Abstract
The novel coronavirus disease 2019 (COVID-19) predisposes patients to venous thromboembolism (VTE) due to risk factors, severe infection, and severe inflammatory responses. The objective is to determine the risk of developing VTE after corticosteroid administration during COVID-19 treatment. Using PRISMA reporting guidelines, a review was conducted from inception until 20 September 2020 with MESH terms including “venous thromboembolism” and “covid-19,” using MEDLINE, Scopus, CINAHL Plus, and WHO Global Database. The inclusion criteria included studies with COVID-19 patients aged 18 years and older with VTE diagnosed by duplex ultrasonography or computed tomography pulmonary angiography (CTPA). Exclusion criteria were studies with non COVID-19 patients and non-VTE patients aged less than 18 years. Quality appraisal was conducted of included studies using the Newcastle-Ottawa Scale (NOS). A random-effect model using 95% confidence intervals, and significance of findings was assessed using Review Manager V5.4.We included 12 observational studies with 2801 patients (VTE n = 434; non-VTE; n = 2367). Patients had a higher risk of presenting with VTE when being administered corticosteroids during treatment of COVID-19 (RR = 1.39, 95% CI = 1.10 to 1.77, I2 = 0%). A positive effect size was found (SMD = 1.00, 95% CI = 0.67 to 1.32, I2 = 85%) for D-dimer laboratory values (µg/mL) in the VTE group. While critically ill COVID-19 patients are more likely to require corticosteroid treatment, it may be associated with increased risk of VTE, and poor clinical prognosis. Risk assessment is warranted to further evaluate patients as case-by-case in reducing VTE and worsening clinical outcomes.
Keywords: venous thromboembolism, pulmonary embolism, deep vein thrombosis, coronavirus, mortality
Introduction
Venous thromboembolism (VTE) is increasingly being observed among Coronavirus disease 2019 (COVID-19) patients. COVID-19 directly and indirectly predisposes patients to VTE, both pulmonary embolism (PE) and deep vein thrombosis (DVT), possibly through critical disease, underlying risk factors and severe inflammatory responses.1,2 However, relevant data reporting the predisposition and outcomes of COVID-19 and VTE are required. Many risk stratification tools are being used for in-hospital prophylaxis including the Caprini and Pauda risk assessment models.3 A Chinese cohort of 1026 hospitalized COVID-19 patients was assessed and established a high risk (40%) of VTE using the Padua prediction score.4 A consensus group of the International Society on Thrombosis and Hemostasis (ISTH) provide clinical guidance on the outcomes of COVID-19 patients related to venous thromboembolism (VTE) suggesting further investigation of the optimal preventive strategy.5
Groups at higher risk for VTE are elderly, hospitalized, obese, cancer, and thrombophilia patients who similarly share a higher risk for severe COVID-19 infections with higher incidences of VTE in these groups.6–9 Most of the risk factors of VTE overlap which illustrates the aggressive nature of thrombotic events in ICU patients of COVID-19. Therefore, it is reasonable to presume a high incidence of VTE in the severe or critically-ill COVID-19 patients. The objectives of this systematic review and meta-analysis are to evaluate the incidence of VTE and compare the baseline characteristics, laboratory parameters and clinical outcomes in patients of COVID-19 with and without VTE. We also aim to identify the risk of VTE among COVID-19 patients prescribed corticosteroid therapy during their hospital stay.
Materials and Methods
Search Strategy and Selection
The systematic review and meta-analysis was conducted adhering to PRISMA guidelines. Databases including MEDLINE, Scopus, CINAHL Plus, and the WHO Global Database were included. No date restrictions were present; the search was conducted from inception until 20 September 2020. We used the following MeSH terms, “venous thromboembolism, and covid-19.” Qualitative primary research articles were added for the purposes of this review. Systematic reviews, meta-analyses, reviews, letters, editorials, case reports, and case series with less than 10 patients were excluded. All duplicates were removed using the software Endnote X9 by 2 independent reviewers (AS and ZS). An independent screening of the titles and abstracts for significance was conducted. The records that were found to be relevant at the stage were selected for further evaluation using full-texts. All discrepancies were resolved by active discussion by 2 reviewers (AS and ZS). During the final stage, discussions were made by all investigators and the third independent reviewer (MS) determined the eligibility of included studies. Studies were also screened by searching reference lists of included studies (umbrella review). Journals including NEJM, The Lancet, The BMJ, JAMA, and Annals of Internal Medicine were manually retrieved for relevant studies. Two investigators (ZS and AS) extracted data from the included studies with tables determined a priori with a third investigator present for any disagreements (MS).
Quality Assessment
For the quality assessment of included studies in the systematic review and meta-analysis, the Newcastle-Ottawa Scale (NOS Scale) was employed to ascertain the quality of studies by 2 reviewers (AS and ZS). If 10 or more studies reported common outcomes, we assessed for publication bias by visually assessing funnel plots.
Outcomes
The primary outcome was to assess whether either group (VTE vs non-VTE) had a higher risk of presenting with VTE, when being administered corticosteroids as treatment of COVID-19. The secondary outcomes were to quantify which group had a higher mean of BMI values, corroborate the mean differences in D-dimer values, and determine the likelihood of mortality in VTE and non-VTE groups.
Data Analysis
Two investigators (AS and ZS) extracted data on a customized spreadsheet for the following variables: incidence, age, gender, BMI, co-morbidities, VTE history, laboratory tests, prophylactic and therapeutic anticoagulation, corticosteroid and antibiotics use, ICU stay, and mortality. Qualitative analysis was conducted and common findings were presented (Supplementary Table 1). Using quantitative analytical methods, a random-effects meta-analysis was conducted to ascertain the differences in the incidence of VTE and non-VTE groups post-administration of corticosteroids. The standardized difference between means and risk ratios (RR) of continuous and dichotomous measures between VTE and non-VTE COVID-19 infected patients was presented to describe characteristics of included studies using 95% confidence intervals. Data analysis was conducted using Review Manager 5.4.
Results
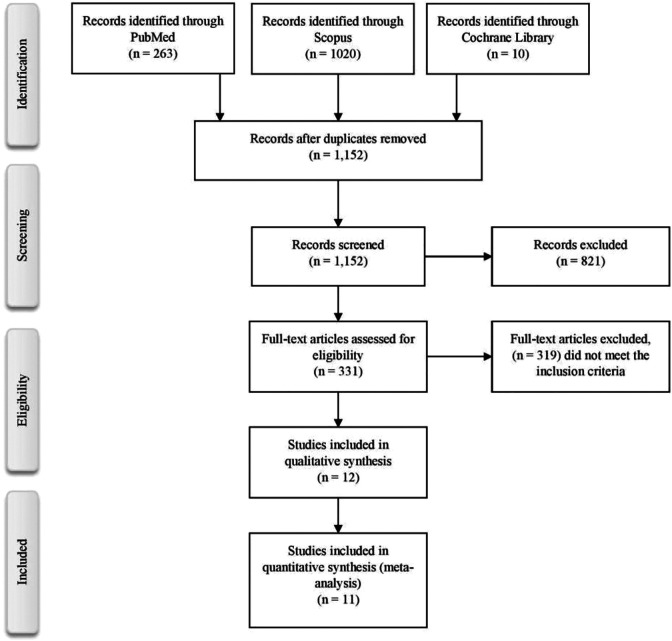
The search process is shown in Figure 1. The first phase of screening yielded 1,293 results. After the duplicates were removed, 1,152 results were assessed for titles and abstracts. In the second phase, 821 results were excluded as they did not fit the inclusion criteria. During the third phase, 331 results were shortlisted, and 12 studies were included in the qualitative and quantitative analysis. We included a total of 2801 patients with 15.5% (n = 434) in the VTE group and 84.5% (n = 2367) in the non-VTE group. The major characteristics of included studies are presented in Supplementary Table 1.
Figure 1.
PRISMA flowchart.
Corticosteroids and VTE
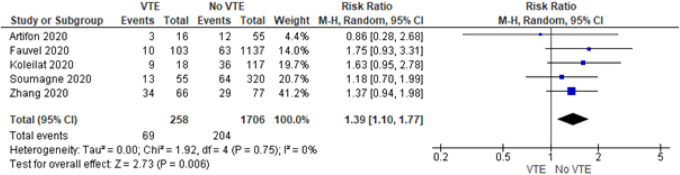
Five of the 12 studies presented data of corticosteroids administration before and during the treatment of COVID-19. We found that patients had a higher risk of presenting with VTE when being administered corticosteroids during treatment of COVID-19 (RR = 1.39, 95% CI = 1.10 to 1.77) (Figure 2). There was no heterogeneity between the studies I2 = 0%).
Figure 2.
Forrest plot for corticosteroids use.
D-Dimer of VTE and Non-VTE Patients
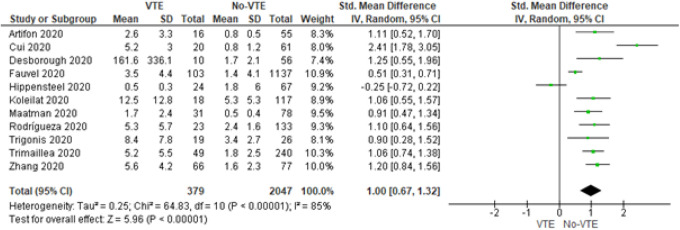
Eleven of the 12 studies reported D-dimer values for VTE and non-VTE groups (Figure 3). A positive effect size was found (SMD = 1.00, 95% CI = 0.67 to 1.32, P < 0.001) for D-dimer laboratory values (µg/mL) in the VTE group. Given the diverse setting of all studies, high heterogeneity was found (I2 = 85%).
Figure 3.
Forrest plot for D-dimer values.
Mortality of VTE and Non-VTE Patients
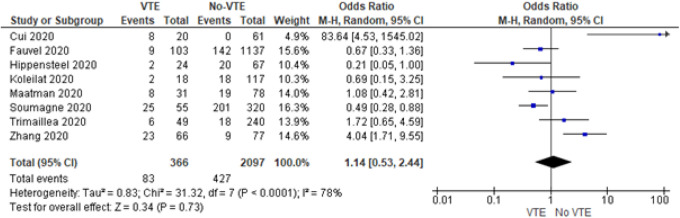
Eight of the 12 studies published mortality data of VTE and non-VTE groups (Figure 4). While the risk of mortality among patients with VTE as compared to the no-VTE group was seemingly high, the results are statistically insignificant (OR = 1.14, 95% CI = 0.53 to 2.44, I2 = 78%, P = 0.73). There was high heterogeneity among the studies (I2 = 78%).
Figure 4.
Forrest plot for mortality.
Publication Bias
Not all studies originated from the same population hence the figure shows 3 deviances from its symmetrical shape for D-dimer (Figure 5). However, the funnel plot for BMI findings is symmetrical. We henceforth conclude that there was mild to moderate presence of publication bias. We acknowledge that a funnel plot may not be the gold standard for this review in comparing the results of the meta-analysis because there is a lack of randomized control trials assessing the risks of corticosteroids in VTE and non-VTE groups.
Figure 5.
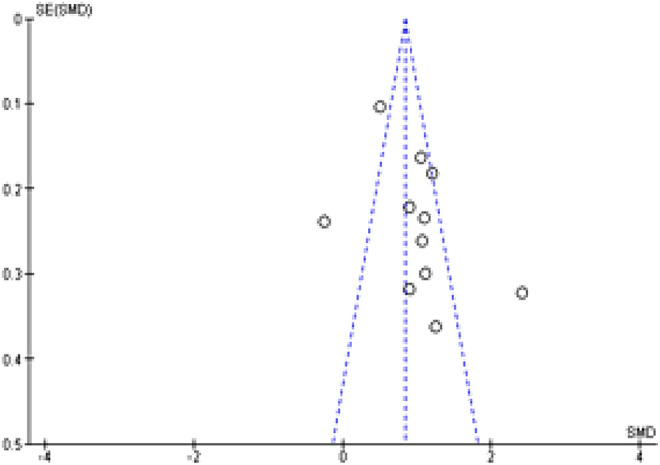
Funnel plot for publication bias.
Discussion
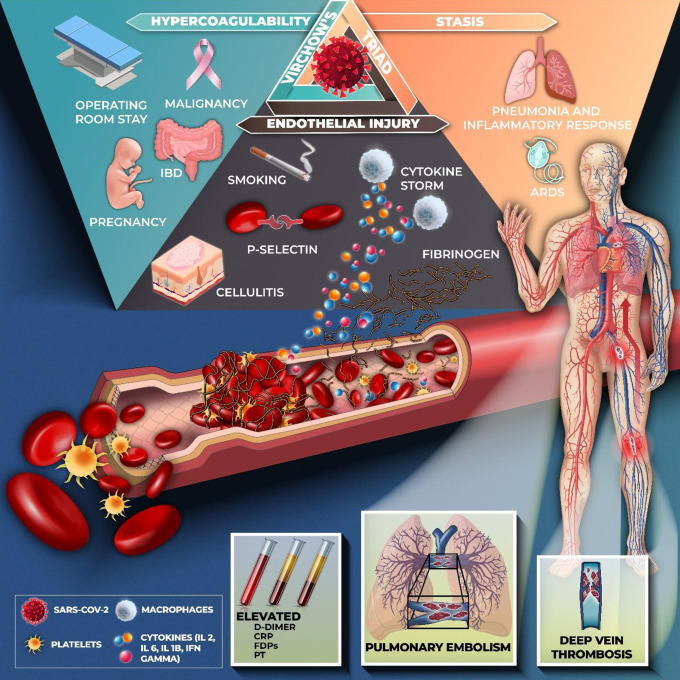
Our findings demonstrate the increased risk of VTE with corticosteroid administration. The mortality rates were higher in the VTE-confirmed patients that correlated with elevated d-dimer. However, no definite findings were available due to the differing corticosteroid regimens and the heterogeneity of the studies. Our results demonstrate that COVID-19 patients with VTE have elevated d-dimer levels. While the drivers of COVID-19 induced VTE are uncertain, overexpression of tissue factor, endothelial disruption, and complement system activation are proposed mechanisms.5 Numerous thrombi have been found in the vessels of the lungs, and those of the liver, heart, and kidneys in patients dying of COVID-19.10 The hypercoagulable state may elucidate the high incidence of VTE reported in patients with COVID-19 regardless of anticoagulant thromboprophylaxis. Figure 6 demonstrates the postulated mechanisms that contribute to the increased incidence of venous thromboembolism in COVID-19.
Figure 6.
Postulated pathophysiology of venous thromboembolism and Covid-19. SARS-CoV2 gains entry into the host cell via the ACE-2 receptor which causes inflammation and cytokine storm associated with the release of interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor-alpha (TNF-α) are released. As a response to viral infection, there is endothelial injury and thrombin formation contributing to a hypercoagulable state. Stasis in critically ill patients with pneumonia and ARDS further contributes to the formation of emboli. Hence, Virchow’s triad drives the development of thrombosis. Down regulation of fibrinolytic and anti-coagulation pathways leads to an increased risk of VTE. Hemostatic abnormalities including elevated levels of d-dimer, C-reactive protein (CRP), fibrin degradation products (FDPs) and prothrombin time (PT) suggesting an underlying coagulopathy process and a high burden of thrombosis.
The anti-inflammatory property of corticosteroids is postulated to have efficacy in COVID-19 associated ARDS and cytokine storm. Interest in the use of corticosteroids rekindled after the UK RECOVERY trial whereby dexamethasone improved the outcome in ICU patients of COVID-19 requiring invasive ventilation. It reduced the mortality rate by 35% in mechanically-ventilated patients (p = 0.0003) and by 20% in patients who were receiving oxygen (p = 0.00021). No benefit was observed in patients with mild disease.11 Consequently, the beneficial effects of corticosteroids were proven in multiple studies with findings suggesting that low dose steroids were more beneficial in contrast to high dose steroids.12–14
However, certain studies reported no benefit from the use of corticosteroids in both severe and moderately ill COVID-19 patients.15,16 High dosage was also significantly associated with elevated mortality rate. A 10 mg rise in hydrocortisone dose was associated with a 4% rise in the mortality rate in COVID-19 patients {adjusted HR: 1.04, 95% CI: 1.01-1.07}.15 Importantly, the majority of these studies were done in critically-ill COVID-19 patients with minimal efficacy in mild illness. Another case control study depicted the effects of adjuvant corticosteroid therapy with antivirals in critically ill patients of COVID-19.15 Multiple organ dysfunction syndrome (MODS) as well as DIC was reported in the steroids group in contrast to the non-steroids group. Furthermore, oral and inhaled glucocorticoids were reported to have an increased risk for recurrent pulmonary thromboembolism.17 Corticosteroids needs to be used with caution along with prudent dosing in critically ill patients of COVID-19. Progressing randomized trials that address key clinical inquiries, particularly corticosteroid dosing and VTE risk, have the potential to diminish the morbidity and mortality from VTE in hospitalized and critically sick patients with COVID-19.
Considerations for Thromboembolic Care
Inflammation may be both a cause and consequence of VTE. Targeting selective inhibitory pathways of inflammation is crucial as the coagulation and immune systems contribute to the formation and resolution of thrombus. Pro-inflammatory cytokines, including interleukin-6 (IL-6) and tumor necrosis alpha (TNF-α), remain higher in VTE patients regardless of the d-dimer levels.18 Among the pro-inflammatory cytokines, IL-1, IL-6, and TNF-α have been observed to induce the expression of tissue factor.18 Selective pharmacological therapy that targets immune mediators in VTE may be considered as a mechanism of effective anti-thromboinflammatory therapy, either prophylactically or therapeutically. Tocilizumab, an IL-6 receptor blocker, may reduce the risk of VTE occurrence in COVID-19 patients. The inflammatory mediators in VTE are activated via the factor XII-inflicted contact system thereby initiating the extrinsic coagulation pathway.19 A randomized control trial reported greater efficacy of recombinant nematode anticoagulant protein c2 (rNAPc2), an 85-amino acid serine protease inhibitor that inhibits the activated factor VII/TF directly, for prevention of VTE in post-operative settings than LMWH.20 Consequently, thromboprotection attributed by targeted anti-thrombo-inflammatory therapy may offer cross-protection through protective mechanisms of inflammation, and coagulation.
Most of the ICU-admitted COVID-19 patients that have been treated with corticosteroids are vulnerable populations. Compounded with immobility, hospitalization and COVID-19 infection, these patients have higher Padua Score and associated signs indicative of underlying coagulopathy. Use of high-dose corticosteroids in these patients can further increase the risk for development of VTE. Risk stratification and cautious use of corticosteroids is of paramount importance to reduce mortality rates in susceptible populations. Misdiagnosis and mistreatment adds to the burden of understanding the treatment algorithm in these patients. In critically ill COVID-19 patients, clinicians need to consider the high likelihood of VTE given the in-situ immunothrombotic nature of the disease. Subsequently, effective VTE prophylaxis based on an individual’s risk factor assessment is essential. Lastly, if corticosteroids prove to be therapeutically effective, identifying the type, dose, and duration of treatment should be cautiously assessed on a case to case basis along with consideration of each patient’s clinical and immunological conditions as well as concomitant administration of antiviral drugs.
Limitations
Studies that analyzed the impact of corticosteroids on VTE incidence rates, use of VTE prophylaxis, and outcomes were limited and many of the studies were retrospective. However, there was no heterogeneity in the use of corticosteroids prior to the occurrence of VTE. Secondly, with multiple comorbidities, VTE may go unnoticed unless conducting a CTPA or ultrasonography. It is likely that the extent of VTE occurrence in COVID-19 is under-reported. Furthermore, data to predict the impact of underlying risk factors such as previous immobility on the incidence VTE occurrence was not available. However, it is pertinent to take into account the risk factors of VTE before administrating higher doses of corticosteroids to avoid predisposing patients to VTE.
Conclusion
The findings revealed a higher likelihood of COVID-19 patients presenting with VTE when administered corticosteroids. While we did not find conclusive findings confirming higher mortality of patients with VTE, elevated d-dimer levels were consistent with a higher incidence of VTE in COVID-19. There is no clear benefit of corticosteroid use in reducing mortality rates over the elevated VTE risk. Consequently, risk assessment of VTE on a case-by-case is strongly suggested and precaution ought to be taken for thromboprophylaxis including limiting the use of corticosteroids in such patients. More critical COVID-19 patients are likely to require corticosteroid treatment which may worsen clinical prognosis.
Supplemental Material
Supplemental Material, sj-pdf-1-cat-10.1177_1076029621993573 for Venous Thromboembolism, Corticosteroids and COVID-19: A Systematic Review and Meta-Analysis by Azza Sarfraz, Zouina Sarfraz, Aminah Abdul Razzack, Gaurav Patel and Muzna Sarfraz in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Authors’ Note: The authors contributed and are assigned authorship as per ICMJE guidelines.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Azza Sarfraz  https://orcid.org/0000-0001-8206-5745
https://orcid.org/0000-0001-8206-5745
Zouina Sarfraz  https://orcid.org/0000-0002-5132-7455
https://orcid.org/0000-0002-5132-7455
Aminah Abdul Razzack  https://orcid.org/0000-0001-7310-6824
https://orcid.org/0000-0001-7310-6824
Gaurav Patel  https://orcid.org/0000-0003-0843-915X
https://orcid.org/0000-0003-0843-915X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu X, Liu C, Chen X, Wu W, Lu G. Comparison between Caprini and Padua risk assessment models for hospitalized medical patients at risk for venous thromboembolism: a retrospective study. Interact Cardiovasc Thorac Surg. 2016;23(4):538–543. [DOI] [PubMed] [Google Scholar]
- 4. Wang T, Chen R, Liu C, et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol [Internet]. 2020;7(5): e632–e363 https://pubmed.ncbi.nlm.nih.gov/32278361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-art review. J Am Coll Cardiol. 2020:75(23):2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in SARS-CoV-2 requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davies NG, Klepac P, Liu Y, Prem K, Jit M, Eggo RM. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205–1211. [DOI] [PubMed] [Google Scholar]
- 8. Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bělohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol. 2013;18(2):129. [PMC free article] [PubMed] [Google Scholar]
- 10. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dexamethasone for COVID-19: preliminary findings. Drug Ther Bull [Internet]. 2020;58(9):133 LP–133. Updated August 25, 2020. Accessed January 4, 2021 http://dtb.bmj.com/content/58/9/133.abstract [DOI] [PubMed] [Google Scholar]
- 12. Fadel R, Morrison AR, Vahia A, et al. Early short-course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. 2020;71(16):2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Jiang W, He Q, et al. Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China. MedRxiv [Internet]. 2020;2020.03.06.20032342 Updated March 12, 2020. Accessed January 4, 2021 http://medrxiv.org/content/early/2020/03/12/2020.03.06.20032342.abstract
- 14. Chroboczek T, Lacoste M, Wackenheim C, et al. Beneficial effect of corticosteroids in severe COVID-19 pneumonia: a propensity score matching analysis. MedRxiv [Internet]. 2020;2020.05.08.20094755. Updated May 13, 2020. Accessed January 4, 2021 http://medrxiv.org/content/early/2020/05/13/2020.05.08.20094755.abstract
- 15. Lu X, Chen T, Wang Y, Wang J, Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit Care. 2020:24(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu J, Huang J, Zhu G, et al. Systemic corticosteroids show no benefit in severe and critical COVID-19 patients in Wuhan, China: a retrospective cohort study. MedRxiv [Internet]. 2020;2020.05.11.20097709. Updated May 14, 2020. Accessed January 4, 2021. http://medrxiv.org/content/early/2020/05/14/2020.05.11.20097709.abstract
- 17. Sneeboer MMS, Hutten BA, Majoor CJ, Bel EHD, Kamphuisen PW. Oral and inhaled corticosteroid use and risk of recurrent pulmonary embolism. Thromb Res. 2016;140:46–50. [DOI] [PubMed] [Google Scholar]
- 18. Saghazadeh A, Hafizi S, Rezaei N. Inflammation in venous thromboembolism: cause or consequence? Int Immunopharmacol [Internet]. 2015;28(1):655–665. Updated September, 2015. Accessed December 5, 2020 http://www.sciencedirect.com/science/article/pii/S1567576915300576 [DOI] [PubMed] [Google Scholar]
- 19. Branchford BR, Carpenter SL. The role of inflammation in venous thromboembolism. Front Pediatr [Internet]. 2018;6:142 Updated 23 May, 2018. Accessed December 5, 2020 https://pubmed.ncbi.nlm.nih.gov/29876337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee A, Agnelli G, Büller H, et al. Dose-response study of recombinant factor VIIa/tissue factor inhibitor recombinant nematode anticoagulant protein c2 in prevention of postoperative venous thromboembolism in patients undergoing total knee replacement. Circulation [Internet]. 2001;104(1):74–78. Updated July 3, 2001. Accessed December 5, 2020 10.1161/hc2601.091386 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-cat-10.1177_1076029621993573 for Venous Thromboembolism, Corticosteroids and COVID-19: A Systematic Review and Meta-Analysis by Azza Sarfraz, Zouina Sarfraz, Aminah Abdul Razzack, Gaurav Patel and Muzna Sarfraz in Clinical and Applied Thrombosis/Hemostasis