Abstract
There is a faction within the chiropractic profession passionately advocating against the routine use of X-rays in the diagnosis, treatment and management of patients with spinal disorders (aka subluxation). These activists reiterate common false statements such as “there is no evidence” for biomechanical spine assessment by X-ray, “there are no guidelines” supporting routine imaging, and also promulgate the reiterating narrative that “X-rays are dangerous.” These arguments come in the form of recycled allopathic “red flag only” medical guidelines for spine care, opinion pieces and consensus statements. Herein, we review these common arguments and present compelling data refuting such claims. It quickly becomes evident that these statements are false. They are based on cherry-picked medical references and, most importantly, expansive evidence against this narrative continues to be ignored. Factually, there is considerable evidential support for routine use of radiological imaging in chiropractic and manual therapies for 3 main purposes: 1. To assess spinopelvic biomechanical parameters; 2. To screen for relative and absolute contraindications; 3. To reassess a patient’s progress from some forms of spine altering treatments. Finally, and most importantly, we summarize why the long-held notion of carcinogenicity from X-rays is not a valid argument.
Keywords: X-ray, chiropractic, low-dose radiation, adult spinal deformity, radiophobia, LNT
Introduction
Despite radiography being a well-established cornerstone of spine and pelvic biomechanical examination in the therapy of spine disorders,1-16 there are pressures to reduce the use of radiological imaging to assess spine structure and function within the chiropractic profession.17-20 This mounting pressure is by a select faction of mostly academics who claim that imaging is over-utilized within chiropractic. In 2017, this movement culminated in a major chiropractic association joining the Choosing Wisely program to denounce the use of X-rays for routine assessment of acute low back pain (within the first 6-weeks of onset) and not to use X-rays for repeated use to monitor patient progress.17 These anti-imaging practice strategies have been shown to lack evidential support and to be antithetical to scientific reality.18,19
Chiropractic anti-imaging activists reiterate common false statements such as “there is no evidence” for biomechanical assessment from X-rays, “there are no guidelines” supporting routine imaging, and that “X-rays are dangerous.” These arguments come in the form of recycled allopathic “red flag only” medical guidelines for spine care,20-22 opinion pieces23,24 and consensus statements.17 All of these claims are factually incorrect as has been extensively pointed out in several publications.13-16,18,19,25-30 X-rays are, in fact, the only practical way to assess a patient’s essential biomechanical spinal parameters (e.g. cervical hyper/hypo-lordosis/kyphosis, thoracic hyper/hypo-kyphosis, lumbar hyper/hypo-lordosis, global/regional sagittal balance, pelvic tilt, sacral slope, thoracic inlet angle, pelvic morphology, etc.).1-12 As well, there are indeed chiropractic guidelines31-34 and rationale13-16,35 that support routine imaging and, as we will discuss later, low-dose radiation exposures from X-rays are now known to be harmless based on updated information.36-48
Problematically, the anti-imaging stance in chiropractic promulgates misinformation that confuses the public and contributes to divisiveness within the profession between those who practice specialty techniques that require routine spine imaging (to guide treatment) and those who practice generalized gross manipulation that, generally, do not use imaging. Further, the anti-imaging narrative gets amplified when reiterated in the literature, such as when mentioned in chiropractic clinical practice guidelines49-51 and when over-stated in low-quality studies.52,53 It has also been thoroughly documented that the anti-imaging stance such as by the American Chiropractor’s Association’s participation in the Choosing Wisely campaign’s Points 1 and 2 anti-imaging stance is fraught with misappropriated medical references (i.e. practice of medicine references) and not supported by any legitimate chiropractic disciplined evidence.18,19 Most problematically, this anti-imaging stance has led to insurance companies capitalizing on the opportunity to adopt policies that discriminate against chiropractors who take X-rays based on these practice strategies that are not representative of the profession, including Blue Cross Blue Shield (BCBS) and others.18
While we acknowledge that traditional “red flags” (malignancy, fracture, inflammatory disorders, etc.) warrant radiographic screening for suspected serious disease processes (this is a concern for all primary care providers), our focus is on the routine use of radiographic imaging for the purposes of screening, assessment, patient management and monitoring of spine and pelvis (spinopelvic) biomechanical parameters from modern and contemporary chiropractic and manual therapy treatment methods. The purpose of this discussion is to assess the validity of common statements made against the use of routine X-ray use in chiropractic practice. Specifically, we show that routine X-ray use is an evidence-based tool to: 1. Assess spinopelvic biomechanical parameters; 2. Screen for relative and absolute contraindications to spine care; 3. Re-assess a patient’s progress to some types of spine rehabilitative treatments. Finally, we provide a synopsis of the most recent evidence showing the long-held assumption of carcinogenicity from X-rays is no longer a valid argument.
Assessing Spinopelvic Parameters
Recent statements made in the chiropractic literature include Jenkins et al. (2018) who stated: “there is currently insufficient evidence to recommend the use of routine spinal X-rays to analyse spinal biomechanics,”20 and Corso et al. (2020) who stated: “We found no evidence that the use of routine or repeat radiographs to assess the function or structure of the spine, in the absence of red flags, improves clinical outcomes and benefits patients.”52 The former article has been critiqued ad nauseam,26 and the latter article was a “rapid review” that did not include any literature published in the last 15 years! It must be mentioned that specifically within the last 15 years (2005-2020) the literature has become replete with high-quality evidence correlating various spinopelvic parameters as measured from X-rays to various health outcomes in the spine care literature.1-16
A simple PubMed search of systematic reviews (SR) of particular spine parameters shows unanimous conclusions favoring significant relationships between altered spine parameters and physiological dysfunction. Forward head posture (i.e. anterior head translation), for example, is associated with headache, altered muscle activity, altered proprioception, altered breathing, pain and disability.54-56 Loss of lumbar lordosis, particularly loss of distal lordosis is associated with and causative for low back pain (LBP).57,58 Osteoarthritis and spondylolisthesis are linked to LBP.58,59 All of these spinal parameters are diagnosed by radiography and, again, these relationships are current scientific consensus based on SRs.54-59
As mentioned, despite factions within chiropractic claiming the opposite, the routine assessment of a patient’s spinopelvic biomechanical parameters is the standard for evaluating adult spinal deformity (ASD) and subluxation/misalignment patterns.1-16,60-62 The International Spine Study Group (ISSG) in fact states: “accurate assessment of ASD requires a thorough radiographic evaluation of both the spine and pelvis, including concomitant assessment of the cervical, thoracic, and lumbar spine, as well as the femoral heads and pelvis.”5 One chiropractic guideline31 cites Hildebrandt63 who outlines what radiographic images encompass a full spine radiographic evaluation, and these include full spine antero-posterior and lateral views, images of the femur heads, sacral base, and upper cervical specific views.
It is important to delineate why assessing spinopelvic parameters is so important to patient treatment. A monumental study was published in 2015 by the European Spine Study Group (ESSG) and a similar study in 2016 by the ISSG.64,65 Pellise et al. showed that the quality of life burden on a sample of 766 ASD patients was significantly more detrimental compared to a database of about 25,000 patients suffering from the common ailments of self-reported arthritis, chronic lung disease, diabetes and congestive heart failure.64 Similarly, Bess et al. demonstrated that the physical component summary scores from the SF-36 quality of life questionnaire in a sample of 497 ASD patients showed similar scores to patients suffering from diabetes, heart disease and rheumatoid arthritis, and that patients with more severe forms of symptomatic ASD “reported physical limitations and health impact as patients with limited use of arms and legs.”65 These studies taken together provide compelling evidence that ASD can have devastating impacts on health that often exceeds the disease burden from well recognized chronic ailments.
It is important to delineate what the ISSG and ESSG considered as ASD; the criteria in these 2 reported studies64,65 were identical and included forward sagittal balance >5 cm, thoracic kyphosis (T4-T12) >60°, pelvic tilt >25° (indicating a decreased distal lumbar lordosis), and scoliosis >20° as measured from full-spine patient radiographs (Figure 1). One may argue that an ASD patient presenting to a spine surgeon may represent a different population than one presenting to a chiropractor or manual therapist and therefore, a surgeon has more of a rationale to utilize X-ray. However, patients having spine deformities exceeding these specific parameter thresholds have been documented to be treated by chiropractors (e.g. anterior sagittal balance >5 cm,66,67 thoracic hyperkyphosis >60°,68-70 pelvic tilt >25° indicating loss of lumbar lordosis,71-75 scoliosis >20°76-80). Indeed, the treatment of ASD patients who have significant disease burden often overlaps between spine surgeons and chiropractors/manual therapists.
Figure 1.

Adult spine deformity specifically refers to the following spine deformity (subluxation) types as assessed with radiographic methods and present in any person 18 years and older: A, Thoracic hyper-kyphosis with a magnitude greater than 60°, green is ideal curve and red is patient kyphosis. B, Anterior (positive) sagittal balance of the C7-S1 plumb-line greater than 5 cm (pink line from centroid C7 to horizontal offset of centroid S1). C, Any frontal view scoliosis of greater than 20°. D, Sagittal pelvic tilt angle (PA) greater than 25°. Note, due to the inverse relationship between this PA measurement and sagittal sacral base angle (SBA), PA is descriptive of a SBA being decreased where normal SBA = 40°, and normal PA is approximately 17-18°. Thus, this measurement represents lumbar hypo-lordosis in the distal lumbar region.
In fact, many spine patients seek non-operative care prior to surgery in attempts to avoid the invasive procedure81-83 (surgical procedures are often viewed as “last resort”) and even after surgical procedures, as there are high rates of surgical failures (i.e. the surgery does not eliminate the pains).84-87 It is also logical that more severe forms of spine deformities preclude lesser forms of spine deformities that ultimately progresses over time.88,89 Thus, chiropractors and manual therapists who specialize in spine-altering treatment methods serve patients who have a myriad of subluxated spine displacement patterns (Figure 2 90,91). If spine patients and their instigating deformities can be treated successfully, this would prevent the later need for surgical procedures from evolving spine deformities (i.e. symptomatic ASD) which has been documented in the literature.81-83 Thus, manual therapists including chiropractors definitively treat ASD patients, and of course, also treat patients having less severe spine deformities.
Figure 2.
Rotations and translations of the head, thorax, and pelvis about the x, y, z-axis Cartesian coordinate system.90,91 All postures have corresponding spine coupling patterns; patients invariably present with multiple posture and spine alterations/deformities/subluxations (Courtesy CBP seminars).
There are many spine and pelvic parameters that can be measured from standing X-rays (Figures 1 and 3).1-16 Cervical lordosis, thoracic kyphosis and lumbar lordosis quantify the sagittal regional curves (Figure 3). Sagittal anterior-posterior displacement (translations) for forward head posture and of the total spine are also typically measured (Figure 1B). Pelvic position including the sacral inclination, pelvic tilt and pelvic incidence are also common measurements (Figure 1D). The scoliosis “Cobb angle” is also measured on the frontal view (Figure 1C). All the mentioned spinopelvic parameters can be easily quantified from radiographs using modern x-ray analysis software, and are amenable to structural change (except pelvic incidence and thoracic inlet angle which are stable morphologic measures in adults). Modern non-surgical spine rehabilitation procedures are becoming popular (e.g. Chiropractic BioPhysics® (CBP®), physiotherapy scoliosis specific exercise (PSSE), etc.) and for providers who practice such methods, full spine radiographic assessment is a necessity to quantify the presenting spinopelvic alignment that in turn, guides patient-specific treatment procedures.
Figure 3.
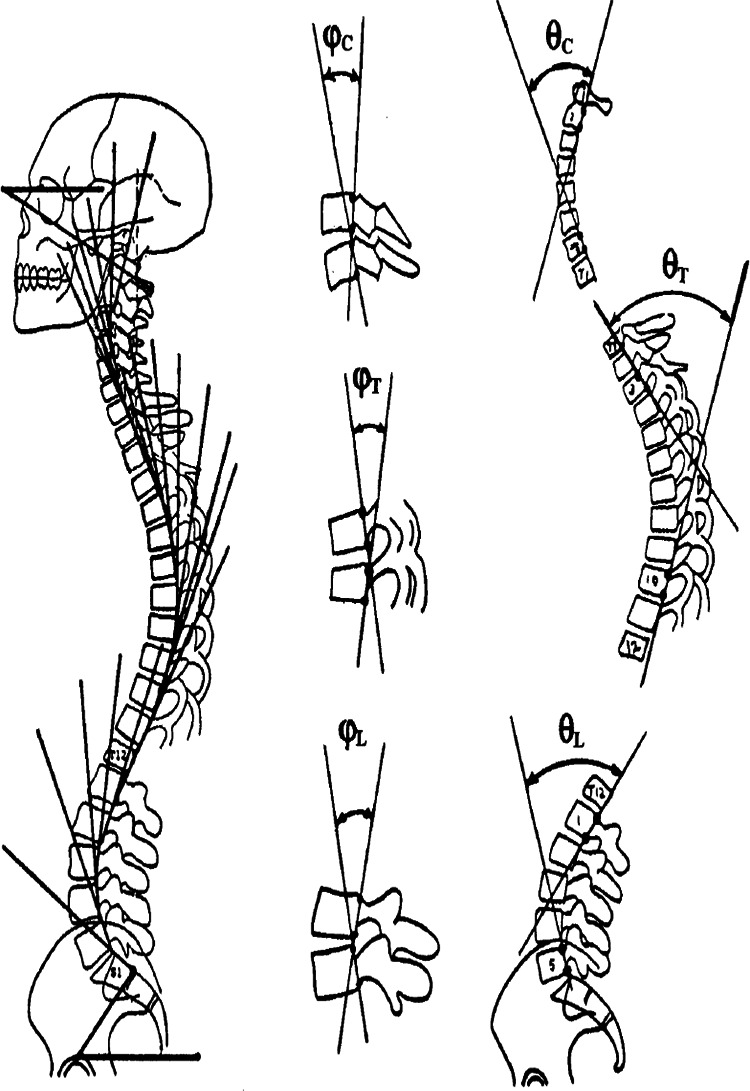
Harrison posterior tangent method used to quantify cervical, thoracic and lumbar lordosis as well as any other sagittal subluxation patterns including individual intersegmental rotation angles (Courtesy CBP seminars).
Screening for Anomalies, Pathologies and Contraindications to Manual Therapies
Since manual therapies are unavoidably “hands-on” that also makes them patient-centric. In other words, manual treatments are ideally applied in a fashion that is custom-tailored to meet the unique needs of the patient and to also match their preferences. Case-in-point is that similar forces of thrusts during spinal manipulation would not be used for infants, children and frail seniors than would be used for healthy adults. More specifically, it is known that congenital anomalies, common pathologies, and relative and absolute contraindications to manual treatments are common92-96 and should be known prior to imparting forces into a patient’s spine and body.
Surprisingly there has been little study of the occurrence of anomalies, contraindications, and certain pathologies that would alter treatment approaches by manual therapists and chiropractors.92-96 Although it has been argued that spinal manipulation treatment is benign20 (i.e. safe to apply to a wide population without comprehensive X-ray screening), mild adverse events are common.97,98 Mild adverse events, although not catastrophic, present real challenges in daily practice and the avoidance of these occurring is important.26 In contrast, there are many popular chiropractic adjusting techniques that are segment-specific (e.g. Atlas Orthogonal, Blair, Diversified, Gonstead, Grostic, Kale, Logan Basic, NUCCA, Palmer upper cervical specific, Toggle-recoil, etc.) and the thrust delivery is based on the initial examination of patient X-rays to ascertain 3-dimensional “thrust vectors.” Further, as mentioned, more recent patient-specific customized structural rehabilitation programs (CBP, PSSE, etc.) also require screening for precision assessment by X-ray and bony anomalies and pathologies including osteoarthritis that may alter treatment approaches unrelated to the necessary spinopelvic parameter relationships.
Of the studies done within the chiropractic literature determining the incidence of bone anomalies, specific pathologies, and contraindications to spinal manipulation, the overall evidential consensus is that these findings are very common (Table 1).92-96 After assessing 847 full spine patient X-rays, for example, Beck et al. determined that 68% of the sample had congenital anomalies, 6% had absolute contraindications (should not receive thrust manipulation), and 0.6-6.6% had “serious” pathology warranting referral for urgent medical assessment/intervention.95 Young et al. diagnosed serious pathology in 44% of 262 patient lumbar radiographs.93 Jenkins et al. found congenital anomalies in 28.5% of 2814 patient cervical X-rays and 18.3% of 1052 patient lumbar X-rays.92 Other studies have found the combination of anomalies and pathologies to range from 66-91% of patient samples.94,96
Table 1.
Incidence of Anomalies, Pathologies and Postural Changes That Could Alter Treatment, and Relative and Absolute Contraindications to Provide Chiropractic Treatment.92-96
| Author | Region | n | Age | Sex | Cohort/Setting | Postural changes | Congenital anomalies | Contraindications | Serious pathology | Anomalies/Pathologies | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Avg (SD) | Relative | Absolute | |||||||||
| Jenkins | Cervical | 2814 | n/r | n/r | Macquarie University | 28.5% | |||||
| Thoracic | 695 | n/r | n/r | Chiro Clinic | 0.7% | ||||||
| Lumbar | 1052 | n/r | n/r | 18.3% | |||||||
| Young | Lumbar | 262 | >/<50 | mix | Chiro Radiologist | 94% | 44% | ||||
| Pryor | Cervical | 413 | n/r | n/r | Chiro College | 91% | |||||
| Thoracic | 403 | n/r | n/r | Clinic | 70% | ||||||
| Lumbar | 402 | n/r | n/r | 79% | |||||||
| Beck | Full spine | 847 | 33 (12) | mix | New Zealand Chiro | 68.1% | 6% | 0.6-6.6% | |||
| College Clinic | |||||||||||
| Bull | Full spine | 1698 | 36 | n/r | Macquarie University | 33% | 14% | 66% | |||
| Chiro Clinic | |||||||||||
In one study reviewing the incidence of radiographic variations in 10,922 “healthy” German air force recruits, it was determined that, amazingly, “In only 2.6% were there no pathological findings.”99 In a study of X-rays among 232 young, healthy Norwegian air force candidates it was determined that “many significant conditions are demonstrated among young healthy individuals” and among the 66% having anomalies, degenerative changes and deviations of posture, there was an average of 3.5 diagnoses per spine X-rayed.100 They also stated “Since the population is highly selected, the figures we present may be minimum numbers in a western industrialized society.”100 As seen, radiological studies of even so-called healthy people show that bone and joint skeletal anomalies and pathologies that translate into relative and absolute contraindications to spine manipulation, show these findings are extremely common and, again, can only be determined by routine X-ray screening.
As a great detriment to the chiropractic profession, most “X-ray guidelines” for chiropractors have dedicated little to no emphasis on the unique aspects of manipulation of the spine and have neglected to provide guidelines reflective of the requirement to screen for important anomalies and relevant pathologies, and only discuss “absolute” contraindications (i.e. red flags).20-22 Such “red flag” guidelines for chiropractic are misleading those who strictly abide by them into having faith in red flag screening. It was pointed out recently that many red flags (i.e. signs of serious pathology) are based on little evidence,101-107 and are not as reliable as has been the enthusiasm for their endorsement.19 Routine X-ray screening would identify important aspects idiosyncratic to the individual patient and allow for better care. We concur with Giles who stated: “because spinal manipulation involves forces to the spine and, in rare circumstances, may result in an adverse reaction it seems justified to image a patient’s painful spinal area when clinical trials involving spinal manipulation are conducted.”108(p130)
A comprehensive list of potentially relevant X-ray findings that could alter treatment approaches has been given by the Practicing Chiropractors’ Committee for Radiology Protocols (PCCRP)31 (Table 2). It should be noted that patients presenting to a manual therapist typically have multiple indications.
Table 2.
Indications for Spine Radiography in Adults and Children Not Associated With “Red Flags.”31
| 1. Abnormal posture; |
| 2. *Spinal Subluxation; |
| 3. Spinal deformity (eg, scoliosis, hyper-kyphosis, hypo-kyphosis, etc…); |
| 4. Trauma, especially trauma to the spine; |
| 5. Birth Trauma (eg, forceps, vacuum extraction, caesarean section etc…); |
| 6. Restricted or abnormal motion; |
| 7. Abnormal gait; |
| 8. Axial pain; |
| 9. Radiating pain (eg, upper extremity, intercostal, lower extremity); |
| 10. Headache; |
| 11. Suspected short leg; |
| 12. Suspected spinal instability; |
| 13. Follow-up for previous deformity, previous abnormal posture, previous spinal subluxation/displacement, previous spinal instability; |
| 14. Suspected osteoporosis; |
| 15. Facial pain; |
| 16. Systemic health problems (eg, skin diseases, asthma, auto-immune diseases, organ dysfunction); |
| 17. Neurological conditions; |
| 18. Delayed developmental conditions; |
| 19. Eye and vision problems other than corrective lenses; |
| 20. Hearing disorders (eg, vertigo, tinnitus, etc…); |
| 21. Spasm, inflammation, or tenderness; |
| 22. Suspected abnormal pelvic morphology; |
| 23. Post-surgical evaluation; |
| 24. Suspected spinal degeneration/arthritis; |
| 25. Suspected congenital anomaly; |
| 26. Pain upon spinal movement; |
| 27. Any “Red Flag Conditions” covered in previous guidelines. |
Re-assessing Structural Alignment Changes
As discussed, the goal of structural-based treatment (as opposed to symptom-based treatment) is to realign the global and segmental morphological shape of the spine and how a person stands relative to their center of balance. Repeating radiological procedures to re-assess spinopelvic response to treatment procedures is an essential practice that often leads to a change in treatment.
Treatment course changes from repeated X-rays may either take on 1 of 4 scenarios (Table 3). Possibilities include positive changes, no change or a worsening of parameters; as can be seen, each scenario has warranted treatment considerations. Logically, continued treatment options for chiropractors utilizing spine altering methods depend on measurement of the spinopelvic parameters as assessed from the follow-up (repeat) X-rays. This is an evidence-based and patient-centered healthcare practice despite the reiteration of anti-imaging narratives from small factions within the profession to not perform repeat imaging (e.g. ACA,17 Corso et al.52).
Table 3.
Differing Treatment Scenarios Based on Findings From Repeat X-rays Following Treatment Trials.
| Scenario | Radiological spinopelvic findings | Treatment alteration |
|---|---|---|
| 1. | Improvement of parameters | None, continue |
| 2. | Correction of parameters to within normal limits | Transition to maintenance |
| 3. | No change in parameters | Reconsider options |
| 4. | Worsening of parameters | Stop/Reconsider options |
As has been outlined recently, there are abundant high-quality, randomized clinical trials (RCTs) that have definitively shown that non-surgical spine rehabilitation procedures can make significant improvements to the spine and posture in those suffering from many different spinal subluxation patterns.72-75,109-125 The spinopelvic parameters having the most accumulated evidence of non-surgical improvements (i.e. RCTs) include the treatment of forward head posture,109-115 cervical hypolordosis/kyphosis,110-117 thoracic hyperkyphosis (hunchback),118-121 lumbar hypolordosis,72-75 and scoliosis.122-125 As can be seen, clinically significant magnitudes of corrections/improvements can be achieved in relatively short durations (e.g. weeks/months).
It must be mentioned that research for musculoskeletal treatments are less developed than other facets of health care research.126 Therefore, the science is not complete, and in this respect, lesser forms of evidence (i.e. case reports/series) should be viewed as important and considered in guidelines created for the manual therapies, particularly chiropractic.127,128 When considering the chiropractic and manual therapy literature showing improvements in other spine and pelvis deformities/subluxation types, there is evidence showing reduction of anterior whole-spine sagittal balance,129,130 reduction of cervical pseudo-scoliosis,131-133 reduction of lumbar pseudo-scoliosis,134,135 increase in thoracic hypokyphosis (straight back syndrome),136-138 reduction of thoracolumbar kyphosis,139 increase in lumbar kyphosis (flat back syndrome),82 reduction of lumbar hyperlordosis and pelvic tilt,140 reduction of cervical spondylolisthesis,141,142 and reduction of lumbar spondylolisthesis.143,144
Pressures to restrict the use of “repeat” (i.e. follow-up) X-rays for assessing patient response to treatment shows a complete disregard for the evidence discussed that definitively illustrates how modern spine rehabilitation techniques and practices successfully re-align the spine and pelvis for a wide variety of presenting subluxation/deformity patterns. The continued anti-X-ray sentiment from “consensus” and opinion within chiropractic needs to stop; it is antithetical to scientific reality and to the practice of contemporary chiropractic practice. We reiterate a quote from the late Michael A. Persinger: “what is happening in recent years is that facts are being defined by consensus. If a group of people think that something is correct, therefore it’s true, and that’s contradictory to science.”145
Synopsis of Why Low-dose Radiation Is Safe
The ubiquitous underpinnings of X-ray restriction campaigns and other practice altering agendas discouraging X-ray use is the prevailing belief in the linear no-threshold (LNT) radiation carcinogenicity ideology.145 Regarding radiation exposures from X-rays and the presumed fears of future untoward health effects, the classic carcinogenicity narrative has been reiterated loud and clear from a small faction within the chiropractic literature.17,20,23,24,52
In a recent “rapid” review concluding no evidence for X-ray use in chiropractic, Corso et al. state: “A known risk for ionizing exposure is the increased frequency of cancer beyond that occurring spontaneously…” and this statement is used to reinforce their conclusions to not X-ray, “…and given the inherent risks of ionizing radiation…”52 In a recent “narrative” review of X-ray use for chiropractic, Jenkins et al. state: “Without definitive thresholds of safe levels of radiation exposure, it should be assumed that some level of risk is associated with the use of X-rays,”20 they go on to endorse the “As Low As Reasonably Achievable” (ALARA) radiation protection principle underpinned by LNT to keep exposures minimized.20 The recent ACA declaration of their participation in Choosing Wisely, their Points 1 and 2 both mention “exposing the patient to radiation” as an additional point for making the case to not X-ray patients.17
We and many others have recently provided details of why low-dose radiation exposures (<200 mGy), including those from X-rays and CT scans are not harmful.13-16,26-31,36-48 The issue of ionizing radiation carcinogenicity is the main underpinning for all anti-imaging rhetoric. Here we sum up many of the arguments against LNT and related concepts that refute the presumptive notion that X-rays cause cancer.
The LNT Model Is Not Valid for Low-Dose Radiation Exposures
The Lifespan study (LSS) of the Hiroshima and Nagasaki survivor data is espoused to be the main evidential support for the LNT model,146 however, the BEIR VII has been heavily criticized for being based on faulty assumptions and analyses.147-149 A recent analysis of the LSS data by Ozasa et al. was published in 2012,150 and, when corrected for a “likely negative bias” in baseline cancer mortality, the data shows a non-linear (quadratic) curve that represents a hormetic pattern rather than the traditional assumed linear (LNT) pattern (Figure 4).151,152 Thus, the persons exposed to the fallout outlived controls.153 Further, others have questioned the validity of the LSS data to support the LNT,154,155 for example, Socol et al. demonstrated that after performing a Monte-Carlo simulation of possible LSS outcomes, they determined that the LSS had insufficient statistical power to support the LNT.155
Figure 4.

Excess relative risk (ERR) correcting for a 20% bias in baseline cancer mortality rate for all solid cancers in atomic bomb survivors from the original data from Ozasa et al.150 Error bars represent 95% confidence intervals. Threshold is at about 0.7 Gy (700 mGy).151,152
On a more fundamental level, recent information has emerged that shows that the LNT model that was first adopted back in the 1950s was spawned based on scientific fraud and misconduct.156-159 The LNT model was adopted based on Hermann J. Muller’s original fruit fly experiments,160 where very high X-ray radiation doses were shown to produce transgenerational phenotypic changes; these changes were claimed by Muller to be gene mutations.158-160 Significantly, the claimed gene mutations were only gene deletions and other chromosomal rearrangements; this misinformation misdirected the radiation genetics research field for decades.158 As it turns out, there is good evidence suggesting that Muller’s nobel prize winning research published in Science in 1927 evaded peer review, and by doing so, he was able to claim primacy for discovering gene mutation.158
To this day, the major governmental and international radiation regulatory bodies continue to support the LNT model for risk assessment despite the exponential increase of evidence showing its invalidity.161-163 Recent reviews of available evidence, not surprisingly, do not support the use of the LNT for risk assessment from the low-dose exposure range. Vaiserman et al., for example, concluded that the “LNT has certainly not been proven to be true” and suggest that, due to the high economic and human costs, the present regulatory burden should be reduced.162 Recently, the Society of Nuclear Medicine and Molecular Imaging convened a task group to examine the validity of the LNT and its applicability for use in risk assessment and radiation protection. The group concluded “the evidence does not support the use of LNT either for risk assessment or radiation protection in the low-dose and dose-rate region.”163
Unfortunately, based on the dubious history surrounding the origins of the LNT and the continued loyalty to the LNT by the major governing bodies despite direct challenges to the continued endorsement of the LNT by these bodies (e.g. BEIR VII147-149), the LNT has evolved to be more political than scientific.164,165 Many have presented possible alternatives to the LNT that may be plausible moving forward.166-171 Indeed, as Cardarelli and Ulsh argue, abandoning use of the traditional LNT model for low dose low-dose rate radiation risk assessment by incorporating updated science into the regulatory process could be successful to ensure science remains the underpinning of decision making, to educate the public on the lack of risks from low-dose radiation exposures, and to “harmonize government policies with the rest of the radiation scientific community.”172(p1)
ALARA Is an Obsolete Radiation Protection Concept
Since the LNT model is not valid for low dose radiation, any concept borne by it is also invalid and obsolete.37,39,41 Many have pleaded for the termination of the ALARA concept and anti-imaging campaigns as used in medical imaging.37,41-44,170,171,173-175 ALARA and the anti-imaging campaigns that endorse it including Image Gently (pediatrics),176 Image Wisely (adults),177 and Choosing Wisely,178 leads to no benefits and ironically may cause more harm (Table 4).37
Table 4.
Synopsis of How ALARA Causes More Harm Than Benefit.37
| Reluctance of doctors to prescribe diagnostic X-rays |
| Constrains practice |
| Adds malpractice risks |
| Delayed diagnosis |
| Missed diagnosis |
| Alternate imaging has harms |
| Reluctance of patients to receive X-rays |
| Shared decision-making leads to X-ray avoidance |
| Constrains medical management |
| Adds malpractice risks |
| Leads to more consultation time |
| Leads to more testing |
| Increased radiation exposures by aligning with ALARA |
| Repeated imaging |
| Missed diagnosis |
| Delayed medical procedures |
| Stifling of low-dose medical research & treatment |
| Ignoring the body’s innate mechanisms |
| Ignoring historic evidence of efficacy |
| Propagation of radiophobia |
| Circular reasoning for continued ALARA |
| Never ending radiophobia narrative |
Actual real harm (and presumed harm by increased radiation exposures) by adhering to the ALARA principle include propagating radiophobia,179 which in turn, increases patient refusal for medically warranted X-rays,180,181 which in turn, increases the burden to medical professionals attempting to deliver high-quality medical care.37 Reduced image quality, by attempting to reduce radiation to the patient,182,183 increases potential missed diagnoses.41,174 Other harms include increased radiation exposures because of retakes (due to using too little exposure or gonadal shielding covering targeted anatomy),37,182,184,185 increased use of alternate imaging methods that presents other unique risks (e.g. sedation during MRI),182,186 and perhaps most importantly, increased liability to physicians.37
In short, the ALARA concept would only be valuable if radiological medical imaging were proven to cause future cancers. However, this is not the case. In a recent study performing a quantitative evaluation of the methodological quality of an originally selected 4,382 studies in order to determine the evidentiary strength either supporting or refuting a causal relationship between low-dose radiation and cancer, it was determined that the majority of articles(84%) having high-quality methods found no increased risk of cancers from exposure less than 200 mSv.187 The authors concluded “The evidence suggests that exposure to multiple CT scans and other sources of low-dose radiation with a cumulative dose up to 100 mSv (approximately 10 scans), and possibly as high as 200 mSv (approximately 20 scans), does not increase cancer risk.”187
The Threshold of Carcinogenesis From Radiation Exposures Is High
As mentioned, when a 20% correction for baseline cancer incidence is accounted for in the Ozasa et al.150 updated analysis of the LSS, the threshold for cancer incidence shows a value of 700 mSv (Figure 4).151,152 Cuttler has reassessed the 1958 UNSCEAR data and found that the threshold for leukemia is at 1100 mGy (95% CI: 0.5-2.6 Gy), much higher than previously thought (Figure 5).188,189 It was also noted than even in the persons who developed leukemia, they accounted for only 0.5% of the exposed cohort.188 As Oakley and Harrison recently stated: “even considering the lower threshold dose of 700 mGy, this represents about 2 to 3 orders of magnitude greater than the amount of radiation given from medical X-rays.”36(p.3) Thus, diagnostic X-rays cannot be expected to cause cancers.
Figure 5.

1958 UNSCEAR data indicates a threshold of about 1.1 Gy (1100 mGy; assuming RBE = 1) for radiogenic leukemia in 95,819 persons exposed to A-bomb radiation from Hiroshima.189
Low-Dose Radiation Upregulates the Adaptive Protection Systems and Prevents Cancers
Although those that push to restrict routine and repeat X-rays for spine care quickly dismiss the concept of radiation hormesis (e.g. Kawchuk et al.23), in our opinion, this reason alone invalidates X-ray fear-mongering from supposed future cancers. The fact is, and as Cuttler astutely reminds us, that experience over the last 120 years has shown that low doses of radiation stimulate the innate protection systems, including the immune system, and these processes involve more than 150 genes.190
Exposures to low-dose radiation incites multiple and multi-hierarchical biopositive mechanisms that prevent, repair or remove damage caused mostly by endogenous reactive oxygen species (ROS) and H2O2 from aerobic metabolism.191-193 Indeed, non-radiogenic (i.e. naturally occurring) molecular damage occurs daily at rates many orders of magnitude greater than the rate of damage caused by low-dose radiation such as diagnostic X-rays.194-196 It is estimated that the endogenous genetic damage caused on a daily basis from simply breathing air is about one million times the damage initially resulting from an X-ray.15 We concur that “it is factually preposterous to have radiophobic cancer concerns from medical X-rays after considering the daily burden of endogenous DNA damage.”18(p7)
Many have presented overviews of the physiological mechanisms that lead to hormetic effects (Figure 6)197-202 and these redundant and efficient processes include DNA repair systems, programed cell death, cell cycle delay, cellular senescence, adaptive memory, bystander effects (exposed cells communicate to non-exposed cells), epigenetics, immune stimulation and tumor suppression (Table 5). The irony is that the stimulation and upregulation of innate protection mechanisms and immune status is what accounts for the curative effects from low-dose irradiation therapies for the treatment of many human ills including inflammatory and neurodegenerative conditions, infections and cancers (Table 6).190, 203-225
Figure 6.
The adaptive response systems very efficiently prevent, repair or remove virtually all DNA alterations.196
Table 5.
Body’s Multiple Adaptive Response Mechanisms That Prevent, Repair and Remove Damage Caused From Mostly Endogenous Reactive Oxygen Species and H2O2 From Aerobic Metabolism. 200
|
|
|
|
|
|
|
|
|
|
Table 6.
Human Diseases, Infections, and Conditions Successfully Treated by Low-Dose Radiotherapy.190,203-225
| Non-cancerous conditions: | Cancers |
|---|---|
| Alzheimer’s disease | Breast |
| Arthritis | Colon |
| Bronchial asthma | Hematological |
| Bursitis | Liver cell |
| Carbuncles | Lung |
| Cervical adenitis | Non-Hodgkin’s lymphoma |
| Deafness | Ovarian |
| Diabetes Type I | Prostate |
| Diabetes Type II | Uterine |
| Furuncles | |
| Gas gangrene | |
| Necrotizing fasciitis | |
| Otitis media | |
| Parkinson’s disease | |
| Pemphigus | |
| Pertussis | |
| Pneumonia | |
| Rheumatoid arthritis | |
| Sinus infection | |
| Tendonitis | |
| Ulcerative colitis |
Historically, it is important to mention that many human diseases were treated by X-rays, so-called “radiotherapy.” Unfortunately, despite radiotherapy showing a 75%-90% efficacy rate,203 the treatment fell out of favor due to the evolution of antibiotics and also from fears of radiation following the atomic bomb droppings during WWII. Radiotherapy was documented to successfully treat arthritis,204 bronchial asthma,205 carbuncles,206 cervical adenitis,207 deafness,207 furuncles,206 gas gangrene,208 necrotizing fasciitis,209 otitis media,207 pertussis,210 pneumonia,211 sinus infection,212 and tendonitis/bursitis.213 These human ailments were treated using typical radiation doses ranging from 30 to 100 roentgen (∼0.3-1.0 mGy) which are fairly high doses, yet there are no reports of ill health effects from these treatments.
Many different cancers have also been successfully treated using radiotherapy.214-220 The typical patient dosing involves exposing the patient to 1.5 Gy over 5-weeks.214-217 It is also noteworthy that radiotherapy (whether given by X-rays or radon) has been making a resurgence in the literature; recent case reports have documented the successful treatment of cancers (e.g. prostate, colon, uterine, lung, and liver cell), ulcerative colitis, rheumatoid arthritis, pemphigus, diabetes types I and II, Alzheimer’s disease and Parkinson’s disease.221-225
The Total Collective Effective Dose (TCD) Concept Is Invalid for Low-Dose Radiation
LNT ideology assumes all radiation is cumulative, that an increase in one’s total collective effective dose (TCD) equates to a linear increase in harm. Thus, dose is used as a surrogate for risk.226 As just discussed, however, low-dose radiation exposures upregulate the innate adaptive response systems, which result in a net zero or less genetic damage (i.e. via over-repair) than was present prior to the initial exposure. This was proven to occur by Löbrich et al. who determined that DNA double-strand breaks (DSBs) occur in human lymphocytes after CT scans; however, these DSBs were repaired between 5 and 24 hours after the scan.227 Most importantly, the endogenous repair mechanisms repaired more than the damage that had initially occurred from the initial scan. Indeed, the final DSB count was less than prior to the scan.
This illustrates how the simple mathematical addition of low-dose exposures to increasing TCDs is theoretical risk and not reflective of reality. A case example is for an adolescent idiopathic scoliosis (AIS) patient, where repeat imaging is necessary during the treatment and monitoring of his/her condition. If we assume the dose threshold for leukemia is 1100 mGy as reported by Cuttler,189 it has been stated: “Since the body’s adaptive response will repair damage done at each X-ray event, X-ray exposures of about 1 to 3 mGy will always remain at a level that is 367 to 1100 times below the carcinogenic dose threshold.”39
It should be noted that even if the LNT were valid, “the calculation of the number of cancer deaths based on collective effective doses from trivial individual doses should be avoided.”228
Risk remains a population-based metric; therefore, its assignment to the individual should never be interpreted deterministically.229 This is because individual effective dose effects have uncertainty, up to plus or minus 40% due to age, gender, mass, etc.230,231 Mitchel argues that the assumption of dose additivity is not supported by the literature and states: “at the low doses and dose rates typical of public and occupational exposures, the radiation protection principle of dose additivity, and the concept that risk can only increase as dose increases are not justified. In general, the use of dose as a surrogate for risk needs re-evaluation.”226 (p287) The TCD concept is flawed and not valid as a model for assessing cancer risk from low-dose exposures.226,232
Aged Cohort Studies Purveying Radiogenic Cancers From Prior Exposures Are Not Generalizable
LNT advocates quickly cite studies that show patient cohorts exposed to radiation during childhood (e.g. CT scans) have also been shown to have increased cancers in adulthood (e.g. Pearce et al.,233 Mathews et al.,234 Hong et al.235). Although on the surface it may seem plausible, this correlation could be causal. The aphorism “correlation is not causation” applies. In fact, the critical flaw to these types of studies is that healthy children do not usually receive CT scans. Therefore, these studies suffer from “reverse causation,” or that the reason for the CT scan in the first place was due to underlying diseases that predisposes one to develop cancer, or actual suspicions of malignancy itself.236,237
Indeed, Journey et al. proved reverse causation after first finding a statistical correlation of increased cancers in a cohort of adults who were CT imaged in youth. When controlling for the reasons for the scans, no statistical correlation prevailed.238 Shibata et al. also determined that of 763 children receiving CT scans, 32% had congenital anomalies.239 Since the normal incidence of congenital anomalies is only about 2.5%, they concluded “the population of children undergoing CT is completely different from that not undergoing CT. The 2 groups should not be compared.”239 Thus, the oft cited and heavily amplified carcinogenic fears of future cancers from medical imaging is not supported.
Conclusions
Routine and repeat X-rays in the nonsurgical treatment of patients with spine disorders is an evidence-based clinical practice that is warranted by those that practice spine-altering methods. The evidence supporting such practices is based on definitive evidence supporting the rationale to assess a patient’s spinopelvic parameters for biomechanical diagnosis, to screen for relative and absolute contraindications for specific spine care methods, and to re-assess the spine and postural response to treatment.
The traditional and underlying presumption of the carcinogenicity from X-rays is not a valid notion because the LNT is not valid for low-dose exposures. The ALARA radiation protection principle is obsolete, the threshold for harm is high, low-dose exposures prevent cancers by stimulating and upregulating the body’s innate adaptive protection mechanisms, the TCD concept in invalid, and aged cohort studies assumed to show cancers resulting from previous X-rays are not generalizable to the wider population because they represent populations predisposed to cancers.
Red flags, or suspected serious underlying disease is a valid consideration warranting screening imaging by all spine care providers. We contend, however, that as long as the treating physician or rehabilitation therapist is practicing evidence-based methods, proven to improve spine and postural parameters in order to provide relief for the myriad of spinal disorders, spinal X-rays are unequivocally justified. Non-surgical spine care guidelines need to account for proven and evolving non-surgical methods that are radiographically guided, patient-centered, and competently practiced by those specialty trained in such methods. This is over and above so-called “red flag only” guidelines. The efforts to universally dissuade chiropractors from routine and repeat X-ray imaging is neither scientifically justified nor ethical.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P.A.O. is a paid research consultant for CBP NonProfit, Inc.; D.E.H. teaches spine rehabilitation methods and sells products to physicians for patient care that require radiography for biomechanical analysis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funds from CBP NonProfit, Inc.
ORCID iD: Paul A. Oakley  https://orcid.org/0000-0002-3117-7330
https://orcid.org/0000-0002-3117-7330
References
- 1. Le Huec JC, Thompson W, Mohsinaly Y, Barrey C, Faundez A. Sagittal balance of the spine. Eur Spine J. 2019;28(9):1889–1905. [DOI] [PubMed] [Google Scholar]
- 2. Afolayan JO, Shafafy R, Maher M, Moon KH, Panchmatia JR. Assessment and management of adult spinal deformities. Br J Hosp Med (Lond). 2018;79(2):79–85. [DOI] [PubMed] [Google Scholar]
- 3. Patwardhan AG, Khayatzadeh S, Havey RM, et al. Cervical sagittal balance: a biomechanical perspective can help clinical practice. Eur Spine J. 2018;27(suppl 1):25–38. [DOI] [PubMed] [Google Scholar]
- 4. Ling FP, Chevillotte T, Leglise A, Thompson W, Bouthors C, Le Huec JC. Which parameters are relevant in sagittal balance analysis of the cervical spine? A literature review. Eur Spine J. 2018;27(Suppl 1):8–15. [DOI] [PubMed] [Google Scholar]
- 5. Celestre PC, Dimar JR, Glassman SD. Spinopelvic parameters: lumbar lordosis, pelvic incidence, pelvic tilt, and sacral slope: what does a spine surgeon need to know to plan a lumbar deformity correction? Neurosurg Clin N Am. 2018;29(3):323–329. [DOI] [PubMed] [Google Scholar]
- 6. Bess S, Protopsaltis TS, Lafage V, et al. International Spine Study Group. Clinical and radiographic evaluation of adult spinal deformity. Clin Spine Surg. 2016;29(1):6–16. [DOI] [PubMed] [Google Scholar]
- 7. Ames CP, Scheer JK, Lafage V, et al. Adult spinal deformity: epidemiology, health impact, evaluation, and management. Spine Deform. 2016;4(4):310–322. [DOI] [PubMed] [Google Scholar]
- 8. Lee SH, Son ES, Seo EM, Suk KS, Kim KT. Factors determining cervical spine sagittal balance in asymptomatic adults: correlation with spinopelvic balance and thoracic inlet alignment. Spine J. 2015;15(4):705–712. [DOI] [PubMed] [Google Scholar]
- 9. Smith JS, Shaffrey CI, Fu KM, et al. Clinical and radiographic evaluation of the adult spinal deformity patient. Neurosurg Clin N Am. 2013;24(2):143–156. [DOI] [PubMed] [Google Scholar]
- 10. Scheer JK, Tang JA, Smith JS, et al. International spine study group. Cervical spine alignment, sagittal deformity, and clinical implications: a review. J Neurosurg Spine. 2013;19(2):141–159. [DOI] [PubMed] [Google Scholar]
- 11. Vrtovec T, Janssen MM, Likar B, Castelein RM, Viergever MA, Pernuš F. Evaluation of pelvic morphology in the sagittal plane. Spine J. 2013;13(11):1500–1509. [DOI] [PubMed] [Google Scholar]
- 12. Lee SH, Kim KT, Seo EM, Suk KS, Kwack YH, Son ES. The influence of thoracic inlet alignment on the craniocervical sagittal balance in asymptomatic adults. J Spinal Disord Tech. 2012;25(2): E41–47. [DOI] [PubMed] [Google Scholar]
- 13. Oakley PA, Ehsani NN, Harrison DE. Repeat radiography in monitoring structural changes in the treatment of spinal disorders in chiropractic and manual medicine practice: evidence and safety. Dose Response. 2019 Dec 6;17(4):1559325819891043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oakley PA, Cuttler JM, Harrison DE. X-ray imaging is essential for contemporary chiropractic and manual therapy spinal rehabilitation: radiography increases benefits and reduces risks. Dose Response. 2018;16(2):1559325818781437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oakley PA, Harrison DE. Radiophobia: 7 reasons why radiography used in spine and posture rehabilitation should not be feared or avoided. Dose Response. 2018;16(2):1559325818781445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oakley PA, Harrison DE. Radiogenic cancer risks from chiropractic X-rays are zero: 10 reasons to take routine radiographs in clinical practice. Ann Vert Sublux Res. 2018;(Mar 10):48–56. https://www.researchgate.net/publication/323687677_Radiogenic_Cancer_Risks_from_Chiropractic_X-rays_are_Zero_10_Reasons_to_Take_Routine_Radiographs_in_Clinical_Practice
- 17. American Chiropractic Association. Five things physicians and patients should question. 2017. Accessed November 2, 2020 http://www.choosingwisely.org/societies/american-chiropractic-association/
- 18. Oakley PA, Harrison DE. Are restrictive medical radiation imaging campaigns misguided? It seems so: a case example of the American Chiropractic Association’s Adoption of “Choosing Wisely.” Dose Response. 2020;18(2):1559325820919321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oakley PA, Harrison DE. American Chiropractic Association’s Participation in Choosing Wisely: close inspection shows no evidence to support its anti-imaging points 1 and 2. A review. Asia-Pac Chiropr J. 2020;1:2: Online only Accessed November 2, 2020 https://apcj.rocketsparkau.com/choosing-wisely-and-the-aca--oakley-and-harrison/ [Google Scholar]
- 20. Jenkins HJ, Downie AS, Moore CS, French SD. Current evidence for spinal X-ray use in the chiropractic profession: a narrative review. Chiropr Man Therap. 2018;26(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor JA, Bussières A. Diagnostic imaging for spinal disorders in the elderly: a narrative review. Chiropr Man Therap. 2012;20(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bussières AE, Taylor JA, Peterson C. Diagnostic imaging practice guidelines for musculoskeletal complaints in adults—an evidence-based approach—part 3: spinal disorders. J Manipulative Physiol Ther. 2008(1):33–88. [DOI] [PubMed] [Google Scholar]
- 23. Kawchuk G, Goertz C, Axén I, et al. X-ray imaging is essential for contemporary chiropractic and manual therapy spinal rehabilitation: radiography increases benefits and reduces risks. Dose Response. 2018;16(2):1559325818811521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bussières AE, Ammendolia C, Peterson C, Taylor JA. Ionizing radiation exposure—more good than harm? The preponderance of evidence does not support abandoning current standards and regulations. J Can Chiropr Assoc. 2006;50(2):103–106. [PMC free article] [PubMed] [Google Scholar]
- 25. Oakley PA, Harrison DE. Letter-to-the-editor regarding Taylor S, Bishop A. Patient and public beliefs about the role of imaging in the management of non-specific low back pain: a scoping review. Physiotherapy. 2020;107:224–233. [DOI] [PubMed] [Google Scholar]
- 26. Oakley PA, Harrison DE. Selective usage of medical practice data, misrepresentations, and omission of conflicting data to support the ‘red flag only’ agenda for chiropractic radiography guidelines: a critical review of the Jenkins et al. article: “current evidence for spinal X-ray use in the chiropractic profession. Ann Vert Sublux Res. 2019;2019(1):141–157. Accessed November 2, 2020. https://www.vertebralsubluxationresearch.com/2019/10/07/selective-usage-of-medical-practice-data-misrepresentations-and-omission-of-conflicting-data-to-support-the-red-flag-only-agenda-for-chiropractic-radiography-guidelines-a-critical-review-of-the/ [Google Scholar]
- 27. Oakley PA, Cuttler JM, Harrison DE. X-ray imaging is essential for contemporary chiropractic and manual therapy spinal rehabilitation: radiography increases benefits and reduces risks. Dose Response. 2018;16(4):1559325818809584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oakley PA, Harrison DD, Harrison DE, Haas JW. A rebuttal to chiropractic radiologists’ view of the 50-year-old, linear-no-threshold radiation risk model. J Can Chiropr Assoc. 2006;50(3):172–181. [PMC free article] [PubMed] [Google Scholar]
- 29. Oakley PA, Harrison DD, Harrison DE, Haas JW. Evidence-based protocol for structural rehabilitation of the spine and posture: review of clinical biomechanics of posture (CBP) publications. J Can Chiropr Assoc. 2005;49(4):270–296. [PMC free article] [PubMed] [Google Scholar]
- 30. Oakley PA, Harrison DD, Harrison DE, Haas JW. On “phantom risks” associated with diagnostic ionizing radiation: evidence in support of revising radiography standards and regulations in chiropractic. J Can Chiropr Assoc. 2005;49(4):264–269. [PMC free article] [PubMed] [Google Scholar]
- 31. Practicing Chiropractors Committee on Radiology Protocols (PCCRP). Published 2009 Accessed November 2, 2020. http://www.chiropractic.org/wp-content/uploads/2018/01/PCCRP-Radiology-Guidelines.pdf
- 32. ACR American College of Radiology. ACR–ASSR–SPR–SSR practice parameter for the performance of spine radiography. Revised 2017 Accessed November 2, 2020 https://www.acr.org/-/media/ACR/Files/Practice-Parameters/Rad-Spine.pdf2
- 33. ICA Committee on Chiropractic Practice Guidelines and Protocols. Diagnostic imaging In: ICA. Recommended Clinical Protocols and Guidelines for the Practice of Chiropractic. Arlington, VA; 2000: 283–314. Chap. 15 Accessed November 2, 2020 https://registerchiropractor.nl/ICA_guidlines.pdf. [Google Scholar]
- 34. Kent C. An evidence-informed approach to spinal radiography in vertebral subluxation centered chiropractic practice. Ann Vert Sublux Res. 2017:142–146. Accessed November 2, 2020 https://www.vertebralsubluxationresearch.com/2017/08/31/an-evidence-informed-approach-to-spinal-radiography-in-vertebral-subluxation-centered-chiropractic-practice/
- 35. Sherman R. Chiropractic x-ray rationale. JCCA. 1986;1(3):33–35. [Google Scholar]
- 36. Oakley PA, Harrison DE. X-ray hesitancy: patients’ radiophobic concerns over medical X-rays. Dose Response. 2020;18(3):1559325820959542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oakley PA, Harrison DE. Death of the ALARA radiation protection principle as used in the medical sector. Dose Response. 2020;18(2):1559325820921641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oakley PA, Navid Ehsani N, Harrison DE 5 Reasons why scoliosis X-rays are not harmful. Dose Response. 2020;18(3):1559325820957797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oakley PA, Ehsani NN, Harrison DE. The scoliosis quandary: are radiation exposures from repeated X-rays harmful? Dose Response. 2019;17(2):1559325819852810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siegel JA, Sacks B. Eliminating use of the linear no-threshold assumption in medical imaging. J Nucl Med. 2017;58(6):1014–1015. [DOI] [PubMed] [Google Scholar]
- 41. Siegel JA, Pennington CW, Sacks B. Subjecting radiologic imaging to the linear no-threshold hypothesis: a non sequitur of non-trivial proportion. J Nucl Med. 2017;58(1):1–6. [DOI] [PubMed] [Google Scholar]
- 42. Siegel JA, McCollough CH, Orton CG. Advocating for use of the ALARA principle in the context of medical imaging fails to recognize that the risk is hypothetical and so serves to reinforce patients’ fears of radiation. Med Phys. 2017;44(1):3–6. [DOI] [PubMed] [Google Scholar]
- 43. Siegel JA, Sacks B, Pennington CW, et al. Dose optimization to minimize radiation risk for children undergoing CT and nuclear medicine imaging is misguided and detrimental. J Nucl Med. 2017;58(6):865–868. [DOI] [PubMed] [Google Scholar]
- 44. Cohen MD. Point: should the ALARA concept and image gently campaign be terminated? J Am Coll Radiol. 2016;13(10):1195–1198. [DOI] [PubMed] [Google Scholar]
- 45. Sacks B, Meyerson G, Siegel JA. Epidemiology without biology: false paradigms, unfounded assumptions, and specious statistics in radiation science. Biol Theory. 2016;11(3):69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ulsh BA. Are risks from medical imaging still too small to be observed or nonexistent? Dose Response. 2015;13(1).pii dose-response.14-030.Ulsh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scott BR, Sanders CL, Mitchel REJ, Boreham DR. CT scans may reduce rather than increase the risk of cancer. J Am Phys Surg. 2008;13(1):8–11. [Google Scholar]
- 48. Scott BR, Di Palma J. Sparsely ionizing diagnostic and natural background radiations are likely preventing cancer and other genomic-instability-associated diseases. Dose Response. 2006;5(3):230–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bussières AE, Stewart G, Al-Zoubi F, et al. Spinal manipulative therapy and other conservative treatments for low back pain: a guideline from the Canadian Chiropractic Guideline initiative. J Manipulative Physiol Ther. 2018;41(4):265–293. [DOI] [PubMed] [Google Scholar]
- 50. Lisi AJ, Salsbury SA, Hawk C, et al. Chiropractic integrated care pathway for low back pain in veterans: results of a Delphi consensus process. J Manipulative Physiol Ther. 2018;41(2):137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hawk C, Schneider M, Ferrance RJ, Hewitt E, Van Loon M, Tanis L. Best practices recommendations for chiropractic care for infants, children, and adolescents: results of a consensus process. J Manipulative Physiol Ther. 2009;32(8):639–647. [DOI] [PubMed] [Google Scholar]
- 52. Corso M, Cancelliere C, Mior S, Kumar V, Smith A, Côté P. The clinical utility of routine spinal radiographs by chiropractors: a rapid review of the literature. Chiropr Man Therap. 2020;28(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shortz SK, Haas M. Relationship between radiographic lumbosacral spine mensuration and chronic low back pain intensity: a cross-sectional study. J Chiropr Med. 2018 Mar;17(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Szczygieł E, Fudacz N, Golec J, Golec E. The impact of the position of the head on the functioning of the human body: a systematic review. Int J Occup Med Environ Health. 2020;33(5):559–568. [DOI] [PubMed] [Google Scholar]
- 55. Elizagaray-Garcia I, Beltran-Alacreu H, Angulo-Díaz S, Garrigós-Pedrón M, Gil-Martínez A. Chronic primary headache subjects have greater forward head posture than asymptomatic and episodic primary headache sufferers: systematic review and meta-analysis. Pain Med. 2020;21(10):2465–2480. [DOI] [PubMed] [Google Scholar]
- 56. Mahmoud NF, Hassan KA, Abdelmajeed SF, Moustafa IM, Silva AG. The relationship between forward head posture and neck pain: a systematic review and meta-analysis. Curr Rev Musculoskelet Med. 2019;12(4):562–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sadler SG, Spink MJ, Ho A, De Jonge XJ, Chuter VH. Restriction in lateral bending range of motion, lumbar lordosis, and hamstring flexibility predicts the development of low back pain: a systematic review of prospective cohort studies. BMC Musculoskelet Disord. 2017;18(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chun SW, Lim CY, Kim K, Hwang J, Chung SG. The relationships between low back pain and lumbar lordosis: a systematic review and meta-analysis. Spine J. 2017;17(8):1180–1191. [DOI] [PubMed] [Google Scholar]
- 59. Raastad J, Reiman M, Coeytaux R, Ledbetter L, Goode AP. The association between lumbar spine radiographic features and low back pain: a systematic review and meta-analysis. Semin Arthritis Rheum. 2015;44(5):571–585. [DOI] [PubMed] [Google Scholar]
- 60. Diebo BG, Shah NV, Boachie-Adjei O, Zhu F, Rothenfluh DA, Paulino CB, Schwab FJ, Lafage V. Adult spinal deformity. Lancet. 2019;394(10193):160–172. [DOI] [PubMed] [Google Scholar]
- 61. Smith JS, Shaffrey CI, Bess S, et al. Recent and emerging advances in spinal deformity. Neurosurgery. 2017;80(3 S): S70–S85. [DOI] [PubMed] [Google Scholar]
- 62. Smith JS, Shaffrey CI, Fu KM, et al. Clinical and radiographic evaluation of the adult spinal deformity patient. Neurosurg Clin N Am. 2013;24(2):143–156. [DOI] [PubMed] [Google Scholar]
- 63. Hildebrandt RW. Chiropractic Spinography. Williams & Wilkins, 1985: 78. [Google Scholar]
- 64. Pellisé F, Vila-Casademunt A, Ferrer M, et al. European Spine Study Group, ESSG. Impact on health related quality of life of adult spinal deformity (ASD) compared with other chronic conditions. Eur Spine J. 2015 Jan;24(1):3–11. [DOI] [PubMed] [Google Scholar]
- 65. Bess S, Line B, Fu KM, et al. International Spine Study Group. The health impact of symptomatic adult spinal deformity: comparison of deformity types to United States population norms and chronic diseases. Spine. 2016;41(3):224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Anderson JM, Oakley PA, Harrison DE. Improving posture to reduce the symptoms of Parkinson’s: a CBP® case report with a 21 month follow-up. J Phys Ther Sci. 2019 Feb;31(2):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Haas JW, Oakley PA, Harrison DE. Non-surgical reduction in anterior sagittal balance subluxation and improvement in overall posture in a geriatric suffering from low back pain and sciatica: a CBP® case report. J Contemporary Chiro. 2020;3(1):45–50. https://journal.parker.edu/index.php/jcc/article/view/101 [Google Scholar]
- 68. Oakley PA, Jaeger JO, Brown JE, Polatis TA, Clarke JG, Whittler CD, Harrison DE. The CBP® mirror image® approach to reducing thoracic hyperkyphosis: a retrospective case series of 10 patients. J Phys Ther Sci. 2018;30(8):1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fedorchuk C, Snow E. Increased lung function & quality of life in asymptomatic subjects following reduction in thoracic hyperkyphosis & vertebral subluxation utilizing Chiropractic Biophysics: A case series. Ann Vert Sublux Res. 2017;(Oct 12):189–200. https://www.vertebralsubluxationresearch.com/2017/10/12/increased-lung-function-quality-of-life-in-asymptomatic-subjects-following-reduction-in-thoracic-hyperkyphosis-vertebral-subluxation-utilizing-chiropractic-biophysics-a-case-series/ [Google Scholar]
- 70. Fortner MO, Oakley PA, Harrison DE. Treating ‘slouchy’ (hyperkyphosis) posture with chiropractic biophysics®: a case report utilizing a multimodal mirror image® rehabilitation program. J Phys Ther Sci. 2017;29(8):1475–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Harrison DE, Harrison DD, Cailliet R, Janik TJ, Holland B. Changes in sagittal lumbar configuration with a new method of extension traction: non-randomized clinical control trial. Arch Phys Med Rehab. 2002;83(11):1585–1591. [DOI] [PubMed] [Google Scholar]
- 72. Diab AAM, Moustafa IM. The efficacy of lumbar extension traction for sagittal alignment in mechanical low back pain: a randomized trial. J Back Musculoskelet Rehabil. 2013;26(2):213–220. [DOI] [PubMed] [Google Scholar]
- 73. Moustafa IM, Diab AA. Extension traction treatment for patients with discogenic lumbosacral radiculopathy: a randomized controlled trial. Clin Rehab. 2012;27(1):51–62. [DOI] [PubMed] [Google Scholar]
- 74. Diab AA, Moustafa IM. Lumbar lordosis rehabilitation for pain and lumbar segmental motion in chronic mechanical low back pain. J Manip Physiol Ther. 2012;35(4):246–253. [DOI] [PubMed] [Google Scholar]
- 75. Oakley PA, Ehsani NN, Moustafa IM, Harrison DE. Restoring lumbar lordosis: a systematic review of controlled trials utilizing Chiropractic Bio Physics® (CBP®) non-surgical approach to increasing lumbar lordosis in the treatment of low back disorders. J Phys Ther Sci. 2020. September;32(9):601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chu ECP, Chakkaravarthy DM, Huang K, Ho VWK, Lo FS, Bhaumik A. Changes in radiographic parameters following chiropractic treatment in 10 patients with adolescent idiopathic scoliosis: a retrospective chart review. Clin Pract. 2020; 10(3):1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Morningstar M, Oslin D. Chiropractic rehabilitation plus nighttime bracing for progressive adolescent idiopathic scoliosis: a case-controlled series. Clin Pract. 2020;9(4):1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Haggard JS, Haggard JB, Oakley PA, Harrison DE. Reduction of progressive thoracolumbar adolescent idiopathic scoliosis by chiropractic biophysics® (CBP®) mirror image® methods following failed traditional chiropractic treatment: a case report. J Phys Ther Sci. 2017;29(11):2062–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Harrison DE, Oakley PA. Scoliosis deformity reduction in adults: a CBP® Mirror Image® case series incorporating the ‘non-commutative property of finite rotation angles under addition’ in five patients with lumbar and thoraco-lumbar scoliosis. J Phys Ther Sci. 2017;29(11):2044–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Morningstar MW, Dovorany B, Stitzel CJ, Siddiqui A. Chiropractic rehabilitation for adolescent idiopathic scoliosis: end-of-growth and skeletal maturity results. Clin Pract. 2017;7(1):911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Weiner MT, Oakley PA, Dennis AK, Shapiro DA, Harrison DE. Increasing the cervical and lumbar lordosis is possible despite overt osteoarthritis and spinal stenosis using extension traction to relieve low back and leg pain in a 66-year-old surgical candidate: a CBP® case report. J Phys Ther Sci. 2018;30(11):1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Harrison DE, Oakley PA. Non-operative correction of flat back syndrome using lumbar extension traction: a CBP® case series of two. J Phys Ther Sci. 2018 Aug;30(8):1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ferrantelli JR, Harrison DE, Harrison DD, Stewart D. Conservative treatment of a patient with previously unresponsive whiplash-associated disorders using clinical biomechanics of posture rehabilitation methods. J Manipulative Physiol Ther. 2005;28(3):e1–8. [DOI] [PubMed] [Google Scholar]
- 84. Harrison DE, Oakley PA, Betz JW. Anterior head translation following cervical fusion—a probable cause of post-surgical pain and impairment: a CBP® case report. J Phys Ther Sci. 2018;30(2):271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Oakley PA, Harrison DE. Alleviation of pain and disability in a post-surgical C4-C7 total fusion patient after reducing a lateral head translation (side shift) posture: a CBP® case report with a 14 year follow-up. J Phys Ther Sci. 2018;30(7):952–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fedorchuk C, Lightstone DF, Andino H. Failed neck surgery: improvement in neck pain, migraines, energy levels, and performance of activities of daily living following subluxation correction using chiropractic BioPhysics® technique: a case study. Ann Vert Sublux Res. 2017;(May 18):93–100. https://www.vertebralsubluxationresearch.com/2017/01/01/failed-neck-surgery-improvement-in-neck-pain-migraines-energy-levels-and-performance-of-activities-of-daily-living-following-subluxation-correction-using-chiropractic-biophysics-technique-a/ [Google Scholar]
- 87. Oakley PA, Berry RH, Harrison DE. A structural approach to the postsurgical laminectomy case. J Vert Sublux Res. 2007;(March 19);1–7. https://www.researchgate.net/publication/237793785_A_Structural_Approach_to_Post-Surgical_Laminectomy_A_Case_Study [Google Scholar]
- 88. Roghani T, Zavieh MK, Manshadi FD, King N, Katzman W. Age-related hyperkyphosis: update of its potential causes and clinical impacts-narrative review. Aging Clin Exp Res. 2017;29(4):567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ailon T, Shaffrey CI, Lenke LG, et al. Progressive spinal kyphosis in the aging population. Neurosurgery. 2015;77(4): S164–S172. [DOI] [PubMed] [Google Scholar]
- 90. Harrison DD, Janik TJ, Harrison GR, Troyanovich S, Harrison DE, Harrison SO. Chiropractic biophysics technique: a linear algebra approach to posture in chiropractic. J Manipulative Physiol Ther. 1996;19(8):525–535. [PubMed] [Google Scholar]
- 91. Harrison DE, Oakley PA. Necessity for biomechanical evaluation of posture, alignment and subluxation. Part I: the 6 subluxation types that satisfy Nelson’s criteria for valid subluxation theory. J Contemp Chiropr. 2018;1(1):9–19. https://journal.parker.edu/index.php/jcc/article/view/16 [Google Scholar] [Google Scholar]
- 92. Jenkins H, Zheng X, Bull PW. Prevalence of congenital anomalies contraindicating spinal manipulative therapy within a chiropractic patient population. Chiro J Australia. 2010;40(2):69–76. [Google Scholar]
- 93. Young KJ, Aziz A. An accounting of pathology visible on lumbar spine radiographs of patients attending private chiropractic clinics in the United Kingdom. Chiro J Australia. 2009;39(2):63–69. [Google Scholar]
- 94. Pryor M, McCoy M. Radiographic findings that may alter treatment identified on radiographs of patients receiving chiropractic care in a teaching clinic. J Chiropractic Education. 2006;20(1):93–94. [Google Scholar]
- 95. Beck RW, Holt KR, Fox MA, Hurtgen-Grace KL. Radiographic anomalies that may alter chiropractic intervention strategies found in a New Zealand population. J Manipulative Physiol Ther. 2004;27(9):554–559. [DOI] [PubMed] [Google Scholar]
- 96. Bull PW. Relative and Absolute Contraindications to Spinal Manipulative Therapy Found on Spinal X-Rays In: Proceedings of the World Federation of Chiropractic 7th Biennial Congress. Orlando, FL, May 2003, p. 376. [Google Scholar]
- 97. Hurwitz EL, Morgenstern H, Vassilaki M, Chiang LM. Frequency and clinical predictors of adverse reactions to chiropractic care in the UCLA neck pain study. Spine. 2005;30(13):1477–1484. [DOI] [PubMed] [Google Scholar]
- 98. Swait G, Finch R. What are the risks of manual treatment of the spine? A scoping review for clinicians. Chiropr Man Therap. 2017;25(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hald HJ, Danz B, Schwab R, Burmeister K, Bähren W. Röntgenologisch nachweisbare Wirbelsäulenveränderungen asymptomatischer junger Männer [Radiographically demonstrable spinal changes in asymptomatic young men]. Rofo. 1995;163(1):4–8. [DOI] [PubMed] [Google Scholar]
- 100. Andersen HT, Wagstaff AS, Sverdrup HU. Spinal X-ray screening of high performance fighter pilots. Aviat Space Environ Med. 1991;62(12):1171–1173. [PubMed] [Google Scholar]
- 101. Parreira PCS, Maher CG, Traeger AC, et al. Evaluation of guideline-endorsed red flags to screen for fracture in patients presenting with low back pain. Br J Sports Med. 2019;53(10):648–654. [DOI] [PubMed] [Google Scholar]
- 102. Premkumar A, Godfrey W, Gottschalk MB, Boden SD. Red flags for low back pain are not always really red: a prospective evaluation of the clinical utility of commonly used screening questions for low back pain. J Bone Joint Surg Am. 2018;100(5):368–374. [DOI] [PubMed] [Google Scholar]
- 103. Verhagen AP, Downie A, Maher CG, Koes BW. Most red flags for malignancy in low back pain guidelines lack empirical support: a systematic review. Pain. 2017;158(10):1860–1868. [DOI] [PubMed] [Google Scholar]
- 104. Verhagen AP, Downie A, Popal N, Maher C, Koes BW. Red flags presented in current low back pain guidelines: a review. Eur Spine J. 2016;25(9):2788–2802. [DOI] [PubMed] [Google Scholar]
- 105. Williams CM, Henschke N, Maher CG, et al. Red flags to screen for vertebral fracture in patients presenting with low-back pain. Cochrane Database Syst Rev. 2013;(1):CD008643. [DOI] [PubMed] [Google Scholar]
- 106. Henschke N, Maher CG, Ostelo RW, de Vet HC, Macaskill P, Irwig L. Red flags to screen for malignancy in patients with low-back pain. Cochrane Database Syst Rev. 2013;(2):CD008686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Downie A, Williams CM, Henschke N, et al. Red flags to screen for malignancy and fracture in patients with low back pain: systematic review. BMJ. 2013;347:f7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Giles L. Prevalence of congenital anomalies contraindicating spinal manipulation therapy within a chiropractic patient population. Chiro J Australia. 2010;40(3):130. [Google Scholar]
- 109. Moustafa IM, Diab AA, Hegazy F, Harrison DE. Does improvement towards a normal cervical sagittal configuration aid in the management of cervical myofascial pain syndrome: a 1-year randomized controlled trial. BMC Musculoskelet Disord. 2018;19(1):396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Moustafa IM, Diab AA, Harrison DE. The effect of normalizing the sagittal cervical configuration on dizziness, neck pain, and cervicocephalic kinesthetic sensibility: a 1-year randomized controlled study. Eur J Phys Rehabil Med. 2017;53(1):57–71. [DOI] [PubMed] [Google Scholar]
- 111. Moustafa IM, Diab AAM, Hegazy FA, et al. Does rehabilitation of cervical lordosis influence sagittal cervical spine flexion extension kinematics in cervical spondylotic radiculopathy subjects? J Back Musculoskelet Rehabil. 2017;30(4):937–941. [DOI] [PubMed] [Google Scholar]
- 112. Moustafa IM, Diab AAM, Taha S, Harrison DE. Demonstration of central conduction time and neuroplastic changes after cervical lordosis rehabilitation in asymptomatic subjects: a randomized, placebo-controlled trial In: Proceedings of the 14th Biennial Congress of the World Federation of Chiropractic. March 15-18, 2017. [Google Scholar]
- 113. Moustafa IM, Diab AA, Taha S, et al. Addition of a sagittal cervical posture corrective orthotic device to a multi-modal rehabilitation program improves short- and long-term outcomes in patients with discogenic cervical radiculopathy. Arch Phys Med Rehabil. 2016;97(12):2034–2044. [DOI] [PubMed] [Google Scholar]
- 114. Moustafa IM, Diab AA, Harrison DE. Does improvement towards a normal cervical sagittal configuration aid in the management of lumbosacral radiculopathy: a randomized controlled trial In: Proceedings of the 13th World Federation of Chiropractic Biennial Congress/ECU Convention. Athens, Greece, May 13-16, 2015; Paper #184 Mediterranean Region Award Winning Paper. [Google Scholar]
- 115. Moustafa IM. Does improvement towards a normal cervical configuration aid in the management of fibromyalgia. A randomized controlled trial. Bull Fac Ph Th Cairo Univ. 2013;18(2):29–41. [Google Scholar]
- 116. Moustafa IM, Diab AM, Ahmed A, Harrison DE. The efficacy of cervical lordosis rehabilitation for nerve root function, pain, and segmental motion in cervical spondylotic radiculopathy. PhysioTherapy. 2011;97:846–847. [Google Scholar]
- 117. Heo SJ, Park SH, Jeong HS, Kim SY. The functional and morphological changes of the cervical intervertebral disc after applying lordotic curve controlled traction: a double-blind randomized controlled study. Int J Environ Res Public Health. 2019;16(12):2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Moustafa IM, Walton LM, Raigangir V, Shousha TM, Harrison D. Reduction of posture hyperkyphosis improves short- and long-term outcomes in patients with neck pain. J Orthop Sports Phys Ther. 2020;50(1):CSM143 Abstract. [Google Scholar]
- 119. Jang HJ, Hughes LC, Oh DW, Kim SY. Effects of corrective exercise for thoracic hyperkyphosis on posture, balance, and well-being in older women: a double-blind, group-matched design. J Geriatr Phys Ther. 2019;42(3):E17–27. doi:10.1519/JPT.0000000000000146 [DOI] [PubMed] [Google Scholar]
- 120. Katzman WB, Vittinghoff E, Lin F, et al. Targeted spine strengthening exercise and posture training program to reduce hyperkyphosis in older adults: results from the study of hyperkyphosis, exercise, and function (SHEAF) randomized controlled trial. Osteoporos Int. 2017;28(10):2831–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kamali F, Shirazi SA, Ebrahimi S, et al. Comparison of manual therapy and exercise therapy for postural hyperkyphosis: a randomized clinical trial. Physiother Theory Pract. 2016;32:92–97. [DOI] [PubMed] [Google Scholar]
- 122. Schreiber S, Parent EC, Hill DL, Hedden DM, Moreau MJ, Southon SC. Schroth physiotherapeutic scoliosis-specific exercises for adolescent idiopathic scoliosis: how many patients require treatment to prevent one deterioration? Results from a randomized controlled trial—“SOSORT 2017 Award Winner”. Scoliosis Spinal Disord. 2017;12(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kuru T, Yeldan İ, Dereli EE, Özdinçler AR, Dikici F, Çolak İ. The efficacy of three-dimensional Schroth exercises in adolescent idiopathic scoliosis: a randomised controlled clinical trial. Clin Rehabil. 2016;30(2):181–190. [DOI] [PubMed] [Google Scholar]
- 124. Schreiber S, Parent EC, Moez EK, et al. The effect of SCHROTH exercises added to the standard of care on the quality of life and muscle endurance in adolescents with idiopathic scoliosis—an assessor and statistician blinded randomized controlled trial: “SOSORT 2015 Award Winner”. Scoliosis. 2015;10(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Noh DK, You JS, Koh JH, et al. Effects of novel corrective spinal technique on adolescent idiopathic scoliosis as assessed by radio-graphic imaging. J Back Musculoskelet Rehabil. 2014;27(3):331–338. [DOI] [PubMed] [Google Scholar]
- 126. Nuckols TK, Lim YW, Wynn BO, et al. Rigorous development does not ensure that guidelines are acceptable to a panel of knowledgeable providers. J Gen Intern Med. 2008;23(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rome P., Waterhouse JD. An evidence-based narrative of the evidence-base concept. Asia-Pacific Chiropr J. 2020;1:004 10.46323/2021004 [DOI] [Google Scholar]
- 128. Ebrall P, Doyle M. The value of case reports as clinical evidence. Chiropr J Australia. 2020;47(1):29–43. www.cjaonline.com.au/index.php/cja/issue/view/15 [Google Scholar]
- 129. Anderson JM, Oakley PA, Harrison DE. Improving posture to reduce the symptoms of Parkinson’s: a CBP® case report with a 21 month follow-up. J Phys Ther Sci. 2019;31(2):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Haas JW, Harrison DE, Oakley PA. Non-surgical reduction in anterior sagittal balance subluxation and improvement in overall posture in a geriatric suffering from low back pain and sciatica: a CBP® case report. J Contemp Chiropr. 2020;3(1):45–50. https://journal.parker.edu/index.php/jcc/article/view/101 [Google Scholar]
- 131. Harrison DE, Cailliet R, Betz J, et al. Conservative methods for reducing lateral translation postures of the head: a nonrandomized clinical control trial. J Rehabil Res Dev. 2004;41(4):631–639. [DOI] [PubMed] [Google Scholar]
- 132. Jaeger JO, Oakley PA, Moore RR, Ruggeroli EP, Harrison DE. Resolution of temporomandibular joint dysfunction (TMJD) by correcting a lateral head translation posture following previous failed traditional chiropractic therapy: a CBP® case report. J Phys Ther Sci. 2018;30(1):103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Haas JW, Oakley PA, Harrison DE. Cervical pseudo-scoliosis reduction and alleviation of dystonia symptoms using Chiropractic BioPhysics® (CBP®) technique: a case report with a 1.5-year follow-up. J Contemp Chiropr. 2019; 2:131–137. [Google Scholar]
- 134. Harrison DE, Cailliet R, Betz JW, et al. Harrison mirror image methods for correcting trunk list: a non-randomized clinical control trial. Eur Spine J. 2005;14(2):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Henshaw M, Oakley PA, Harrison DE. Correction of pseudoscoliosis (lateral thoracic translation posture) for the treatment of low back pain: a CBP® case report. J Phys Ther Sci. 2018;30(9):1202–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Fortner MO, Oakley PA, Harrison DE. Chiropractic biophysics management of straight back syndrome and exertional dyspnea: a case report with follow-up. J Contemp Chiropr. 2019;2:115–122. https://journal.parker.edu/index.php/jcc/issue/view/2 [Google Scholar]
- 137. Betz JW, Oakley PA, Harrison DE. Relief of exertional dyspnea and spinal pains by increasing the thoracic kyphosis in straight back syndrome (thoracic hypo-kyphosis) using CBP® methods: a case report with long-term follow-up. J Phys Ther Sci. 2018;30(1):185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mitchell JR, Oakley PA, Harrison DE. Nonsurgical correction of straight back syndrome (thoracic hypokyphosis), increased lung capacity and resolution of exertional dyspnea by thoracic hyperkyphosis mirror image® traction: a CBP® case report. J Phys Ther Sci. 2017;29(11):2058–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gubbels CM, Werner JT, Oakley PA, Harrison DE. Reduction of thoraco-lumbar junctional kyphosis, posterior sagittal balance, and increase of lumbar lordosis and sacral inclination by Chiropractic BioPhysics® methods in an adolescent with back pain: a case report. J Phys Ther Sci. 2019 Oct;31(10):839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Oakley PA, Ehsani NN, Harrison DE. Non-surgical reduction of lumbar hyperlordosis, forward sagittal balance and sacral tilt to relieve low back pain by Chiropractic BioPhysics® methods: a case report. J Phys Ther Sci. 2019;31(10):860–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Fedorchuk C, Lightstone DF, DeVon Comer R, Katz E, Wilcox J. Improvements in cervical spinal canal diameter and neck disability following correction of cervical lordosis and cervical spondylolistheses using chiropractic BioPhysics technique: a case series. J Radiol Case Rep. 2020;14(4):21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Fedorchuk C, Lightstone D. Reduction in cervical anterolisthesis & pain in a 52-year-old female using chiropractic biophysics® technique: a case study and selective review of literature. Ann Vert Sublux Res. 2016;2016(3):118–124. https://www.chiroindex.org/?search_page=articles&action&articleId=24805 [Google Scholar]
- 143. Fedorchuk C, Lightstone DF, McRae C, Kaczor D. Correction of grade 2 spondylolisthesis following a non-surgical structural spinal rehabilitation protocol using lumbar traction: a case study and selective review of literature. J Radiol Case Rep. 2017;11(5):13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Fedorchuk CA, Lightstone DF, Oakley PA, Harrison DE. Correction of a double spondylolisthesis of the lumbar spine utilizing chiropractic biophysics® technique: a case report with 1-year follow-up. J Phys Ther Sci. 2021;33(1):89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Persinger MA. Human library project: Dr. Michael Persinger, Sudbury. 6:16 minute recording on Canadian broadcast corporation website. 2012. Accessed November 2, 2020 https://www.cbc.ca/player/play/2324819676
- 146. National Research Council of the National Academies. Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIRVII Phase 2. The National Academies Press; 2006. [Google Scholar]
- 147. Siegel JA, Greenspan BS, Maurer AH, et al. The BEIR VII estimates of low-dose radiation health risks are based on faulty assumptions and data analyses: a call for reassessment. J Nucl Med. 2018;59(7):1017–1019. [DOI] [PubMed] [Google Scholar]
- 148. O’Connor MK. Risk of low-dose radiation and the BEIR VII report: a critical review of what it does and doesn’t say. Phys Med. 2017;43:153–158. [DOI] [PubMed] [Google Scholar]
- 149. Calabrese EJ, O’Connor MK. Estimating risk of low radiation doses—a critical review of the BEIR VII report and its use of the linear no-threshold (LNT) hypothesis. Radiat Res. 2014;182(5):463–474. [DOI] [PubMed] [Google Scholar]
- 150. Ozasa K, Shimizu Y, Suyama A, et al. Studies of the mortality of atomic bomb survivors, report 14, 1950-2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177(6):229–243. [DOI] [PubMed] [Google Scholar]
- 151. Doss M. Evidence supporting radiation hormesis in atomic bomb survivor cancer mortality data. Dose Response. 2012;10:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Doss M. Linear no-threshold model vs radiation hormesis. Dose Response. 2013;11:480–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Sutou S. Low-dose radiation from A-bombs elongated lifespan and reduced cancer mortality relative to un-irradiated individuals. Genes Environ. 2018;40:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sutou S. Black rain in Hiroshima: a critique to the life span study of a-bomb survivors, basis of the linear no-threshold model. Genes Environ. 2020;42:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Socol Y, Dobrzynski L. Atomic bomb survivors life-span study: insufficient statistical power to select radiation carcinogenesis model. Dose Response. 2015;13(1):pii: dose-response.14-034.Socol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Calabrese EJ. On the origins of the linear no-threshold (LNT) dogma by means of untruths, artful dodges and blind faith. Environ Res. 2015;142:432–442. [DOI] [PubMed] [Google Scholar]
- 157. Calabrese EJ. LNTgate: how scientific misconduct by the U.S. NAS led to governments adopting LNT for cancer risk assessment. Environ Res. 2016;148:535–546. [DOI] [PubMed] [Google Scholar]
- 158. Calabrese EJ. Was Muller’s 1946 Nobel Prize research for radiation-induced gene mutations peer-reviewed? Philos Ethics Humanit Med. 2018;13(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Calabrese EJ. Muller’s Nobel Prize Lecture: when ideology prevailed over science. Toxicol Sci. 2012;126(1):1–4. [DOI] [PubMed] [Google Scholar]
- 160. Muller HJ. Artificial transmutation of the gene. Science. 1927; 66(1699):84–87. [DOI] [PubMed] [Google Scholar]
- 161. Jaworowski Z. Radiation hormesis—a remedy for fear. Human Exper Toxicol. 2010;29(4):263–270. [DOI] [PubMed] [Google Scholar]
- 162. Vaiserman A, Koliada A, Zabuga O, Socol Y. Health impacts of low-dose ionizing radiation: current scientific debates and regulatory issues. Dose Response. 2018;16(3):doi:10.117/1559325818796331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Siegel JA, Brooks AL, Fisher DR, et al. A critical assessment of the linear no-threshold hypothesis: its validity and applicability for use in risk assessment and radiation protection. Clin Nucl Med. 2019;44(7):521–525. [DOI] [PubMed] [Google Scholar]
- 164. Cuttler JM. The LNT issue is about politics and economics, not safety. Dose Response. 2020;18(3). doi:10.117/1559325820949066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Cuttler JM. Remedy for radiation fear—discard the politicized science. Dose Response. 2014;12(2):170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Cara YK, Michael D, Joseph MK. Replacing LNT: the integrated LNT-hormesis model. Dose Response. 2020;18(2). doi:10.117/1559325820913788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Sohrabi M. Universal radiation protection system (URPS); a natural global standardised trend for human exposure control in 21st century. Radiat Prot Dosimetry. 2019;184(3-4):277–284. [DOI] [PubMed] [Google Scholar]
- 168. Doss M. Future of radiation protection regulations. Health Phys. 2016;110(3):274–275. [DOI] [PubMed] [Google Scholar]
- 169. Calabrese EJ. Model uncertainty via the integration of hormesis and LNT as the default in cancer risk assessment. Dose Response. 2015;13(4). doi:10.117/1559325815621764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Siegel JA, Sacks B, Welsh JS. Time to terminate LNT: radiation regulators should adopt LT. J Radiol Oncol. 2017;1(5):49–53. [Google Scholar]
- 171. Siegel JA, Sacks B, Welsh JS. Time to eliminate LNT: The NRC needs to adopt LT and eliminate ALARA. Nucl Med Biomed Imag. 2017;2(3):1–5. [Google Scholar]
- 172. Cardarelli JJ, Ulsh BA. It is time to move beyond the linear no-threshold theory for low-dose radiation protection. Dose Response. 2018;16(3). doi:10.117/1559325818779651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Doss M. Should the ALARA concept and the image gently campaign be terminated? Paper presented at the International Pediatric Radiology; Chicago, IL, May 17, 2016. Accessed March 25, 2020 http://www.pedrad.org/LinkClick.aspx?fileticket.3EHiVxngKs%3d&portalid.5 [Google Scholar]
- 174. Siegel JA, Welsh JS. Does imaging technology cause cancer? Debunking the linear no-threshold model of radiation carcinogenesis. Technol Cancer Res Treat. 2016;15(2):249–256. [DOI] [PubMed] [Google Scholar]
- 175. Cohen MD. Reply to Dr. Andronikou: disavowing the ALARA concept in pediatric imaging. Pediatr Radiol. 2017;47(1):116–117. [DOI] [PubMed] [Google Scholar]
- 176. Image Gently Alliance. Alliance for radiation safety in pediatric imaging image gently. Published 2007 Accessed November 2, 2020. https://www.imagegently.org/About-Us/The-Alliance
- 177. Joint Task Force on Adult Radiation Protection. The Imaging Wisely Campaign. Published 2009 Accessed November 2, 2020 https://www.imagewisely.org/About-Us
- 178. American Board of Internal Medicine. Choosing Wisely. ABIM Foundation © 2020 Accessed November 2, 2020 https://www.abimfoundation.org/what-we-do/choosing-wisely
- 179. Angelidis G, Tsougos I, Valotassiou V, Georgoulias P. Low-dose radiation cancer risk hypothesis may lead to ‘radiophobia’-driven imaging avoidance? J Nucl Cardiol. 2020;27(3):1050. [DOI] [PubMed] [Google Scholar]
- 180. Dauer LT, Thornton RH, Hay JL, Balter R, Williamson MJ, St Germain J. Fears, feelings, and facts: interactively communicating benefits and risks of medical radiation with patients. AJR Am J Roentgenol. 2011;196(4):756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Boutis K, Cogollo W, Fischer J, Freedman SB, Ben David G, Thomas KE. Parental knowledge of potential cancer risks from exposure to computed tomography. Pediatrics. 2013;132(2):305–311. [DOI] [PubMed] [Google Scholar]
- 182. Brody AS, Guillerman RP. Don’t let radiation scare trump patient care: 10 ways you can harm your patients by fear of radiation induced cancer from diagnostic imaging. Thorax. 2014;69(8):782–784. [DOI] [PubMed] [Google Scholar]
- 183. Goske MJ, Strauss KJ, Coombs LP, et al. Diagnostic reference ranges for pediatric abdominal CT. Radiology. 2013;268(1):208–218. [DOI] [PubMed] [Google Scholar]
- 184. Walker MR, El Naga AN, Atassi OH, Perkins CH, Mitchell SA. Effect of initial emergency room imaging choice on time to hip reduction and repeat imaging. Injury. 2019;50(3):686–689. [DOI] [PubMed] [Google Scholar]
- 185. McKenney S, Gingold E, Zaidi H. Gonadal shielding should be discontinued for most diagnostic imaging exams. Med Phys. 2019;46(3):1111–1114. [DOI] [PubMed] [Google Scholar]
- 186. Jevtovic-Todorovic V, Absalom AR, Blomgren K, et al. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth. 2013;111(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Schultz CH, Fairley R, Murphy LS, Doss M. The risk of cancer from CT scans and other sources of low-dose radiation: a critical appraisal of methodologic quality. Prehosp Disaster Med. 2020;35(1):3–16. [DOI] [PubMed] [Google Scholar]
- 188. Cuttler JM. Evidence of a dose threshold for radiation-induced leukemia. Dose Response. 2018;16(4):1559325818811537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Cuttler JM. Evidence of dose threshold for radiation-induced leukemia: absorbed dose and uncertainty. Dose Response. 2019;17(1):1559325818820973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Cuttler JM. Application of low doses of ionizing radiation in medical therapies. Dose Response. 2020;18(1):1559325819895739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Sies H, Berndt C, Jones DP. Oxidative stress. Ann Rev Biochem. 2017;86(7):715–748. [DOI] [PubMed] [Google Scholar]
- 192. Sies H, Feinendegen LE. Radiation hormesis: the link to nanomolar hydrogen peroxide. Antioxid Redox Signal. 2017;27(9):596–598. [DOI] [PubMed] [Google Scholar]
- 193. Billen D. Spontaneous DNA damage and its significance for the “negligible dose” controversy in radiation protection. Radiat Res. 1990;124(2):242–245. [PubMed] [Google Scholar]
- 194. Feinendegen LE, Cuttler JM. Biological effects from low doses and dose rates of ionizing radiation: science in the service of protecting humans, a synopsis. Health Phys. 2018;114(6):623–626. [DOI] [PubMed] [Google Scholar]
- 195. Feinendegen LE, Pollycove M, Neumann RD. Hormesis by low dose radiation effects: low-dose cancer risk modeling must recognize up-regulation of protection In: Baum RP, ed. Therapeutic Nuclear Medicine. Springer; 2012:789–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Pollycove M, Feinendegen LE. Radiation-induced versus endogenous DNA damage: possible effect of inducible protective responses in mitigating endogenous damage. Human Exp Toxicol. 2003;22(6):290–306. [DOI] [PubMed] [Google Scholar]
- 197. Tharmalingam S, Sreetharan S, Brooks AL, Boreham DR. Reevaluation of the linear no-threshold (LNT) model using new paradigms and modern molecular studies. Chem Biol Interact. 2019;301:54–67. [DOI] [PubMed] [Google Scholar]
- 198. Brooks AL. The impact of dose rate on the linear no threshold hypothesis. Chem Biol Interact. 2019;301(5):68–80. [DOI] [PubMed] [Google Scholar]
- 199. Brooks AL. Low dose radiation: the history of the US Department of Energy Research Program Washington State University Press; 2018. [Google Scholar]
- 200. Hoffmann GR. A perspective on the scientific, philosophical, and policy dimensions of hormesis. Dose Response. 2009;7(1):1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Liu SZ. Cancer control related to stimulation of immunity by low dose radiation. Dose Response. 2007;5(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Pollycove M. Radiobiological basis of low-dose irradiation in prevention and therapy of cancer. Dose-Response. 2006;5(1):26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Calabrese EJ, Dhawan G, Kapoor R, Kozumbo WJ. Radiotherapy treatment of human inflammatory diseases and conditions: optimal dose. Hum Exp Toxicol. 2019;38(8):888–898. [DOI] [PubMed] [Google Scholar]
- 204. Kuhns JG, Morrison SL. Twelve years’ experience in roentgenotherapy for chronic arthritis. N Engl J Med. 1946;235:399–405. [DOI] [PubMed] [Google Scholar]
- 205. Calabrese EJ, Dhawan G, Kapoor R. The use of X rays in the treatment of bronchial asthma: a historical assessment. Radiat Res. 2015;184(2):180–192. [DOI] [PubMed] [Google Scholar]
- 206. Calabrese EJ. X-ray treatment of carbuncles and furuncles (boils): a historical assessment. Hum Exp Toxicol. 2013;32(8):817–827. [DOI] [PubMed] [Google Scholar]
- 207. Calabrese EJ, Dhawan G. Historical use of X-rays: treatment of inner ear infections and prevention of deafness. Hum Exp Toxicol. 2014;33(5):542–553. [DOI] [PubMed] [Google Scholar]
- 208. Calabrese EJ, Dhawan G. The role of X-rays in the treatment of gas gangrene: a historical assessment. Dose Response. 2012;10(4):626–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Dhawan G, Kapoor R, Dhamija A, Singh R, Monga B, Calabrese EJ. Necrotizing fasciitis: low-dose radiotherapy as a potential adjunct treatment. Dose Response. 2019;17(3):1559325819871757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Calabrese EJ, Dhawan G, Kapoor R. Radiotherapy for pertussis: an historical assessment. Dose Response. 2017;15(2):1559325817704760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Calabrese EJ, Dhawan G. How radiotherapy was historically used to treat pneumonia: could it be useful today? Yale J Biol Med. 2013;86(4):555–570. [PMC free article] [PubMed] [Google Scholar]
- 212. Calabrese EJ, Dhawan G. The historical use of radiotherapy in the treatment of sinus infections. Dose Response. 2013;11(4):469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Calabrese EJ, Dhawan G, Kapoor R. Use of X-rays to treat shoulder tendonitis/bursitis: a historical assessment. Arch Toxicol. 2014;88(8):1503–1517. [DOI] [PubMed] [Google Scholar]
- 214. Oakley PA. Is use of radiation hormesis the missing link to a better cancer treatment? J Cancer Ther. 2015;6(7):601–605. [Google Scholar]
- 215. Cuttler JM, Pollycove M, Welsh JS. Application of low doses of radiation for curing cancer. Can Nucl Soc Bull. 2000:21(2):45–50. [Google Scholar]
- 216. Sakamoto K. Radiobiological basis for cancer therapy by total or half-body irradiation. Nonlinearity Biol Toxicol Med. 2004;2(4):293–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Sakamoto K, Myogin M, Hosoi Y, et al. Fundamental and clinical studies on cancer control with total or upper half body irradiation. JASTRO. 1997;9(3):161–175. [Google Scholar]
- 218. Richaud PM, Soubeyran P, Eghbali H, et al. Place of low-dose total body irradiation in the treatment of localized follicular non-Hodgkin’s lymphoma: results of a pilot study. Int J Radiat Oncol Biol Phys. 1998;40(2);387–390. [DOI] [PubMed] [Google Scholar]
- 219. Choi NC, Timothy AR, Kaufman SD, Carey RW, Aisenberg AC. Low dose fractionated whole body irradiation in the treatment of advanced non-Hodgkin’s lymphoma. Cancer. 1979;43(5):1636–1642. [DOI] [PubMed] [Google Scholar]
- 220. Chaffey JT, Rosenthal DS, Moloney WC, Hellman S. Total body irradiation as treatment for lymphosarcoma. Int J Radiat Oncol Biol Phys. 1976;1(5-6):399–405. [DOI] [PubMed] [Google Scholar]
- 221. Kojima S, Tsukimoto M, Shimura N, Koga H, Murata A, Takara T. Treatment of cancer and inflammation with low-dose ionizing radiation: three case reports. Dose Response. 2017;15(1):1559325817697531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Kojima S, Thukimoto M, Cuttler JM, et al. Recovery from rheumatoid arthritis following 15 months of therapy with low doses of ionizing radiation: a case report. Dose Response. 2018;16(3):1559325818784719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kojima S, Cuttler JM, Shimura N, Koga H, Murata A, Kawashima A. Radon therapy for autoimmune diseases pemphigus and diabetes: 2 case reports. Dose Response. 2019;17(2):1559325819850984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Kojima S, Cuttler JM, Inoguchi K, et al. Radon therapy is very promising as a primary or an adjuvant treatment for different types of cancers: 4 case reports. Dose Response. 2019;17(2):1559325819853163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Cuttler JM, Moore ER, Hosfeld VD, Nadolski DL. Second update on a patient with Alzheimer disease treated by CT scans. Dose Response. 2018;16(1):1559325818756461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Mitchel RE. Cancer and low dose responses in vivo: implications for radiation protection. Dose Response. 2007;5(4):284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Löbrich M, Rief N, Kühne M, Heckmann M, Fleckenstein J, Rübe C, Uder M. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci USA. 2005;102(25):8984–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. ICRP. The 2007 recommendations of the International Commission on Radiological Protection. ICRP publication 103; Ann ICRP. 2020;37(2-4):1–332. [DOI] [PubMed] [Google Scholar]
- 229. Samei E, Tian X, Paul Segars W, Frush DP. Radiation risk index for pediatric CT: a patient-derived metric. Pediatr Radiol. 2017; 47(13):1737–1744. [DOI] [PubMed] [Google Scholar]
- 230. Martin CJ. The application of effective dose to medical exposures. Radiat Prot Dosimetry. 2008;128(1):1–4. [DOI] [PubMed] [Google Scholar]
- 231. Martin CJ. Effective dose: how should it be applied to medical exposures? Br J Radiol. 2007;80(956):639–647. [DOI] [PubMed] [Google Scholar]
- 232. Calabrese EJ. Flaws in the LNT single-hit model for cancer risk: an historical assessment. Environ Res. 2017;158:773–788. [DOI] [PubMed] [Google Scholar]
- 233. Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Hong JY, Han K, Jung JH, Kim JS. Association of exposure to diagnostic low-dose ionizing radiation with risk of cancer among youths in south Korea. JAMA Netw Open. 2019;2(9):e1910584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Walsh L, Shore R, Auvinen A, Jung T, Wakeford R. Risks from CT scans—what do recent studies tell us? J Radiol Prot. 2014;34(1):E1–5. [DOI] [PubMed] [Google Scholar]
- 237. Dahal S, Budoff MJ. Low-dose ionizing radiation and cancer risk: not so easy to tell. Quant Imaging Med Surg. 2019;9(12):2023–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Journy N, Rehel JL, Ducou Le Pointe H, et al. Are the studies on cancer risk from CT scans biased by indication? Elements of answer from a large-scale cohort study in France. Br J Cancer. 2015;112(1):185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Shibata S, Shibamoto Y, Maehara M, Hobo A, Hotta N, Ozawa Y. Reasons for undergoing CT during childhood: can CT exposed and CT-naive populations be compared? Dose Response. 2020;18(1):1559325820907011. [DOI] [PMC free article] [PubMed] [Google Scholar]




