Abstract
Background:
Aromatase inhibitor-induced arthralgia (AIA) is the most common side effect of aromatase inhibitors (AIs) used in breast cancer patients and is related to the rate of adherence to AIs. The clinical effects of acupuncture on AIA have been assessed by some randomized controlled trials (RCTs). However, some studies reported that acupuncture was effective, while others claimed that it was ineffective. To clarify the clinical and placebo effects of acupuncture in treating AIA, we conducted this meta-analysis.
Methods:
Two reviewers (XL and GW) independently searched for RCTs in 5 English databases (PubMed, Web of Science, Embase, Springer, Cochrane Library) and 4 Chinese databases (China National Knowledge Infrastructure Database (CNKI), SinoMed, VIP and Wanfang Database) from their inception to 30 November 2019. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, this meta-analysis was performed by fixed or random-effects models, and data were pooled with mean differences (MDs).
Results:
Seven trials involving 603 patients were reviewed. The primary outcome, the Brief Pain Inventory (BPI) score, significantly differed between the acupuncture and control groups [pain-related interference: MD = −1.89, 95% confidence interval (CI) [−2.99, −0.79], Z = 3.36 (P = .008 < .05), pain severity: MD = −1.57, 95% CI [−2.46, −0.68], Z = 3.45 (P = .0006 < .05), worst pain: MD = −2.31, 95% CI [−3.15, −1.48], Z = 5.47 (P < .0001 < .05)]. No severe adverse events were reported in any study.
Conclusion:
This meta-analysis showed that acupuncture is a safe and effective treatment for breast cancer patients with AIA. Additional research with improved blinding methods is warranted to further explore the nature of non-specific and placebo effects in true and sham acupuncture.
Keywords: acupuncture, aromatase inhibitor-induced arthralgia, breast cancer, meta-analysis
Background
Breast cancer is the most widespread cancer and is the second leading cause of death among women.1 Aromatase inhibitors (AIs), the standard treatment for early-stage breast cancer, can reduce the risk of recurrence in postmenopausal and hormone receptor-positive patients.2 The types of AIs include steroidal inhibitors (exemestane) and nonsteroidal inhibitors (anastrozole and letrozole). The American Society of Clinical Oncology (ASCO) Clinical Practice Guideline recommends that women with node-positive breast cancer are offered extended AI therapy for up to a total of 10 years of adjuvant endocrine treatment.3 Nevertheless, side effects caused by AIs, such as severe aromatase inhibitor-induced arthralgia (AIA), may cause poor adherence to AIs. One study4 revealed that up to 50% of patients terminated the use of AIs within the first year of use. Another prospective study5 including 1916 patients receiving upfront anastrozole concluded that AIA was related to treatment noncompliance.
At present, the interventions for relieving AIA include drugs and exercise. A review6 suggested that exercise, weight loss, vitamin D and bisphosphonate can be beneficial for mild arthralgia. However, their clinical effects are still unclear. In addition, some drugs, such as bisphosphonate, have nonnegligible side effects, including acute-phase reactions, gastrointestinal sequelae and nephrotoxicity.7 Some experts have suggested that prednisolone or nonsteroidal anti-inflammatory drugs are taken for AIA,6,8,9 but clinicians have argued that these drugs are associated with a risk of heart attack and stroke.10
Considering these unfavorable side effects, alternative approaches, such as acupuncture, yoga or exercise, have been used to treat AIA in recent years. Acupuncture has been confirmed to have a positive effect on AIA by some randomized controlled trials (RCTs).11-17 The Clinical Practice Guidelines18 also recommended that acupuncture is used to relieve side effects caused by conventional treatments for breast cancer. However, the effect of acupuncture on AIA still needs to be further confirmed by high-quality studies or related meta-analyses.
By November 2019, 4 meta-analyses19-22 on the effect of acupuncture on AIA had been published. However, they did not assess the inconsistent placebo effects of acupuncture, and they did not include articles published in China, where acupuncture originated. In addition, a multicenter study11 with 226 patients suggested the effect of acupuncture on AIA, which may affect the results of previous meta-analyses. Therefore, it was necessary to perform additional research to comprehensively assess the effect of acupuncture on AIA.
In general, the primary aim of this study was to clarify the clinical and placebo effects of acupuncture with respect to those of a control intervention. In addition, we aimed to provide suggestions for the design of future studies. The comprehensive searches and rigorous eligibility criteria strengthened the validity and generalizability of our review.
Methods
Study Eligibility Criteria
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines23 to perform this meta-analysis. The inclusion criteria consisted of RCTs that evaluated the effects of acupuncture on AIA in patients with breast cancer. The participants were (1) aged 18 years or older; (2) patients diagnosed with breast cancer on the basis of pathology, cytology, or histological features; and (3) patients taking AIs for more than 1 month. For the interventions of the experimental group, all types, doses, and regimens of acupuncture, such as electroacupuncture and auricular acupuncture, were included. For the control intervention, sham acupuncture, drugs and the absence of treatment were included. The primary outcome was the severity of joint pain, as assessed by the Brief Pain Inventory (BPI), and the secondary outcomes were the scores for the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), visual analog scale (VAS), functional assessment of cancer therapy (FACT), and other assessment tools. Nonrandomized studies, review articles, repeated publications, commentaries, letters, case reports, meeting abstracts, guidelines and nonpeer-reviewed articles were excluded.
Search Strategy
Two reviewers (XL and GW) independently searched for articles in 5 English databases (PubMed, Web of Science, Embase, Springer, Cochrane Library) and 4 Chinese databases (China National Knowledge Infrastructure Database (CNKI), SinoMed, VIP, and Wanfang) from their inception to 30 November 2019. The following English search terms were used for titles, abstracts and keywords: (“acupuncture” or “acupressure” or “acupoint” or “electroacupuncture” or “ear acupuncture” or “auricular acupuncture” or “warm needling” or “moxibustion”) and (“aromatase inhibitor”). The following Chinese medical subject heading (MeSH) terms were used for the electronic searches: (“acupuncture (针灸)” or “acupuncture (针刺)” or “electroacupuncture (电针)” or “ear acupuncture (耳针)” or “scalp acupuncture (头针)” or “moxibustion (艾灸)” or “acupoint (穴位)” or “acupoint (腧穴)”) and MeSH (“aromatase inhibitor (芳香化酶抑制剂)”). All searches were performed by two independent reviewers, and disagreements were resolved by consensus or, if necessary, by consulting with a third party (LJ). All the search strategies were developed and adapted for each database. The search strategies used for PubMed were as follows:
#1 Aromatase Inhibitor [MeSH Terms] OR Aromatase Inhibitor [Title/Abstract]
#2 Acupuncture [MeSH Terms] OR Electroacupuncture [MeSH Terms]
#3 Acupuncture [Title/Abstract] OR acupressure [Title/Abstract] OR acupoint [Title/Abstract] OR Electroacupuncture [Title/Abstract] OR ear acupuncture [Title/Abstract] OR auricular acupuncture [Title/Abstract] OR warm needling [Title/Abstract] OR moxibustion [Title/Abstract] OR stimulat [Title/Abstract] OR electrostimulat [Title/Abstract] OR neurostimula [Title/Abstract] OR Zhen Jiu [Title/Abstract] OR meridian [Title/Abstract] OR Jing Luo [Title/Abstract]
#4 #2 OR #3
#5 #1 AND #4.
Selection of Studies and Data Extraction
Two authors (XL and GW) independently evaluated all titles and abstracts to identify all eligible studies. The full texts of candidate articles were subsequently screened to determine whether the articles were relevant to AIA. Discrepancies in this process were settled by discussion, with a third party (JL) if necessary. JL did not participate in the screening or data extraction processes. The study selection process was documented with a flow diagram according to the Cochrane handbook. XL and GW collected the data and recorded the data in Microsoft Excel. The data included the study design, sample size, age of the patients, eligibility criteria, details of the acupuncture and control groups (methods, acupoints, session, etc.), outcomes such as the BPI and WOMAC scores, side effects and conclusions. We emailed study authors if data were missing or unclear.
Quality Assessment
The risk of bias of the articles selected was independently assessed by 2 reviewers (XL and GW) based on the Cochrane Collaboration’s risk of bias tool.24 Any discrepancies were discussed with a third party (JL). This tool addressed sequence generation, allocation concealment, the blinding of the participants and personnel, the blinding of the outcome assessment, incomplete outcome data, selective reporting, and other bias. Each type of bias was graded as having a low, high or unclear level or risk by those 2 reviewers for all articles.
Statistical Analyses
We used Review Manager software (version 5.3, Cochrane Collaboration, UK) to statistically analyze the data and generate forest plots.25 The severity of AIA in the included studies was measured by several scales with continuous data (eg, BPI and WOMAC). The changes in the continuous variables were measured by mean differences (MDs) and standard deviations (SDs). MDs were used to pool the measurement data. Statistical heterogeneity was examined with the Cochrane Q statistic and I2 statistic. The overall effect differences were considered statistically significant when P ≤ .05. If P ≥ .10 and I2 ≤ 50%, we adopted a fixed-effects model to account for expected heterogeneity; otherwise, a random-effects model was used.26 If the level of heterogeneity was substantial, post hoc subgroup analyses were performed according to the characteristics of different studies or patients. If it was inappropriate to pool data because of heterogeneity, only descriptive analyses were performed.
Results
Study Selection
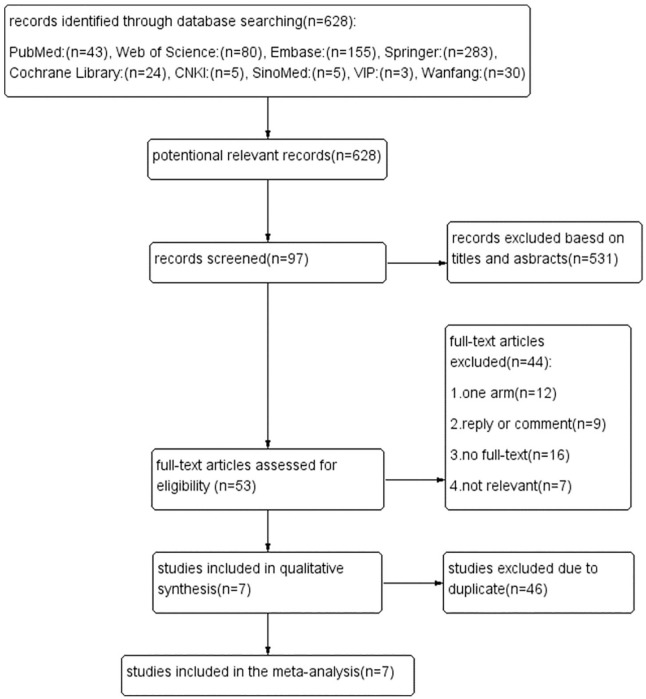
We extracted 628 studies from 5 English databases and 4 Chinese databases. A total of 531 articles were excluded after the titles and abstracts were screened. We excluded 44 articles according to the eligibility criteria by reading the full texts. Of the remaining 53 articles, 12 were single-arm studies, 9 were commentaries, 16 did not have full-text versions available, and 7 articles were not related to our study. After 46 duplicate articles were excluded, 7 articles11-17 were included in the final meta-analysis (Figure 1).
Figure 1.
Flow chart.
Among these 7 articles, the study by Oh et al.13 had incomplete data, and we failed to make contact with the author to retrieve the missing data. Bao et al.15 used medians to describe the results, while other articles used averages and standard deviations. Li et al.17 used the VAS to evaluate the severity of AIA, while other articles used the BPI. Because of these inconsistencies, it was difficult to analyze these 3 articles together with the other 4 articles, so we only described their results and did not perform meta-analyses.
Study Characteristics
Basic characteristics
The 7 articles included a total of 603 patients. The study characteristics are shown in Table 1. Four articles13-15,17 had 2 arms, 211,12 had 3 arms, and 117 had 4 arms. Only 1 study11 had a sufficient sample size of over 50 in each group. The average age of the included participants ranged from 41 to 85 years. All patients were diagnosed with breast cancer stages Ⅰ-Ⅲ and hormone receptor-positive cancer and took AIs for more than 1 month. The drop-out rate was less than 12% in all 7 articles.
Table 1.
Study characteristics.
| Study design | Sample size | Age | Inclusion criteria | Drop out rate (%) | Outcome Measurement tool | Conclusion | |||
|---|---|---|---|---|---|---|---|---|---|
| Acupuncture | Control | Acupuncture | Control | ||||||
| Oh et al.13 | Two arms | 15 | 14 | <45 12 (86%) ≥45 2 (14%) | <45 14 (93%) ≥45 1 (7%) | Postmenopausal; stage I, II or IIIa; hormone receptor-positive; third generation AI ≥ 6 months; BPI-SF ≥ 3; | 9.4 | (1) Pain: BPI, WOMAC. (2) Functional ability: FACT-G, Grip test. (3) Inflammation biomarker: CRP, ESR | TA versus SA: non-significant findings. TA was well tolerated and potential |
| Mao et al.12 | Three arms | 22 | SA: 22 WLC:23 | 57.5 ± 10.1 | SA: 60.9 ± 6.5. WLC:60.6 ± 8.2 | Stages I–III; AI ≥ 3 months; numerical rating scale ≥ 3 | 11.9 | (1) Pain: BPI, WOMAC. (2) Functional ability: DASH, PPT. (3) Global Impression of Change | (1) TA > WLC: significantly effective. (2) SA > WLC: significantly effective. (3) TA versus SA: nonsignificant |
| Hershman et al.11 | Three arms | 110 | SA: 59 WLC: 57 | 60.8 (34.1-80.6) | SA: 57.0 (40.6-77.5) WLC: 60.6 (27.1-76.0) | Postmenopausal or premenopausal with gonadotropin-releasing hormone agonist; stages I-III; primary invasive estrogen receptor-positive; third generation AI ≥ 30 days to continue for at least one additional year; Zubrod performance 0-1; BPI-SF ≥ 3 | 11.9 | (1) Pain: BPI, WOMAC, PROMIS PI-SF. (2) Functional ability: M-SACRAH, FACT-ES | joint pain at 6 weeks. (1) TA > WLC: statistically significant reduction. (2) TA vs SA: statistically significant reduction. (3) Uncertain clinical importance |
| Crew et al.14 | Two arms | 20 | 18 | 58(44-77) | 57(37-77) | Postmenopausal; Stage I-III;Third-generation AI ≥ 3 months; BPI-SF ≥ 3 | 11.6 | (1) Pain: WOMAC, BPI-SF. (2) Functional ability: M-SACRAH, FACT-G | TA > SA:significant. |
| Bao et al.15 | Two arms | 23 | 24 | 61 (45–85) | 61 (44–82) | Postmenopausal; Stage 0-III; ER and/or PR positive; Third-generation AI ≥ 1 month; HAQ-DI ≥ 0.3 and/or VAS ≥ 20 | 7.8 | (1) Pain: VAS. (2) Functional ability:HAQ-DI. (3) Serum:estradiol, cytokine profile, and b-endorphin | (1) TA versus SA: nonsignificant. (2) Positive trends were observed. |
| Ye et al.16 | Four arms | ①:30. ②:31 | ③:33. ④:30 | ①:44~84. ②:41~78 | ③:50~80. ④:41~77 | Postmenopausal; Stage I-III; ER and/or PR positive; Third-generation AI ≥ 1 month; BPI-SF ≥ 3 | 11.4 | (1) Pain:BPI-SF. (2) BMD of lumber vertebrae | BPI-SF: (1) ① versus ②: nonsignificant. (2) ① ② Versus ③④: significant after 6 weeks but nonsignificant after 12 weeks. BMD T-score:nonsignificant |
| Li et al.17 | Two arms | 36 | 36 | 58 (47-70) | 57 (45-68) | postmenopausal or ovariectomy; stage I-III; third-generation AI ≥ 1 month; VAS ≥ 3 | 0 | (1) Pain: VAS. (2) Activty of daily living: BI. (3) BMD of lumber vertebrae. (4) Serum: estradiol,IFN-γ,IL-4 | VAS, BI, BMD T-score, IFN-γ, IL-4: Canggui Tanxue > Caltrate: significantly effective. E2: Canggui Tanxue versus Caltrate nonsignificant |
Abbreviations: SA: sham acupuncture group; WLC: waitlist control group; TA: true acupuncture group; BPI: Brief Pain Inventory; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; DASH: Quick Disability of Arm, Shoulder, Hand scale; PPT: Physical Performance Test; FACT: Functional Assessment of Cancer Therapy; M-SACRAH: Modified Score for the Assessment and Quantification of Chronic Rheumatoid Affections of the Hands; VAS: Visual Analogue Scale; HAQ: Health Assessment Questionnaire.
Interventions
The interventions included acupuncture (auricular acupuncture,16 body acupuncture11-15,17), sham acupuncture,11-15 drugs16,17 and no treatment.11,12
Auricular acupuncture was administered in 1 study16 for 3 minutes 18 times a week for 12 weeks. In the body acupuncture groups, the duration of each session ranged from 20 to 45 minutes, the frequency of treatment ranged from twice to 8 times each week, and the entire study lasted for 6 to 12 weeks. Standard acupoints were used in 4 studies.11,13-15 Li et al.17 used the “Ashi Point (阿是穴)” in the most painful area, while Mao et al.12 used 4 local points around the most painful joint and 4 distant points to regulate the whole body. In the sham-acupuncture groups, needles were inserted into the skin in 2 studies11,14 and were not inserted in 3 studies.12,13,15
Outcomes
The severity of joint pain was mainly assessed by the BPI in 5 articles,11-14,16 by the WOMAC in 4 articles11-14 and by the VAS in 2 articles.15,17 Five articles11-15 evaluated functional ability with the FACT,11,13,14 quick disabilities of the arm, shoulder, hand (DASH) scale,12 physical performance test (PPT),12 modified score for the assessment and quantification of chronic rheumatoid affections of the hands (M-SACRAH)11,14 and health assessment questionnaire (HAQ).15 Laboratory indices, including the C-reactive protein (CRP) level, erythrocyte sedimentation rate (ESR), estradiol level, cytokine profile, β-endorphin level, and interferon-γ (IFN-γ) and interleukin 4 (IL-4) levels, were detected in 3 articles.13,15,17
The BPI was used to assess the worst pain, worst stiffness and pain severity associated with AIA in breast cancer patients. The WOMAC was used to evaluate the severity of osteoarthritis in the knees or hips. The VAS is a standard measure of clinical musculoskeletal disorder severity than ranges from 0 (no pain) to 100 (severe pain). For assessing hand pain, stiffness, and functional status, the M-SACRAH was used. The FACT was used to assess physical ability and endocrine symptoms. The DASH scale was used to assess upper extremity function. The PPT included assessments of both lower and upper extremity function, as well as balance and endurance. In the HAQ-DI, dressing, rising, eating, walking, grooming, reaching gripping, and performing errands were assigned scores of 0 (no difficulty), 1 (some difficulty), 2 (much difficulty), or 3 (unable to do).
Results
In total, 5 studies11-15 compared the effect of acupuncture and sham acupuncture; 3 of these studies12,13,15 showed that the difference was statistically significant. Two studies11,12 showed that the difference between the acupuncture group and the no treatment group was significant. Two studies16,17 compared the effect of acupuncture with that of drugs. One study16 showed a significant difference after 6 weeks but no significant difference after 12 weeks, while the other showed a significant difference during treatment. Seven articles11-17 reported few and minor adverse reactions that did not severely harm patients.
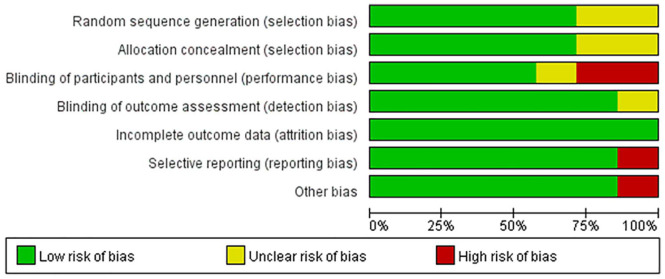
Risk of Bias Assessment
The risk of bias assessment for all studies is shown in Figure 2. Figure 3 shows the risk of bias for each RCT according to the Cochrane risk of bias tool.
Figure 2.
Risk of bias summary.
Figure 3.
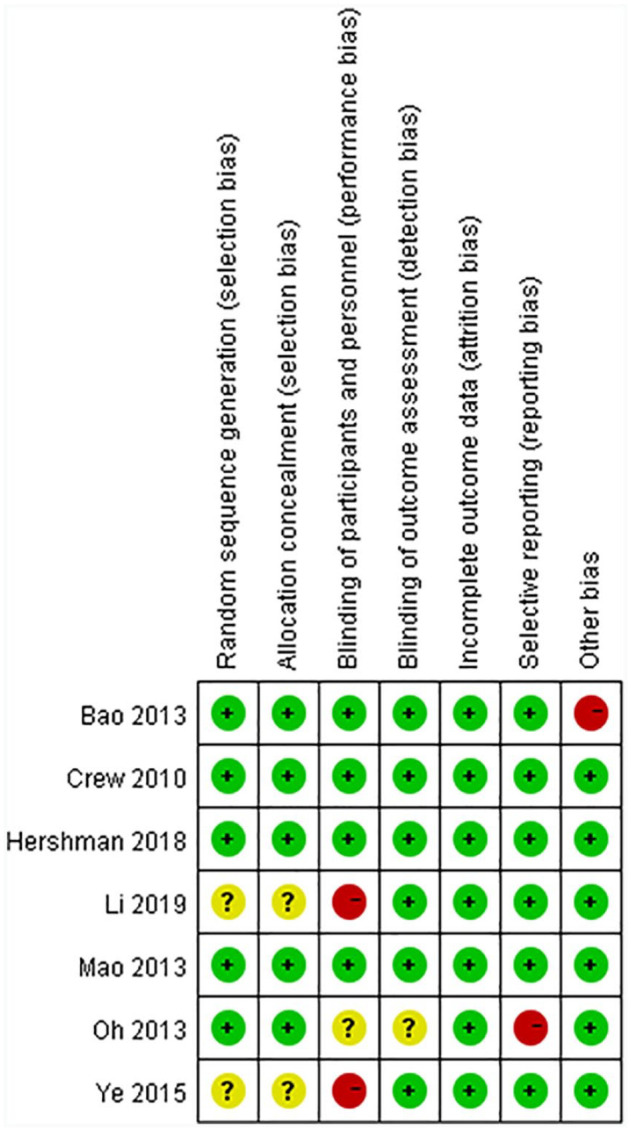
Risk of bias.
Adequate sequence generation
Five studies11-15 reported the methods used for randomization clearly; studies that used computer-generated randomization tables were judged to have a low risk of bias, whereas the remaining 2 studies16,17 did not report how random numbers were generated and were judged to have an unclear risk of bias.
Allocated concealment
Three studies12-14 achieved concealment by using sealed, opaque envelopes, and 2 studies11,15 achieved concealment by using a central trial center. Therefore, these 5 studies were judged to have a low risk of bias. The remaining 2 studies16,17 did not report whether group allocation was adequately concealed and were judged to have an unclear risk of bias.
Blinding methods
Because of the specificity of acupuncture, it is difficult to blind acupuncturists. Therefore, four studies11,12,14,15 performed blinding for patients and were judged as having a low risk of bias. Two studies16,17 compared the effect of acupuncture with that of drugs, and the included patients were definitely aware which group (acupuncture group or drug group) they belonged to; these studies were judged as having a high risk of bias. The level of risk was unclear for 1 study.13
Five studies11,12,14-16 performed blinding for the investigator or outcome assessor and were judged as having a low risk of bias, whereas the remaining 2 studies13,17 did not mention this type of blinding and were judged has having an unclear risk of bias.
Incomplete outcome data and selective outcome reporting
Only 1 study13 provided insufficient data and was judged as having a high risk of bias. The remaining six11,12,14-17studies were judged as having a low risk of bias.
Other bias
The baseline HAQ score in the real acupuncture group was significantly higher than that in the sham-acupuncture group in 1 study,15 which was judged as having a high risk of bias. The remaining six studies11-14,16,17 were judged as having a low risk of bias.
Outcomes
BPI
The BPI consists of 3 subscales: pain-related interference, pain severity, and worst pain. In this part, we analyzed each subscale. In 5 articles11-14,16 that used the BPI to assess the severity of pain, the study by Oh et al.13 stated that there were no significant differences in pain severity or interference with daily functioning only between the sham and real electroacupuncture groups, and complete data were not provided, so it was difficult to include this article in the meta-analyses. Finally, 4 eligible articles11,12,14,16 were included in this part.
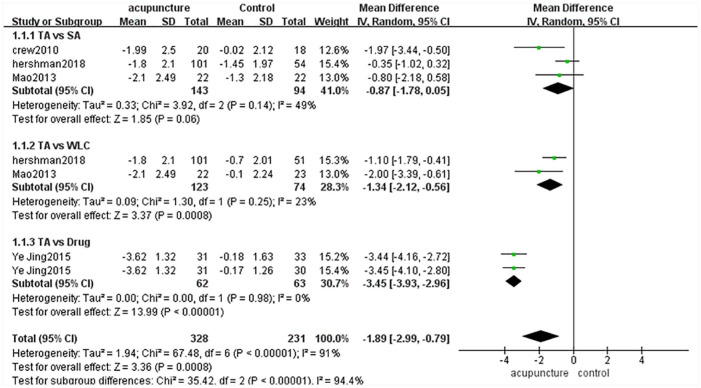
BPI Pain-Related Interference
There were 174 cases in the acupuncture group and 231 cases in the control group (sham-acupuncture group, waitlist group or drug group).11-14,16 The heterogeneity of these 4 articles was high (P < .00001, I2 = 91%), so we used a random-effects model in combined-effect analyses. The acupuncture group was superior to the control group [MD = −1.89, 95% CI [−2.99, −0.79], Z = 3.36 (P = .008 < .05)] (Figure 4).
Figure 4.
BPI pain-related interference.
Because of the high heterogeneity among these 4 studies, we divided them into 3 subgroups according to the control method used. There were no significant differences between the acupuncture group and sham-acupuncture group [MD = −0.87, 95% CI [−1.78,0.05], Z = 1.85 (P = .06 > .05)], while there were significant differences between the acupuncture group and waitlist group [MD = −1.34, 95% CI [−2.12, 0.56], Z = 3.37 (P = .008 < .05)] and between the acupuncture group and drug group [MD = −3.45, 95% CI [−3.93, 2.96], Z = 3.36 (P < .00001)]. Subgroup analyses showed that the heterogeneity among subgroups was high (P < .00001, I2 = 96.8%) (Figure 4).
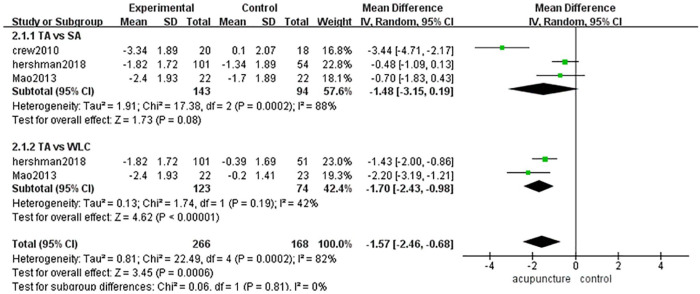
BPI Pain Severity
There were 143 cases in the acupuncture group and 168 cases in the control group (sham-acupuncture group or waitlist group).11,12,14 The heterogeneity among these 3 articles was high (P = .0002, I2 = 82%), so we used a random-effects model in combined-effect analyses. Acupuncture was more effective than sham acupuncture or the placebo [MD = −1.57, 95% CI [−2.46, −0.68], Z = 3.45 (P = .0006 < .05)] (Figure 5).
Figure 5.
BPI pain severity.
Because of the high heterogeneity among these 3 studies, we divided them into 2 subgroups according to the control method used. There were no significant differences between the acupuncture group and sham-acupuncture group [MD = −1.48, 95% CI [−3.15,0.19], Z = 1.73 (P = .08 > .05)], while there were significant differences between the acupuncture group and waitlist group [MD = −1.70, 95% CI [−2.43, −0.98], Z = 4.62 (P < .00001)]. Subgroup analyses showed that the heterogeneity among subgroups was low (P = .81, I2 = 0%) (Figure 5).
BPI Worst Pain
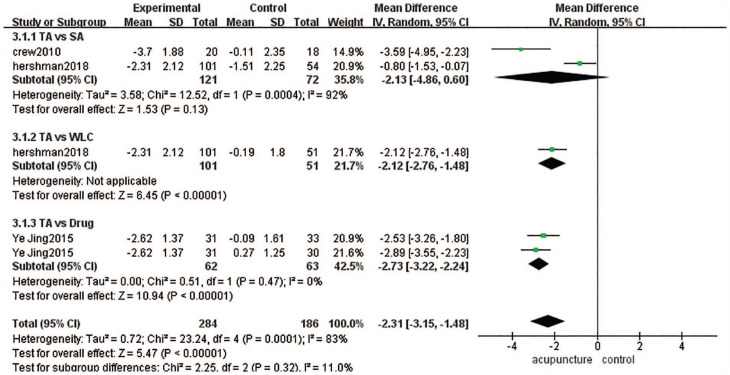
There were 152 cases in the acupuncture group and 186 cases in the control group (sham-acupuncture group, waitlist group or drug group).11,14,16 The heterogeneity among these 3 articles was high (P = .00001, I2 = 83%), so we used a random-effects model in combined-effect analyses. There were significant differences between the acupuncture group and control group [MD = −2.31, 95% CI [−3.15, −1.48], Z = 5.47 (P < .0001 < .05)] (Figure 6).
Figure 6.
BPI worst pain.
Because of the high heterogeneity among these 3 studies, we divided them into 3 subgroups according to the kind of control method used. There were no significant differences between the acupuncture group and sham-acupuncture group [MD = −2.13, 95% CI [−4.86,0.60], Z = 1.53 (P = .13 > .05)], while there were significant differences between the acupuncture group and waitlist group [MD = −2.12, 95% CI [−2.76, 1.48], Z = 6.45 (P < .00001)] and between the acupuncture group and drug group [MD = −2.73, 95% CI [−3.22, 2.24, Z = 10.94 (P < .00001)]. Subgroup analyses showed the heterogeneity among subgroups was low (P = .32, I2 = 11%) (Figure 6).
WOMAC
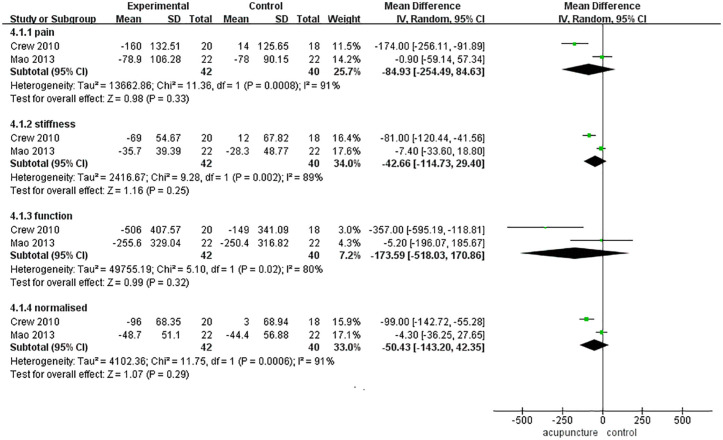
The WOMAC consists of 4 subscales: the pain, stiffness, function and normalized subscales. In this part, we planned to analyze the subscores respectively. However, in the 4 articles11-14 that used the WOMAC as an assessment tool, Hershman et al.11 reported only a total score, and Oh et al.13 did not report the specific WOMAC results. Therefore, two eligible articles12,14 were included in this part.
There were 42 cases in the acupuncture group and 40 cases in the control group (sham acupuncture). There were no significant differences between the 2 groups in the pain score [MD = −84.93, 95% CI [−254.49, 84.63], Z = 0.98 (P = .33 > .05)], stiffness score [MD = −42.66, 95% CI [−114.73, 29.40], Z = 1.16 (P = .25 > .05)], functional score [MD = −173.59, 95% CI [−518.03, 170.86], Z = 0.99 (P = .32 > .05)] or normalized score [MD = −50.43, 95% CI [−143.20, 42.35], Z = 1.07 (P = .29 > .05)] (Figure 7).
Figure 7.
WOMAC.
Adverse Effects
No severe adverse events were reported in any study. Three studies reported adverse events, such as bruising and presyncope,11 pain,12 and minor bruising.13 Two studies reported there were no adverse events.15,17 However, the other 2 studies did not mention adverse events14,16 (Table 1).
Discussion
This meta-analysis assessed the effect of acupuncture on AIA in breast cancer patients. The results showed that acupuncture can significantly improve the pain-related interference score, pain severity score and worst pain score for the BPI compared with drugs and no treatment. Furthermore, no severe adverse events were reported in any of the studies. Therefore, we conclude that acupuncture can be an effective and safe treatment for AIA.
The effect of acupuncture on AIA has been preliminarily confirmed, but the mechanism is still unclear. The main cause of arthralgia is the lack of estrogen,27 which may decrease the generation of endogenous opioids, thereby leading to a lowered pain threshold.28 Acupuncture has been demonstrated to enhance endogenous opiates, such as dynorphin, endorphin, and encephalin. In addition, polymodal receptor hypothesis,29 purinergic signaling30 and other mechanotransduction-based responses31 to acupuncture may also contribute to pain relief.
In the eligible studies in this meta-analysis, 3 studies tested blood samples from patients to explore the mechanism of acupuncture. Mao et al.12 and Li et al.17 reported that there were no significant changes in the serum estrogen level between the acupuncture and control groups. As mentioned above, Bao et al.15 showed a significant reduction in the interleukin 17 (IL-17) level in both the real and sham-acupuncture groups. The IL-17 pathway is associated with the development of AIA.32 Therefore, we hypothesize that acupuncture may treat AIA by modulating IL-17.
Compared with drugs and no treatment, acupuncture is effective in treating AIA. However, when we compared acupuncture with sham acupuncture, there were no significant differences in the pain-related interference score, pain severity score or worst pain score for the BPI. According to the pain, stiffness, functional and normalized WOMAC scores (Figure 7), compared with sham acupuncture, acupuncture did not significantly improve the symptoms.
In 5 articles that used sham acupuncture as a control method, Mao et al.,12 Oh et al.,13 and Bao et al.14 used sham needles that did not penetrate the skin. All the authors found that compared with sham acupuncture, acupuncture does not statistically significantly improve the symptoms of AIA. Hershman et al.14 and Crew et al.17 used minimally invasive needles to penetrate the skin in the sham-acupuncture groups. The authors found that the effects of acupuncture were statistically significantly better than those of sham acupuncture.
The purpose of including a sham-acupuncture group in a clinical trial on acupuncture is to reduce the differences in outcomes that are caused by non-specific effects.33 However, as the analyses above show, whether the effect of acupuncture is better than that of sham acupuncture is still unclear and controversial.
Acupuncture has been used in China and many other countries for several decades. Some clinical experts argue that acupuncture is definitely effective according to their experiences. However, if we want to demonstrate the effects of acupuncture scientifically to a broad audience, we need to follow the basic guidelines34 of how to conduct scientific clinical research. The placebo group is necessary.
As mentioned before, sham acupuncture is considered a placebo intervention. However, how to perform sham acupuncture correctly to successfully reduce the placebo effect or psychological effects of acupuncture remains unclear. Some experts who used sham needles that do not penetrate the skin12,13,15 indicated that the effect of sham acupuncture is equivalent to that of acupuncture; others who used slightly more invasive needs that penetrate the skin when performing sham acupuncture11,14 showed that the effect of acupuncture is better than that of sham acupuncture. In other studies, a systematic review reported that sham acupuncture may be as effective as real acupuncture.35 Other studies have indicated that both real and sham acupuncture can result in the binding of μ opioids to receptors in the brain36 and activate the pain-related neuromatrix.37
Given that this meta-analysis has shown that acupuncture is effective, perhaps in the future, all clinical and methodological experts should focus on finding a proper sham-acupuncture intervention to be used in acupuncture trials to concretely and scientifically show the effects of acupuncture; then, the medical community would have evidence that acupuncture is an acceptable and effective treatment for some symptoms such as pain and disorders such as insomnia and mood disorders.
Before this study, a previous meta-analysis18 assessed the double-blinded studies (Mao et al.,12 Crew et al.,14 and Bao et al.15). The assessment is worth considering.
In double-blind studies, both the patients and doctors are unaware of which group the patient belongs to,38-40 which makes it easier to carry out a pharmaceutical trial. However, in interventional clinical trials, such as those on operations and acupuncture, the operator will definitely know which kind of intervention he or she should perform for a given participant,41 which means he or she knows the group allocation of the patient. Therefore, these studies are single-blind rather than double-blind studies.
Therefore, in our meta-analysis, we assessed 5 articles11,12,14-16 that were blinded rather than double blinded and 2 articles13,16 that did not clearly report a method of blinding.
Blinding is critical for acupuncture trials.42 We suggest that the acupuncturist talks to the patient as little as possible, preventing the patient from knowing which group he or she belongs to, and an “acupuncture robot”43 can be used in the future to ensure that the acupuncturist is blinded.
The level of heterogeneity was high among all 7 studies. Although subgroup analyses were carried out, the heterogeneity level was still high. We considered that different kinds of control interventions may be the reason for heterogeneity [the heterogeneity among subgroups (P < .00001, I2 = 94.4%)] (Figure 4). In addition, differences in factors such as the acupoints, needle type, number of treatment sessions, and period between treatments may contribute to heterogeneity. The BPI scores, WOMAC scores and other scores are patient-reported outcomes (PROs). Although an increasing number of clinical trials regard PROs as the most important outcomes in clinical trials,44 the subjectivity of PROs can reduce the consistency of results.
Limitations
First, the number of included RCTs was small, and there were only 603 patients in our meta-analysis. Second, the results of PROs, which were the primary outcomes of all the articles, were not as objective as some experimental results. This subjectivity may reduce the accuracy of the results of each article. Finally, the heterogeneity of the studies was high, which prevented us from drawing a clear conclusion.
Conclusion
Compared with drugs and no treatment, acupuncture significantly improved BPI scores in breast cancer patients with AIA. However, there were no significant differences between the acupuncture group and sham-acupuncture group in the BPI scores or WOMAC scores. No significant side effects were associated with acupuncture treatment. Therefore, this meta-analysis showed that acupuncture is a safe and effective treatment for breast cancer patients with AIA. Future studies with better blinding methods are warranted to further explore the nature of non-specific and placebo effects in true and sham acupuncture.
Acknowledgments
The current study was funded by the First Affiliated Hospital of Nanjing Medical University.
Footnotes
Abbreviations: AIA: aromatase inhibitor-induced arthralgia; AIs: aromatase inhibitors; RCTs: randomized controlled trials; CNKI: China National Knowledge Infrastructure Database; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; MD: mean difference; ASCO: American Society of Clinical Oncology; BPI: brief pain inventory; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; SD: standard deviation; VAS: visual analog scale; DASH: quick disability of arm, shoulder, hand scale; PPT: physical performance test; FACT: functional assessment of cancer therapy; M-SACRAH: modified score for the assessment and quantification of chronic rheumatoid affections of the hands; VAS; HAQ: health assessment questionnaire; PROs: patient-reported outcomes.
Authors’ Contributions: XL, GW, and JL conceived the study together, analyzed and interpreted the data, and drafted the manuscript. They contributed equally to the work. HX, JH and XC revised the manuscript. MX and JT supervised the development of the work, reviewed the manuscript and served as corresponding authors. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Key Research and Development Program of China (No. 2016YFC0905900), National Natural Science Foundation of China (No. 81872365), Jiangsu Provincial Key Research Development Program (No. BE2019731), Jiangsu Provincial Key Funding Project for Maternal and Child Health Research (No. F201821) and the Clinical Research Project of Jiangsu Province Hospital (No. JSPH-511C-2018-8).
Competing Interests: The authors declare that they have no competing interests.
ORCID iD: Xiaomeng Liu  https://orcid.org/0000-0002-1212-2772
https://orcid.org/0000-0002-1212-2772
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
References
- 1. Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev 2016;17:43-46. [DOI] [PubMed] [Google Scholar]
- 2. Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor–positive breast cancer. J Clin Oncol 2010;28:3784-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline focused update. J Clin Oncol 2014;32:2255-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat 2012;134:459-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hadji P, Jackisch C, Bolten W, et al. COMPliance and arthralgia in clinical therapy: the COMPACT trial, assessing the incidence of arthralgia, and compliance within the first year of adjuvant anastrozole therapy. Ann Oncol 2014;25:372-327. [DOI] [PubMed] [Google Scholar]
- 6. Niravath P. Aromatase inhibitor-induced arthralgia: a review. Ann Oncol 2013;24:1443-1449. [DOI] [PubMed] [Google Scholar]
- 7. Kothawala P, Badamgarav E, Ryu S. Systematic review and meta-analyses of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc 2007;82:1493-1501. [DOI] [PubMed] [Google Scholar]
- 8. Nahm N, Mee S, Marx G. Efficacy of management strategies for aromatase inhibitor induced arthralgia in breast cancer patients: a systematic review. Asia Pac J Clin Oncol 2018;30:12845. [DOI] [PubMed] [Google Scholar]
- 9. Roberts K, Rickett K, Greer R, et al. Management of aromatase inhibitor induced musculoskeletal symptoms in postmenopausal early breast cancer: a systematic review and meta-analyses. Crit Rev Oncol/Hematol 2017;111:66-80. [DOI] [PubMed] [Google Scholar]
- 10. Papaioannou A, Kennedy CC, Dolovich L, et al. Patient adherence to osteoporosis medications: problems, consequences and management strategies. Drugs Aging 2007;24:37-55. [DOI] [PubMed] [Google Scholar]
- 11. Hershman DL, Unger JM, Greenlee H, et al. Effect of Acupuncture vs Sham Acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer: a randomized clinical trial. JAMA 2018;320:167-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mao JJ, Xie SX, Farrar JT, et al. A randomised trial of electro-acupuncture for arthralgia related to aromatase inhibitor use. Eur J Cancer 2014;50:267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oh B, Kimble B, Costa DSJ, et al. Acupuncture for treatment of arthralgia secondary to aromatase inhibitor therapy in women with early breast cancer: pilot study. Acupunct Med 2013;31:264-271. [DOI] [PubMed] [Google Scholar]
- 14. Crew KD, Capodice JL, Greenlee H, et al. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J Clin Oncol 2010;28:1154-1160. [DOI] [PubMed] [Google Scholar]
- 15. Bao T, Cai L, Giles JT, et al. A dual-center randomized controlled double blind trial assessing the effect of acupuncture in reducing musculoskeletal symptoms in breast cancer patients taking aromatase inhibitors. Breast Cancer Res Treat 2013;138:167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ye J, Wang B, Lv XA, et al. Clinical study of acupuncture intervention in muscle, bone and joint pain caused by aromatase inhibitors in the treatment of breast cancer. Shanghai J Acup Moxib 2015;34:642-646. [Google Scholar]
- 17. Li J, Huang M, Lin M, et al. Clinical effect of Canggui Tanxue acupuncture at ashi point in the treatment of muscle, bone and joint pain induced by aromatase inhibitor of breast cancer. China Med Herald 2019;16:132-135. [Google Scholar]
- 18. Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin 2017;67:194-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bae K, Yoo H-S, Lamoury G, et al. Acupuncture for aromatase inhibitor-induced arthralgia: a systematic review. Integr Cancer Ther 2015;14:496-502. [DOI] [PubMed] [Google Scholar]
- 20. Halsey EJ, Xing M, Stockley RC. Acupuncture for joint symptoms related to aromatase inhibitor therapy in postmenopausal women with early-stage breast cancer: a narrative review. Acupunct Med 2015;33:188-195. [DOI] [PubMed] [Google Scholar]
- 21. Chen L, Lin CC, Huang TW, et al. Effect of acupuncture on aromatase inhibitor-induced arthralgia in patients with breast cancer: a meta-analysis of randomized controlled trials. Breast 2017;33:132-138. [DOI] [PubMed] [Google Scholar]
- 22. Anand K, Niravath P. Acupuncture and vitamin D for the management of aromatase inhibitor-induced arthralgia. Curr Oncol Rep 2019;21:51. [DOI] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta analyses: the PRISMA statement. Ann Intern Med 2009;151:264-269. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane 2020. www.training.cochrane.org/handbook.
- 25. The Nordic Cochrane Centre TCC. Copenhagen: review manager (RevMan) [Computer program]. Version 5.3; 2014. [Google Scholar]
- 26. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analyses. Stat Med 2002;21:1539-1558. [DOI] [PubMed] [Google Scholar]
- 27. Müller ST, Keiler AM, Kräker K, et al. Influence of estrogen on individual exercise motivation and bone protection in ovariectomized rats. Lab Anim 2018;52:479-489. [DOI] [PubMed] [Google Scholar]
- 28. Felson DT, Cummings SR. Aromatase inhibitors and the syndrome of arthralgias with estrogen deprivation. Arthritis Rheum 2005;52:2594-2598. [DOI] [PubMed] [Google Scholar]
- 29. Kawakita K, Shinbara H, Imai K, et al. How do acupuncture and moxibustion act? - Focusing on the progress in Japanese acupuncture research. J Pharmacol Sci 2006;100:443-459. [DOI] [PubMed] [Google Scholar]
- 30. Tang Y, Yin HY, Liu J, et al. P2X receptors and acupuncture analgesia. Brain Res Bull 2019;151:144-152. [DOI] [PubMed] [Google Scholar]
- 31. Qin W, Tian J, Bai L, et al. FMRI connectivity analyses of acupuncture effects on an amygdalaassociated brain network. Mol Pain 2008;4:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ingle JN, Schaid DJ, Goss PE, et al. Genome-wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitors. J Clin Oncol 2010;28:4674-4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vase L, Baram S, Takakura N, et al. Can acupuncture treatment be double-blinded? An evaluation of double-blind acupuncture treatment of postoperative pain. PLoS One 2015;10:e0119612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen H, Yang M, Ning Z, et al. A guideline for randomized controlled trials of acupuncture. Am J Chin Med 2019;47:1-18. [DOI] [PubMed] [Google Scholar]
- 35. Moffet HH. Sham acupuncture may be as efficacious as true acupuncture: a systematic review of clinical trials. J Altern Complement Med 2009;15:213-216. [DOI] [PubMed] [Google Scholar]
- 36. Harris RE, Zubieta JK, Scott DJ, et al. Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs). NeuroImage 2009;47:1077-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu MT, Sheen JM, Chuang KH, et al. Neuronal specificity of acupuncture response: a fMRI study with electroacupuncture. Neuroimage 2002;16:1028-1037. [DOI] [PubMed] [Google Scholar]
- 38. Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet 2002;359:696Y700. [DOI] [PubMed] [Google Scholar]
- 39. Boutron I, Guittet L, Estellat C, et al. Reporting methods of blinding in randomized trials assessing nonpharmacological treatments. PLoS Med 2007;4:370-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Armijo-Olivo S, Fuentes J, da Costa BR, et al. Blinding in physical therapy trials and its association with treatment effects: a meta-epidemiological study. Am J Phys Med Rehabil 2017;96:34-44. [DOI] [PubMed] [Google Scholar]
- 41. Fregni F, Imamura M, Chien HF, et al. Challenges and recommendations for placebo controls in randomized trials in physical and rehabilitation medicine: a report of the international placebo symposium working group. Am J Phys Med Rehabil 2010;89:160Y72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lan KC, Litscher G. Robot-controlled acupuncture—an innovative step toward modernization of the ancient traditional medical treatment method. Medicines 2019;6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vodicka E, Kim K, Devine EB, et al. Inclusion of patient-reported outcome measures in registered clinical trials: evidence from ClinicalTrials.gov (2007–2013). Contemp Clin Trials 2015;43:1-9. [DOI] [PubMed] [Google Scholar]