Abstract
Objective
To determine the appropriate amount of indocyanine green for bronchial insufflation.
Methods
We enrolled 20 consecutive patients scheduled for anatomical segmentectomy in the Kochi Medical School Hospital. After inducing general anesthesia, 6 to 60 mL of 200-fold-diluted indocyanine green (0.0125 mg/mL) was insufflated into the subsegmental bronchi in the targeted pulmonary segmental bronchus. The volume of the targeted pulmonary segments was calculated using preoperative computed tomography. Fluorescence spread in the segmental alveoli was visualized using a dedicated near-infrared thoracoscope.
Results
The targeted segment was uniformly visualized by indocyanine green fluorescence in 16/20 (80.0%) cases after insufflating indocyanine green. A receiver operating characteristic curve indicated that the area under the curve was 0.984; the optimal cut-off volume of diluted indocyanine green for insufflation was 8.91% of the calculated targeted pulmonary segment volume.
Conclusions
The setting for indocyanine green insufflation was optimized for near-infrared fluorescence image-guided anatomical segmentectomy. By injecting the correct amount of indocyanine green, fluorescence-guided anatomical segmentation may be performed more appropriately.
Keywords: Anatomical segmentectomy, bronchoscopic insufflation, indocyanine green fluorescence, intrasegmental plane, lung cancer, minimally invasive surgery
Introduction
Lung cancer is the leading cause of cancer death worldwide.1 This cancer is categorized into two main histological types: small cell lung carcinoma (SCLC; 15% of all lung cancers) and non-SCLC (NSCLC; 85% of all lung cancers). NSCLCs are generally subcategorized into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, and surgical resection is recommended for clinical stage I–II NSCLC.2,3 Pulmonary lobectomy has been the standard surgical treatment for NSCLC.4 However, there has been a recent increase in the identification of ground-glass nodules (GGNs) using computed tomography (CT). Patients with pulmonary GGNs are diagnosed pathologically with early-stage lung adenocarcinoma, such as adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA).5 AIS and MIA exhibit alveolar replacement proliferation and poor stromal infiltration and have excellent 5-year overall survival rates after sublobar limited pulmonary resection.6,7
When a GGN is present in the middle layer or in the center of the lung, where wide wedge resection is not technically applicable, anatomical segmentectomy is performed. Visual recognition of the intersegmental plane; i.e., identifying the border between the pulmonary segment targeted for resection and the adjoining pulmonary segments, is an important practical factor in anatomical segmentectomy. The intersegmental pulmonary veins serve as anatomical landmarks when dissecting the central portion of the intersegmental plane. To determine the peripheral portion of the intersegmental plane, demarcation lines are created either by inflating or deflating the targeted segment.8,9 However, inflation of the intended pulmonary segment may reduce the surgical working space during video-assisted thoracic surgery. In addition, a combination of indocyanine green fluorescence (ICG-FL) imaging and systemic injection of ICG can be used to identify the intersegmental plane.10–14
Another recently developed ICG-FL-based method involves bronchoscopic ICG insufflation into the targeted segmental bronchus during general anesthesia induction, which makes the targeted segment positive for ICG-FL (Figure 1). However, there have been few reports on bronchoscopic ICG insufflation.15,16 Although this method is superior to the systemic administration method, in that it allows continued visualization of the pulmonary segment targeted for anatomical segmentectomy for several hours during surgery, it is a technically challenging method. Specifically, the capacity of the targeted pulmonary segment differs in each segment, and the appropriate volume of ICG required for insufflation into the targeted segment has not been scientifically verified.
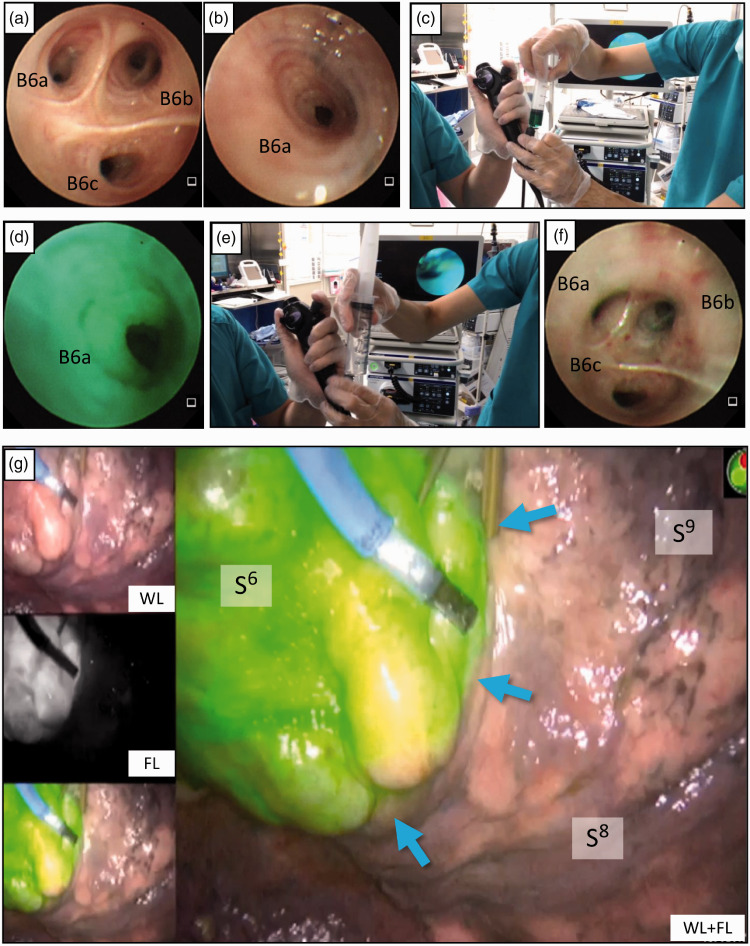
Figure 1.
Indocyanine green (ICG)-fluorescence (FL) intersegmental plane visualization by bronchoscopic ICG insufflation. After inducing general anesthesia and endotracheal intubation, the bronchoscope is advanced to the targeted segmental bronchus. (a) Bronchoscopic view of left subsegmental bronchus B6a, B6b, and B6c before ICG insufflation. (b) The orifice of the left B6a wedged by the tip of the flexible bronchoscope before ICG insufflation. (c) A bolus of ICG (0.0125 mg/mL) is insufflated into each subsegmental bronchus through the bronchoscope’s utility channel. (d) Bronchoscopic view of B6a during ICG insufflation. (e) Immediately following the ICG bolus, 150 mL or more of air is pushed into the bronchus. (f) Bronchoscopic view of the left B6 after ICG insufflation into B6a, B6b, and B6c. (g) Subsequent video-assisted thoracoscopy showing that the left S6 segmental lung exhibits uniform ICG-FL, and the intersegmental plane (blue arrows) between S6 and S8 is visualized.
WL, white light-only image; FL, fluorescence-only image; Cr, cranial; Ca, caudal; Ve, ventral; Do, dorsal.
In this study, we determined the appropriate volume of diluted ICG for bronchoscopic insufflation into the resection-targeted pulmonary segmental bronchus.
Materials and methods
Patient recruitment
We enrolled patients who were scheduled for anatomical pulmonary segmental resection and who provided preoperative written informed consent to participate in the study and for their individual data to be published. This study was approved by our institutional review board.
Bronchoscopic ICG insufflation into the targeted segmental bronchus
ICG insufflation into the targeted segmental bronchus was performed as previously reported.15 Briefly, after inducing general anesthesia, endotracheal intubation was performed using a single-lumen endotracheal tube. A bronchoscope with a distal end diameter of 4.0 mm (BF-MP160F; Olympus, Shinjuku, Japan) was then inserted. To identify the targeted subsegmental bronchi, we wedged the tip of the bronchoscope into each sub-subsegmental bronchus, injected 3 mL of 200-fold diluted ICG from the bronchoscope’s accessory channel, and immediately injected 50 mL of air three times (150 mL of air in total) with a 50-mL syringe to push the ICG solution to the periphery. A similar procedure was performed in each sub-subsegmental bronchus in the targeted pulmonary segment (Figure 1). After confirming that ICG fluorescence was evenly distributed in the targeted pulmonary segment following the first three insufflations, the total volume of diluted ICG insufflated into the segmental bronchi was gradually reduced to 9 mL. Additionally, after confirming the dose at which the fluorescence distribution became non-uniform, the insufflation volume was increased to approximately half of the initial volume.
Calculating the targeted pulmonary segment volume
The volume of the targeted pulmonary segment was calculated using the previously captured CT data and the Synapse Vincent volume analyzer (Fujifilm, Minato, Japan) (Figure 2). The ratio of the diluted ICG injection volume to the volume of the target lung area was calculated, and the results are presented as percentages in Table 1.
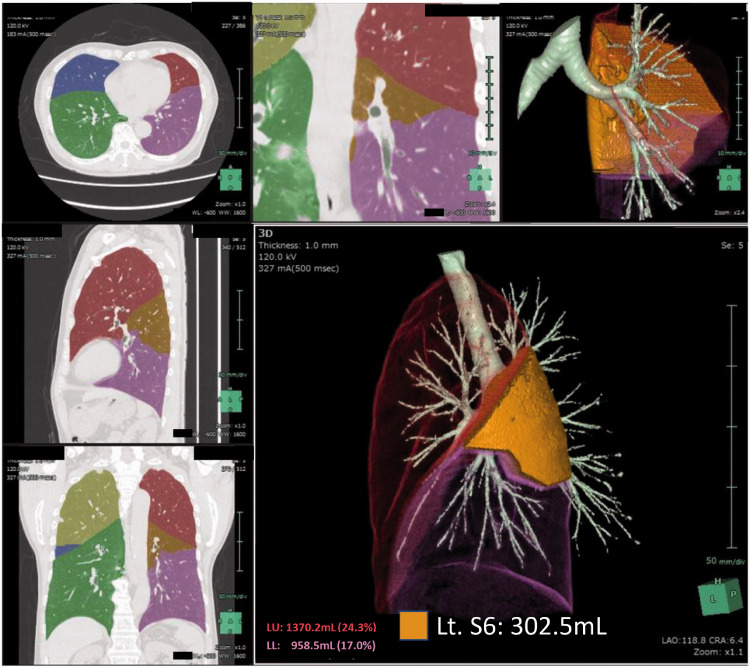
Figure 2.
Calculating the capacity of the targeted pulmonary segment. From the computed tomography data, the volume of the targeted pulmonary segment is calculated using the Synapse Vincent software (Fujifilm, Minato, Japan). In the presented case, the volume of the left S6 is calculated as 302.5 mL.
Table 1.
Bronchoscopic indocyanine green insufflation volume, capacity of the targeted segment, and indocyanine green-fluorescence distribution status.
| Case # | Age (years) | Sex | Targeted segment | ICG volume (mL) | Segmental lung capacity (mL) | ICG vol./Segmental lung capacity (%) | Uniform ICG-FL distribution |
|---|---|---|---|---|---|---|---|
| #1 | 64 | M | Lt. S6 | 60 | 302.5 | 19.8 | Y |
| #2 | 36 | F | Lt. S6 | 60 | 74.4 | 80.6 | Y |
| #3 | 44 | M | Lt. S6 | 60 | 260.0 | 23.1 | Y |
| #4 | 69 | F | Rt. S6 | 20 | 168.1 | 11.9 | Y |
| #5 | 68 | M | Rt. S7, S8, S9, S10a | 40 | 697.6 | 5.7 | Y |
| #6 | 69 | M | Rt. S6 | 9 | 101.0 | 8.9 | Y |
| #7 | 61 | F | Rt. S8 | 6 | 257.0 | 2.3 | N |
| #8 | 63 | M | Rt. S9+S10 | 12 | 654.1 | 1.8 | N |
| #9 | 71 | M | Lt. S10 | 36 | 372.3 | 9.7 | Y |
| #10 | 80 | M | Rt. S10 | 9 | 472.0 | 1.9 | N |
| #11 | 71 | M | Lt. S3 | 30 | 371.2 | 8.1 | N |
| #12 | 74 | M | Rt. S1 | 38 | 380.0 | 10.0 | Y |
| #13 | 78 | M | Lt. S1 + 2, S3 | 60 | 597.5 | 10.0 | Y |
| #14 | 82 | F | Rt. S6 | 21 | 130.0 | 16.2 | Y |
| #15 | 90 | M | Lt. S4, S5 | 32 | 301.9 | 10.6 | Y |
| #16 | 76 | F | Lt. S1 + 2Sc | 16 | 146.9 | 10.9 | Y |
| #17 | 82 | F | Rt. S10c | 12 | 113.0 | 10.6 | Y |
| #18 | 70 | F | Lt. S8 | 13 | 124.2 | 10.5 | Y |
| #19 | 86 | F | Lt. S1 + 2c | 9 | 76.0 | 11.8 | Y |
| #20 | 69 | F | Lt. S1 + 2c | 13 | 128.0 | 10.2 | Y |
M, male; F, female; Lt, left; Rt, right; ICG, indocyanine green; FL, fluorescence; Y, Uniform; N, non-uniform.
Thoracoscopic ICG-FL detection
ICG-FL of the targeted pulmonary segment was visualized using the PINPOINT® endoscopic fluorescence imaging system (Novadaq, Mississauga, Canada). Successful ICG-FL visualization of the targeted pulmonary segment was confirmed by four surgeons, namely the operator and three observing thoracic surgeons.
Cut-off value determination
The ideal cut-off value for the minimum required amount of insufflating ICG was determined using a receiver operating characteristic (ROC) curve and by determining the Youden index using JMP version 12.0.1 for Windows (SAS institute Inc., Cary, NC, USA).
Results
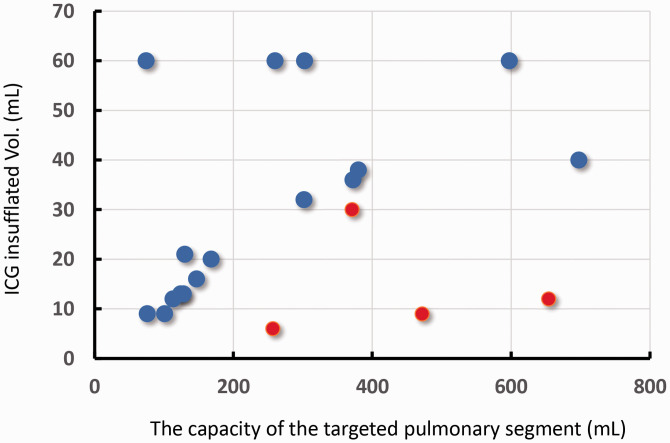
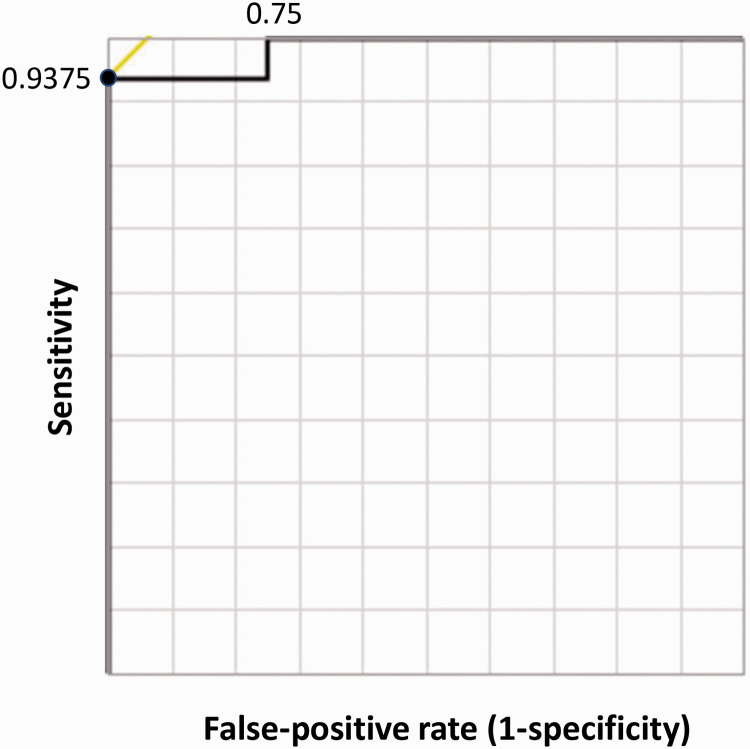
Twenty patients were enrolled in this study. Their clinical information, ICG insufflation amount, ratio of ICG insufflation volume to the volume of the targeted pulmonary segment (expressed as percentages), and fluorescence distribution conditions are shown in Table 1. In 16 of the 20 cases, the targeted pulmonary segment was uniformly visualized by ICG-FL (80.0%); 4 cases exhibited a non-uniform ICG-FL distribution (Figure 3). The relationship between ICG insufflation volume and target lung volume is shown in Figure 4. The ROC curve determined the optimal cut-off volume proportion for uniform fluorescence visualization of the target segmental lung as 0.089 mL of diluted ICG per unit volume (mL) of the targeted segmental lung, with a sensitivity of 0.938 and specificity of 1.00 (Figure 5). No complications associated with this study were observed in any of the cases.
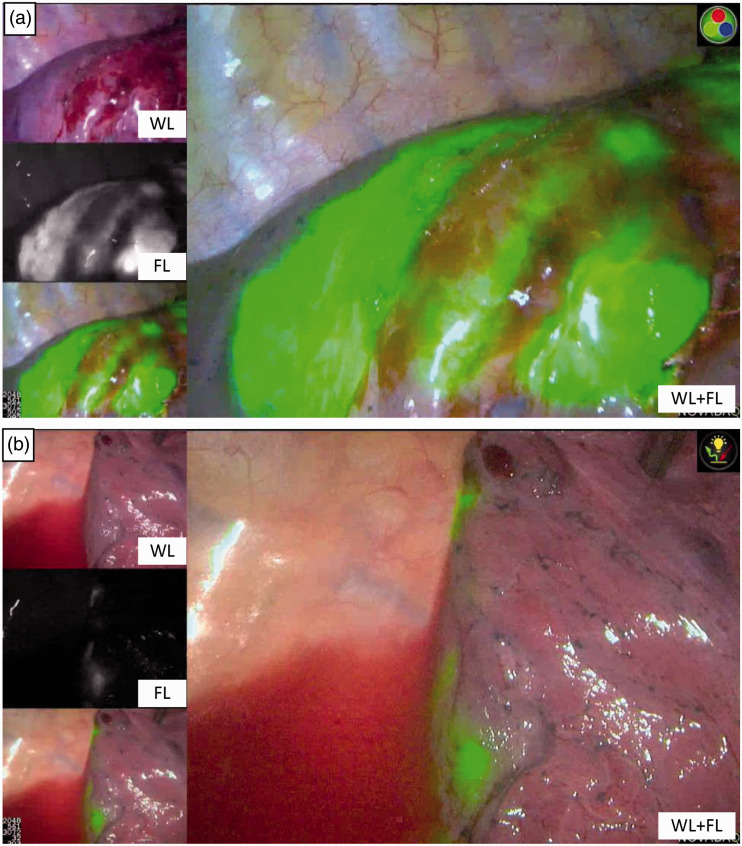
Figure 3.
Two representative cases of unsuccessful indocyanine green (ICG-FL) insufflation. The targeted segment is non-uniformly stained with ICG-FL. (a) Case #7. (b) Case #8.
WL, white light.
Figure 4.
Relationship between the volume of the targeted segments and bronchoscopic indocyanine green (ICG) injection volume. The cases in which the targeted pulmonary segment exhibited uniform fluorescence are plotted as blue dots, and the cases with uneven fluorescence are plotted as red dots.
Figure 5.
Receiver operating characteristic (ROC) curve analysis to determine the diluted indocyanine green (ICG) insufflation volume per unit lung volume. We performed a ROC curve analysis to calculate the optimal amount of ICG volume per target pulmonary segmental lung volume for successful uniform fluorescence visualization. The area under the curve (AUC) was 0.984, and the cut-off value was 8.91%.
Discussion
In this study, we determined the appropriate volume of ICG for insufflation into the segmental bronchus of the pulmonary segment to depict the targeted segment by ICG-FL. The optimal injection volume was estimated by beginning with a sufficient injection volume, gradually reducing the injection volume to a level where the fluorescence distribution became non-uniform, and gradually increasing the injection volume again.
Anatomical pulmonary segmentectomy requires intraoperative identification of the margins of the pulmonary segments (intersegmental plane) to be resected. The resected segment inflation method is a useful technique for visualizing the intersegmental demarcation line during segmentectomy.16 Although this method can be performed without the need for special equipment, the inflated pulmonary segment may obstruct the field of view in thoracoscopic surgery. As a method of identifying the intersegmental plane without changing the field of view in endoscopic surgery, systemic administration of ICG has been used. By ligating the segmental pulmonary artery feeding the target pulmonary segment to be excised in advance, the target pulmonary segment is visualized as a fluorescence-deficient area, while other segments positively exhibit fluorescence.17 Although this method is simple, the visualization time of the intersegmental plane by ICG fluorescence is as short as several minutes, which is considered a disadvantage. The advantage of using the ICG endobronchial insufflation method for visualizing the targeted segmental bronchus is that once ICG insufflation is performed at induction of general anesthesia, ICG-FL of the targeted segment is long-lasting and can be observed continuously during surgery. Therefore, the surgeon can confirm the intersegmental plane intraoperatively at any time. The disadvantage of this procedure is that it is cumbersome to perform during anesthesia induction.
This study has some limitations. Successful bronchoscopic insufflation depends on whether the distal end of the bronchoscope and the inner diameter of the targeted segmental bronchus match. If some of the insufflated ICG leaks backward, the net insufflated ICG volume might be less than the estimated volume, which may result in non-uniform insufflation of ICG. By sealing the bronchial lumen with a balloon catheter, such as a Fogarty arterial embolectomy catheter,17 it is possible to insufflate ICG into the targeted bronchus without ectopic distribution of ICG. Other limitations in this study are the small sample size and the single-center design, which was highly dependent on the operator’s bronchoscopic technique.
In conclusion, the current study determined the minimum required volume of ICG for bronchoscopic insufflation to enable ICG-FL visualization of the intersegmental plane for anatomical pulmonary segmentectomy. Using an appropriate volume of ICG for insufflation into the bronchus makes it possible to reliably visualize the targeted pulmonary segment. The current technique can also be applied in anatomical segmentectomies.
Acknowledgements
The authors express their gratitude to Mr. Katumi Noguchi (Mizuho Medical Co., Ltd., Tokyo), Mr. Joseph Kletzel, and Mr. Fumi Koshino, Novadaq (Mississauga, ON, Canada) for providing PINPOINT©.
Footnotes
Declaration of conflicting interest: The authors have no conflicts of interest.
Funding: This work was supported by a Grant-in-aid for Scientific Research, Japanese Society for the Promotion of Science KAKENHI (15K01294).
ORCID iD: Takashi Anayama https://orcid.org/0000-0002-3989-6268
References
- 1.World Health Organization. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (2002, accessed 10 July 2010).
- 2.Asamura H, Goya T, Koshiishi Y, et al. A Japanese lung cancer registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol 2008; 3: 46–52. [DOI] [PubMed] [Google Scholar]
- 3.Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer 2005; 50: 227–234. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg 1995; 60: 615–623. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical Ia lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011; 6: 751–756. [DOI] [PubMed] [Google Scholar]
- 6.Yamato Y, Tsuchida M, Watanabe T, et al. Early results of a prospective study of limited resection for bronchioloalveolar adenocarcinoma of the lung. Ann Thorac Surg 2001; 71: 971–974. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida J, Nagai K, Yokose T, et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg 2005; 129: 991–996. [DOI] [PubMed] [Google Scholar]
- 8.Tsubota N. An improved method for distinguishing the intersegmental plane of the lung. Surg Today 2000; 30: 963–964. [DOI] [PubMed] [Google Scholar]
- 9.Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007; 133: 753–758. [DOI] [PubMed] [Google Scholar]
- 10.Misaki N, Chang SS, Gotoh M, et al. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg 2009; 138: 613–618. [DOI] [PubMed] [Google Scholar]
- 11.Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010; 140: 752–756. [DOI] [PubMed] [Google Scholar]
- 12.Kasai Y, Tarumi S, Chang SS, et al. Clinical trial of new methods for identifying lung intersegmental borders using infrared thoracoscopy with indocyanine green: comparative analysis of 2- and 1-wavelength methods. Eur J Cardiothorac Surg 2013; 44: 1103–1107. [DOI] [PubMed] [Google Scholar]
- 13.Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014; 46: 112–115. [DOI] [PubMed] [Google Scholar]
- 14.Mun M, Okumura S, Nakao M, et al. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J Vis Surg 2017; 3: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012; 143: 1330–1335. [DOI] [PubMed] [Google Scholar]
- 16.Endoh M, Oizumi H, Kato H, et al. How to demarcate intersegmental plane with resected-segments inflation method using the slip knot technique in thoracoscopic anatomic segmentectomy. J Vis Surg 2017; 3: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekine Y, Itoh T, Toyoda T, et al. Precise anatomical sublobar resection using a 3D medical image analyzer and fluorescence-guided surgery with transbronchial instillation of indocyanine green. Semin Thorac Cardiovasc Surg 2019; 31: 595–602. [DOI] [PubMed] [Google Scholar]