Summary
Population‐based prevalence surveys of Covid‐19 contribute to establish the burden of infection, the role of asymptomatic and mild infections in transmission, and allow more precise decisions about reopen policies. We performed a systematic review to evaluate qualitative aspects of these studies, assessing their reliability and compiling practices that can influence the methodological quality. We searched MEDLINE, EMBASE, bioRxiv and medRxiv, and included cross‐sectional studies using molecular and/or serological tests to estimate the prevalence of Covid‐19 in the general population. Survey quality was assessed using the Joanna Briggs Institute Critical Appraisal Checklist for Prevalence Studies. A correspondence analysis correlated methodological parameters of each study to identify patterns related to higher, intermediate and lower risks of bias. The available data described 37 surveys from 19 countries. The majority were from Europe and America, used antibody testing, and reached highly heterogeneous sample sizes and prevalence estimates. Minority communities were disproportionately affected by Covid‐19. Important risk of bias was detected in four domains: sample size, data analysis with sufficient coverage, measurements in standard way and response rate. The correspondence analysis showed few consistent patterns for high risk of bias. Intermediate risk of bias was related to American and European studies, municipal and regional initiatives, blood samples and prevalence >1%. Low risk of bias was related to Asian studies, nationwide initiatives, reverse‐transcriptase polymerase chain reaction tests and prevalence <1%. We identified methodological standards applied worldwide in Covid‐19 prevalence surveys, which may assist researchers with the planning, execution and reporting of future population‐based surveys.
Keywords: Covid‐19, cross‐sectional studies, epidemiology, infectious diseases, prevalence, SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Abbreviations
- BioS
biological sample
- JBI
Joanna Briggs Institute
- N/A
not applicable
- NPS
nasopharyngeal swabs
- MERS
Middle East respiratory syndrome
- P
prevalence
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- RT‐PCR
reverse‐transcriptase polymerase chain reaction
- S
sensitivity
- SARS
severe acute respiratory syndrome
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviation
1. INTRODUCTION
In December 2019, the third most important coronavirus in the twenty‐first century (severe acute respiratory syndrome coronavirus 2 – SARS‐CoV‐2) was identified as the causative agent of SARS outbreak in Wuhan, Hubei province, China.1, 2 SARS‐CoV‐2 has spread rapidly around the world leading the disease (Covid‐19) to acquire pandemic status on 11 March 2020. 3 As of 14 October 2020, there are ∼38 million confirmed cases and ∼1.1 million reported deaths in 216 countries, areas or territories. More than 50% of these cases were reported in the United States, India and Brazil, the worst‐hit countries.4, 5
According to the current evidence, the main form of SARS‐CoV‐2 spreading is through human‐to‐human transmission via respiratory droplets and contact routes. 6 The standard diagnostic testing method is the reverse‐transcriptase polymerase chain reaction (RT‐PCR) test,7, 8 which is able to detect current infections, and it is recommended for people with Covid‐19 symptoms and for all close contacts of the confirmed cases. A complementary approach is to use antibody tests (e.g., point‐of‐care test or enzyme‐linked immunosorbent assay) to detect a past infection and the production of antibodies (IgM and/or IgG) against SARS‐CoV‐2. 8
Covid‐19 causes diverse degrees of illness, ranging from asymptomatic infection to severe pneumonia. 9 However, surveillance is only based on the confirmed cases, which can represent an underestimation of total cases due to non‐testing in mildly affected or asymptomatic individuals. Population‐based prevalence surveys can help to establish the disease epidemiology, the burden of infection, the role that asymptomatic and mild infections play in the transmission, and to enable precise evidence‐based decisions about control and reopen policies, while no pharmacological intervention is available. 10 Moreover, accurate estimates of the basic reproduction number, of exposed and susceptible populations, and the fatality rates can be obtained.11, 12
Statistical extrapolations will only be reliable for the population if (i) the sample of individuals is sufficient, random and representative of the general population; (ii) if the measurements are standardized and (iii) if the tests used have adequate sensitivity and specificity, among other factors. 13 For example, a recent systematic review and meta‐analysis evaluated the diagnostic accuracy of serological tests in 40 studies. The conclusion indicated that the use of existing point‐of‐care serological tests is not supported by available evidence due to low performance. 14 Thus, a critical evaluation of these parameters is necessary to verify the reliability of the population‐based surveys of Covid‐19.
We performed a systematic review to evaluate and summarize the main results regarding the Covid‐19 prevalence obtained through population‐based surveys, their reliability and biases. Our main aims were to evaluate the qualitative aspects of these studies and to compile practices that can influence positively or negatively the methodological quality.
2. METHODS
2.1. Registration and reporting
The protocol for this systematic review was registered on PROSPERO (ID: CRD42020202186). Reporting was conducted according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) (Supplementary Checklist).
2.2. Search strategy
Systematic literature searches for published and unpublished (preprint) articles were conducted from 15 July to 05 September 2020. MEDLINE (accessed via PubMed), EMBASE, bioRxiv and medRxiv databases were searched using the following controlled vocabulary heading and terms: ’seroprevalence’, ’prevalence’, ’serology’, ’immunoassay’, ’enzyme linked immunosorbent assay’, ’real time polymerase chain reaction’, ’cross‐sectional study’, ’population screening’, ’severe acute respiratory syndrome coronavirus 2’ and ’Covid‐19’. These terms and their synonyms were combined using logical operators and adapted according to the searched database. Only articles published in English were retrieved. The complete search strategy for each database is on Table S1.
2.3. Inclusion and exclusion criteria
The review included cross‐sectional or repeated cross‐sectional studies using molecular or serological tests to estimate the prevalence of Covid‐19 in municipalities, regions, states or countries around the world. Studies were excluded based on the following criteria: (i) non‐cross‐sectional studies, (ii) studies with correlation between Covid‐19 and other diseases or health determinants, (iii) non‐random selection of participants (e.g., convenience sampling), (iv) inclusion of a specific group of participants only (e.g., with comorbidities, pregnant, elderly, healthcare workers, pediatric patients), and (v) non‐human samples.
2.4. Article screening and data extraction
Four pairs of authors (AMM and CLML, ABG and JGK, ASS and VBF, and GDC and JCP) independently reviewed the titles and abstracts, in parallel, and included publications identified by either author for full‐text review. These authors also reviewed full texts to determine which publications met the inclusion criteria and then re‐analysed the texts and supplemental materials to extract the following relevant information, when available: (i) authors, (ii) study location, (iii) coverage, (iv) study type, (v) random sampling method, (vi) period of testing, (vii) number of tests, (viii) biological samples, (ix) type of test used, (x) if test validation was performed, (xi) the test sensitivity and specificity, (xii) prevalence and (xiii) statistical methods (Table 1). Disagreements in the screening and data extraction were discussed among the reviewers and, if consensus cannot be reached, a third reviewer (ATW) made the ultimate decision.
TABLE 1.
Characteristics of 37 population‐based prevalence surveys during the Covid‐19 pandemic until September 2020
| Continent and region | Coverage | No. rounds | Period (2020) | Sample selection method | No. of tests | Biological samples | Test(s) used | Test validation a | Sensitivity (95% CI) b | Specificity (95% CI) b | Prevalence (95% CI) | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Africa | ||||||||||||
| Niger state/Nigeria | State | 1 | June 26–30 | Random selection using clustered‐stratified strategy covering the 3 geopolitical zones in the state | 185 | Whole blood | LFIA for IgG/IgM | Yes, RT‐PCR confirmed cases | 100% | 100% | IgG: 25.41% | ( 16 ) |
| IgM: 2.16% | ||||||||||||
| Asia | ||||||||||||
| Wuhan/Hubei/China | Municipality | 1 | May 14–June 1 | Screening programme (not detailed) | 9,899,828 | NPS | RT‐PCR and mixed testing via sample pooling | NA | ‐ | ‐ | 0.303/10,000 (0.270–0.339) | ( 17 ) |
| Mumbai/India | Municipality | 1 | Slums: June 29–July 14 | Random selection of households using geographically‐spaced community sampling in areas classified as slums and as non‐slums | 6904 | Serum | CLIA for IgG | No, by other studies | 90% (74.4–96.5)–96.9% (89.5–99.5) | 100% (95.4–100) | Slums: 54.1% (52.7–55.6) | ( 18 ) |
| Non‐slums: July 3–July 19 | Slums: 4202 | Non‐slums: 16.1% (14.9–17.4) | ||||||||||
| No‐slums: 2702 | ||||||||||||
| Guilan province/Iran | Province | 1 | April | Random selection of households using multistage cluster strategy | 528 | Capillary blood | LFIA for IgG/IgM | No, by manufacturer and other study | 63.30% | 100% | Raw: 22% (19–26) | ( 19 ) |
| Weighted: 21% (14–29) | ||||||||||||
| Test adjusted: 33% (28–39) | ||||||||||||
| Utsunomiya/Japan | Municipality | 1 | 14 June–5 July | Random selection of households from basic resident registry | 742 | Uninformed | CLIA for IgG | No, by manufacturer | 97.30% | 96.30% | Unweighted: 0.40% (0.08–1.18) | ( 20 ) |
| Weighted: 1.23% (0.17–2.28%) | ||||||||||||
| West bank/Palestina | County | 1 | June 15–30 | Random selection of households based on census tracts with probability proportional to size sampling | 1319 | Serum | CLIA for IgG/IgM | Yes, RT‐PCR confirmed cases | 100% (88.1–100) (14 days post PCR confirmation) | 99.81% (99.65–99.9) | 0% (0–0.0036) | ( 21 ) |
| Europe | ||||||||||||
| Faroe Islands/Denmark | Region | 1 | April 27–May 1 | Random selection based on the population registry | 1075 | Serum | Automated (ELISA) | No, by manufacturer | 94.4% (90.9–6.8) | 100% (98.8–100.0) | 0.7% (0.3–1.3) | ( 22 ) |
| England | Country | 1 | May 01–June 01 | Random selection using the NHS patient list | 120,620 | Self‐administered NPS | RT‐PCR | NA | 70% | ‐ | 0.13% (0.11–0.15) | ( 23 ) |
| England | Country | 1 | June 20–13 July | Random selection of adults using the NHS patient list | 105,651 | Finger‐prick blood | LFIA | Yes, RT‐PCR confirmed cases | 84.4% (70.5–93.5) | 98.6% (97.1–99.4) | Raw: 5.6% (5.4–5.7). | ( 24 ) |
| Test adjusted: 6.0% (5.8–6.1) | ||||||||||||
| Neustadt‐am‐Rennsteig/Germany | Municipality | 1 | May 12–22 | All households from the community were invited | 620 | Pharyngeal washes | RT‐PCR, 2 ELISAs, 2 CLIAs for IgG, 1 CMIA for IgG | Uninformed | ‐ | ‐ | RT‐PCR: 0% | ( 25 ) |
| Blood drawn | Antibody: 8.4% | |||||||||||
| Hungary | Country | 1 | May 1–16 | Random selection based on settlements using two‐stage stratified strategy, and stratification by age from the population registry | 10,575 | NPS | RT‐PCR | Uninformed | ‐ | ‐ | 68/10.000 (50–86) | ( 26 ) |
| Blood | Automated antibody test | |||||||||||
| Iceland | Country | 1 | April 1–4 | Random selection (not detailed) | 2283 | NPS | RT‐PCR | NA | 6 genome copies per reaction | No cross‐reactivity observed | 0.6% (0.3–0.9) | ( 27 ) |
| Castiglione d'Adda/Lombardy/Italy | Municipality | 1 | May 18–June 7 | Random selection stratifying by sex and age classes from the municipal registry list | 509 | NPS | CLIA for IgG | Uninformed | ‐ | ‐ | 22.6% (17.2–29.1%) | ( 28 ) |
| Serum blood drawn | ||||||||||||
| Vó/Vêneto/Italy | Municipality | 2 | Beginning of lockdown: February 21–29, | Sampling from the majority of the municipality population | Beginning of lockdown: 2812 | NPS | RT‐PCR | NA | E gene: 5 genome copies per reaction | ‐ | Beginning of lockdown: 2.6% (2.1–3.3) | ( 29 ) |
| End of lockdown: March 7 | End of lockdown: 2343 a | RdRp gene: 50 genome copies per reaction | End of lockdown: 1.2% (0.8–1.8) | |||||||||
| Luxembourg | Country | 1 | April 15–May 5 | Random selection defined by the crossing of the 3 stratification variables through a deterministic random bit generator within strata | RT‐PCR: 1842 | NPS | RT‐PCR | Yes, cohort of hospitalized patients | Combined (IgA/IgG): 85.7% | Combined (IgA/IgG) 99.5% | RT‐PCR: 0.3% | ( 30 ) |
| IgA and IgG: 1820 | Blood drawn | CE‐labelled ELISA for IgA/IgG | IgA: 11.0% | |||||||||
| IgG: 1.9% | ||||||||||||
| Both IgA and IgG: 1.6% | ||||||||||||
| Slovenia | Country | 1 | April 20–May 1 | Random selection of a representative sample using central population register data | 1366 | NPS | Two‐target PCR‐based assay | Yes | 100% | 100% | 0.15% (posterior mean 0.18%, 95% Bayesian CI: 0.03–0.47; 95% highest density region 0.01–0.41) | ( 31 ) |
| Spain | Country | 1 | April 27–May 11 | Random selection of households based on census tracts using stratified two‐stage strategy and performed by national institute of statistics | 61,075 | Finger‐prick blood | LFIA for IgG/IgM | Yes, RT‐PCR‐positive individuals for both tests | IgG: 82.1% | IgG: 100% | Point‐of‐care test: 5.0% (4.7–5.4) | ( 32 ) |
| Blood drawn | CLIA for IgG | IgM: 69.6% | IgM: 99% | Immunoassay: 4.6% (4.3–5.0) | ||||||||
| Barcelona/Spain | Municipality | 1 | April 21–24 | Random selection from individuals registered at a primary health care facility | 311 | Capillary blood | LFIA for IgG/IgM | Uninformed | ‐ | ‐ | 5.47% (3.44–8.58) | ( 33 ) |
| Stockholm/Sweden | Municipality | 1 | June 17–18 | Random selection (not detailed) | 213 | Uninformed | LFIA for IgG/IgM | Yes, serum from negative and RT‐PCR‐positive individuals | ≅100% | 100%–95.5% | Norra djurgårdsstaden: 4.1% (±3.5%) | ( 34 ) |
| Norra djurgårdsstaden: 123 | Tensta: 30% (±9.7%) | |||||||||||
| Tensta: 90 | ||||||||||||
| Stockholm/Sweden | Municipality | 2 | April 01–May 31 | Random selection of adults in households and mail distribution of home‐sampling kits | 878 | Finger‐prick blood | Multiplexed serology assay (developed in this paper) | Yes, compared to ELISA assays (EuroImmun AG) against the S1 and N proteins | 100% | 96%–100% (depending on the antigen used) | Set 1: 10.11% (7.31–12.92) | ( 35 ) |
| Set 1: 435 | Set 2: 10.84% (7.94–13.73) | |||||||||||
| Set 2: 443 | ||||||||||||
| Geneva/Switzerland | Municipality | 5 | April 6–May 9 (once every week for 5 weeks) | Random selection based on an already existing representative sample of the general population (bus santé study) | 2766 | Peripheral venous blood | Automated (ELISA) | Yes, sera from pre‐pandemic negative controls and RT‐PCR‐positive individuals | 93% | 100% | R1: 4.8% (2.4–8.0) | ( 36 ) |
| R2: 8.5% (5.9–11.4) | ||||||||||||
| R3: 10.9% (7.9–14.4) | ||||||||||||
| R4: 6.6% (4.3–9.4) | ||||||||||||
| R5: 10.8% (8.2–13.9) | ||||||||||||
| North America | ||||||||||||
| Los Angeles/California/USA | County | 1 | April 10–14 | Random selection with stratification in subgroups based on age, sex, race, and ethnicity distribution | 863 | Uninformed | LFIA | Yes, RT‐PCR‐positive individuals | 82.7% (76–88.4) | 99.5% (99.2–99.7) | 4.06% (exact binomial: 2.84–5.60) | ( 37 ) |
| Santa Clara/California/USA | County | 1 | April 3–4 | Facebook ads targeting a sample of individuals living within the county by demographic and geographic characteristics and stratification | 3330 | Capillary blood | LFIA | Yes, RT‐PCR‐positive individuals | 82.8% (76.0–88.4) | 99.5% (99.2–99.7) | Raw: 1.5% (exact binomial: 1.1–2.0) | ( 38 ) |
| Test adjusted: 1.2% (0.7–1.8) | ||||||||||||
| Census‐weighted: 2.8% (1.3–4.7) | ||||||||||||
| Connecticut/USA | State | 1 | June 10–July 6 | Random selection from landline and cell phone numbers and re‐stratification by census designations | 505 | Serum | Automated immunodiagnostic system | Yes, RT‐PCR‐positive individuals | 94% (81–99) | ‐ | 3.1% (90% CI: 1.4–4.8) | ( 39 ) |
| DeKalb and Fulton counties/Georgia/USA | County | 1 | April 28–May 3 | Random selection of households based on two‐stage cluster strategy | 696 | Plasma | Automated immunodiagnostic system | No, by CDC testing 1laboratory | 93.2% | 99% | 2.5% (1.4–4.5) | ( 40 ) |
| Blaine/Idaho/USA | County | 1 | May 4–19 | Random selection of volunteers after stratification by ZIP code, age and gender within ZIP code | 917 | Plasma | CLIA for IgG | No, by other studies | 92.9%–100% (14 days after symptom onset) | 99.6%–100% | 22.7% (20.1–25.5) | ( 41 ) |
| Indiana/USA | State | 1 | April 25–29 | Random selection based on a list of residents derived from tax returns, and stratification using public health preparedness districts as sampling strata | 3658 | NPS | RT‐PCR | No, by manufacturer | 100% (14 days after symptom onset) | 99.6% (14 days after symptom onset) | RT‐PCR raw: 1.74% (1.10–2.54) | ( 42 ) |
| Peripheral venous blood | CLIA for IgG | Antibody raw: 1.01% (0.76–1.45) | ||||||||||
| Overall estimate: 2.79% (2.02– 3.70) | ||||||||||||
| Baton Rouge/Louisiana/USA | Region | 1 | July 15–31 | Random selection using a method developed by public democracy, choosing between residents with digital ads for recruitment, and re‐stratification of volunteers by census designations | 2138 | NPS | RT‐PCR | Uninformed | ‐ | ‐ | 6.6% | ( 43 ) |
| Blood drawn | Automated (qualitative immunoglobulin for IgG) | |||||||||||
| Orleans and Jeferson Parishes/Louisiana/USA | County | 1 | May 9–15 | Random selection based on a novel 2‐step system developed by public democracy considering >50 characteristics, including social determinants of health and census population data | 2640 | NPS | RT‐PCR | No, by CDC and other studies | 100% (95.1–100) (17 days after symptom onset) (Bryan et al., 2020) | 99.90% (Bryan et al., 2020) | Raw: 6.9% (6.0%–.0%) | ( 44 ) |
| Blood drawn | Automated (qualitative immunoglobulin for IgG) | Census‐weighted: 7.8% (7.8%–7.9%) | ||||||||||
| South America | ||||||||||||
| Barrio Mugica/Buenos Aires city/Argentina | Municipality | 1 | June 10–July 1 | Random selection using two‐stage strategy using geographical areas determined by the department of statistic and census | 873 | Finger‐prick blood | Automated (ELISA) | Yes, RT‐PCR confirmed cases | 95% (after 21 days of symptom onset) | 100% | Weighted IgG: 53.4% (52.8–54.1) | ( 45 ) |
| Brazil | Country | 1 | May 14–21 | Random selection of households based on census tracts from sentinel cities in all Brazilian states | 24,995 | Finger‐prick blood | LFIA for IgG/IgM | No, pooled estimate based on four validation studies | 84.8% (95% CI: 81.4–87.8) | 99% (95% CI: 97.8–99.7) | 1.4% (1.3–1.6) | ( 46 ) |
| Espírito santo/Brazil | State | 1 | May 13–15 | Random selection based on census tracts using most populous municipalities in the state and lesser populous municipalities | 5775 | Finger‐prick blood | LFIA for IgG/IgM | No, by manufacturer | 86.4% | 97.63% | Prevalence step: 2.1% (1.67–2.52) | ( 47 ) |
| Prevalence step: 4612 | Extension step: 0.26% (0.05–0.75) | |||||||||||
| Extension step: 1163 | ||||||||||||
| Maranhão/Brazil | State | 1 | 27 July–8 August | Random selection of households based on census tracts in three stratified stages in four regions | 3156 | Serum | Automated CLIA for IgG/IgM | No, by other studies | ‐ | 99.7% | 40.4% (35.6–45.3) | ( 48 ) |
| Teresina/Piauí/Brazil | Municipality | 7 | April 19–May 31 (1‐week interval) | Random selection of households based on the registry of 78 basic health units and stratification by sex, age, and geographical distribution | 6300 | Uninformed | LFIA for IgG/IgM | No, by manufacturer | 86% | 99% | R1: 0.56% (0.18–1.3) | ( 49 ) |
| R2: 0.89% (0.39–1.75) | ||||||||||||
| R3: 1.44% (0.77–2.45) | ||||||||||||
| R4: 2% (1.19–3.14) | ||||||||||||
| R5: 3.78% (2.63–5.24) | ||||||||||||
| R6: 5.78% (4.3–7.3) | ||||||||||||
| R7: 8.33% (6.61–10.33) | ||||||||||||
| Rio Grande do Sul/Brazil | State | 3 | April 11–May 11 (2‐week interval) | Random selection of households based on census tracts using multi‐stage sampling strategy in sentinel cities | 13,111 | Finger‐prick blood | LFIA for IgG/IgM | Yes, RT‐PCR confirmed cases | 84.8% (81.4–87.8%) – pooled | 99.0% (97.8–99.7%) – pooled | R1: 0.048% (0.006–0.174) | ( 50 ) |
| R1: 4151 | R2: 0.135% (0.049–0.293) | |||||||||||
| R2: 4460 | R3: 0.222% (0.107–0.408) | |||||||||||
| R3: 4500 | ||||||||||||
| São Paulo/Brazil | Municipality | 1 | May 4–12 | Random selection of households in six districts | 517 | Serum blood drawn | CLIA for IgG/IgM | No, by other studies | IgM: 100% | IgM: 94.1% | Census‐weighted: 4.7% (3.0–6.6) | ( 51 ) |
| Randomly‐selected: 299 | IgG: 100% (20 days after symptom onset) | IgG: 99.5% (20 days after symptoms onset) | ||||||||||
| Cohabitants: 218 | ||||||||||||
| Baixada Santista/são Paulo/Brazil | Region | 1 | Uninformed | Random selection of households based on census tracts and stratification by age, gender and living conditions | 2342 | Uninformed | LFIA for IgG/IgM | Yes, RT‐PCR confirmed cases after more than 14 days of symptoms | ‐ | ‐ | 1.4% (0.93–1.93) | ( 52 ) |
Abbreviations: 95% CI, 95% confidence interval; CLIA, chemiluminescent microparticle immunoassay; ELISA, enzyme‐linked immunosorbent assay; LFIA, lateral flow immunoassay; NHS, National Health Service; NPS, nasopharyngeal and oropharyngeal swabs; R, Round.
Repeated people between the first and second rounds.
If test validation was performed internally by the study or in a publication performed by the same authors. N/A was considered when the RT‐PCR method (gold standard) was applied.
Sensitivity and specificity reported by the study or the reference cited, according to the ‘Test validation’ column.
2.5. Survey quality
We assessed each survey quality by using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Prevalence Studies. 13 This tool evaluates nine domains: (D1) sample frame adequacy, (D2) recruitment method, (D3) sample size, (D4) study subjects and the setting, (D5) coverage, (D6) diagnostic methods, (D7) the reliability and standardization of measurements, (D8) statistical analysis, and (D9) the response rate. For each study, ’yes’, ’no’ and ’unclear’ options were selected, meaning ‘low’, ‘high’ and ‘unclear’ risk of bias, respectively. The number of ’yes’ answers to these nine domains was counted, with a higher number of yes representing less risk of bias. Graphs considering each risk of bias domain across all studies were prepared using the robvis R package v. 0.3.0.900. 15
2.6. Definitions
Additional objective criteria were adopted for the survey quality assessment. For D4, the prevalence estimates should be stratified by conventional sex and age classes minimally. For D5, ’no’ was chosen when there was a lack of a subgroup representativity. If the response rate >70% or <70% with adequate sample size, ’yes’ was chosen. The option ‘unclear’ was selected only if there was no information about the response rate in the article. For D6, a method was considered valid if the sensitivity >70%. For D7, self‐sampling was considered as a practice of high risk of bias. In the case of a collection described by health professionals or trained individuals and using standardized methods, we assumed a low risk of bias. For D8, a minimum description of statistical methods was sufficient to classify the study as low risk of bias. For D9, if the response rate <70% without stratification or statistical management, the study was considered to have a high risk of bias. Response rate >70% or appropriate management of low response rate was related to a low risk of bias, while missing information about the proportion of tested in relation to the recruited individuals was associated with unclear.
2.7. Data analysis
A correspondence analysis was performed to visualize the relationships among categories of the row and column variables in a low‐dimensional graphic. The row variables (respective categories) were (i) study continent (Africa, Asia, Europe, North America and South America); (ii) coverage (country, region and municipality); (iii) biological samples (BioS) (uninformed, blood only, both swab and blood, serum/plasma, and swab only); (iv) test validation (external, uninformed, yes [internal] and RT‐PCR [N/A (not applicable): gold‐standard]); (v) test sensitivity (S) (<80%, 80%–90%, 90%–100%, unavailable and RT‐PCR [N/A: gold‐standard]). The column variables (respective categories) were (vi) prevalence (<1%, 1%–3%, 3%–5%, 5%–20% and >20%) and (vii) risk of bias (low [≤1 high risk], intermediate [1 < high risk ≤ 3], and high [>3 high risk]) (see Survey Quality). Two studies18, 34 were split due to widely divergent prevalences reported in each part of the municipalities investigated. Therefore, despite the 37 studies included, 39 records were considered in this analysis. The PROC CORRESP from SAS Studio (Release 3.8, Enterprise Edition) available on the SAS OnDemand for Academics platform was used to perform the correspondence analysis.
3. RESULTS
Of 49 full‐text articles screened, we excluded 12 (Table S2), and identified 37 eligible for extensive review (Figure 1, Table 1). Of these, 23 (62.2%) were preprint, while 14 (37.8%) were peer reviewed and published. Fifteen articles (40.5%) were from Europe, 8 (21.6%) from North America, 8 (21.6%) from South America, 5 (13.5%) from Asia and 1 (2.7%) from Africa. The countries with the vast majority of population‐based prevalence study initiatives were the United States (n = 8; 21.6%), Brazil (n = 7; 18.9%) and the United Kingdom (n = 3; 8.1%). Importantly, 15 of the 16 studies in the Americas were conducted in the United States or Brazil, which are included in the TOP three of confirmed cases and deaths worldwide. In total, 19 countries had studies included in this analysis (Figure 2). Considering the coverage of these studies, 16 (43.2%) had regional (state/province/county) scope, 13 (35.1%) were restricted to municipalities and 8 (21.6%) were nationwide studies.
FIGURE 1.
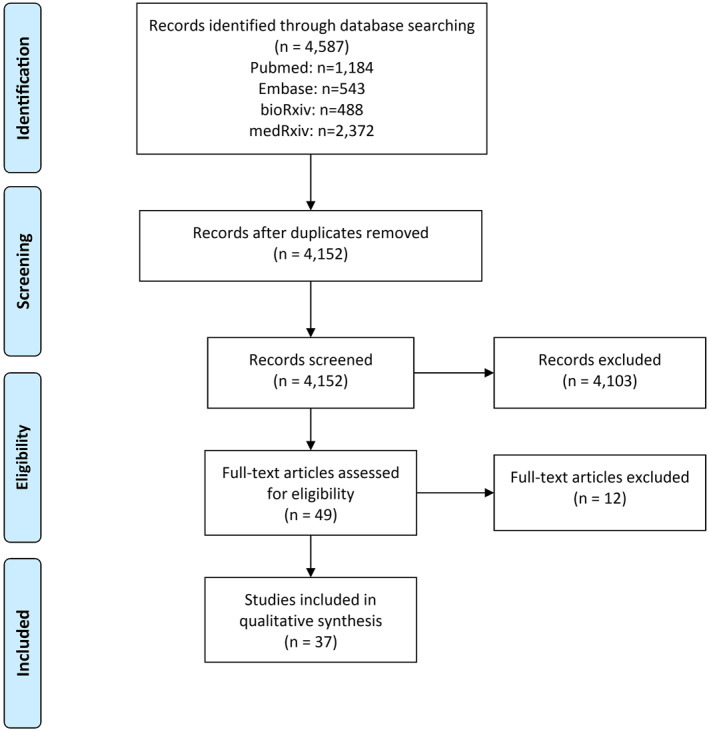
PRISMA flowchart of the literature search
FIGURE 2.
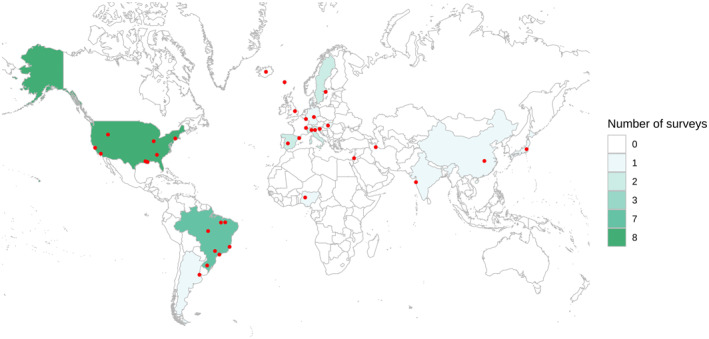
Map of countries and specific regions with prevalence surveys. Red dots represent regions and cities where the initiatives were performed. In nationwide studies, the point was placed in the centre of the country
The vast majority of studies (n = 25; 67.6%) reported only antibody testing, while the exclusive use of RT‐PCR was presented in 5 (13.5%), and both tests were conducted in 7 (18.9%) studies. The authors of 15 (46.9%) of the 32 studies that used serological tests reported their own validation test performance, while in 13 (40.6%), the validation performed by other studies or by the manufacturer was described. Excluding Wuhan's (China) screening programme 17 that tested 9,899,828, at least 394,090 individuals were tested in the other 36 studies that reported the number of tests. However, this number was highly variable among studies (mean: 10,946.94, median: 1990, standard deviation (SD): 27,382.34). Considering the periods of these surveys, most of them were conducted between April and July 2020 (Figure 3).
FIGURE 3.
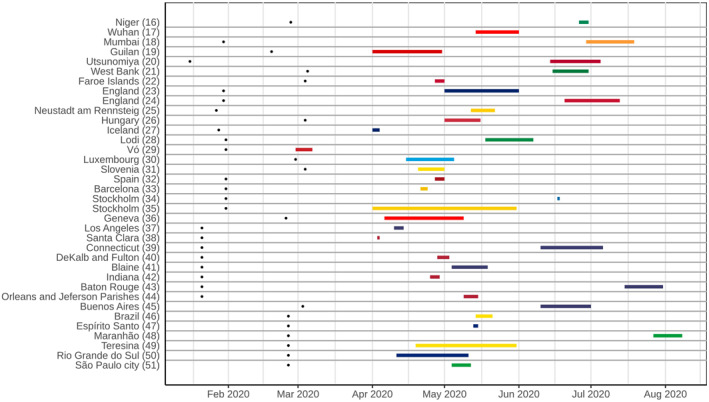
Timeline of population‐based Covid‐19 prevalence surveys conducted worldwide, with the duration of each survey and an overview of the most represented periods. Black dots on the left represent the date of the first confirmed case in the country of each survey
Most studies (n = 35; 94.6%) presented low risk of bias overall, but only one had low risk of bias in all nine domains. 32 Two studies showed overall unclear risk of bias.34, 49 Apart from these, another three studies had a sum of high and unclear risk of bias higher than the low risk of bias16, 20, 27 (Figure S1). Considering the nine domains established and three possible answers (low, unclear and high), on average, 6.35, 1.43 and 1.19 of each option were chosen, respectively. The median values were 6.0, 1.0 and 1.0, while the SDs were 1.44, 1.26 and 1.08. Considering the sum of the results with some risk of bias (unclear and high), the mean, median and SD were 2.62, 3.0 and 1.46, respectively. Considering each domain in all studies, >75% low risk of bias across the studies was observed in five domains. On the other hand, three criteria (data analysis with sufficient coverage, measurements in standard way, and response rate adequacy) were adequate in <50% of the studies. The remaining domain (sample size) was adequate in ∼70% of the studies (Figure 4).
FIGURE 4.
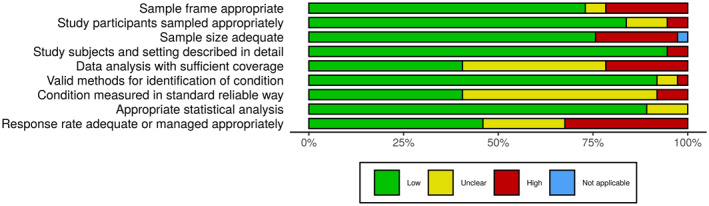
Risk of bias assessment summary table across all studies. *No weights were applied for different studies. †Not applicable was selected in ‘sample size adequate’ because the study had zero prevalence (impossible to calculate the sample size required)
Considering the analysis of correspondence performed (Figures 5 and S2) among seven main variables (continent, coverage, biological samples, test validation, sensitivity, prevalence and risk of bias), we found some important correlations. European, North‐ and South‐American studies presented, in general, an intermediate risk of bias, while Asian studies tended to a low risk of bias. Regarding the coverage of the studies, regional and municipal studies presented an association with intermediate risk of bias, while nationwide studies were related to low risk of bias.
FIGURE 5.
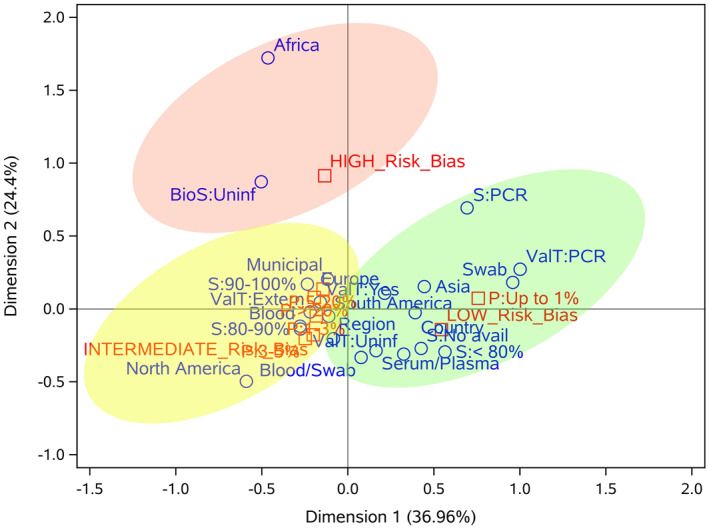
Correspondence analysis of seven important variables of population‐based Covid‐19 prevalence surveys. The categories of row (continent, coverage, biological samples, test validation and sensitivity) and column (risk of bias and prevalence) variables are represented in blue and red, respectively. Light red, yellow and green ellipses represent high, intermediate and low risk of bias, respectively. BioS, biological sample; S, sensitivity; P, prevalence; ValT: test validation
Studies that performed molecular tests on nasopharyngeal swabs (NPS) tended to have a low risk of bias, while those with blood samples were related to intermediate risk. Regarding prevalence, the majority of the studies with swab samples (RT‐PCR) showed prevalence (P) < 1%, while studies using only blood, or swab and blood, exhibited P > 1%. Validation in serological tests had no significant impact on the quality of studies, since both external and internal validation were related to intermediate risk of bias. On the other hand, the use of the gold standard (RT‐PCR) was associated with low risk of bias. P < 1% was more frequent in studies with low risk of bias, while P > 1% was associated with intermediate risk of bias. Some categories presented in Figure 5 have not been reported here after manual examination due to their low frequency (e.g., Africa, BioS: Uninformed, S: <80%).
4. DISCUSSION
We observed that important limitations of the studies were the low sample size and the low response rate (Figure 4). These factors influence heavily on reliable prevalence estimates. 53 Moreover, the recruitment by letter, by mail or online may play a significant role in reducing the response rate and inadequately address the target population.54, 55
For example, in the Icelandic study, 27 the authors discussed the small variation in the prevalence estimates between open invitation and random selection recruitments. However, the random selection methods were not detailed and the sample size to detect the estimated prevalence was not adequate (<2529 individuals). 56 In the Slovenian study, 31 despite being considered nationwide, the sample size was 1366, which represented ∼7× less than necessary (10,179), 56 and there was no management of the low response rate (<50%). Some authors seem to have not been concerned with managing this issue because even though the response rate was low, there was still an adequate sample.23, 35, 36, 40, 42 Repeated cross‐sectional studies featured a widely distinct prevalence estimate on each round.29, 36, 49, 50 This trend might be caused by the ascending curve of infected people, following the epidemic's natural course. Therefore, there was a need for different sample sizes for each period. Unfortunately, some studies did not yield adequate sample size in all rounds.49, 50
The same proportion of studies validated their methods internally to report accuracy16, 21, 23, 24, 30, 32, 34, 35, 36, 37, 38, 39, 45, 50, 52 or used sensitivity and specificity given by manufacturers or other external studies.18, 19, 20, 22, 36, 40, 41, 42, 44, 45, 46, 47, 48, 49, 50, 51 We also noticed that it is quite unclear if the field teams followed standardized protocols for the data collection and testing. The absence of complete information resulted in a loss of quality in the methodological analysis, 13 and we speculate that one rationale would be the editing process of these articles, which were published as letters or comments. In some studies,23, 24, 35 the samples were collected by the participants themselves, which causes an increase in the number of discarded samples and can reduce the sensitivity, especially of RT‐PCR, highly influenced by a well done sample collection procedure. 57
However, the studies presented several strengths to highlight. Valid methods consistently stated the identification of the condition and the manufacturer indicated this accuracy. The majority of the sampling methods was conducted appropriately regarding randomness, and the participants were well described and stratified, thus mitigating possible selection bias.18, 19, 24, 26, 28, 30, 33, 37, 39, 43, 44, 46, 48, 49, 50, 51 A strong trend was observed in relation to the sampling procedures used in Brazil. All studies used a standardized household sampling method based on census tracts46, 47, 48, 50, 51, 52 or healthcare units. 49
An interesting method of sample selection was the use of social network ads targeting individuals by demographic and geographic characteristics and stratification, which despite being convenient inserts the biases of technology usage and the participation of people most likely to be infected. However, in these cases, statistical management seems to have been adequate to accommodate the sampling issues in the prevalence estimates.38, 43 Biases were also introduced when volunteers were recruited, but data analysis was conducted properly in these cases.41, 43 Nevertheless, these practices cover up important methodological issues despite minimizing the biases of studies and they should be avoided.
Covid‐19 has an extensive spectrum of manifestation, including asymptomatic infection, mild disease, severe pneumonia and death.2, 9 Asymptomatic individuals may play an important role in viral transmission. 10 The prevalence of asymptomatic infection in the community is still unclear, but essential to estimate the true Covid‐19 prevalence. Generally, infection rates are calculated based on tests in symptomatic patients, and it may cause serious underestimations in prevalence.10, 11, 12 This issue can be circumvented by surveying randomly recruited populations. 11
In fact, the asymptomatic rate of infection is quite hard to estimate. Nevertheless, we can consider some relevant observations. The proportion of symptom‐free patients with Covid‐19 in most studies is higher than SARS 58 and Middle East respiratory syndrome (MERS) 59 coronavirus epidemics, which was reported to lay between 0% and 7.5%. However, in the case of Covid‐19, these rates were widely variable among PCR‐positive and/or seropositive, ranging from 19.6% 47 to 69%. 23 The burden of the disease among symptomatic individuals was higher in older age groups,28, 29 and there was no statistical difference in the viral load of symptomatic versus asymptomatic. 29 On the other hand, RT‐PCR‐ and antibody‐negative participants also reported symptoms,25, 27 raising the possibility of infection by other respiratory aetiological agents. However, the comparability of asymptomatic rate estimates is hindered by different approaches applied, since the period of symptoms screening before the sampling ranged from 1 week 23 to several months. 26
Some studies demonstrated a disproportionate seroprevalence in black communities,24, 40, 42, 43, 44, 46, 47, 51 multiracial, Hispanic, Indigenous, and Asian persons,24, 34, 38, 39, 44, 46 as well as in public‐facing workers17, 24, 43 and slums population.18, 45 These data show the disparities that minority communities face to access healthcare systems, arisen from a complex relationship of social, environmental, economic and structural inequities.60, 61 Therefore, a priori knowledge of these trends in seroprevalence is essential for the sample design and for the instruction of field teams regarding protective measures in these surveys.
In the study from Stockholm, 34 it was observed a significant difference in seroprevalence between the two areas (4.1% in middle‐ to high‐income suburb and 30% in lower income suburb). The authors related this high prevalence with cramped accommodation, which enhances cluster transmission, and with a majority of public‐facing workers in the suburb. In Mumbai, 18 the authors found a higher seroprevalence in the slums (54.1%) compared with non‐slums (16.1%). Thus, it is discussed that the epidemic may be in advanced stages in slums due to higher population density.
The data from Brazilian studies46, 47, 48, 49, 50, 51, 52 suggest that Covid‐19 pandemic was highly heterogeneous in the country, with rapid growth in North and Northeast regions, and slow progression in the South and Centre‐West regions. These data demonstrate the impact of differences in demographics, urban infrastructure and income on the viral transmission and seroprevalence, emphasizing health inequality.62, 63
It is important to note that the data presented here are based on the articles until 5 September 2020. Therefore, more recent articles are not included in the analysis and a future investigation may identify whether or not these patterns will continue to be observed. In addition, previous preprint articles can be currently published. In general, we believe that the peer review process should contribute to increase the quality of these unpublished articles with a higher risk of bias.
We have decided not to conduct a meta‐analysis because of the prevalence heterogeneity among studies and the different stages of pandemic faced in the countries and continents at the time of each survey. Thus, a summary measure of meta‐analysis would not be able to generalize overall findings sufficiently. In contrast, we found that a correspondence analysis was more able to detect the correlation among variables.
In this analysis, few consistent patterns were observed for studies with a high risk of bias, indicating that particular methodological choices of each study may affect its quality, not choices that are being made in many studies worldwide. The high number of ‘unclear’ reported (n = 53; 15.9%) may be related to the accelerated speed of publication, the forgetfulness of these items in the writing process of the manuscript or the lack of knowledge of checklists like the one used in this work. 13 Therefore, we recommend the use of standardized checklists for the planning, execution and reporting of prevalence studies. Intermediate risk of bias was associated with American and European studies, municipal and regional initiatives, blood samples, P > 1%, and internal/external validation. Low risk of bias was associated with Asian studies, nationwide initiatives, P < 1%, NPS samples and RT‐PCR tests. As correspondence analysis is a descriptive statistical analysis, we carefully examined the patterns observed and their frequency to detect only patterns that are effectively consistent.
5. CONCLUSION
To our knowledge, this is the first systematic review to summarize Covid‐19 prevalence surveys in the general population by correlating practices that can influence positively or negatively the methodological quality. Although the number of studies included were relatively low (n = 37) and the correspondence analysis presents some outliers due to the low representativeness of some categories, our findings allowed the identification of practices applied worldwide in Covid‐19 prevalence studies associated with the methodological quality. These data may assist researchers in the planning, execution and reporting of future population‐based surveys with high methodological quality.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Conceptualization: Claudia Elizabeth Thompson, Liane Nanci Rotta, Vinícius Bonetti Franceschi. Methodology: All authors. Formal analysis: Alvaro Vigo, Claudia Elizabeth Thompson, Liane Nanci Rotta, Vinícius Bonetti Franceschi. Investigation: Andressa Barreto Glaeser, Amanda de Menezes Mayer, Andressa Schneiders Santos, Ana Trindade Winck, Carem Luana Machado Lessa, Gabriel Dickin Caldana, Julia Gonçalves Küchle, Julia Gonçalves Küchle, Vinícius Bonetti Franceschi. Data curation: Vinícius Bonetti Franceschi. Writing – original draft: All authors. Writing – review and editing: Andressa Schneiders Santos, Ana Trindade Winck, Alvaro Vigo, Claudia Elizabeth Thompson, Liane Nanci Rotta, Paulo Ricardo Gazzola Zen, Vinícius Bonetti Franceschi. Visualization: Alvaro Vigo, Vinícius Bonetti Franceschi. Supervision: Ana Trindade Winck, Alvaro Vigo, Claudia Elizabeth Thompson, Liane Nanci Rotta, Paulo Ricardo Gazzola Zen.
6.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the included studies for the data provided and for important contributions in facing the pandemic in their countries.
This work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Amparo à Pesquisa do Estado do RS (FAPERGS) (Brazilian Government Agencies). The funders had no role in the study design, data generation and analysis, decision to publish or the preparation of the manuscript.
Franceschi VB, Santos AS, Glaeser AB, et al. Population‐based prevalence surveys during the Covid‐19 pandemic: a systematic review. Rev Med Virol. 2021;31(4):e2200. 10.1002/rmv.2200
DATA AVAILABILITY STATEMENT
The authors affirm that the processed data supporting our findings are available within the article and its supplementary materials. Raw data are accessible upon request to the corresponding author (Claudia Elizabeth Thompson).
References
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO Director‐General's opening remarks at the media briefing on COVID‐19. 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed October 21, 2020. [Google Scholar]
- 4. WHO coronavirus disease (COVID‐19) dashboard. https://covid19.who.int. Accessed October 21, 2020. [Google Scholar]
- 5. COVID‐19 MAP. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed October 21, 2020. [Google Scholar]
- 6. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;29. 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(3):2000045. 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention (CDC) . Coronavirus Disease 2019 (COVID‐19). 2020. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html. Published February 11, Accessed October 21, 2020. [Google Scholar]
- 9. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 382:1708‐1720. 10.1056/NEJMoa2002032. Published online February 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid‐19 – studies needed. N Engl J Med. 2020;382(13):1194‐1196. 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization . Population‐based age‐stratified seroepidemiological investigation protocol for COVID‐19 Virus Infection. 2020. https://apps.who.int/iris/handle/10665/331656. Published online 2020. Accessed October 21, 2020. [Google Scholar]
- 12. Tang Y‐W, Schmitz JE, Persing DH, Stratton CW. Laboratory diagnosis of COVID‐19: current issues and challenges. J Clin Microbiol. 2020;58(6). 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. JBI Evid Implement. 2015;13(3):147‐153. 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 14. Bastos ML, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for Covid‐19: systematic review and meta‐analysis. Br Med J. 2020;370:m2516. 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGuinness LA, Higgins JPT. Risk‐of‐bias VISualization (robvis): an R package and Shiny web app for visualizing risk‐of‐bias assessments. Res Synth Methods. 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 16. Majiya H, Aliyu‐Paiko M, Balogu VT, et al. Seroprevalence of COVID‐19 in Niger state. medRxiv. 2020. 10.1101/2020.08.04.20168112. [DOI] [Google Scholar]
- 17. Cao S, Gan Y, Wang C, et al. Citywide nucleic acid screening of SARS‐CoV‐2 infections in post‐lockdown Wuhan, China: results and implications. medRxiv. 2020. 10.1101/2020.06.29.20142554. Published online June 30. [DOI] [Google Scholar]
- 18. Malani A, Shah D, Kang G, et al. Seroprevalence of SARS‐CoV‐2 in slums and non‐slums of Mumbai, India, during June 29‐July 19, 2020. medRxiv. 2020. 10.1101/2020.08.27.20182741. Published online September 1, 2020. [DOI] [Google Scholar]
- 19. Shakiba M, Nazari SSH, Mehrabian F, Rezvani SM, Ghasempour Z, Heidarzadeh A. Seroprevalence of COVID‐19 virus infection in Guilan province, Iran. medRxiv. 2020. 10.1101/2020.04.26.20079244. Published online May 1, 2020. [DOI] [Google Scholar]
- 20. Nawa N, Kuramochi J, Sonoda S, et al. Seroprevalence of SARS‐CoV‐2 IgG antibodies in Utsunomiya city, Greater Tokyo, after first pandemic in 2020 (U‐CORONA): a household‐ and population‐based study. medRxiv. 10.1101/2020.07.20.20155945. Published online July 26, 2020. [DOI] [Google Scholar]
- 21. Qutob N, Awartani F, Salah Z, et al. Seroprevalence of SARS‐CoV‐2 in Palestine: a cross‐sectional seroepidemiological study. medRxiv. 10.1101/2020.08.28.20180083. Published online September 2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petersen MS, Strøm M, Christiansen DH, et al. Seroprevalence of SARS‐CoV‐2–specific antibodies, Faroe Islands. Emerg Infect Dis. 2020;26(11):2761‐2763. 10.3201/eid2611.202736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riley S, Ainslie KEC, Eales O, et al. Community prevalence of SARS‐CoV‐2 virus in England during May 2020: REACT study. medRxiv. 10.1101/2020.07.10.20150524. Published online July 11, 2020. [DOI] [Google Scholar]
- 24. Ward H, Atchison CJ, Whitaker M, et al. Antibody prevalence for SARS‐CoV‐2 in England following first peak of the pandemic: REACT2 study in 100,000 adults. medRxiv. 10.1101/2020.08.12.20173690. Published online August 21, 2020. [DOI] [Google Scholar]
- 25. Weis S, Scherag A, Baier M, et al. Seroprevalence of SARS‐CoV‐2 antibodies in an entirely PCR‐sampled and quarantined community after a COVID‐19 outbreak ‐ the CoNAN study. medRxiv. 10.1101/2020.07.15.20154112. Published online July 17, 2020. [DOI] [Google Scholar]
- 26. Merkely B, Szabó AJ, Kosztin A, et al. Novel coronavirus epidemic in the Hungarian population, a cross‐sectional nationwide survey to support the exit policy in Hungary. GeroScience. 2020;42(4):1063‐1074. 10.1007/s11357-020-00226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS‐CoV‐2 in the Icelandic population. N Engl J Med. 2020;382(24):2302‐2315. 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pagani G, Conti F, Giacomelli A, et al . Seroprevalence of SARS‐CoV‐2 significantly varies with age: results from a mass population screening. J Infect. 2020;S0163‐4453(20):30629. 10.1016/j.jinf.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lavezzo E, Franchin E, Ciavarella C, et al . Suppression of a SARS‐CoV‐2 outbreak in the Italian municipality of Vo. Nature. 2020;584 (7821):425–429. 10.1038/s41586-020-2488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Snoeck CJ, Vaillant M, Abdelrahman T, et al. Prevalence of SARS‐CoV‐2 infection in the Luxembourgish population: the CON‐VINCE study. medRxiv. 10.1101/2020.05.11.20092916. Published online May 18, 2020. [DOI] [Google Scholar]
- 31. Vodičar PM, Valenčak AO, Zupan B, et al. Low prevalence of active COVID‐19 in Slovenia: a nationwide population study of a probability‐based sample. Clin Microbiol Infect. 2020. 10.1016/j.cmi.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pollán M, Pérez‐Gómez B, Pastor‐Barriuso R, et al. Prevalence of SARS‐CoV‐2 in Spain (ENE‐COVID): a nationwide, population‐based seroepidemiological study. Lancet. 2020. 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brotons C, Serrano J, Fernandez D, et al. Seroprevalence against COVID‐19 and follow‐up of suspected cases in primary health care in Spain. medRxiv. 10.1101/2020.06.13.20130575. Published online June 16, 2020. [DOI]
- 34. Lundkvist Å, Hanson S, Olsen B. Pronounced difference in Covid‐19 antibody prevalence indicates cluster transmission in Stockholm, Sweden. Infect Ecol Epidemiol. 2020;10(1):1806505. 10.1080/20008686.2020.1806505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roxhed N, Bendes A, Dale M, et al. A translational multiplex serology approach to profile the prevalence of anti‐SARS‐CoV‐2 antibodies in home‐sampled blood. medRxiv. 10.1101/2020.07.01.20143966. Published online July 2, 2020. [DOI] [Google Scholar]
- 36. Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti‐SARS‐CoV‐2 IgG antibodies in Geneva, Switzerland (SEROCoV‐POP): a population‐based study. Lancet. 2020. 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sood N, Simon P, Ebner P, et al. Seroprevalence of SARS‐CoV‐2–specific antibodies among adults in Los angeles county, California, on April 10‐11, 2020. J Am Med Assoc. 2020;323(23):2425‐2427. 10.1001/jama.2020.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bendavid E, Mulaney B, Sood N, et al. COVID‐19 antibody seroprevalence in Santa Clara county, California. medRxiv. 10.1101/2020.04.14.20062463. Published online April 30, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mahajan S, Srinivasan R, Redlich CA, et al . Seroprevalence of SARS‐CoV‐2‐Specific IgG Antibodies Among Adults Living in Connecticut: post‐Infection Prevalence (PIP) Study. Am J Med. 2020. 10.1016/j.amjmed.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Biggs HM. Estimated community seroprevalence of SARS‐CoV‐2 antibodies – two Georgia Counties. MMWR Morb Mortal Wkly Rep. 2020;69. 10.15585/mmwr.mm6929e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McLaughlin C, Doll MK, Morrison KT, et al. High community SARS‐CoV‐2 antibody seroprevalence in a Ski resort community, Blaine county, Idaho, US. Preliminary results. medRxiv. 10.1101/2020.07.19.20157198. Published online July 21, 2020. [DOI] [Google Scholar]
- 42. Menachemi N. Population point prevalence of SARS‐CoV‐2 infection based on a statewide random sample — Indiana, April 25–29, 2020. MMWR Morb Mortal Wkly Rep. 2020;69. 10.15585/mmwr.mm6929e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Feehan AK, Velasco C, Fort D, et al . Racial and Workplace Disparities in Seroprevalence of SARS‐CoV‐2, Baton Rouge, Louisiana, USA. Emerging Infect Dis. 2021;27(1). 10.3201/eid2701.203808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feehan AK, Fort D, Garcia‐Diaz J, et al . Seroprevalence of SARS‐CoV‐2 and Infection Fatality Ratio, Orleans and Jefferson Parishes, Louisiana, USA, May 2020. Emerging Infect Dis. 2020;26(11):2765–2768. 10.3201/eid2611.203029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Figar S, Pagotto V, Luna L, et al. Community‐level SARS‐CoV‐2 seroprevalence survey in urban slum dwellers of Buenos aires city, Argentina: a participatory research. medRxiv. 10.1101/2020.07.14.20153858. Published online July 18, 2020. [DOI] [Google Scholar]
- 46. Hallal P, Hartwig F, Horta B, et al. Remarkable variability in SARS‐CoV‐2 antibodies across Brazilian regions: nationwide serological household survey in 27 states. medRxiv. 10.1101/2020.05.30.20117531. Published online May 30, 2020. [DOI] [Google Scholar]
- 47. Gomes CC, Cerutti C, Zandonade E, et al. A population‐based study of the prevalence of COVID‐19 infection in Espirito Santo, Brazil: methodology and results of the first stage. medRxiv. 10.1101/2020.06.13.20130559. Published online June 16, 2020. [DOI] [Google Scholar]
- 48. Silva AAMda, Neto LGL, Azevedo CMPS, et al. Population‐based seroprevalence of SARS‐CoV‐2 is more than halfway through the herd immunity threshold in the State of Maranhao, Brazil. medRxiv. 10.1101/2020.08.28.20180463. Published online September 1, 2020. [DOI] [Google Scholar]
- 49. Vieira MA da C e S, Vieira CP de B, Borba A de S, et al. Sequential serological surveys in the early stages of the coronavirus disease epidemic: limitations and perspectives. Rev Soc Bras Med Trop. 2020;53. 10.1590/0037-8682-0351-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Silveira MF, Barros AJD, Horta BL, et al. Population‐based surveys of antibodies against SARS‐CoV‐2 in Southern Brazil. Nat Med. 2020:1‐4. 10.1038/s41591-020-0992-3. Published online July 8. [DOI] [PubMed] [Google Scholar]
- 51. Tess BH, Granato CFH, Alves MCGP, et al. SARS‐CoV‐2 seroprevalence in the municipality of Sao Paulo, Brazil, ten weeks after the first reported case. medRxiv. 10.1101/2020.06.29.20142331. Published online June 29, 2020. [DOI] [Google Scholar]
- 52. Calife K, Caseiro MM, Barrosdos CRS, et al. COVID‐19 seroprevalence in Baixada Santista metropolitan area, Sao Paulo, Brazil. medRxiv. 10.1101/2020.08.28.20184010. Published online September 1, 2020. [DOI] [Google Scholar]
- 53. Draugalis JR, Coons SJ, Plaza CM. Best practices for survey research reports: a synopsis for authors and reviewers. Am J Pharm Educ. 2008;72(1). 10.5688/aj720111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. So R, Shinohara K, Aoki T, Tsujimoto Y, Suganuma AM, Furukawa TA. Effect of recruitment methods on response rate in a web‐based study for primary care physicians: factorial randomized controlled trial. J Med Internet Res. 2018;20(2):e28. 10.2196/jmir.8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cook C, Heath F, Thompson RL. A Meta‐Analysis of Response Rates in Web‐ or Internet‐Based Surveys. Educ Psychol Meas. 2000;60 (6):821–836. 10.1177/00131640021970934. [DOI] [Google Scholar]
- 56. Naing L, Winn T, Rusli BN. Practical issues in calculating the sample size for prevalence studies. Arch Orofacial Sci. 2006;1:9‐14. [Google Scholar]
- 57. Tahamtan A, Ardebili A. Real‐time RT‐PCR in COVID‐19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453‐454. 10.1080/14737159.2020.1757437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leung GM, Lim WW, Ho L‐M, et al. Seroprevalence of IgG antibodies to SARS‐coronavirus in asymptomatic or subclinical population groups. Epidemiol Infect. 2006;134(2):211‐221. 10.1017/S0950268805004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Degnah AA, Al‐amri SS, Hassan AM, et al. Seroprevalence of MERS‐CoV in healthy adults in western Saudi Arabia, 2011–2016. J Infect Public Health. 2020;13(5):697‐703. 10.1016/j.jiph.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Abuelgasim E, Saw LJ, Shirke M, Zeinah M, Harky A. COVID‐19: unique public health issues facing Black, Asian and minority ethnic communities. Curr Probl Cardiol. 2020;45(8):100621. 10.1016/j.cpcardiol.2020.100621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Millett GA, Jones AT, Benkeser D, et al. Assessing differential impacts of COVID‐19 on black communities. Ann Epidemiol. 2020;47:37‐44. 10.1016/j.annepidem.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID‐19 in Brazil: a cross‐sectional observational study. Lancet Glob Health. 2020;8(8):e1018‐e1026. 10.1016/S2214-109X(20)30285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pinto AS, Rodrigues CA, Sobrinho CL, et al. Covid‐19 epidemic curve in Brazil: a sum of multiple epidemics, whose income inequality and population density in the states are correlated with growth rate and daily acceleration. medRxiv. 10.1101/2020.09.09.20191353. Published online September 14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The authors affirm that the processed data supporting our findings are available within the article and its supplementary materials. Raw data are accessible upon request to the corresponding author (Claudia Elizabeth Thompson).


