, 2 = daytime napping as before, 3 = more daytime napping than before and 4 = less daytime napping than before.
Changes in sleep patterns between before and after the pandemic
Changes in sleep patterns between before and after the pandemic were obtained by subtracting the sleep conditions before the pandemic from the sleep conditions during the stay‐at‐home order. For example, the difference between wake‐up times on weekdays before and after the pandemic was obtained by subtracting the wake‐up times on weekdays before the pandemic from the wake‐up times on weekdays during the stay‐at‐home order. The same method was used to obtain the differences between wake‐up times on weekends, time falling asleep on weekdays, time falling asleep on weekends, sleep duration on weekdays, sleep duration on weekends, sleep latency on weekdays and sleep latency on weekends.
Lifestyle
For lifestyle, three items were used to evaluate changes in exercise levels, learning/working efficiency and using electronic devices before and after the pandemic. A 5‐point Likert‐type scale was used for exercise level and learning/working efficiency: 1 = substantially worsened, 2 = slightly worsened, 3 = basically unchanged, 4 = slightly improved and 5 = substantially improved. A 5‐point Likert‐type scale was used for time using electronic devices: 1 = substantially reduced, 2 = slightly reduced, 3 = basically unchanged, 4 = slightly increased and 5 = substantially increased.
Negative emotion
For negative emotion, seven items were used to evaluate changes in subjective feelings (annoyance, anxiety, helplessness, lack of interest in other things, sense of control, fatigue and anger) in the participants during the stay‐at‐home order (e.g., "Compared with before the pandemic, your feelings of being annoyed during the stay‐at‐home order have…"). A 5‐point Likert‐type scale was used: 1 = significantly reduced, 2 = slightly reduced, 3 = basically unchanged, 4 = slightly aggravated and 5 = severely aggravated.
Demographic variables
Demographic variables included gender, age, body mass index (BMI) and occupation (including undergraduate students, graduate students and employed people).
2.3. Data analysis
Jamovi 1.2.6 was applied to perform descriptive statistics on the major variables, including sleep (such as time of waking up and falling asleep, sleep duration, sleep latency and daytime napping), lifestyle and negative emotions. Changes in individual sleep patterns on weekdays and weekends before and after the pandemic and the effects of daytime naps on changes in sleep patterns were investigated by repeated measures analysis of variance (ANOVA). SPSS 25.0 was used to conduct a partial correlation analysis of the major variables when the demographic variables were controlled. The effects of changes in sleep patterns and daytime napping on lifestyle and negative emotions were investigated through hierarchical regression analysis. In the hierarchical regression analysis, the first layer was the demographic variables, the second layer was the independent variables, including changes in sleep patterns and changes in the type of nap before and after the pandemic, and changes in lifestyle and negative emotions were the dependent variables. The categorical variables (daytime napping and occupation) were coded as dummy variables, and taking daytime naps as before (original code of 2) and undergraduate student (original code of 1) were coded as 0. A bilateral α < 0.05 was considered statistically significant.
3. RESULTS
3.1. Changes in lifestyle and negative emotions under the stay‐at‐home order during the pandemic
In terms of lifestyle, compared with before the pandemic, 37% of the participants reported that their exercise levels were slightly worse, 43.2% of the participants reported that their learning/working efficiency was slightly worse and approximately half (50.2%) of the participants reported that time using electronic devices increased significantly during the stay‐at‐home order. In terms of negative emotions, only anxiety and annoyance were slightly aggravated, accounting for 46.8% and 53.1%, respectively. In terms of sleep quality, 51.7% of the participants reported that sleep quality remained unchanged during the stay‐at‐home order compared with before the pandemic (see Table 2).
Table 2.
Descriptive statistics of changes in sleep quality, lifestyles and negative emotions (n = 930)
| Item | Options | Mean (SD) | ||||
|---|---|---|---|---|---|---|
| Substantially worse | Slightly worse | Basically unchanged | Slightly improved | Substantially improved | ||
| Compared with before the pandemic, your sleep quality during the stay‐at‐home order has been…. | 25 (2.69%) | 223 (23.98%) | 481 (51.72%) | 147 (15.81%) | 54 (5.81%) | 2.98 (0.86) |
| Compared with before the pandemic, your physical activity level during the stay‐at‐home order has been…. | 137 (14.73%) | 351(37.74%) | 236(25.38%) | 143(15.38%) | 63 (6.77%) | 2.62 (1.12) |
| Compared with before the pandemic, your learning (working) efficiency during the stay‐at‐home order has been…. | 123 (13.23%) | 402 (43.23%) | 237 (25.48%) | 127 (13.66%) | 41 (4.41%) | 2.53 (1.03) |
| Substantially reduced | Slightly reduced | Basically unchanged | Slightly increased | Substantially increased | ||
|---|---|---|---|---|---|---|
| Compared with before the pandemic, time spent using electronic devices (including computer, TV, mobile phone, etc.) time during the stay‐at‐home order has been…. | 6 (0.65%) | 30 (3.23%) | 85 (9.14%) | 342 (36.77%) | 467 (50.22%) | 4.33 (0.82) |
| Significantly reduced | Slightly reduced | Basically unchanged | Slightly aggravated | Severely aggravated | ||
|---|---|---|---|---|---|---|
| Compared with before the pandemic, your feelings of being annoyed during the stay‐at‐home order have been…. | 33 (3.55%) | 69 (7.42%) | 348 (37.42%) | 427 (45.91%) | 53 (5.70%) | 3.43 (0.85) |
| Compared with before the pandemic, your feelings of anxiety during the stay‐at‐home order have been…. | 41 (4.41%) | 54 (5.81%) | 333 (35.81%) | 435 (46.77%) | 67 (7.20%) | 3.47 (0.88) |
| Compared with before the pandemic, your feeling of helpless during the stay‐at‐home order have been…. | 48 (5.16%) | 53 (5.70%) | 529 (56.88%) | 260 (27.96%) | 40 (4.30%) | 3.21 (0.82) |
| Compared with before the pandemic, your interest in other things during the stay‐at‐home order has been…. | 51 (5.48%) | 92 (9.89%) | 470 (50.54%) | 290 (31.18%) | 27 (2.90%) | 3.16 (0.85) |
| Compared with before the pandemic, your feelings of control during the stay‐at‐home order have been…. | 35 (3.76%) | 61 (6.56%) | 493 (53.01%) | 303 (32.58%) | 38 (4.09%) | 3.27 (0.80) |
| Compared with before the pandemic, your feelings of tiredness during the stay‐at‐home order have been…. | 39 (4.19) | 79 (8.49%) | 392 (42.15%) | 371 (39.89%) | 49 (5.27%) | 3.34 (0.87) |
| Compared with before the pandemic, your feelings of anger during the stay‐at‐home order have been…. | 50 (5.38%) | 93 (10.00%) | 494 (53.12%) | 254 (27.31%) | 39 (4.19%) | 3.15 (0.86) |
A partial correlation analysis was performed to preliminarily examine the relationships between sleep pattern changes, daytime napping, lifestyle and negative emotions. The partial correlation analysis results between the major variables after controlling the demographic variables are shown in Table 3. Changes in lifestyle were significantly correlated with differences in wake‐up times, falling asleep times, sleep latency and sleep quality before and after the pandemic, and were not significantly correlated with differences in sleep duration. Negative emotions were not significantly correlated with differences in wake‐up times on weekends and sleep duration on weekdays, and significantly correlated with changes in other sleep patterns. Daytime napping was significantly associated with lifestyle and negative emotions. These results provided preliminary support for further analysis.
Table 3.
Partial correlation between the main variables
| Variable (No.) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| The difference in wake‐up time on weekdays (1) | |||||||||||||||||||
| The difference in wake‐up time on weekends (2) | 0.52*** | ||||||||||||||||||
| The difference in sleep time on weekdays (3) | 0.49*** | 0.41*** | |||||||||||||||||
| The difference in sleep time on weekends (4) | 0.25*** | 0.31*** | 0.50*** | ||||||||||||||||
| The difference in sleep duration on weekdays (5) | 0.58*** | 0.15*** | −0.43*** | −0.21*** | |||||||||||||||
| The difference in sleep duration on weekends (6) | 0.18*** | 0.58*** | −0.20*** | −0.38*** | 0.38*** | ||||||||||||||
| The difference in sleep latency on weekdays (7) | 0.25*** | 0.20*** | 0.27*** | 0.20*** | 0.00 | −0.02 | |||||||||||||
| The difference in sleep latency on weekends (8) | 0.15*** | 0.16*** | 0.16*** | 0.15*** | 0.01 | −0.02 | 0.68*** | ||||||||||||
| Sleep quality (9) | −0.09** | −0.07* | −0.22*** | −0.16*** | 0.11*** | 0.16*** | −0.20*** | −0.17*** | |||||||||||
| Daytime nap (10) | 0.10** | 0.02 | 0.10** | 0.02 | 0.02 | −0.02 | 0.01 | −0.01 | −0.03 | ||||||||||
| Exercise (11) | −0.17*** | −0.12*** | −0.21*** | −0.08** | 0.01 | 0.02 | −0.15*** | −0.14*** | 0.23*** | −0.08* | |||||||||
| Learning (work) efficiency (12) | −0.14*** | −0.09** | −0.18*** | −0.10** | 0.02 | 0.04 | −0.11*** | −0.09** | 0.32*** | −0.10** | 0.37*** | ||||||||
| Electronic device use time (13) | 0.15*** | 0.08* | 0.13*** | 0.06 | 0.04 | −0.04 | 0.14*** | 0.07* | −0.12*** | 0.13*** | −0.25*** | −0.20*** | |||||||
| Annoyed (14) | 0.09** | 0.02 | 0.06 | 0.02 | 0.04 | −0.01 | 0.10** | 0.07* | −0.29*** | 0.11*** | −0.23*** | −0.35*** | 0.19*** | ||||||
| Anxious (15) | 0.06 | −0.01 | 0.10** | 0.04 | −0.03 | −0.06 | 0.10** | 0.08* | −0.29*** | 0.13*** | −0.23*** | −0.33*** | 0.17*** | 0.79*** | |||||
| Helpless (16) | 0.10** | 0.02 | 0.11** | 0.05 | 0.00 | −0.04 | 0.10** | 0.07* | −0.29*** | 0.11*** | −0.22*** | −0.33*** | 0.10** | 0.68*** | 0.70*** | ||||
| Uninterested (17) | 0.10** | 0.04 | 0.09** | 0.07* | 0.02 | −0.04 | 0.10** | 0.09** | −0.20*** | 0.07* | −0.20*** | −0.32*** | 0.09** | 0.41*** | 0.40*** | 0.41*** | |||
| Losing control (18) | 0.08* | 0.04 | 0.09** | 0.07* | 0.00 | −0.03 | 0.11*** | 0.08* | −0.23*** | 0.13*** | −0.21*** | −0.38*** | 0.13*** | 0.44*** | 0.44*** | 0.45*** | 0.56*** | ||
| Fatigue (19) | 0.04 | 0.02 | 0.11** | 0.08* | −0.06 | −0.07* | 0.15*** | 0.13** | −0.40*** | 0.11*** | −0.25*** | −0.30*** | 0.16*** | 0.47*** | 0.52*** | 0.49*** | 0.44*** | 0.41*** | |
| Angry (20) | 0.06 | 0.00 | 0.08* | 0.04 | −0.01 | −0.04 | 0.14*** | 0.14*** | −0.31*** | 0.11*** | −0.19*** | −0.30*** | 0.12*** | 0.51*** | 0.54*** | 0.52*** | 0.42*** | 0.44*** | 0.54*** |
The control variables were demographic variables, including gender, age, body mass index (BMI) and education level.
p < .05.
p < .01.
p < .001.
3.2. Changes in sleep patterns under the stay‐at‐home order during the COVID‐19 pandemic
To explore changes in the sleep patterns of the participants before and after the pandemic, wake‐up time, time falling asleep, sleep duration and sleep latency were used as dependent variables to perform a 2 (pandemic: before the pandemic, stay at home) × 2 (weeks: weekdays and weekends) repeated‐measures ANOVA. The main effects of the pandemic were significant: F wake‐up time (1,929) = 106.02, p < .001; F time falling asleep (1,929) = 22.05, p < .001; F sleep duration (1,929) = 38.14, p < .001; F sleep latency (1,929) = 54.99, p < .001. Overall, wake‐up time and time falling asleep during the stay‐at‐home order were significantly shifted to later compared to wake‐up time and time falling asleep before the pandemic (meanwake‐up time before pandemic = 8:11 AM, meanwake‐up time during the stay‐at‐home order = 8:30 AM; meantime falling asleep before pandemic = 00:10 AM, meantime falling asleep during the stay‐at‐home order = 00:18 AM), and sleep duration and sleep latency during the stay‐at‐home order were significantly longer than before the pandemic (meansleep duration before pandemic = 8.02 h, meansleep duration during the stay‐at‐home order = 8.20 h; meansleep latency before the pandemic = 22.53 min, Meansleep latency during the stay‐at‐home order = 26.36 min). Additionally, the interactions between the pandemic and weeks were significant: F wake‐up time (1,929) = 122.50, p < .001; F time falling asleep (1,929) = 34.71, p < .001; F sleep duration (1,929) = 34.79, p < .001; F sleep latency (1,929) = 13.23, p < .001. Under the stay‐at‐home order during the COVID‐19 pandemic, changes in wake‐up time, time of falling asleep, sleep duration and sleep latency on weekdays were significantly greater than on weekends. The detailed results and variation tendencies are shown in Table 4 and Figure 1.
Table 4.
Simple effect of the interaction between the pandemic and weeks
| Weekdays | Mean difference | t | Weekends | Mean difference | t | |||
|---|---|---|---|---|---|---|---|---|
| Before the pandemic | Stay at home | Before the pandemic | Stay at home | |||||
| Wake‐up time | 7.68 AM | 8.17 AM | −0.49 hr | −14.40*** | 8.71 AM | 8.83 AM | −0.12 h | −3.56** |
| Time falling asleep | 23.97 PM | 24.18 PM | −0.21 hr | −6.69*** | 24.37 PM | 24.43 PM | −0.06 h | −1.88 |
| Sleep duration | 7.71 h | 7.99 h | −0.28 h | −8.41*** | 8.34 h | 8.40 h | −0.06 h | −1.87 |
| Sleep latency | 20.36 min | 25.02 min | −4.65 min | −8.25*** | 24.69 min | 27.70 min | −3.01 min | −5.34*** |
p < .05.
p < .01.
p < .001.
Figure 1.
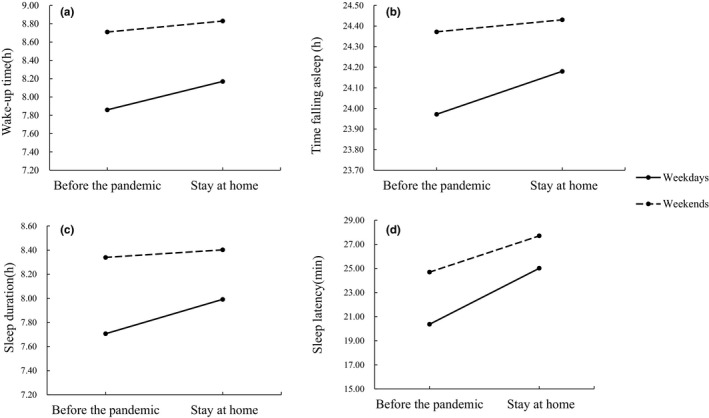
(a) Interaction between the pandemic and weeks for wake‐up time. (b) Interaction between the pandemic and weeks for time falling asleep. (c) Interaction between the pandemic and weeks for sleep duration. (d) Interaction between the pandemic and weeks for sleep latency
3.3. The effect of daytime napping on sleep pattern changes under the stay‐at‐home order during the COVID‐19 pandemic
To further investigate the effect of daytime napping on changes in sleep patterns, wake‐up time, time of falling asleep, sleep duration and sleep latency were used as dependent variables to perform 4 (type of napping changes) × 2 (pandemic: before pandemic, stay at home) × 2 (weeks: weekdays and weekends) repeated‐measures ANOVAs. For wake‐up time, time falling asleep and sleep latency, the interactions between the pandemic and daytime napping were significant (see Figure 2): F wake‐up time (3,926) = 6.48, p < .001; F time falling asleep (3,926) = 3.96, p < .01; F sleep latency (3,926) = 3.78, p < .05 (see Table 5). In detail, in the group with no daytime napping and less daytime napping than before, compared to before the pandemic outbreak, the wake‐up time was later and the sleep duration and sleep latency were longer during stay‐at‐home orders. In the group with more daytime napping than before, the differences in wake‐up time, time falling asleep, sleep duration and sleep latency before and after the pandemic were significant. In the group taking daytime napping as before, the differences in wake‐up time, time falling asleep, sleep duration and sleep latency before and after the pandemic were not significant. For wake‐up time and sleep latency, the interactions of pandemic, weeks and daytime napping were significant (see Figure 3): F wake‐up time (3,926) = 3.27, p < .05; F sleep latency (3,926) = 5.53, p < .001. The results showed that in the group taking daytime napping as before, the differences in wake‐up time, time falling asleep and sleep duration between before and after the pandemic were significant on weekdays, but were not significant on the weekends (see Table 6). In the group of no daytime napping, the differences in wake‐up time and sleep duration between before and after the pandemic were significant on the weekdays, but on weekends, only the difference in sleep latency between before and after the pandemic was significant. In the group taking more daytime naps, there were significant differences in wake‐up time, time falling asleep, sleep duration and sleep latency between before and after the pandemic on weekdays, and significant difference in wake‐up time between before and after the pandemic on weekends. In the group with less daytime napping, there were significant differences in wake‐up time, time falling asleep and sleep duration between before and after the pandemic on weekdays. Although sleep patterns became later on the weekdays during stay‐at‐home orders in all groups, the group taking daytime naps as before had minimal variation. There were no significant differences in sleep quality between before and after the pandemic in each group.
Figure 2.
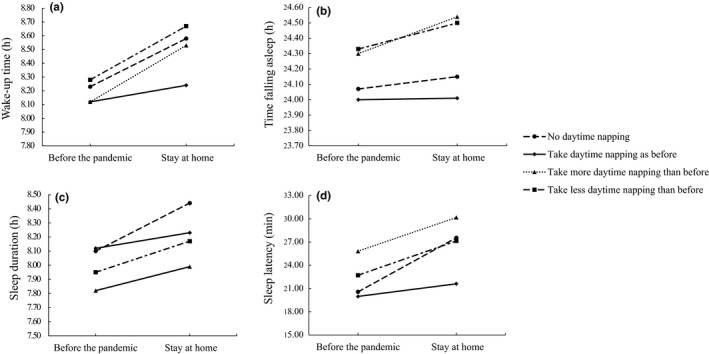
(a) The interaction between the pandemic and daytime naps for wake‐up time. (b) The interaction between the pandemic and daytime naps for falling asleep time. (c) The interaction between the pandemic and daytime naps for sleep duration. (d) The interaction between the pandemic and daytime naps for sleep latency
Table 5.
Simple effect test of the interaction between the pandemic and daytime napping
| Napping type | Before the pandemic | Stay at home | t | |
|---|---|---|---|---|
| Wake‐up time | No daytime napping | 8.23 AM | 8.58 AM | −4.31*** |
| Daytime napping as before | 8.12 AM | 8.24 AM | −2.28 | |
| More daytime napping than before | 8.12 AM | 8.53 AM | −8.63*** | |
| Less daytime napping than before | 8.28 AM | 8.67AM | −5.39*** | |
| Time falling asleep | No daytime napping | 24.06 PM | 24.14 PM | −0.97 |
| Daytime napping as before | 23.99 PM | 24.00 PM | −0.24 | |
| More daytime napping than before | 24.30 PM | 24.53 PM | −5.19*** | |
| Less daytime napping than before | 24.32 PM | 24.49 PM | −2.48 | |
| Sleep duration | No daytime napping | 8.16 h | 8.44 h | −3.56** |
| Daytime napping as before | 8.12 h | 8.23 h | −2.16 | |
| More daytime napping than before | 7.82 h | 7.99 h | −3.86** | |
| Less daytime napping than before | 7.95 h | 8.17 h | −3.17* | |
| Sleep latency | No daytime napping | 20.57 min | 27.55 min | −4.87*** |
| Daytime napping as before | 19.98 min | 21.61 min | −1.79 | |
| More daytime napping than before | 25.82 min | 30.17 min | −5.29*** | |
| Less daytime napping than before | 22.71 min | 27.14 min | −3.46* |
p < .05.
p < .01.
p < .001.
Figure 3.

(a) The interaction of the pandemic, daytime naps and weeks for wake‐up time. (b) The interaction of the pandemic, daytime naps and weeks for time falling asleep. (c) The interaction of the pandemic, daytime naps and weeks for sleep duration. (d) The interaction of the pandemic, daytime naps and weeks for sleep latency
Table 6.
Simple effect of the interactions among the pandemic, daytime napping and weeks
| Napping type | Weeks | Before the pandemic | Stay at home | t | |
|---|---|---|---|---|---|
| Wake‐up time | No daytime napping | Weekdays | 7.78 AM | 8.29 AM | −5.49*** |
| Weekends | 8.68 AM | 8.87 AM | −2.02 | ||
| Daytime napping as before | Weekdays | 7.66 AM | 7.92 AM | −4.29** | |
| Weekends | 8.58 AM | 8.56 AM | 0.32 | ||
| More daytime napping than before | Weekdays | 7.59 AM | 8.18 AM | −10.93*** | |
| Weekends | 8.66 AM | 8.89 AM | −4.10** | ||
| Less daytime napping than before | Weekdays | 7.65 AM | 8.35 AM | −8.19*** | |
| Weekends | 8.90 AM | 9.00AM | −1.20 | ||
| Time falling asleep | No daytime napping | Weekdays | 23.89 PM | 24.05 PM | −1.79 |
| Weekends | 24.25 PM | 24.24 PM | 0.02 | ||
| Daytime napping as before | Weekdays | 23.85 PM | 23.89 PM | −0.78*** | |
| Weekends | 24.15 PM | 24.13 PM | 0.35 | ||
| More daytime napping than before | Weekdays | 24.06 PM | 24.39 PM | −6.66*** | |
| Weekends | 24.55 PM | 24.69 PM | −2.82 | ||
| Less daytime napping than before | Weekdays | 24.12 PM | 24.40 PM | −3.69* | |
| Weekends | 24.54 PM | 24.60 PM | −0.84 | ||
| Sleep duration | No daytime napping | Weekdays | 7.88 h | 8.25 h | −3.87* |
| Weekends | 8.44 h | 8.63 h | −2.05 | ||
| Daytime napping as before | Weekdays | 7.81 h | 8.02 h | −3.59* | |
| Weekends | 8.43 h | 8.43 h | −0.00 | ||
| More daytime napping than before | Weekdays | 7.53 h | 7.79 h | −4.89*** | |
| Weekends | 8.12 h | 8.20 h | −1.54 | ||
| Less daytime napping than before | Weekdays | 7.54 h | 7.94 h | −4.84*** | |
| weekends | 8.36 h | 8.40 h | −0.43 | ||
| Sleep latency | No daytime napping | Weekdays | 19.07 min | 26.52 min | −4.77*** |
| Weekends | 22.07 min | 28.57 min | −4.16** | ||
| Daytime napping as before | Weekdays | 18.84 min | 20.63 min | −1.80 | |
| Weekends | 21.12 min | 22.58 min | −1.47 | ||
| More daytime napping than before | Weekdays | 22.24 min | 28.53 min | −7.00*** | |
| Weekends | 29.40 min | 31.82 min | −2.69 | ||
| Less daytime napping than before | Weekdays | 21.40 min | 25.55 min | −2.96 | |
| Weekends | 24.02 min | 28.73 min | −3.37 |
p < .05.
p < .01.
p < .001.
3.4. Effects of changes in sleep patterns and daytime napping on lifestyle and psychological feelings during the pandemic
To further investigate the effects of changes in sleep (wake‐up time, time of falling asleep, sleep duration, sleep latency and sleep quality) and daytime napping on lifestyle and negative emotions during the pandemic, hierarchical regression analysis was performed (see Tables 7 and 8). After controlling for demographic variables, the R‐squared had significantly improved in all the regression equations. In the regression model with lifestyle as the dependent variable: ΔR 2 exercise = 0.09, p < .001; ΔR 2 learning/working efficiency = 0.12, p < .001; ΔR 2 electronic device using time = 0.07, p < .001. In the regression model with negative emotions as the dependent variable: ΔR 2 annoyed = 0.11, p < .001; ΔR 2 anxious = 0.11, p < .001; ΔR 2 helpless = 11, p < .001; ΔR 2 uninterested = 0.06, p < .001; ΔR 2 losing control = 0.07, p < .001; ΔR 2 exhausted = 0.17, p < .001; ΔR 2 angry = 0.12, p < .001.
Table 7.
Results of hierarchical regression analysis of the changes in sleep patterns, daytime napping and lifestyles between before and after the epidemic
| Factor | Exercise (β) | Learning (working) efficiency (β) | Electronic device use time (β) | |||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| 1. Demographic variables | ||||||
| Gender | 0.04 | 0.04 | −0.06 | −0.05 | 0.03 | 0.00 |
| Age | 0.13* | 0.10* | 0.13* | 0.10* | −0.20*** | −0.20*** |
| Body mass index | −0.04 | −0.05 | 0.00 | −0.01 | −0.03 | −0.03 |
| Graduate | 0.01 | 0.03 | −0.14*** | −0.12** | 0.05 | 0.03 |
| Worked | 0.04 | 0.05 | 0.04 | 0.05 | 0.02 | 0.03 |
| 2. Independent variables | ||||||
| The difference in wake‐up time on weekends | −0.02 | 0.00 | 0.13* | |||
| The difference in time falling asleep on weekdays | −0.19*** | −0.14** | 0.06 | |||
| The difference in time falling asleep on weekends | 0.05 | 0.02 | −0.09 | |||
| The difference in sleep duration on weekdays | −0.08*** | −0.07 | 0.09* | |||
| The difference in sleep duration on weekends | 0.01 | −0.01 | −0.16* | |||
| The difference in sleep latency on weekdays | −0.01 | 0.01 | 0.11* | |||
| The difference in sleep latency on weekends | −0.08 | −0.03 | −0.04 | |||
| Sleep quality | 0.18*** | 0.28*** | −0.07* | |||
| No daytime napping | 0.01 | 0.03 | 0.03 | |||
| More daytime napping than before | −0.04 | −0.10** | 0.17*** | |||
| Less daytime napping than before | −0.05 | −0.02 | 0.10** | |||
| F | 5.23*** | 7.86*** | 8.39*** | 11.07*** | 6.65*** | 6.86*** |
| R 2 | 0.03 | 0.12 | 0.04 | 0.16 | 0.04 | 0.11 |
| ΔR 2 | 0.03*** | 0.09*** | 0.04*** | 0.12*** | 0.04*** | 0.07*** |
The difference in wake‐up time on weekdays does not enter any regression model.
Data results are kept to two decimal places.
p﹤0.05.
p﹤0.01.
p﹤0.001.
Table 8.
Hierarchical regression analysis results for changes in sleep patterns, daytime napping and negative emotions between before and after the epidemic
| Factor | Annoyed (β) | Anxious (β) | Helpless (β) | |||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| 1. Demographic variables | ||||||
| Gender | 0.01* | 0.01 | 0.02 | 0.02 | −0.02 | −0.02 |
| Age | −0.15 | −0.11* | −0.018*** | −0.15*** | −0.16*** | −0.13* |
| Body mass index | 0.00 | 0.02 | −0.01 | 0.01 | 0.01 | 0.03 |
| Graduate | 0.11* | 0.09* | 0.18*** | 0.16*** | 0.13*** | 0.10* |
| Working | 0.01 | 0.00 | 0.02 | 0.02 | 0.05 | 0.04 |
| 2. Independent variables | ||||||
| The difference in wake‐up time on weekends | −0.12 | −0.13* | −0.12 | |||
| The difference in time falling asleep on weekdays | 0.06 | 0.09 | 0.10* | |||
| The difference in time falling asleep on weekends | 0.02 | 0.01 | 0.02 | |||
| The difference in sleep duration on weekdays | 0.08* | 0.03 | 0.06 | |||
| The difference in sleep duration on weekends | 0.10 | 0.08 | 0.09 | |||
| The difference in sleep latency on weekdays | 0.03 | 0.02 | 0.03 | |||
| The difference in sleep latency on weekends | 0.00 | 0.01 | −0.00 | |||
| Sleep quality | −0.29*** | −0.27*** | −0.28*** | |||
| No daytime napping | 0.08* | 0.08* | 0.06 | |||
| More daytime napping than before | 0.12*** | 0.12*** | 0.10** | |||
| Less daytime napping than before | 0.16*** | 0.17*** | 0.14*** | |||
| F | 4.03** | 8.87*** | 7.99*** | 10.23*** | 3.61** | 8.25*** |
| R 2 | 0.02 | 0.13 | 0.04 | 0.15 | 0.02 | 0.13 |
| ΔR 2 | 0.02** | 0.11*** | 0.04*** | 0.11*** | 0.02** | 0.11*** |
| Factor | Uninterested (β) | Losing control (β) | Fatigue (β) | Angry (β) | ||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| 1. Demographic variables | ||||||||
| Gender | 0.04 | 0.03 | 0.04 | 0.03 | −0.01 | −0.01 | 0.02 | 0.03 |
| Age | −0.10 | −0.09 | −0.08 | −0.06 | −0.15** | −0.11* | −0.11* | −0.08 |
| Body mass index | 0.05 | 0.06 | 0.01 | 0.02 | 0.02 | 0.05 | 0.04 | 0.07* |
| Graduate | 0.07 | 0.05 | 0.10* | 0.08 | 0.05 | 0.02 | 0.08 | 0.06 |
| Working | −0.05 | −0.05 | −0.05 | −0.05 | −0.08 | −0.08 | −0.08 | −0.09 |
| 2. Independent variables | ||||||||
| The difference in wake‐up time on weekends | −0.05 | −0.04 | −0.04 | −0.11 | ||||
| The difference in time falling asleep on weekdays | 0.07 | 0.04 | −0.01 | 0.04 | ||||
| The difference in time falling asleep on weekends | 0.03 | 0.02 | 0.02 | 0.02 | ||||
| The difference in sleep duration on weekdays | 0.08* | 0.04 | −0.03 | 0.03 | ||||
| The difference in sleep duration on weekends | 0.01 | 0.02 | 0.02 | 0.07 | ||||
| The difference in sleep latency on weekdays | −0.00 | 0.04 | 0.03 | 0.03 | ||||
| The difference in sleep latency on weekends | 0.05 | 0.01 | 0.04 | 0.06 | ||||
| Sleep quality | −0.18*** | −0.21*** | −0.37*** | −0.30*** | ||||
| No daytime napping | 0.06 | 0.02 | 0.06 | 0.06 | ||||
| More daytime napping than before | 0.11*** | 0.13*** | 0.16*** | 0.10** | ||||
| Less daytime napping than before | 0.07 | 0.11*** | 0.11** | 0.14*** | ||||
| F | 4.20*** | 4.94*** | 4.25*** | 6.08*** | 8.63*** | 15.82*** | 6.71*** | 10.51*** |
| R 2 | 0.02 | 0.08 | 0.02 | 0.10 | 0.05 | 0.22 | 0.04 | 0.16 |
| ΔR2 | 0.02*** | 0.06*** | 0.02*** | 0.07*** | 0.05*** | 0.17*** | 0.04*** | 0.12*** |
The difference in wake‐up time on weekdays was not entered into any regression model.
Data results are kept to 2 decimal places.
p < .05.
p < .01.
p < .001.
A delayed sleep–wake pattern, increased sleep duration and sleep latency had significant impacts on lifestyles and emotions (the specific results are presented in Table 7 and Table 8). Change in sleep quality was one of the important factors affecting lifestyle and negative emotions. Specifically, greater sleep quality during stay‐at‐home orders positively predicted exercise level (β = 0.18, p < .001) and learning/working efficiency (β = 0.28, p < .001), and negatively predicted electronic device use (β = −0.07, p < .05) and negative emotions (β annoyance = −0.29, p < .001; β anxiety = −0.27, p < .001; β helplessness = −0.28, p < .001; β uninterested = −0.18, p < .001; β losing control = −0.21, p < .001; β fatigue = −0.37, p < .001; β anger = −0.30, p < .001).
Regarding changes in daytime napping, learning/working efficiency in the group taking more daytime naps was significantly lower than that in the group taking daytime naps as before (β = −0.10, p < .01), and electronic device use in the groups taking more daytime naps and taking fewer daytime naps was significantly greater than in the group taking daytime naps as before (β taking more daytime naps = 0.17, p < .001; β taking less daytime naps = 0.10, p < .01). Negative emotions in the group that reported taking more daytime naps were significantly higher than in the group that reported taking daytime naps as before (β annoyance = 0.12, p < .001; β anxiety = 0.12, p < .001; β helplessness = 0.10, p < .01; β uninterested = 0.11, p < .001; β losing control = 0.13, p < .001; β fatigue = 0.16, p < .001; β anger = 0.10, p < .01), and negative emotions in the group that reported taking fewer daytime naps were significantly higher than those in the group that reported taking daytime naps as before (β annoyance = 0.16, p < .001; β anxiety = 0.17, p < .001; β helplessness = 0.14, p < .001; β losing control = 0.11, p < .001; β fatigue = 0.11, p < .01; β anger = 0.14, p < .001). Annoyance and anxiety in the group that reported no daytime napping were higher than in the group that reported taking daytime naps as before (β annoyance = 0.08, p < .05; β anxiety = 0.08, p < .05).
4. DISCUSSION
As a public health emergency of international concern, COVID‐19 caused individuals to experience acute psychological stress, and affected their sleep, lifestyles and emotions. Stress responses without care or treatment may ultimately lead to acute stress disorder (ASD) and post‐traumatic stress disorder (PTSD, Friedman, 2015). This study investigated the sleep, lifestyles and psychological well‐being of young adults during the stay‐at‐home orders and demonstrated that isolation decreased their exercise levels and learning/working efficiency (compared with before the pandemic), increased electronic device use, and elevated annoyance and anxiety levels. During the pandemic, the stay‐at‐home order delayed individuals' sleep–wake schedules, increased sleep duration and prolonged sleep latency, which could further affect individuals' lifestyles and negative emotions during the pandemic. Importantly, the present findings indicated that maintaining stable daytime napping was helpful in maintaining sleep and psychological health under the stay‐at‐home orders during the pandemic, which may provide evidence for preventing and intervening in acute psychological stress caused by emergencies such as the COVID‐19 pandemic.
4.1. The relationship between sleep and individual lifestyles and negative emotions during the stay‐at‐home order
Studies have shown that increased digital media usage near bedtime and decreased physical activity occurred during the COVID‐19 pandemic (Cellini et al., 2020; Ong et al., in press). In line with this research, the present study demonstrated that acute stress as a result of the pandemic affected not only individual lifestyles (increased use of electronic devices, decreased exercise levels and low learning/working efficiency), but also affected individual psychological well‐being (increased levels of anxiety and annoyance) during the stay‐at‐home orders. It has been shown that the SARS pandemic, which was similar to the COVID‐19 pandemic, affected people's mental health and led to negative emotions, such as fear, loneliness and annoyance (Tam et al., 2004; Yu et al., 2005). A review published in The Lancet proposed that the pandemic would aggravate negative emotions, such as anxiety, depression and panic (Xiang et al., 2020). Moreover, during the stay‐at‐home orders, news about the pandemic may aggravate people's fears and other negative emotions (Léger et al., 2020; Montemurro, 2020). Therefore, much attention should be paid to people's mental health under the stress of a pandemic.
Since the outbreak of COVID‐19, a series of studies have focused on individuals' sleep changes during the pandemic (Cellini et al., 2020; Léger et al., 2020; Sanford et al., 2014). One study found that among 1,005 adults, 54% reported that their sleep worsened during the period of lockdown (Léger et al., 2020). Another study investigating 1,310 young adults showed that sleep rhythms were significantly delayed, with increased sleep duration and lower sleep quality (Cellini et al., 2020). Investigators also proposed that acute stress responses triggered by the pandemic promoted the secretion of stress‐related neuropeptides and hormones, thereby promoting wakefulness and affecting sleep architecture (Sanford et al., 2014). In the current study, we found that the sleep rhythms of young adults were delayed, sleep duration was increased and sleep latency was prolonged during the pandemic. Generally, a late sleep–wake schedule is linked with poor mental and physical health (Dong et al., 2019; Roenneberg et al., 2012). We further investigated the influence of the changes in sleep–wake schedules on individual lifestyles and negative emotions, and found that the delayed sleep–wake times could positively predict the participants' negative emotions and electronic device use, but negatively predict the exercise levels during the stay‐at‐home orders. Previous literature had proved that sleep loss and late nocturnal sleep are related to deterioration in physical health, mental health and quality of life (Haraden et al., 2017; Kayaba et al., 2020; Krističević et al., 2018; Morita et al., 2015). Delayed sleep–wake patterns can negatively predict positive emotions and social experiences (Asaoka et al., 2004; Segura‐Jiménez et al., 2015; Totterdell et al., 1994). During the pandemic, time of falling asleep and wake‐up time were later than before the pandemic, which may also cause people to feel stronger negative emotions and suffer from unsatisfactory lifestyles, such as low working/learning efficiency.
Besides the adverse effects of delayed sleep phases, the consequences of poor sleep quality also require attention. In the present study, up to 26.67% of participants reported that their sleep worsened (slightly or substantially) between before and after the pandemic. Our results revealed that poor sleep quality could also positively predict the participants' negative emotions and electronic device use, but negatively predict their exercise levels during the stay‐at‐home orders. Studies have demonstrated that compared to sleep quantity, sleep quality is closely related to health, mood, life satisfaction, tension and exhaustion (Pilcher et al., 1997). The current research proved that even though sleep duration increased during the pandemic, late sleep–wake schedules and poor sleep quality negatively influenced working/learning efficiency, physical activity, emotions, etc. Therefore, during the stress of a pandemic, interventions and healthcare that highlight both biological rhythms and sleep quality may facilitate individual lifestyles and psychological well‐being.
4.2. The stabilizing effect of regular daytime napping on nocturnal sleep patterns, lifestyle and emotions during stay‐at‐home orders
In addition to the benefits of nocturnal sleep, appropriate daytime napping can improve individuals' mood, alertness and performance (Brooks & Lack, 2006; Jones, 2009; Tietzel & Lack, 2001). These improvements are found to be significant in habitual daytime nappers but not non‐nappers (Evans et al., 1977; Milner et al., 2006). Our results testified that taking regular daytime naps during the stay‐at‐home order also had the same advantages, and appropriate daytime napping can reduce negative emotions caused by pandemic‐induced acute stress. Furthermore, maintaining regular daytime naps as they were before the pandemic effectively reduces negative emotions, enhances learning (working) efficiency and decreases electronic device use Research also indicated that the ability to fall asleep during daytime, sleep quality and benefits accrued from napping may be responsible for the frequency of an individual choosing to take a nap (Milner & Cote, 2009). Our study found that individuals who seldom take naps or experience lower nap frequency could attribute to the aforementioned reason and the intense pandemic atmosphere. Eventually, in the absence of effective countermeasures to cope with stress, these two groups of people became more emotionally distressed and had unsatisfactory lifestyles.
Moreover, naps of long duration might lead to severe sleep inertia that negatively affects daily performance (ÅKerstedt et al., 1989). Converging evidence indicated that daytime napping and nocturnal sleep seem to affect each other mutually. Studies have suggested that long naps can interfere with sleep architecture, resulting in reduced duration of nocturnal sleep and poor sleep quality due to the increased proportion of slow‐wave sleep (ÅKerstedt et al., 1989; Dinges, 1989). Furthermore, increased daytime napping causes reduced sleep pressure at night. Therefore, more time is required to accumulate a sufficient sleep drive to initiate sleep, causing a delayed sleep rhythm and increased sleep latency (Lovato & Lack, 2010). Poor nocturnal sleep and impaired daytime function, which in turn could lead to a greater need for daytime napping, create a long‐term vicious cycle.
The current study focused on changes in daytime napping caused by emergencies and explores the effects of changes in daytime napping on sleep patterns, lifestyle and negative emotions. The results indicated that stabilizing habitual daytime naps after the pandemic could maintain normal sleep patterns, improve learning efficiency and reduce negative emotions during the pandemic. These findings could provide a theoretical basis for future research. The impact of the COVID‐19 pandemic is still present. Therefore, addressing the pandemic's negative impacts is a main concern for school healthcare professionals, social workers and clinical practitioners. Maintaining regular daytime naps may be an effective way to alleviate the effect of acute psychological stress and to prevent and control mental disorders during the pandemic.
4.3. Limitations and future research directions
This study has the following limitations. First, because the COVID‐19 pandemic is an emergency, the researchers could not precisely collect information before the pandemic. Therefore, this study utilized personal recollection to collect information regarding behaviours and emotions before the pandemic, which to some extent achieves the purpose of exploring differences between before and after the pandemic. However, the bias in memory still exists and the cross‐sectional nature of this study cannot accurately reflect the causal relationship between the variables. Second, in the selection of participants, this study only focused on young adults with stable physical and mental health and did not explore the psychological and behavioural impacts of the COVID‐19 pandemic on people of other ages (such as adolescents, middle‐aged adults and older adults) during the stay‐at‐home order, which could be a research area for future studies. Finally, this study preliminarily explored changes in sleep patterns, lifestyle and psychological well‐being during the stay‐at‐home order and revealed the stabilizing effect of regular daytime napping on sleep patterns, lifestyle and psychological well‐being, and provided information regarding maintaining psychological health and sleep health during the pandemic. However, it should be noted that unhealthy lifestyles and negative emotions could also affect sleep adversely. The European Academy for Cognitive Behavioral Therapy for Insomnia (CBT‐I Academy) explored individuals' changes in sleep during COVID‐19 stay‐at‐home orders and proposed that reductions in duration of exposure to sunshine and exercise levels, and negative psychological status are the main factors that affect sleep health (Altena et al., 2020). Another study also suggested that changes in social zeitgebers (such as regular work schedules, social activities and living environments) greatly disrupted individuals' sleep patterns during the pandemic (Cellini et al., 2020). Future clinical studies and interventions may consider the bidirectional relationship of sleep problems, individual lifestyle and emotions.
5. CONCLUSION
Our study found that the stay‐at‐home orders delayed the sleep–wake phase, prolonged sleep duration and sleep latency, changed lifestyles and aggravated negative emotions among young adults during the COVID‐19 pandemic. Delayed sleep after the pandemic outbreak affected lifestyles and negative emotions. The current study has some noteworthy findings regarding regular daytime napping being an effective way to stabilize sleep patterns and biological rhythms, maintain good habits, alleviate the effect of acute psychological stress and prevent and control mental disorders during the pandemic.
CONFLICT OF INTERESTS
No conflicts of interest declared.
AUTHOR CONTRIBUTIONS
DW and ZJ: data collection, data analysis, and writing and revision of the manuscript. LG and ZB: data analysis and editing the manuscript. MN: conceptualization, funding acquisition, methodology, supervision and review and editing of the manuscript.
Funding information
This work was supported by Guangdong Basic and Applied Basic Research Foundation, China (No. 2019A1515012182).
Dai W, Zhou J, Li G, Zhang B, Ma N. Maintaining normal sleep patterns, lifestyles and emotion during the COVID‐19 pandemic: The stabilizing effect of daytime napping. J Sleep Res.2021;30:e13259. 10.1111/jsr.13259
W. Dai and J. Zhou contributed to this manuscript equally.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- ÅKerstedt, T. , Torsvall, L. , & Gillberg, M. (1989). Shift work and napping. In Dinges D. F., & Broughton R. J. (Eds.), Sleep and alertness: Chronobiological, behavioural and medical aspects of napping (pp. 205–220). New York, NY: Raven Press. [Google Scholar]
- Altena, E. , Baglioni, C. , Espie, C. A. , Ellis, J. , Gavriloff, D. , Holzinger, B. , Schlarb, A. , Frase, L. , Jernelöv, S. , & Riemann, D. (2020). Dealing with sleep problems during home confinement due to the COVID‐19 outbreak: Practical recommendations from a task force of the European CBT‐I Academy. Journal of Sleep Research, 29(4), e13052. 10.1111/jsr.13052 [DOI] [PubMed] [Google Scholar]
- Asaoka, S. , Fukuda, K. , & Yamazaki, K. (2004). Effects of sleep‐wake pattern and residential status on psychological distress in university students. Sleep and Biological Rhythms, 2(3), 192–198. 10.1111/j.1479-8425.2004.00138.x [DOI] [Google Scholar]
- Bai, Y. , Lin, C.‐C. , Lin, C.‐Y. , Chen, J.‐Y. , Chue, C.‐M. , & Chou, P. (2004). Survey of stress reactions among health care workers involved with the SARS outbreak. Psychiatric Services, 55(9), 1055–1057. 10.1176/appi.ps.55.9.1055 [DOI] [PubMed] [Google Scholar]
- Brooks, A. , & Lack, L. (2006). A brief afternoon nap following nocturnal sleep restriction: Which nap duration is most recuperative? Sleep, 29(6), 831–840. 10.1093/sleep/29.6.831 [DOI] [PubMed] [Google Scholar]
- Carmona, F. , Nieto, D. , Meléndez, J. , & Martínez, A. (2016). Acute stress among healthcare staff during a public health emergency in México. International Journal of Emergency Mental Health and Human Resilience, 18(1), 747–752. 10.4172/1522-4821.1000312 [DOI] [Google Scholar]
- Cellini, N. , Canale, N. , Mioni, G. , & Costa, S. (2020). Changes in sleep pattern, sense of time, and digital media use during COVID‐19 lockdown in Italy. Journal of Sleep Research, 29(4):e13074 (preprint). 10.31234/osf.io/284mr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, S. , Janicki‐Deverts, D. , & Miller, G. E. (2007). Psychological stress and disease. JAMA, 298(14), 1685. 10.1001/jama.298.14.1685 [DOI] [PubMed] [Google Scholar]
- Cohen, S. , Kessler, R. C. , & Gordon, U. L. (1995). Strategies for measuring stress in studies of psychiatric and physical disorder. In Cohen S., Kessler R. C., & Gordon U. L. (Eds.), Measuring Stress: A Guide for Health and Social Scientists (pp. 3–26). New York, NY: Oxford University Press. [Google Scholar]
- Dinges, D. F. (1989). Napping patterns and effects in human adults. In Dinges D. F., & Broughton R. J. (Eds.), Sleep and alertness: Chronobiological, behavioural and medical aspects of napping (pp. 171–204). New York, NY: Raven Press. [Google Scholar]
- Dong, L. , Martinez, A. J. , Buysse, D. J. , & Harvey, A. G. (2019). A composite measure of sleep health predicts concurrent mental and physical health outcomes in adolescents prone to eveningness. Sleep Health, 5(2), 166–174. 10.1016/j.sleh.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, M. , & Zheng, J. (2020). Letter to the editor: Headline stress disorder caused by Netnews during the outbreak of COVID‐19. Health Expectations, 23(2), 259–260. 10.1111/hex.13055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, F. , Cook, M. , Cohen, H. , Orne, E. , & Orne, M. (1977). Appetitive and replacement naps: EEG and behavior. Science, 197(4304), 687–689. 10.1126/science.17922 [DOI] [PubMed] [Google Scholar]
- Friedman, M. J. (2015). Strategies for acute stress reactions and acute stress disorder (ASD). In Friedman M. J. (ed). Posttraumatic and acute stress disorders (pp. 115–135). Cham, Switzerland: Springer International Publishing. 10.1007/978-3-319-15066-6_6 [DOI] [Google Scholar]
- Haraden, D. A. , Mullin, B. C. , & Hankin, B. L. (2017). The relationship between depression and chronotype: A longitudinal assessment during childhood and adolescence. Depression and Anxiety, 34(10), 967–976. 10.1002/da.22682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, S. (2009). Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Yearbook of Pulmonary Disease, 2009, 279–280. 10.1016/S8756-3452(08)79243-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaba, M. , Matsushita, T. , Enomoto, M. , Kanai, C. , Katayama, N. , Inoue, Y. , & Sasai‐Sakuma, T. (2020). Impact of sleep problems on daytime function in school life: A cross‐sectional study involving Japanese university students. BMC Public Health, 20(1), 371. 10.1186/s12889-020-08483-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krističević, T. , Štefan, L. , & Sporiš, G. (2018). The associations between sleep duration and sleep quality with body‐mass index in a large sample of young adults. International Journal of Environmental Research and Public Health, 15(4), 758. 10.3390/ijerph15040758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léger, D. , Beck, F. , Fressard, L. , Verger, P. , Peretti‐Watel, P. , Peretti‐Watel, P. , Seror, V. , Cortaredona, S. , Fressard, L. , Launay, O. , Raude, J. , Verger, P. , Beck, F. , Legleye, S. L’Haridon, O. , Ward, J. , & Léger, D. (2020). Poor sleep associated with overuse of media during the COVID‐19 lockdown. Sleep, 43(10), zsaa125. 10.1093/sleep/zsaa125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson, D. J. (1986). A conception of adult development. American Psychologist, 41(1), 3–13. 10.1037/0003-066X.41.1.3 [DOI] [Google Scholar]
- Lo Martire, V. , Caruso, D. , Palagini, L. , Zoccoli, G. , & Bastianini, S. (in press). Stress & sleep: A relationship lasting a lifetime. Neuroscience & Biobehavioral Reviews, S0149763419301496, 10.1016/j.neubiorev.2019.08.024 [DOI] [PubMed] [Google Scholar]
- Lovato, N. , & Lack, L. (2010). The effects of napping on cognitive functioning. Kerkhof G. & van Dongen H. In Progress in brain research. (Vol. 185, pp. 155–166). Amsterdam, Netherlands: Elsevier. 10.1016/B978-0-444-53702-7.00009-9 [DOI] [PubMed] [Google Scholar]
- Milner, C. E. , & Cote, K. A. (2009). Benefits of napping in healthy adults: Impact of nap length, time of day, age, and experience with napping. Journal of Sleep Research, 18(2), 272–281. 10.1111/j.1365-2869.2008.00718.x [DOI] [PubMed] [Google Scholar]
- Milner, C. E. , Fogel, S. M. , & Cote, K. A. (2006). Habitual napping moderates motor performance improvements following a short daytime nap. Biological Psychology, 73(2), 141–156. 10.1016/j.biopsycho.2006.01.015 [DOI] [PubMed] [Google Scholar]
- Montemurro, N. (2020). The emotional impact of COVID‐19: From medical staff to common people. Brain, Behavior, and Immunity, 87, 23–24. 10.1016/j.bbi.2020.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, Y. , Sasai‐Sakuma, T. , Asaoka, S. , & Inoue, Y. (2015). Prevalence and correlates of insufficient sleep syndrome in Japanese young adults: A web‐based cross‐sectional study. Journal of Clinical Sleep Medicine, 11(10), 1163–1169. 10.5664/jcsm.5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, J. , Lau, T. , Massar, S. , Chong, Z. , Ng, B. , Koek, D. , Zhao, W. , Yeo, B. , Cheong, K. , & Chee, M. (in press). COVID‐19‐related mobility reduction: Heterogenous effects on sleep and physical activity rhythms. Sleep, Online First. 10.1093/sleep/zsaa179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacák, K. , & Palkovits, M. (2001). Stressor specificity of central neuroendocrine responses: implications for stress‐related disorders. Endocrine Reviews, 22(4), 502–548. 10.1210/edrv.22.4.0436 [DOI] [PubMed] [Google Scholar]
- Pilcher, J. J. , Ginter, D. R. , & Sadowsky, B. (1997). Sleep quality versus sleep quantity: Relationships between sleep and measures of health, well‐being and sleepiness in college students. Journal of Psychosomatic Research, 42(6), 583–596. 10.1016/S0022-3999(97)00004-4 [DOI] [PubMed] [Google Scholar]
- Roenneberg, T. , Allebrandt, K. V. , Merrow, M. , & Vetter, C. (2012). Social jetlag and obesity. Current Biology, 22(10), 939–943. 10.1016/j.cub.2012.03.038 [DOI] [PubMed] [Google Scholar]
- Sanford, L. D. , Suchecki, D. , & Meerlo, P. (2014). Stress, arousal, and sleep. In Meerlo P., Benca R. M., & Abel T. (Eds.), Sleep, Neuronal Plasticity and Brain Function, Vol. 25 (pp. 379–410). Berlin, Heidelberg: Springer. 10.1007/7854_2014_314 [DOI] [Google Scholar]
- Segura‐Jiménez, V. , Carbonell‐Baeza, A. , Keating, X. D. , Ruiz, J. R. , & Castro‐Piñero, J. (2015). Association of sleep patterns with psychological positive health and health complaints in children and adolescents. Quality of Life Research, 24(4), 885–895. 10.1007/s11136-014-0827-0 [DOI] [PubMed] [Google Scholar]
- Tam, C. W. C. , Pang, E. P. F. , Lam, L. C. W. , & Chiu, H. F. K. (2004). Severe acute respiratory syndrome (SARS) in Hong Kong in 2003: Stress and psychological impact among frontline healthcare workers. Psychological Medicine, 34(7), 1197–1204. 10.1017/S0033291704002247 [DOI] [PubMed] [Google Scholar]
- Tietzel, A. J. , & Lack, L. C. (2001). The short‐term benefits of brief and long naps following nocturnal sleep restriction. Sleep, 24(3), 293–300. 10.1093/sleep/24.3.293 [DOI] [PubMed] [Google Scholar]
- Totterdell, P. , Reynolds, S. , Parkinson, B. , & Briner, R. B. (1994). Associations of sleep with everyday mood, minor symptoms and social interaction experience. Sleep, 17(5), 466–475. 10.1093/sleep/17.5.466 [DOI] [PubMed] [Google Scholar]
- Wood, D. , Crapnell, T. , Lau, L. , Bennett, A. , Lotstein, D. , Ferris, M. , & Kuo, A. (2018). Emerging adulthood as a critical stage in the life course. In Halfon N., Forrest C. B., Lerner R. M., & Faustman E. M. (Eds.), Handbook of life course health development (pp. 123–143). Cham, Switzerland: Springer; International Publishing. 10.1007/978-3-319-47143-3_7 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2020). Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019‐nCoV). Retrieved August 2, 2020, from https://www.who.int/news‐room/detail/30‐01‐2020‐statement‐on‐the‐second‐meeting‐of‐the‐international‐health‐regulations‐(2005)‐emergency‐committee‐regarding‐the‐outbreak‐of‐novel‐coronavirus‐(2019‐ncov) [Google Scholar]
- Wright, K. P. , Linton, S. K. , Withrow, D. , Casiraghi, L. , Lanza, S. M. , de la Iglesia, H. , Vetter, C. , & Depner, C. M. (2020). Sleep in university students prior to and during COVID‐19 stay‐at‐home orders. Current Biology, 30(14), R797–R798. 10.1016/j.cub.2020.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, Y.‐T. , Yang, Y. , Li, W. , Zhang, L. , Zhang, Q. , Cheung, T. , & Ng, C. H. (2020). Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. The Lancet Psychiatry, 7(3), 228–229. 10.1016/S2215-0366(20)30046-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H. Y. R. , Ho, S. C. , So, K. F. E. , & Lo, Y. L. (2005). The psychological burden experienced by Hong Kong midlife women during the SARS epidemic. Stress and Health, 21(3), 177–184. 10.1002/smi.1051 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


