Angiotensin converting enzyme‐2 (ACE‐2) is the cell‐surface receptor enabling viral uptake of corona virus 2019 (SARS‐CoV‐2), thus ACE‐2 is a first step towards COVID‐19 disease. ACE‐2 is a metalloenzyme located primarily on the apical surface, and serves as the entry point also for other coronaviruses, including HCoV‐NL63 and SARS‐CoV. Throughout evolution, ACE‐2 precedes renin, suggesting that ACE‐2’s role changed over time. 1
The renin angiotensin system (RAS) and COVID‐19 are intertwined. 2 Thus, various concerns come up regarding the safety of angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) for COVID‐19 patients. Interestingly, analogous concerns have not been raised for the Middle‐East Respiratory Syndrome (MERS), although MERS is also caused by a corona virus that is incorporated into cells, yet not by ACE‐2, but by dipeptidyl peptidase‐4. 3
Novel coronavirus pneumonia is a new type of infectious disease, caused by SARS‐CoV‐2. The lungs are the first and primarily affected target organs of SARS‐CoV‐2. After infecting the lungs, the infiltrating viruses may enter the circulation and reach the kidneys, where they can accumulate and damage resident cells. 4 Impaired glomerular filtration also occurs, usually manifested by increased blood creatinine and urea nitrogen levels, and moderate proteinuria. The incidence of reported acute kidney injury varies widely because of the different populations included in different studies, and is approximately 0.1%‐29%. High expression of ACE‐2 in the kidney, especially in podocytes and proximal tubular epithelial cells, may be related to the susceptibility of renal parenchymal cells and direct viral invasion of the renal parenchyma after COVID‐19 infection. However, in fact live virus have not been recovered from urine and viral mRNA has not been amplified from kidney biopsies.
Under physiological conditions, ACE‐2 converts ANGII to angiotensin (1‐7). Angiotensin (1‐7) changes the balance from ANGII‐induced vasoconstriction to Mas receptor‐activated vasodilation after extensive binding to Mas receptors. In addition, activation of the angiotensin (1‐7)/ACE‐2 axis inhibits the production of reactive oxygen species and downregulates secretion of pro‐inflammatory cytokines. Accordingly, binding of SARS‐CoV‐2 to ACE‐2 may weaken the activity of the latter and shift ACE/ACE‐2 balance to a state of enhanced ANGII activity, which leads to vasoconstriction and inflammatory 5 and oxidative organ damage, and further promotes damage. 6
The use of RAS inhibitors can increase expression of ACE‐2 on the tissue and apical surface to a certain extent. Therefore, the use of ACEIs or ARBs to treat diseases is considered likely to increase the risk of SARS‐CoV‐2 exposure. However, so far, there is no experimental evidence indicating that ACEIs or ARBs augment the susceptibility to SARS‐CoV‐2 or aggravate the outcomes and severity of COVID‐19. Accordingly, large observational studies have not shown any association with increased COVID‐19 risk and patients who are receiving ACE inhibitors or ARBs. 7
After SARS‐CoV infection in mice, ACE‐2 protein levels are greatly reduced, depending on virus replication, which is consistent with the situation in host cells. 8 Additionally, SARS‐CoV‐2 can weaken the activity of ACE‐2 by binding to ACE‐2. In consequence, elevated ANGII further aggravates kidney injury by activating AT1R, causing inflammation and fibrosis. Inhibiting RAS by ACEIs/ARBs or recombinant ACE‐2 increases expression of ACE‐2, thereby reducing ANGII levels and enhancing Ang‐(1‐7) generation. Increased ACE‐2 expression promotes the activation of Mas receptor (MasR), which in turn attenuates inflammation and fibrosis, and therefore attenuates kidney injury. 9 Moreover, the increase in the level of soluble ACE‐2 may also neutralize SARS‐CoV‐2, limit virus entry and reduce tissue damage. Therefore, providing RAS inhibitors seems advantageous for COVID‐19 patients (Figure 1A). Indeed, observational cohort studies, and randomized trials for other endpoints provide no evidence for adverse effects RAS inhibition in COVID‐19. 7 Thus, RAS inhibitors should not be withheld from patients because of any SARS‐CoV‐2 concerns. 10 , 11 The contrary may hold true.
FIGURE 1.
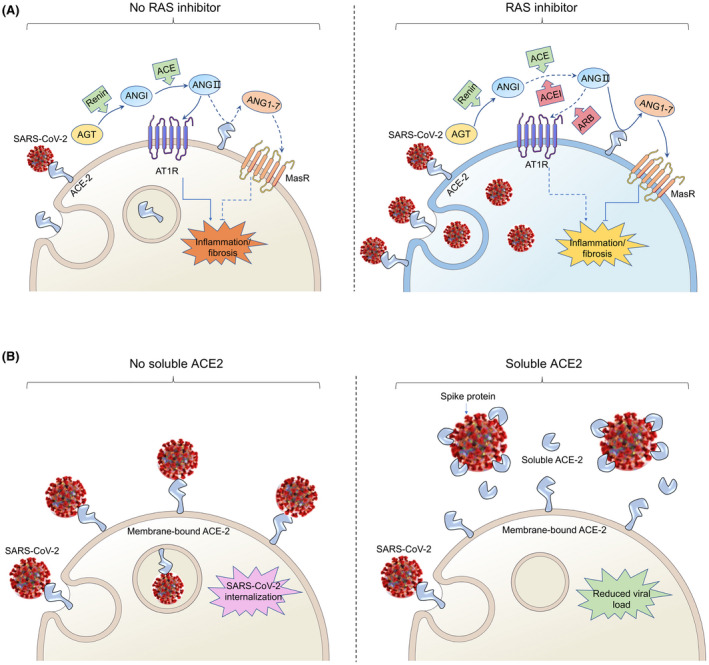
Possible effects of COVID‐19 on kidney RAS and its treatment. A, SARS‐CoV‐2 gains entry into the cell by binding to angiotensin‐converting enzyme 2 (ACE‐2) and weakens the activity of ACE‐2, then elevates angiotensin II (ANGII), which drives kidney injury by activating the angiotensin II type 1 receptor (AT1R), causing inflammation and fibrosis (left panel). The addition of angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) could increase ACE‐2 abundance and thus enhance viral entry, and diminish the production of ANGII. Enhanced angiotensin‐(1‐7) generation takes place by ACE‐2, which promotes the activation of Mas receptor (MasR), thus attenuating inflammation and fibrosis (right panel). B, SARS‐CoV‐2 gains entry into the cell by binding to angiotensin‐converting enzyme 2 (ACE‐2) (left panel). Human recombinant soluble ACE‐2 (hrsACE‐2) binds to Spike protein can reduce binding to ACE‐2 at the membrane, thus inhibiting SARS‐CoV‐2 internalization (right panel)
Monteil et al 12 reported that infection with SARS‐CoV‐2 can be inhibited with a human recombinant soluble ACE‐2 (hrsACE‐2). The hrsACE‐2 reduced SARS‐CoV‐2 recovery from Vero cells by a factor of 1000‐5000. The investigators also showed that SARS‐CoV‐2 can directly infect engineered human blood vessel organoids and human kidney organoids, which can be inhibited by hrsACE‐2. Other sophisticated approaches are suggested by Chan et al 13 By using deep mutagenesis, that group identified mutations in ACE‐2 that increased spike protein binding across the interaction surface, in the asparagine 90‐glycosylation motif, and at buried sites. The mutational landscape provided the group with a blueprint for understanding the specificity of the interaction between ACE‐2 and spike protein, as well as for engineering high‐affinity decoy receptors. Combining mutations brought up ACE‐2 variants affinities that rival those of monoclonal antibodies. A stable dimeric variant reveals potent SARS‐CoV‐2 and SARS‐CoV neutralization in vitro. The engineered soluble ACE‐2 receptor was catalytically active, and its close similarity with the native receptor could limit the potential for viral escape (Figure 1B). These novel approaches could help us therapeutically in the future.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENT
We would like to thank the support of Zhejiang Provincial Key Laboratory of Immunity and Inflammatory diseases.
Contributor Information
Pontus B. Persson, Email: pontus.persson@charite.de.
Jianhua Mao, Email: maojh88@zju.edu.cn.
REFERENCES
- 1. Fournier D, Luft FC, Bader M, Ganten D, Andrade‐Navarro MA. Emergence and evolution of the renin‐angiotensin‐aldosterone system. J Mol Med (Berl). 2012;90(5):495‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Imai Y, Kuba K, Rao S, et al. Angiotensin‐converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang N, Shi X, Jiang L, et al. Structure of MERS‐CoV spike receptor‐binding domain complexed with human receptor DPP4. Cell Res. 2013;23(8):986‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun J, Zhu A, Li H, et al. Isolation of infectious SARS‐CoV‐2 from urine of a COVID‐19 patient. Emerg Microbes Infect. 2020;9(1):991‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID‐19. J Infect. 2020;80(6):607‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu N, Jiang S, Persson PB, Persson EAG, Lai EY, Patzak A. Reactive oxygen species in renal vascular function. Acta Physiol (Oxf). 2020;229(4):e13477. [DOI] [PubMed] [Google Scholar]
- 7. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with covid‐19. N Engl J Med. 2020;382(17):1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11(8):875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ali Q, Dhande I, Samuel P, Hussain T. Angiotensin type 2 receptor null mice express reduced levels of renal angiotensin II type 2 receptor/angiotensin (1–7)/Mas receptor and exhibit greater high‐fat diet‐induced kidney injury. J Renin Angiotensin Aldosterone Syst. 2016;17(3):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bozkurt B, Kovacs R, Harrington B. Joint HFSA/ACC/AHA statement addresses concerns Re: using RAAS antagonists in COVID‐19. J Card Fail. 2020;26(5):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiong S, Liu L, Lin F, et al. Clinical characteristics of 116 hospitalized patients with COVID‐19 in Wuhan, China: a single‐centered, retrospective, observational study. BMC Infect Dis. 2020;20(1):787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monteil V, Kwon H, Prado P, et al. Inhibition of SARS‐CoV‐2 infections in engineered human tissues using clinical‐grade soluble human ACE2. Cell. 2020;181(4):905‐913.e907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan KK, Dorosky D, Sharma P, et al. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science. 2020;369(6508):1261‐1265. [DOI] [PMC free article] [PubMed] [Google Scholar]


