Abstract
Microdosimetry is a tool for the investigation of microscopic energy deposition of ionizing radiation. This work used Caenorhabditis elegans as a model to estimate the microdosimetric deposition level at the 60Co gamma radiation. Monte Carlo software PHITS was employed to establish irradiated nematodes model. The dose deposition of the entire body and gonad irradiated to 100 Gy was calculated. The injury levels of radiation were evaluated by the detection of biological indicators. The result of microdosimetric experiment suggested that the dose of whole body of nematodes was estimated to be 99.9 ± 57.8 Gy, ranging from 19.6 to 332.2 Gy. The dose of gonad was predicted to be 129.4 ± 558.8 Gy (9.5-6597 Gy). The result of biological experiment suggested that there were little changes in the length of nematodes after irradiation. However, times of head thrash per minute and the spawning yield in 3 consecutive days decreased 27.1% and 94.7%, respectively. Nematodes in the irradiated group displayed heterogeneity. Through contour analysis, trends of behavior kinematics and reproductive capacity of irradiated nematodes proved to be consistent with the dose distribution levels estimated by microdosimetric model. Finally, C. elegans presented a suitable combined model of microdosimetry and biology for studying radiation.
Keywords: microdosimetry, caenorhabditis elegans, animal model, radiation
Introduction
The biological effect of ionizing radiation generally depends on the actual energy deposition of rays in organisms.1-4 Due to the randomness of interaction between rays and substances, the macroscopic dosimetry such as absorbed dose and dose rate can only reflect the average level of the energy deposition of the irradiated substance. When the scale is reduced to micron dimension or even smaller, a remarkable deviation occurs between the practical radiation energy deposition and the absorbed dose.5-7 In order to study the microscopic distribution of radiation energy, the basic concept of micro dosimetry was put forward.5 Dose deposition distribution and fluctuation of ionizing radiation in microscopic levels such as cell and subcell were surveyed. Microdosimetry has been widely utilized in the study of radiation biological effect, solid radiation effect, radiation protection, radiation therapy and so on.8-10
The combination of microdosimetry and biology is of significance. Biological effect of radiation is induced by radiation energy deposition. For the systematic comprehension of the relationship between radiation dose and biological effect, microdosimetry and biological effect should be combined organically. Cell or animal models used previously in biological study were not practicable in physical modeling owing to excessive cells. Moreover, there are various kinds of cells in common animal models, which are lack of uniform evaluation standard. Therefore, an animal model with simple structure is required to establish a combined model of microdosimetry and biology.
As one of the classical model animals, Caenorhabditis elegans is characterized by transparent body, fast life cycle and high homology with human genes. It has become an ideal model animal for the study of biological effect of ionizing radiation.11-14 Compared with other model animals such as mouse and zebrafish, C. elegans is smaller in body size. Throughout the development cycle, the body size of nematodes is close to that of human cells ranging from 25 µm to 1 mm, which makes them dominant models for microdomestry estimation. Unlike cells, nematodes are complete living organisms with multiple biosystems such as nervous, digestive and reproductive system, which are helpful for the study of biological changes caused by ionizing radiation on animal scale. Previous reports had showed that some damage effects of C. elegans induced by ionizing radiation exposure displayed a dose-dependent manner, such as vulva-protruding deformity, vulvaless deformity, decreased spawning capacity and limited movement ability.15-17 The biological indicators are important for the determination of the distribution of microscopic energy deposition. Therefore, C. elegans is an ideal animal for microdosimetry biology model.
In the study of biological effect of ionizing radiation, the absorbed dose of C. elegans used to be obtained by indirectly measurement with thermoluminescence dosimeter, nanometer dosimeter etc. 16,18 The specific absorbed dose of each worm could not be accurately detected, and the difference of radiation effect between individuals could not be explained from the perspective of microdosimetry. In this work, Monte Carlo software PHITS was applied to establish C. elegans physical model upon 60Co irradiation exposure for the first time. The dose distribution of the entire body and gonad of individual nematode in the petri dish was simulated and calculated when subjected to gamma irradiation. Alterations of the dose estimation results of physical model were evaluated from the aspects of irradiation growth, behavior activity levels and reproductive ability of nematodes on irradiation.
Materials and Methods
Culture and Maintenance of Nematodes
The N2 strain was obtained from the group of Huimin Zhang, College of Basic Medicine and Life Sciences, Soochow University. It was used as the wild-type C. elegans background in this study. Before performing the experiments, nematodes were maintained at 20°C in NGM solid medium (1.7% agar, 2.5 mg·mL-1 peptone, 25 mM NaCl, 50 mM KH2PO4 pH 6.0, 5 μg·mL-1 cholesterol, 1 mM CaCl2, 1 mM MgSO4) supplemented with fresh Escherichia coli OP50.
The petri dish with sufficient adult worms was selected and embryos were washed off the cell-strainer with M9 buffer. After being digested with bleach solution and washed with M9 buffer for thrice, embryos were incubated on non-seeded NGM plates without OP50 for 24 h at 20°C. The following day, synchronous L1 nematodes were transferred onto a prepared agar pad on a glass slide.
Irradiation
The external gamma radiation exposure was performed at the State Key Laboratory of Radiation Medicine and Protection, School of Radiation Medicine and Protection, Soochow University. The entire body of L1 nematodes was exposed to 25 Gy/min of gamma radiation (total doses ∼100 Gy). The worms were transferred to a fresh NGM agar medium containing OP50 at 20°C immediately after irradiation.
Effects on Somatic Growth and Reproduction
L1 nematodes were photographed with a microscope (Normaski). For the establish of nematode model, geometric parameters of the whole body and gonad of 30 nematodes were measured with imageJ software. The length (size) of nematodes was determined at 48 h of exposure onward from L1 stage.
The young adult nematodes were then placed on NGM plates and immersed with K-medium solution. A head thrash was defined when the length of body was bent to half. Times of head thrash per minute was randomly recorded with 15 nematodes in each group.
After exposure, the young adult nematodes were isolated and placed in NGM medium with one in each dish, subsequently transferred to fresh petri dish every 24 hours. The egg-laying amount for 3 consecutive days was recorded with 30 nematodes in each group using stereomicrograph. Nematodes died during the experiment were excluded in this study.
Model Establishment and Monte Carlo Simulation
In this work, a model was constructed and Monte Carlo simulation was performed on the irradiated nematodes to obtain the absorbed dose distribution of nematodes and gonads. Irradiation source, culture dish, culture medium, body and gonad of nematodes were taken into account in the model. Gamma photons with energy of 1.17 and 1.33 MeV emitted by 60Co decay were the irradiation source. The petri dish made up from PMMA was used with a diameter of 6 cm. For simplification, the medium was simulated as liquid water. According to the measured result of the size of whole body and gonads, cuboid with 384.0 µm in length, 25.8 µm in both height and width were simulated for nematode size, while cuboid with 20.3 µm in length, 10.0 µm in both height and width were simulated for gonadal size.
In this study, 200 nematodes were simulated to distribute randomly as a first approximation. That is, nematodes were cultured in a random distribution in the medium and they were assumed to have no intersections. The recent version (version 3.20) of the Particle and Heavy-Ion Transport code System (PHITS) was used in Monte Carlo simulation,19,20 which has been widely used for various applications, such as low-energy neutron interaction,21 beam transport functions,22 and a microdosimetric tally function.23 The cut-off energy of photon and electron were both set at 1 keV. To improve efficiency, variance of forced collision in the nematodes was declined. The simulated photon number were 20E8 with the maximum relative error less than 4.8%, and the error was calculated by more than 5 times. Simulations were executed on a system with 44 Intel(R) Xeon(R) E5-2696 and 256 GB RAMs.
Statistical Analysis
SPSS 20.0 software was used for statistical analysis. Non-paired t-test and Mann-Whitney U test were applied for normal distribution and non-normal distribution, respectively. The frequency distribution diagram of biological data and dose estimation was divided into 10 frequency areas averagely. The inter-subject effect MEASURE was applied to compare contour of the 2 groups to verify whether they were consistent. Statistical significance was considered when p-values was lower than 0.05. Each experiment was repeated at least 3 times.
Results
Establishment of Microdosimetry Model and Dose Estimation for C. Elegans
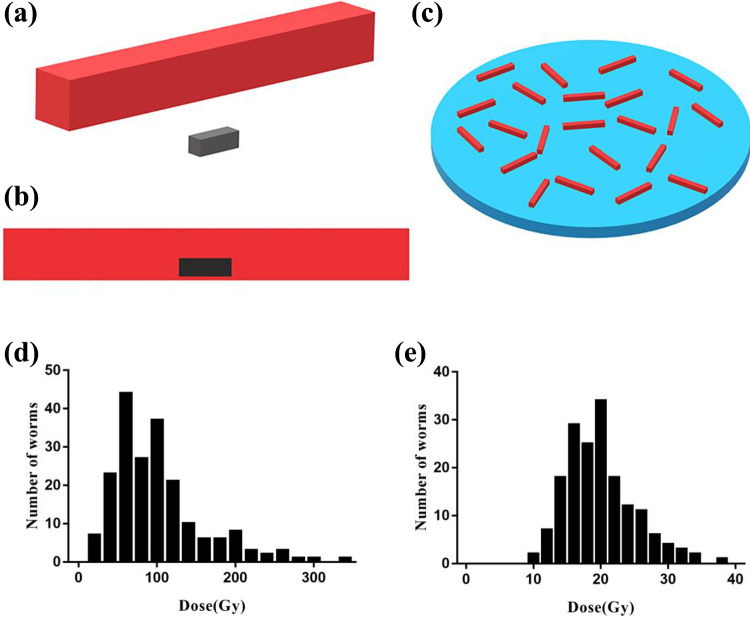
To reveal the potential reason for the changes of potential biological effects after irradiation, nematodes model upon 60Co irradiation exposure was established by Monte Carlo software PHITS (Figure 1.a, b, c). Nematodes were presumed to be in water layer. When the average absorbed dose of the water layer (nematodes were not included) reached 100 Gy, the simulated absorbed doses for the whole body and gonad of 200 nematodes were listed as follows: the absorbed dose of body was 99.9 ± 57.8 Gy (19.6-332.2 Gy), that of gonads were 129.4 ± 558.8 Gy (9.5-6597 Gy). To reflect the general level, the extremely high doses of gonads were excluded, finally the gonad absorbed dose was determined to be 19.8 ± 5.1 Gy. Meanwhile, the frequency distribution of the estimated dose suggested that the dose of 50% nematodes body ranged from 50 to 110 Gy, and that of 11 cases (5.5%) exceeded the extreme value 210 Gy (Figure 1.d).After excluding extremly high doses, the dose of 50% gonads distributed between 16.0 and 22.7 Gy (Figure 1.e).
Figure 1.
Microdosimetric model Establishment and dose estimation of C. elegans. a. Schematic diagram of body (red) and gonad (black) of C. elegans (cuboids). b. Schematic diagram of the relative position of body and gonad. The gonad was located in the ventral body. c. Schematic diagram of irradiation conditions. Nematodes were distributed relatively uniformly on the medium (blue). d. Frequency distribution diagram for dose of nematodes body. e. Frequency distribution diagram for nematodes gonads (with outliers excluded).
Fitting of Growth and Changes of Movement Behavior With the Dose of Nematodes
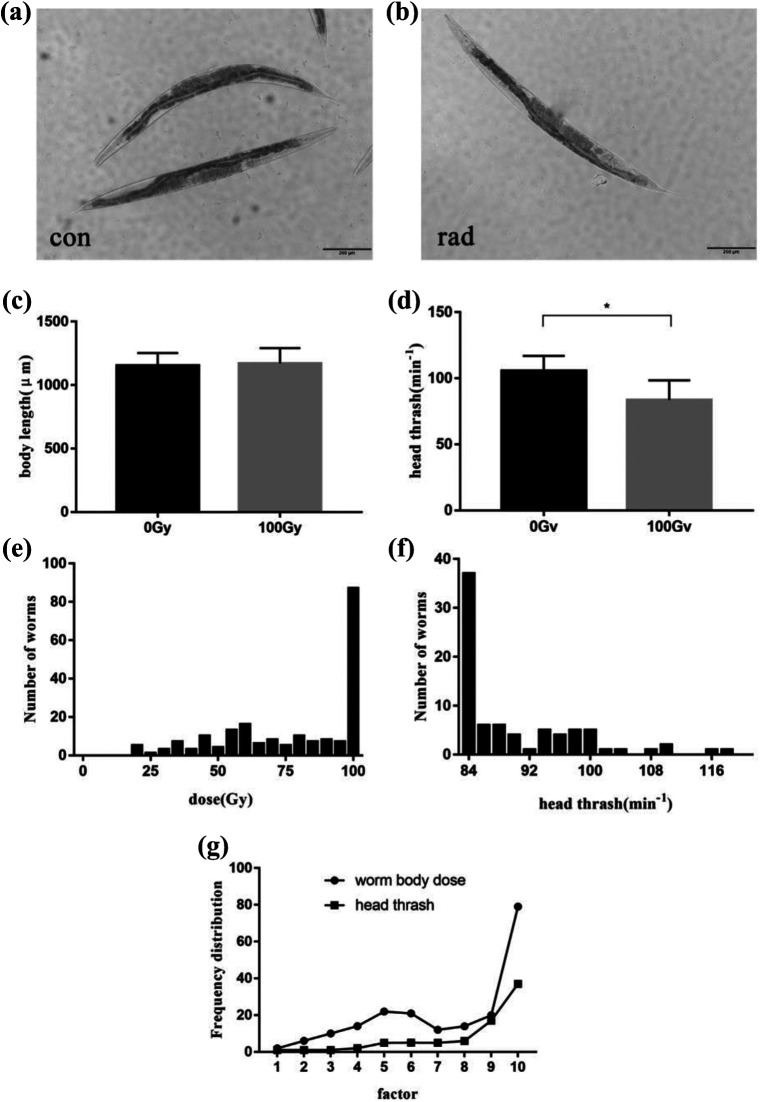
The effects of gamma radiation on C. elegans were illustrated by changes of the body length and head thrash frequency. After 48 hours post irradiation, nematodes developed to adults (Figure 2.a, b). Nematodes in the control group and the irradiation group displayed a length of 1152.6 ± 100.1 µm and 1171.2 ± 120.1 μm, respectively(Figure 2.c). There was no statistically significant between these 2 groups (p > 0.05). Nematodes developed to L4 stage at 36 hours post exposure to gramma radiation, and the frequency of nematode head thrash was investigated. Head swing frequency of nematodes in control group reached 105.6 ± 11.3 times/min, while that of the irradiation group fell 27.1% to 84.0 ± 14.8 times/min. There were statistically distinct differences between them (p < 0.05) (Figure 2.d), and the result indicated the ionizing radiation could affect the movement of C. elegans. The behavior and movement ability of C. elegans may be more easily affected by radiation than the level of growth and development. Considering the estimated dose, the head swing frequency of C. elegans could be inferred as 84 times/min when the average dose of C. elegans was 99.9 Gy.
Figure 2.
Effects of ionizing radiation on growth and movement of C. elegans. a. Size of nematode in the control group. b. Size of nematode in irradiation group. c. Effects of ionizing radiation on the length of C. elegans body. d. Effects of ionizing radiation on the head thrash frequency of C. elegans. e. Frequency distribution for dose of irradiated nematodes. f. Frequency distribution of head thrash frequency in the irradiation group. g. Frequency distribution profile of worm body dose and head thrash frequency. Asterisks indicate significant difference from control treatment (p-value < 0.05). Scale bar: 200 μm.
The relationship between the changes of head thrash after irradiation and the estimated microdose of C. elegans was investigated. Nematodes receiving the radiation dose higher than 99.9 Gy was assigned to one group based on the microdose frequency distribution. According to the frequency distribution of the head swing, nematodes with the frequency less than 84 times/min were allocated to one group. Finally, the contour analysis of the 2 processed frequency distribution curves demonstrated that there was no statistical difference between them (p > 0.05) (Figure 2.g), implying that there was connection between the frequency distribution of head swing and the estimated worm dose.
Fitting of the Egg Laying Amount Variation of Nematode With the Gonad dose
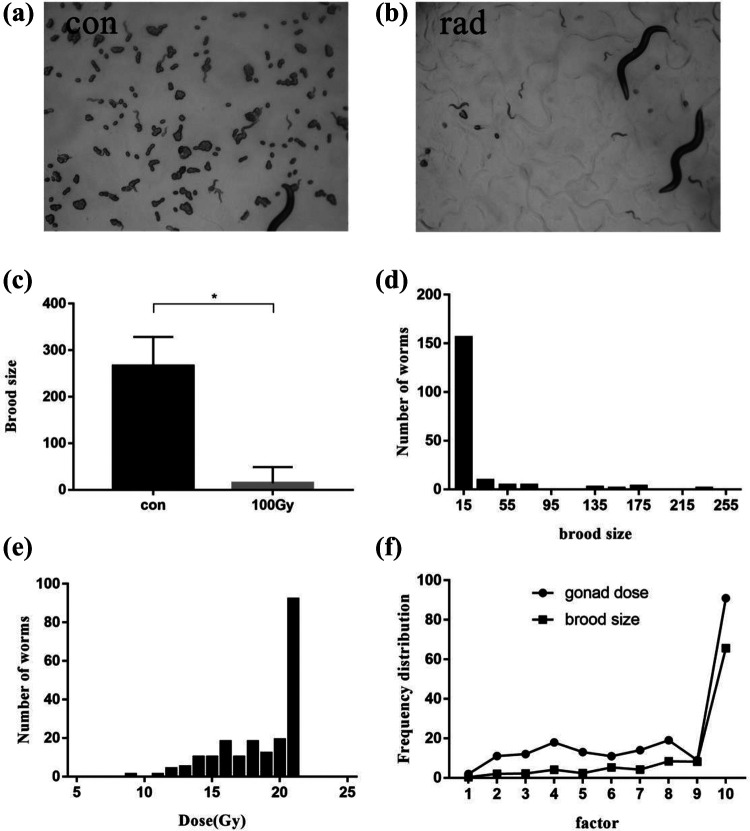
Injury of germ cells induced by ionizing radiation directly resulted in the decrease of the egg laying amount (Figure 3a, b). The total egg laying amounts of C. elegans were 266.1 ± 62.5 in the control group and 14.1 ± 34.69 in the irradiation group. Compared with the control group, the spawning amount in the irradiation group fell 94.72% upon exposure to radiation. These 2 groups showed statistically significant difference (p < 0.05) (Figure 3.c). Given the estimated dose, it could be speculated that the total spawns of C. elegans in 3 consecutive days were 14 when the average gonad dose was 19.8 Gy.
Figure 3.
Effects of ionizing radiation on C. elegans reproductive system and analysis. a. Fecundity of nematodes in the control group. b. Fecundity of nematodes in the irradiation group. c. Effects of ionizing radiation on egg laying amount. d. Frequency distribution of egg laying amount in irradiation group. e. Frequency distribution for dose of gonads after irradiation. f. Frequency distribution profile of gonadal dose and egg laying amount. Asterisks indicate significant difference from control treatment (p-value < 0.05).
The connection between alterations of spawning amounts after irradiation and gonad estimated result by microdosimetry was studied. According to the frequency distribution of dose, gonads exposed to high doses (≥19.8 Gy) of radiation were assigned to one group (Figure 3.d). Based on the frequency distribution of spawning amount from the results of reproductive capacity, nematodes with the spawning amount less than 14 were classified to one group (Figure 3.e). The contour analysis of the 2 processed frequency distribution curves showed there was no statistical difference between these 2 groups (p > 0.05) (Figure 3.f), indicative of the association between the distribution of egg-laying amount and the gonad dose.
Discussion
In this study, a microdosimetry model of Caenorhabditis elegans irradiated by 60Co irradiation device was established by using Monte Carlo software PHITS for the first time. The difference of dose micro-distribution was verified by the determination of head thrash and brood size. Head thrash is an indicator of swimming behavior of nematodes, which is mainly regulated by cholinergic neurons. Among the cholinergic related genes, ric-3 is located in pharynx and body wall,24 while lev-10 and cam-1 are located in muscle cells.25,26 From the expression pattern of each gene, it can be seen that cholinergic neurons are relatively evenly distributed in the whole body. It has been reported that ionizing radiation inhibits movement through systemic mechanisms, which may involve motor neurons and / or body wall muscle cells.27 Therefore, head thrash was used as an indicator to measure the dose of the worm body. The brood size is a recognized biological end point,28 which is often used in toxicological studies and can reflect the damage of the reproductive system to a certain extent.29,30 The brood size is mainly related to the development of germ cells of nematodes. It was reported that ionizing radiation can induce apoptosis of damaged germ cells through DNA damage checkpoints, resulting in reduction of the brood size.16 Therefore, the brood size was selected as an indicator to reflect the gonadal dose in this study. In the study of radiobiology, it was noted that the bystander effect should not be ignored. The bystander effect refers to the phenomenon that the biological effects of ionizing radiation can be observed in unexposed neighboring cells to an irradiated cell or group of cells,31-33 which had been confirmed in C.elegans. Although the irradiation condition of this study was performed on whole body, which is different from the local irradiation used to study the bystander effect, the side effect may still affect the experimental results. Moreover, with the deepening of the later study, the irradiation conditions may change from whole body irradiation to local irradiation, so it is necessary to consider the bystander effect and distinguish the radiation biological effect of the irradiated tissues from the bystander effect.
In the establishment of microdosimetric model, the overall dose was concerned, so the overall geometric shape of worm body and gonad was mainly considered. However, the internal structure of nematode and gonad was not distinguished, which are regarded as homogeneous tissues. Due to the low simulated volume and the difficulty of tangent calculation, particles in surface transport may easily “leak” particles (“particle lost”). Thus, this study adopted a rectangle for simulation instead of slender ellipsoid and the cylinder.
The average dose of the worm was estimated to be 99.9 Gy by the microdosemetry model, which was consistent with the set radiation dose 100 Gy. However, there were remarkable differences between individual worms in the dose as for the microscopic dose distribution. The distinguish was more visible for gonads (may be one thousand times), implying that the difference of dose distribution may increase with the decrease of size of the objective. This result was consistent with the conclusion of Kellerer et al.6 However, the individual dose deposition did not cohere with the set dose in the radiobiological study of C. elegans on the micron scale. The model of entire body and gonad of C. elegans was constructed by Monte Carlo method, which compensated for the lack of the model for C. elegans dose estimation. The result indicated the heterogeneity of biological dose distribution in small organisms.
At present, cell model was adopted in the study of microdosimetry. Nevertheless, except for weak maneuverability, cells can only be cultured in vitro, and there is a certain difference between the damage caused by irradiation and in living cells. In order to verify the rationality of the construction of the Monka model and the accuracy of the microdosimetric estimation results, the animal model was established. The whole body and gonad of C. elegans larvae are about 384.0 µm and 20.3 µm in length, respectively, which makes it an ideal animal model for the study of microdosimetry. As a result, the head thrash frequency and egg laying amount of C. elegans could reflect the radiation injury level induced by the energy deposition of the entire body and gonad of worms. The contour analysis illustrated that the frequency distribution of head thrash and egg laying amount had good correspondence with the dose of body and gonad of C. elegans, respectively. Therefore, C. elegans is an appropriate animal model for microdosimetric study, and head thrash frequency and spawning amount can be used as parameters to confirm the dose of the body and gonad of worms.
The possibility of C. elegans to be used as a combined model of microdosimetry and biology was preliminarily explored. Indicators to verify the results of microdosimetric dose estimation were discovered, which may provide a solution for microdosimetry study on the whole animal level. Yet there were still some limitations for this work. The model was a bit simple and different with the actual shape of nematode. In the future study, it is necessary to calculate the dose deposition of internal organs more precisely, including the gonad, nerve, intestine and other major organs. Modeling and Monte Carlo simulation with high accuracy are required. Therefore, more exact surface models may be the tendency of research to make it close to the irregular geometry of C. elegans.
Conclusion
C.elegans is known to be sensitivity of to ionizing gamma radiation. On the microscopic scale, the energy deposition of ionizing radiation is significant to study the biological effects of ionizing radiation. C. elegans was utilized as a model to determine the microdosimetric deposition level of nematodes at the 60Co gamma irradiation. Monte Carlo software PHITS was used for the modeling of irradiated nematodes. The dose of whole body and gonad of nematodes were estimated to be 99.9 ± 57.8 Gy (19.6-332.2 Gy) and 129.4 ± 558.8 Gy (9.5-6597 Gy), respectively. The length of nematodes made little change upon irradiation. Yet frequency of head thrash and the egg laying in 3 consecutive days decreased 27.1% and 94.7%, respectively. Variation trend of growth and reproduction of irradiated nematodes agreed with the dose distribution levels estimated by microdosimetric model. All in all, C. elegans proved to be an ideal model of microdosimetry and biology to investigate radiation.
Acknowledgments
We are grateful to the group of Huimin Zhang, College of Basic Medicine and Life Sciences, Soochow University for providing the C. elegans strain.
Authors’ Note: Na Chen and Liang Sun conceived and designed the experiment. Wentao Yu and Huiqiang Long performed the biological experiments. Yidi Wang and Liang Sun performed the Microdosimetric experiments. Jin Gao analyzed the data. Wentao Yu wrote the article. Wentao Yu and Huiqiang Long and Jin Gao contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant numbers 82003391, U186720), the Natural Science Research Projects of Colleges and Universities in Jiangsu Province (grant numbers 20KJB310007), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Collaborative Innovation Center of Radiological Medicine of Jiangsu Higher Education Institutions, China and the Nuclear Energy Development Project, China (No. 2016-1295).
ORCID iD: Na Chen  https://orcid.org/0000-0002-6165-8071
https://orcid.org/0000-0002-6165-8071
References
- 1. Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. Effects of ionizing radiation on biological molecules—mechanisms of damage and emerging methods of detection. Antioxid Redox Signal. 2014;21(2):260–292. doi:10.1089/ars.2013.5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haines GA. Germ cell and dose-dependent DNA damage measured by the comet assay in murine spermatozoaa after testicular x-irradiation. Biol Reprod. 2002;67(3):854–861. doi:10.1095/biolreprod.102.004382 [DOI] [PubMed] [Google Scholar]
- 3. Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of125IdU-induced DNA double-strand breaks with γ-H2AX antibody. Radiat Res. 2002;158(4):486–492. doi:10.1667/0033-7587(2002)158[0486: Qdoiid]2.0.Co;2 [DOI] [PubMed] [Google Scholar]
- 4. Grudzenski S, Raths A, Conrad S, Rube CE, Lobrich M. Inducible response required for repair of low-dose radiation damage in human fibroblasts. Proc Natl Acad Sci U S A. 2010;107(32):14205–14210. doi:10.1073/pnas.1002213107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rossi HH, Zaider M, Bolch W. Microdosimetry and its applications. Physics Today. 1997;50(12):70–71. doi:10.1063/1.882031 [Google Scholar]
- 6. Kellerer AM, Chmelevsky D. Concepts of microdosimetry. Radiat Environ Biophys. 1975;12(1):61–69. doi:10.1007/bf02339810 [DOI] [PubMed] [Google Scholar]
- 7. Barberet P, Vianna F, Karamitros M, et al. Monte-Carlo dosimetry on a realistic cell monolayer geometry exposed to alpha particles. Phys Med Biol. 2012;57(8):2189–2207. doi:10.1088/0031-9155/57/8/2189 [DOI] [PubMed] [Google Scholar]
- 8. Ballarini F, Alloni D, Facoetti A, Ottolenghi A. Heavy-ion effects: from track structure to DNA and chromosome damage. N J Physics. 2008;10(7):075008 doi:10.1088/1367-2630/10/7/075008 [Google Scholar]
- 9. Anon. Microdosimetry. ICRU report 36. International Commission on Radiation Units & Measurements; 1983. [Google Scholar]
- 10. Matsuya Y, Fukunaga H, Omura M, Date H. A model for estimating dose-rate effects on cell-killing of human melanoma after boron neutron capture therapy. Cells. 2020;9(5):1117 doi:10.3390/cells9051117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng Y, Zhang M, Zheng L, et al. Cysteine protease cathepsin B mediates radiation-induced bystander effects. Nature. 2017;547(7664):458–462. doi:10.1038/nature23284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng XZ, Yin XL, Allan R, et al. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C-elegans. Science. 2008;322(5898):110–115. doi:10.1126/science.1158111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deng X, Hofmann ER, Villanueva A, et al. Caenorhabditis elegans ABL-1 antagonizes p53-mediated germline apoptosis after ionizing irradiation. Nat Genet. 2004;36(8):906–912. doi:10.1038/ng1396 [DOI] [PubMed] [Google Scholar]
- 14. Herman RK, Albertson DG, Brenner S. Chromosome rearrangements in Caenorhabditis elegans. Genetics. 1976;83(1):91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cui F, Ma N, Han X, et al. Effects of (60)Co gamma irradiation on the reproductive function of Caenorhabditis elegans. Dose Response. 2019;17(1). doi:10.1177/1559325818820981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maremonti E, Eide DM, Oughton DH, et al. Gamma radiation induces life stage-dependent reprotoxicity in Caenorhabditis elegans via impairment of spermatogenesis. Sci Total Environ. 2019;695:133835 doi:10.1016/j.scitotenv.2019.133835 [DOI] [PubMed] [Google Scholar]
- 17. Maremonti E, Eide DM, Rossbach LM, Lind OC, Salbu B, Brede DA. In vivo assessment of reactive oxygen species production and oxidative stress effects induced by chronic exposure to gamma radiation in Caenorhabditis elegans. Free Radic Biol Med. 2020;152:583–596. doi:10.1016/j.freeradbiomed.2019.11.037 [DOI] [PubMed] [Google Scholar]
- 18. Weidhaas JB, Eisenmann DM, Holub JM, Nallur SV. A Caenorhabditis elegans tissue model of radiation-induced reproductive cell death. Proc Natl Acad Sci U S A. 2006;103(26):9946–9951. doi:10.1073/pnas.0603791103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwamoto Y, Sato T, Hashimoto S, et al. Benchmark study of the recent version of the PHITS code. J Nucl Sci Technol. 2017;54(5):617–635. doi:10.1080/00223131.2017.1297742 [Google Scholar]
- 20. Sato T, Iwamoto Y, Hashimoto S, et al. Features of Particle and Heavy Ion Transport code System (PHITS) version 3.02. J Nucl Sci Technol. 2018;55(6):684–690. doi:10.1080/00223131.2017.1419890 [Google Scholar]
- 21. Niita K, Iwamoto Y, Sato T, et al. Event generator models in the particle and heavy ion transport code system; PHITS. J Korean Phys Soc. 2011;59(2(3)):827–832. doi:10.3938/jkps.59.827 [Google Scholar]
- 22. Nose H, Niita K, Hara M, et al. Improvement of three-dimensional Monte Carlo code PHITS for heavy ion therapy. J Nucl Sci Technol. 2005;42(2):250–255. doi:10.3327/jnst.42.250 [Google Scholar]
- 23. Takada K, Sato T, Kumada H, et al. Validation of the physical and RBE-weighted dose estimator based on PHITS coupled with a microdosimetric kinetic model for proton therapy. J Radiat Res. 2018;59(1):91–99. doi:10.1093/jrr/rrx057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen M, Alfonso A, Johnson CD, Rand JB. Caenorhabditis elegans mutants resistant to inhibitors of acetylcholinesterase. Genetics. 1995;140(2):527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fiser J, Chiu C, Weliky M. Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature. 2004;431(7008):573–578. doi:10.1038/nature02907 [DOI] [PubMed] [Google Scholar]
- 26. Forrester WC, Dell M, Perens E, Garriga G. A C. elegans Ror receptor tyrosine kinase regulates cell motility and asymmetric cell division. Nature. 1999;400(6747):881–885. doi:10.1038/23722 [DOI] [PubMed] [Google Scholar]
- 27. Suzuki M, Hattori Y, Sakashita T, Yokota Y, Kobayashi Y, Funayama T. Region-specific irradiation system with heavy-ion microbeam for active individuals of Caenorhabditis elegans. J Radiat Res. 2017;58(6):881–886. doi:10.1093/jrr/rrx043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson GL, Boyd WA, Williams PL. Assessment of sublethal endpoints for toxicity testing with the nematode Caenorhabditis elegans. Environ Toxicol Chem. 2001;20(4):833–838. doi:10.1002/etc.5620200419 [PubMed] [Google Scholar]
- 29. Mohan N, Chen C-S, Hsieh H-H, Wu Y-C, Chang H-C. In vivo imaging and toxicity assessments of fluorescent nanodiamonds in Caenorhabditis elegans. Nano Lett. 2010;10(9):3692–3699. doi:10.1021/nl1021909 [DOI] [PubMed] [Google Scholar]
- 30. Wu Q, He K, Liu P, Li Y, Wang D. Association of oxidative stress with the formation of reproductive toxicity from mercury exposure on hermaphrodite nematode Caenorhabditis elegans. Environ Toxicol Pharmacol. 2011;32(2):175–184. doi:10.1016/j.etap.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 31. Bertucci A, Pocock RDJ, Randers-Pehrson G, Brenner DJ. Microbeam irradiation of the C. elegans nematode. Journal of Radiation Research. 2009;50(Suppl A):A49–A54. doi:10.1269/jrr.08132 s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo X, Sun J, Bian P, et al. Radiation-induced bystander signaling from somatic cells to germ cells in Caenorhabditis elegans. Radiat Res. 2013;180(3):268–275. doi:10.1667/RR3218.1 [DOI] [PubMed] [Google Scholar]
- 33. Tang H, Chen L, Chen L, et al. Interaction between radioadaptive response and radiation-induced bystander effect in Caenorhabditis elegans: a unique role of the DNA damage checkpoint. Radiat Res. 2016;186(6):662–668. doi:10.1667/RR14548.1 [DOI] [PubMed] [Google Scholar]