Abstract
Introduction
The OM‐85 (Broncho‐Vaxom) consumption has drawn considerable attention in the prevention of recurrent respiratory tract infections. However, it has been reported that the relationship between OM‐85 consumption and recurrent respiratory tract infections is variable. This meta‐analysis was performed to evaluate this relationship.
Methods
A systematic literature search up‐to May 2020 was performed and 14 studies were detected with 1859 paediatric subjects, of them 890 consumed OM‐85. They were reporting relationships between OM‐85 consumption and recurrent respiratory tract infections. Odds ratio (OR) or mean differences (MD) with 95% confidence intervals (CIs) was calculated to evaluate the prognostic role of OM‐85 consumption and recurrent respiratory tract infections using the dichotomous or continuous method with a random or fixed‐effect model.
Results
OM‐85 consumption was significantly related to lower frequency of respiratory tract infections (MD, −1.16; 95% CI, −1.66 to −0.65, P < .001); lower total duration of respiratory tract infections (MD, −19.51; 95% CI, −23.00 to −16.01, P < .001); lower incidence of respiratory tract infections (OR, 0.40; 95% CI, 0.21‐0.77, P = .006); lower number of antibiotic courses (MD, −1.40; 95% CI, −2.63 to 0.17, P = .03); and lower antibiotic use (OR, 0.38; 95% CI, 0.29‐0.52, P < .001). However, OM‐85 consumption was not significantly related to adverse event rate (OR, 1.02; 95% CI, 0.52‐2.03, P = .94); or to wheezing attacks frequency (MD, −0.25; 95% CI, −0.59 to 0.08, P = .14).
Conclusions
The impact of OM‐85 consumption on recurrent respiratory tract infections may have a great effect as a tool to improve subjects’ immunity against recurrent respiratory tract infections, which could be helpful in crucial situations, eg, COVID‐19 pandemic. OM‐85 non‐consumers had an independent risk relationship with recurrent respiratory tract infections. This relationship forces us to recommend OM‐85 consumption with those with a high risk of recurrent respiratory tract infections to avoid any possible complications.
What’s new
The OM‐85 (Broncho‐Vaxom) consumption has drawn considerable attention in the prevention of recurrent respiratory tract infections. However, it has been reported that the relationship between OM‐85 consumption and recurrent respiratory tract infections is variable. This meta‐analysis was performed to evaluate this relationship.
What’s known
The impact of OM‐85 consumption on recurrent respiratory tract infections may have a great effect as a tool to improve subjects’ immunity against recurrent respiratory tract infections, which could be helpful in crucial situations, eg, COVID‐19 pandemic. OM‐85 non‐consumers had an independent risk relationship with recurrent respiratory tract infections. This relationship forces us to recommend OM‐85 consumption with those with a high risk of recurrent respiratory tract infections to avoid any possible complications.
1. BACKGROUND
A respiratory tract infection is an infectious disease in the respiratory tract, upper respiratory tract infection, or lower respiratory tract infection. Respiratory tract infections can affect the sinuses, throat, airways, or lungs. Lower respiratory infections tend to be far more serious conditions than upper respiratory infections. 1 Most respiratory tract infections get better without treatment, but sometimes patients may need critical care. 1 Recurrent respiratory tract infections are one of the common diseases in children. It is upper or lowers respiratory tract infections that occur usually per year, though, the perception of repetition remains unclear. According to the guidelines of the Dutch College of General Practitioners referral for recurrent respiratory tract infection is designated if acute otitis media occurs >4 times per year, sore throat occurs >5 times per year, or if otitis media with effusion continues for >6 months. 1 The period of recurrent respiratory tract infections is longer and it may disturb children's growth and increase the suffering from other respiratory diseases through adulthood.
The pathogenesis of recurrent respiratory tract infections is complex by the variability of microbial causes, immunology, and respiratory diseases. 2 These days, there is a great concern about respiratory tract infection prevention due to the COVID‐19 pandemic. Some studies recommend an association between the Bacillus Calmette‐Guérin (BCG) vaccine and the malaria treatment to the prevention of SARS‐CoV‐2 infection. Other related infant's lower vulnerability to the BCG vaccine. Also, some other reports claimed the possible use of chloroquine and hydroxychloroquine as prophylactic in such a pandemic. 3
OM‐85 (Broncho‐Vaxom) is not directly related to COVID‐19 but can help to reduce the incidence of respiratory tract infections. OM‐85 is an orally administered immunomodulator consists of the lyophilised bacterial lysate of eight pathogenic bacteria of the respiratory tract. OM‐85 stimulates immune defences and the manufacture of salivary IgA, bronchoalveolar IgA, and serum IgA and IgG 4 ; it has been used since 1980 to both adults and children to stop recurrences of respiratory tract infections; also, to increasing IgA and IgG; OM‐85 has shown other immunomodulating properties, such as inducing the terminal maturation of human dendritic cells with an enhanced T cell‐stimulatory capacity, 5 up‐regulating the Th1‐specific cytokine IFN‐γ, and the down‐regulating the Th2‐specific cytokine IL‐4. 6 All these properties could activate various systems in the chain of immunologic defence reactions.
Meanwhile, the outcomes of most of the studies on OM‐85 are inconsistent and the sample sizes are small; this meta‐analysis of randomised controlled trials of OM‐85 used in paediatric respiratory tract infections was to assess the efficacy and safety of OM‐85 consumption and to deliver evidence for clinical use of OM‐85 in paediatric respiratory tract infections suggest its use in crucial situations, eg, the COVID‐19 pandemic.
2. METHODS
The study performed here followed the meta‐analysis of studies in the epidemiology statement, 7 which was conducted following an established protocol.
2.1. Study selection
Studies included were randomised controlled trials assessing the efficacy and safety of OM‐85 (Broncho‐Vaxom) on paediatric recurrent respiratory tract infections. Studies included were that stated statistical measures of relationship (odds ratio [OR], incidence rate ratio, or relative risk, with 95% confidence intervals [CIs]) between OM‐85 consumption and its efficacy and safety in paediatric recurrent respiratory tract infections.
Only human studies in the English language were considered. Inclusion was not limited by study size or publication type. Publications excluded were review articles and commentary and studies that did not deliver a measure of association. Figure 1 shows the whole study process.
FIGURE 1.
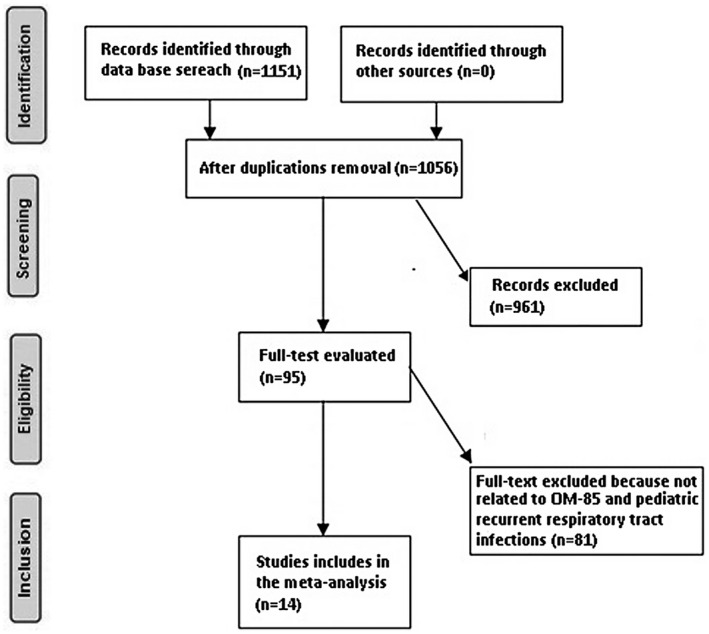
Schematic diagram of the study procedure
2.2. Identification
First, we conducted a systematic search of OVID, Embase, Cochrane Library, PubMed, and Google scholar, till May 2020, using a blend of keywords and similar words for OM‐85, Broncho‐Vaxom, paediatric recurrent respiratory tract infections, antibiotic use, adverse drug reactions, and wheezing. All identified studies were pooled in an EndNote file, duplicates were omitted, and the title and abstracts were reviewed to exclude studies that did not report the association between OM‐85 consumption and its efficacy and safety in paediatric recurrent respiratory tract infections, based on pre‐specified inclusion and exclusion criteria. The remaining articles were reviewed for associated information.
2.3. Screening
Data were abridged on the following bases; study‐related and subject‐related characteristics onto a standardised form. last name of the primary author, period of study, year of publication, country, region of the studies, and study design; population type, total number and number of OM‐85 consumers, demographic data and clinical and treatment characteristics; paediatric recurrent respiratory tract infections characteristics, the period of evaluating OM‐85 consumption related to paediatric recurrent respiratory tract infections, qualitative and quantitative method of evaluation, information source, and outcome evaluation; and statistical analysis OR or relative risk, with 95% CI, of association between OM‐85 efficacy and safety in paediatric recurrent respiratory tract infections. 8 When there were different data from one study, we extracted them independently. The risk of bias in these studies; individual studies were evaluated using the quality in prognosis studies tool, which evaluates validity and bias in studies of prognostic factors across six domains: participation, attrition, prognostic factor measurement, confounding measurement, and account, outcome measurement, and analysis and reporting. 9 Any inconsistencies were addressed by a reevaluation of the original article.
2.4. Eligibility
The main outcome focused on the efficacy and safety of OM‐85 on paediatric recurrent respiratory tract infections. Evaluation of efficacy and safety of OM‐85 consumption compared with the control of paediatric recurrent respiratory tract infections was extracted to form a summary.
2.5. Inclusion
Sensitivity analyses were limited only to studies reporting the relationship between OM‐85 consumption and its efficacy and safety in paediatric recurrent respiratory tract infections subjects. For subcategory and sensitivity analysis, we used OM‐85 consumers compared with control for comparisons between paediatric recurrent respiratory tract infections as reference.
2.6. Statistical analysis
The dichotomous or continuous method with a random‐effect model or fixed‐effect was used to calculate the OR or Mean differences (MD) and 95% CI. The I 2 index was calculated; the I 2 index is between 0% and 100%. Values of about 0%, 25%, 50%, and 75% indicate no, low, moderate, and high heterogeneity, respectively. 10 When I 2 was higher than 50%, we chose the random effect model; when it was lower than 50%, we used the fixed‐effect model. A subcategory analysis was completed by stratifying the original evaluation per outcome categories as described before. In this analysis, a P‐value for differences between subcategories of <.05 was considered statistically significant. Publication bias was evaluated quantitatively using the Egger regression test (publication bias considered present if P ≥ .05), and qualitatively, by the visual examination of funnel plots of the logarithm of ORs or MD vs their standard error (SE). 8 All P‐values were two‐tailed. All calculations and graphs were performed using reviewer manager version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
3. RESULTS
A total of 1151 unique studies were identified, of which 14 studies (between 1984 and 2020) fulfilled the inclusion criteria and were included in the study. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25
The 14 studies included 1859 paediatrics with 890 OM‐85 paediatric consumers. All studies had paediatric recurrent respiratory tract infections.
Study size ranged from 43 to 400 paediatric followed for any possible recurrent respiratory tract infections at the start of the study as shown in Table 1. Fifteen studies reported data stratified subjects by the OM‐85 consumption related to the frequency of respiratory tract infections; three studies related to the total duration of respiratory tract infections; four studies related to the incidence of respiratory tract infections; five studies related to the adverse event rate; four studies related to the number of antibiotic courses; four studies related to antibiotic use; and three studies for the frequency of wheezing attacks. The impact of OM‐85 consumption on recurrent respiratory tract infections was observed in all populations studied.
TABLE 1.
Characteristics of the selected studies for the meta‐analysis
| Study | Total | OM‐85 consumers | Country |
|---|---|---|---|
| Maestroni 17 | 20 | 11 | Switzerland |
| Zagar 18 | 55 | 29 | Yugoslavia |
| Paupe 19 | 116 | 61 | France |
| Jara‐Pérez 20 | 200 | 99 | Mexico |
| Esposito 2 12 | 288 | 100 | Italy |
| Gutiérrez‐Tarango 21 | 54 | 26 | Mexico |
| Schaad 22 | 232 | 120 | Switzerland and Germany |
| Del‐Río‐Navarro 23 | 43 | 22 | Mexico |
| Razi 24 | 75 | 35 | Turkey |
| Bitar 16 | 177 | 87 | Lebanon |
| Chen 25 | 96 | 48 | China |
| Esposito 1 14 | 400 | 200 | Italy |
| Sly 15 | 49 | 25 | Australia |
| Souza 13 | 54 | 27 | Brazil |
| Total | 1859 | 890 | — |
OM‐85 consumption was significantly related to lower frequency of respiratory tract infections (MD, −1.16; 95% CI, −1.66 to −0.65, P < .001) with high heterogeneity (I 2 = 92%); lower total duration of respiratory tract infections (MD, −19.51; 95% CI, −23.00 to −16.01, P < .001) with no heterogeneity (I 2 = 0%); lower incidence of respiratory tract infections (OR, 0.40; 95% CI, 0.21‐0.77, P = .006) with moderate heterogeneity (I 2 = 72%); lower number of antibiotic courses (MD, −1.40; 95% CI, −2.63 to 0.17, P = .03) with high heterogeneity (I 2 = 96%); and lower antibiotic use (MD, 0.38; 95% CI, 0.29‐0.52, P < .001) with no heterogeneity (I 2 = 14%)as shown in Figures 2 and 3.
FIGURE 2.
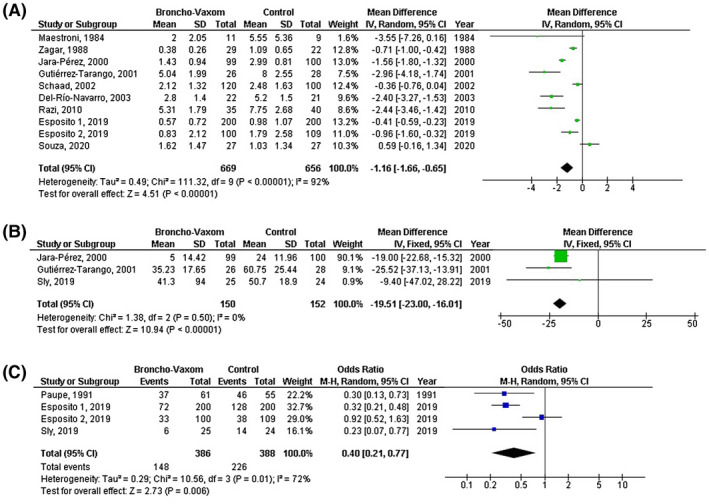
Forest plot of the OM‐85 consumption compared with the control of paediatric recurrent respiratory tract infections related to A) frequency of respiratory tract infections, B) total duration of respiratory tract infections, C) incidence of respiratory tract infections
FIGURE 3.
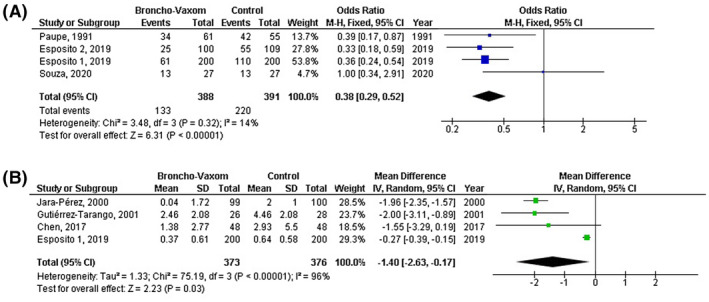
Forest plot of the OM‐85 consumption compared with the control of paediatric recurrent respiratory tract infections related to A) antibiotic uses, B) number of antibiotic courses
However, OM‐85 consumption was not significantly related to higher or lower adverse event rate (OR, 1.02; 95% CI, 0.52‐2.03, P = .94) with no heterogeneity (I 2 = 0%); or to wheezing attacks frequency (MD, −0.25; 95% CI, −0.59 to 0.08, P = .14) with high heterogeneity (I 2 = 90%) as shown in Figure 4.
FIGURE 4.
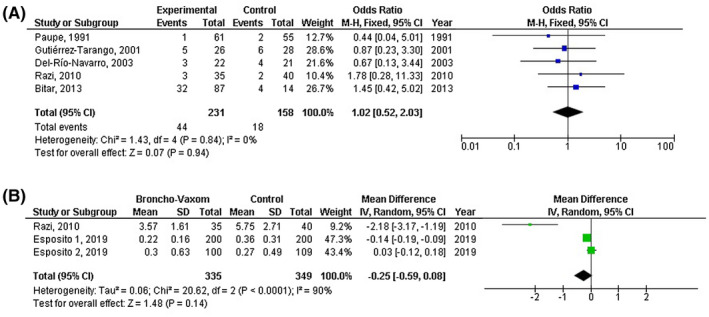
Forest plot of the OM‐85 consumption compared with the control of paediatric recurrent respiratory tract infections related to A) adverse event rate, B) wheezing attacks frequency
Stratified analysis of studies adjust for OM‐85 consumption linked to reduction in fever rate, 22 reduction in incidence acute nasopharyngitis, 12 , 24 and effect on IgM and IgG were not performed because the studies reported or adjusted for these factors were few.
Based on the visual inspection of the funnel plot as well as on quantitative measurement using the Egger regression test, there was no evidence of publication bias (P = .84).
4. DISCUSSION
The OM‐85 consumption was examined for its efficacy and safety of paediatric recurrent respiratory tract infections with diverse results. Previous studies reported the efficacy and safety of OM‐85 consumption in recurrent respiratory tract infections; however, their results were conflicting. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25
In this meta‐analysis study based on 14 studies in 1859 paediatrics with 842 OM‐85 paediatric consumers, we showed that OM‐85 consumption is significantly related to a lower frequency of respiratory tract infections, a lower total duration of respiratory tract infections, and lower incidence of respiratory tract infection. This effect was observed primarily in all populations studied. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 This finding suggests that OM‐85 consumption protects against recurrent respiratory tract infections and could be of help in a pandemic such as the COVID‐19 pandemic to reduce and accompanied supra‐infection.
The results also suggested that the OM‐85 consumption was positively related to a lower number of antibiotic courses, and lower antibiotic use reflecting higher efficacy against recurrent respiratory tract infections. However, no relation was found between OM‐85 consumption and high adverse event rate or frequency wheezing attacks. That could be due to the low number of studies that fulfilled the requirement of our meta‐analysis with the relationship between OM‐85 consumption and wheezing attack frequency (three studies). The frequency wheezing attacks results may differ if more studies were found with such a relationship since the P‐value was low (P = .14) although for the relation between OM‐85 consumption and high adverse event rate (five studies) it is not expected to find any possible significant relation since the P‐value is almost equal 1 (P = .94). A previous meta‐analysis found OM‐85 consumption with a more adverse even rate; however, we could not find this significant here. That might be because their study comprises 53 studies, 42 of them were from China. Hence the adverse events found could be specific for Chinese paediatrics and that worth further study. 26
Respiratory tract infections are vital causes of illness, death, and disability in children 27 , 28 and consequently are one of the major costs load on the healthcare system. 29 The pathogenesis of recurrent respiratory tract infections is complex and has many factors, eg, anatomical and physiological features of the respiratory system, lack of vitamins, trace elements or calcium, genetic, environmental factors, and reduced immunity. 30 To treat recurrent respiratory tract infections in children, probable pathogenic factors such as environmental and other manageable factors should be stopped to improve nutrition and physical fitness and add trace elements and vitamins; anti‐bacterial or antiviral regiments could be started in the acute stage. 30 So, immunomodulator agents, such as OM‐85 are recommended in recurrent respiratory tract infections. 31
Most of the recurrent respiratory tract infections are caused by viruses, but these are often followed by bacterial supra‐infection. 32 Irrespective of whether they are viruses or bacteria, the occurrence of pathogens in the respiratory tract activates the innate and adaptive immune systems. 33 A key event amongst the actions of the adaptive immune system is the production of IgA molecules. 33 As a result, patients with impaired immunity and chronic inflammation are at greater risk of respiratory tract infections. 34
OM‐85 is an immunomodulator that consists of lyophilised bacterial lysates from 21 different bacterial strains from the eight‐major species that are most often related to respiratory tract infections. 35 , 36 Previous studies indicate that OM‐85 decreased the frequency of recurrent respiratory tract infections and improved immune function. The production of antigen‐specific memory CD4+ T cells could up‐regulate T helper type 1 (Th1) immune responses, increasing more effective anti‐microbial defences in the long‐term. 35 , 36 previous studies have reported the advantage of OM‐85 in respiratory tract infection reduction; however, the sample sizes of most studies were small. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 A systematic quantitative review of 13 clinical trials evaluating OM‐85 reported favouring OM‐85 against acute respiratory tract infection in children. 37 In 2010 Schaad completed a systematic review to evaluate the effectiveness of OM‐85 for stopping the incidence of respiratory tract infections in paediatric populations. 38 They showed a 26.2% reduction of recurrent respiratory tract infections with OM‐85. In a recent meta‐analysis, 19 studies, the results indicated that through a whole year, compared with the routine regiment, the inclusion of OM‐85 reduced the occurrence of acute respiratory tract infection. 39
The reasons for the effect of OM‐85 consumption on paediatric recurrent respiratory tract infections are likely to be multi‐factorial. Unfortunately, no study adjusted for further relations, so it was hard to draw out any of these multi‐factors effects of OM‐85 consumption on paediatric recurrent respiratory tract infections. Few studies answered whether an OM‐85 consumption is linked to a reduction in fever rate, 22 reduction in incidence acute nasopharyngitis, 12 , 24 and effect on IgM and IgG, 17 so, we could not draw solid outcomes related to these factors.
From the study presented here, we recommend increasing the use of OM‐85 consumption against paediatric recurrent respiratory tract infections since the OM‐85 is easily available and safe especially in pandemics related to respiratory tract infections such as the COVID‐19 pandemic to oppose the possible negative result as early as possible.
4.1. Limitations
The sample size was small in most of the included studies. The 14 included studies recruited subjects in each study were mostly with one ethnicity. There may be selection bias in this study since so many of the studies found were excluded from the meta‐analysis. However, the studies excluded did not satisfy the inclusion criteria of our meta‐analysis. Also, the included studies recruited subjects with different disease ages, and treatment strategies, which could result in heterogeneity in the meta‐analysis. The subgroup analysis showed that significant heterogeneity still exists in different sample size groups.
Also, we could not answer whether OM‐85 consumption is linked to a reduction in fever rate, reduction in the incidence of acute nasopharyngitis, and an effect on IgM and IgG.
5. CONCLUSIONS
The impact of OM‐85 consumption on paediatric recurrent respiratory tract infections may have a positive effect as a tool to improve subjects’ immunity against paediatric recurrent respiratory tract infections. OM‐85 non‐consumers had an independent risk relationship with recurrent respiratory tract infections. This relationship forces us to recommend OM‐85 consumption with those with a high risk of recurrent respiratory tract infections to avoid any possible complications especially in such a COVID‐19 pandemic that mainly affects the respiratory tract.
AVAILABILITY OF DATA AND MATERIALS
The datasets analysed during the current study are available from the corresponding author on reasonable request.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
Mohamed EA Abdelrahim, Yi Zhu: Conception and design. All authors involved in the Administrative support, Provision of study materials or patients, Data analysis and interpretation; Manuscript writing; Final approval of manuscript. All authors have read and approved the manuscript. Changqing Cao, Jinghua Wang, Yuning Li, Yumei Li, Liyan Ma: Collection and assembly of data.
Cao C, Wang J, Li Y, et al. Efficacy and safety of OM‐85 in paediatric recurrent respiratory tract infections which could have a possible protective effect on COVID‐19 pandemic: A meta‐analysis. Int J Clin Pract. 2021;75:e13981. 10.1111/ijcp.13981
Funding Information
There was no external funding for this study itself. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
DATA AVAILABILITY STATEMENT
The datasets analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Damoiseaux R, et al. NHG‐standaard otitis media acuta bij kinderen. Huisarts Wet. 2006;49:615‐621. [Google Scholar]
- 2. Schaad UB, Esposito S, Razi CH. Diagnosis and management of recurrent respiratory tract infections in children: a practical guide. Arch Pediatr Infect Dis. 2016;4:e31039. [Google Scholar]
- 3. Osama El‐Gendy A, et al. Bacillus Calmette‐Guérin vaccine, antimalarial, age and gender relation to COVID‐19 spread and mortality. Vaccine. 2020;38:5564‐5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koatz AM, et al. Clinical and immunological benefits of OM‐85 bacterial lysate in patients with allergic rhinitis, asthma, and COPD and recurrent respiratory infections. Lung. 2016;194:687‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zelle‐Rieser C, et al. A clinically approved oral vaccine against pneumotropic bacteria induces the terminal maturation of CD83+ immunostimulatory dendritic cells. Immunol Lett. 2001;76:63‐67. [DOI] [PubMed] [Google Scholar]
- 6. Huber M, Mossmann H, Bessler W. Th1‐orientated immunological properties of the bacterial extract OM‐85‐BV. Eur J Med Res. 2005;10:209‐217. [PubMed] [Google Scholar]
- 7. Stroup DF, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008‐2012. [DOI] [PubMed] [Google Scholar]
- 8. Gupta A, et al. Obesity is independently associated with increased risk of hepatocellular cancer‐related mortality. Am J Clin Oncol. 2018;41:874‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayden JA, et al. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280‐286. [DOI] [PubMed] [Google Scholar]
- 10. Higgins JP, et al. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Esposito S, et al. Nonspecific immunomodulators for recurrent respiratory tract infections, wheezing and asthma in children: a systematic review of mechanistic and clinical evidence. Curr Opin Allergy Clin Immunol. 2018;18:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esposito S, et al. A randomized, placebo‐controlled, double‐blinded, single‐centre, phase IV trial to assess the efficacy and safety of OM‐85 in children suffering from recurrent respiratory tract infections. J Transl Med. 2019;17:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Souza, FCD , et al. OM‐85 BV for primary prevention of recurrent airway infections: a pilot randomized, double‐blind, placebo‐controlled study. Einstein (São Paulo). 2020;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Esposito S, et al. Impact of OM‐85 given during two consecutive years to children with a history of recurrent respiratory tract infections: a retrospective study. Int J Environ Res Public Health. 2019;16:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sly PD, et al. Primary prevention of severe lower respiratory illnesses in at‐risk infants using the immunomodulator OM‐85. J Allerg Clin Immunol. 2019;144:870‐872.e11. [DOI] [PubMed] [Google Scholar]
- 16. Bitar MA, Saade R. The role of OM‐85 BV (Broncho‐Vaxom) in preventing recurrent acute tonsillitis in children. Int J Pediatr Otorhinolaryngol. 2013;77:670‐673. [DOI] [PubMed] [Google Scholar]
- 17. Maestroni GJ, Losa GA. Clinical and immunobiological effects of an orally administered bacterial extract. Int J Immunopharmacol. 1984;6:111‐117. [DOI] [PubMed] [Google Scholar]
- 18. Zagar S, Löfler‐Badzek D. Broncho‐Vaxom® in children with rhinosinusitis: a double‐blind clinical trial. ORL. 1988;50:397‐404. [DOI] [PubMed] [Google Scholar]
- 19. Paupe J. Immunotherapy with an oral bacterial extract (OM‐85 BV) for upper respiratory infections. Respiration. 1991;58:150‐154. [DOI] [PubMed] [Google Scholar]
- 20. Jara‐Pérez JV, Berber A. Primary prevention of acute respiratory tract infections in children using a bacterial immunostimulant: a double‐masked, placebo‐controlled clinical trial. Clin Ther. 2000;22:748‐759. [DOI] [PubMed] [Google Scholar]
- 21. Gutiérrez‐Tarango MD, Berber A. Safety and efficacy of two courses of OM‐85 BV in the prevention of respiratory tract infections in children during 12 months. Chest. 2001;119:1742‐1748. [DOI] [PubMed] [Google Scholar]
- 22. Schaad UB, et al. Immunostimulation with OM‐85 in children with recurrent infections of the upper respiratory tract: a double‐blind, placebo‐controlled multicenter study. Chest. 2002;122:2042‐2049. [DOI] [PubMed] [Google Scholar]
- 23. Del‐Río‐Navarro BE, et al. Use of OM‐85 BV in children suffering from recurrent respiratory tract infections and subnormal IgG subclass levels. Allergol Immunopathol. 2003;31:7‐13. [DOI] [PubMed] [Google Scholar]
- 24. Razi CH, et al. The immunostimulant OM‐85 BV prevents wheezing attacks in preschool children. J Allerg Clin Immunol. 2010;126:763‐769. [DOI] [PubMed] [Google Scholar]
- 25. Chen J, et al. Bacterial lysate for the prevention of chronic rhinosinusitis recurrence in children. J Laryngol Otol. 2017;131:523. [DOI] [PubMed] [Google Scholar]
- 26. Yin J, et al. Broncho‐Vaxom in pediatric recurrent respiratory tract infections: a systematic review and meta‐analysis. Int Immunopharmacol. 2018;54:198‐209. [DOI] [PubMed] [Google Scholar]
- 27. Kabra S, Verma I. Acute lower respiratory tract infection: the forgotten pandemic. Ind J Pediatr. 1999;66:873‐875. [DOI] [PubMed] [Google Scholar]
- 28. Shann F, et al. Introduction: acute respiratory tract infections: The forgotten pandemic. Clin Infect Dis. 1999;28:189‐191. [DOI] [PubMed] [Google Scholar]
- 29. Gates GA. Cost‐effectiveness considerations in otitis media treatment. Otolaryngol Head Neck Surg. 1996;114:525‐530. [DOI] [PubMed] [Google Scholar]
- 30. Nokso‐Koivisto J, et al. Viral etiology of frequently recurring respiratory tract infections in children. Clin Infect Dis. 2002;35:540‐546. [DOI] [PubMed] [Google Scholar]
- 31. Cardinale F, et al. Epithelial dysfunction, respiratory infections and asthma: the importance of immunomodulation. A focus on OM‐85. Expert Rev Respir Med. 2020;14:1019‐1026. [DOI] [PubMed] [Google Scholar]
- 32. Yui I, et al. Novel clinical features of recurrent human respiratory syncytial virus infections. J Med Virol. 2014;86:1629‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Z, Wang F‐S. Plasmacytoid dendritic cells act as the most competent cell type in linking antiviral innate and adaptive immune responses. Cell Mol Immunol. 2005;2:411‐417. [PubMed] [Google Scholar]
- 34. Rantala A, Jaakkola JJ, Jaakkola MS. Respiratory infections in adults with atopic disease and IgE antibodies to common aeroallergens. PLoS One. 2013;8:e68582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng X, Yang X, Shen J. Influence of iron deficiency on serum IgG subclass and pneumococcal polysaccharides specific IgG subclass antibodies. Chin Med J. 1994;107:813. [PubMed] [Google Scholar]
- 36. Pu F, et al. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: a randomized controlled open‐label trial. Clin Interv Aging. 2017;12:1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steurer‐Stey C, et al. Oral purified bacterial extracts in acute respiratory tract infections in childhood: a systematic quantitative review. Eur J Pediatr. 2007;166:365‐376. [DOI] [PubMed] [Google Scholar]
- 38. Schaad UB. OM‐85 BV, an immunostimulant in pediatric recurrent respiratory tract infections: a systematic review. World J Pediatr. 2010;6:5‐12. [DOI] [PubMed] [Google Scholar]
- 39. Cazzola M, Anapurapu S, Page CP. Polyvalent mechanical bacterial lysate for the prevention of recurrent respiratory infections: a meta‐analysis. Pulm Pharmacol Ther. 2012;25:62‐68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.
The datasets analysed during the current study are available from the corresponding author on reasonable request.


