Abstract
Background
At the end of December 2019, a novel coronavirus tentatively named SARS‐CoV‐2 in Wuhan, a central city in China, was announced by the World Health Organization. SARS‐CoV‐2 is an RNA virus that has become a major public health concern after the outbreak of the Middle East Respiratory Syndrome‐CoV (MERS‐CoV) and Severe Acute Respiratory Syndrome‐CoV (SARS‐CoV) in 2002 and 2012, respectively. As of 29 October 2020, the total number of COVID‐19 cases had reached over 44 million worldwide, with more than 1.17 million confirmed deaths.
Discussion
SARS‐CoV‐2 infected patients usually present with severe viral pneumonia. Similar to SARS‐CoV, the virus enters respiratory tract cells via the angiotensin‐converting enzyme receptor 2. The structural proteins play an essential role in budding the virus particles released from different host cells. To date, an approved vaccine or treatment option of a preventive character to avoid severe courses of COVID‐19 is still not available.
Conclusions
In the present study, we provide a brief review of the general biological features of CoVs and explain the pathogenesis, clinical symptoms and diagnostic approaches regarding monitoring future infectivity and prevent emerging COVID‐19 infections.
Keywords: coronavirus, diagnostic methods, genome structure, pathogenesis
The essential structural proteins play an important role in budding the virus particles released from different host cells. Notably, reported cases confirm human‐to‐human transmission, along with numerous cases of exported virus infections all over the world. However, to date, an approved vaccine or treatment option of preventive character to avoid severe courses of COVID‐19 is still not available. In the present study, we provide a brief review of the general biological features of CoVs and explain the pathogenesis, clinical symptoms and diagnostic approaches with regard to monitoring future infectivity and preventing emerging COVID‐19 infections.
1. INTRODUCTION
In December 2019, a novel coronavirus (nCoV) termed “SARS‐CoV‐2”, announced by the World Health Organization (WHO) as being responsible for the outbreak of COVID‐19, was
reported. 1 , 2 The incidence of the SARS‐CoV (Severe Acute Respiratory Syndrome‐coronavirus) in 2002 and 2003 and the MERS‐CoV (Middle East Respiratory Syndrome‐coronavirus) in 2012 showed the potential for the transmission of newly emerging CoVs from animal to human and person to person. 3 , 4 , 5 In total, seven human coronaviruses (HCoVs) have now been discovered, including HCoV229E, HCoV‐OC43, HCoV‐NL63, HKU1, SARS‐CoV, MERS‐CoV and SARS‐CoV‐2. 6 , 7
In the last two decades, SARS‐CoV and MERS‐CoV have caused epidemics with mortality rates of approximately 9.5% and 34.4%, respectively. 8 COVID‐19 was the third highly epidemic disease to be detected, with a lower mortality rate than SARS and MERS, differing from country to country. According to WHO statistics, there are 45,678,440 (1 November 2020) confirmed cases in 219 countries caused by the high transmission capacity of SARS‐CoV‐2. 9 Hence, to characterize the acute infection in humans as a result of the SARS‐CoV‐2, scientists and governments have urgently taken decisive steps to monitor its outbreak and carry out etiological research. 10 In combination with advances in molecular technologies, the development of a viable vaccine appears to be imminent. 11 , 12
The higher transmissibility, varied clinical manifestations and lower pathogenicity of COVID‐19 could be a result of diversity in the biology and genome structure of the SARS‐CoV‐2 compared to SARS‐CoV and MERS‐CoV. 13 , 14 This review focuses on the virus biology, clinical symptoms and potential diagnostic routes for achieve efficient prevention and reduction of COVID‐19 mortality.
2. GENOMICS STRUCTURE AND BIOLOGICAL FEATURES OF SARS‐COV‐2
Coronaviruses belong to the order Nidovirales in the family coronaviridae. Coronavirinae and Torovirinae subfamilies are divided from the family. The subfamily Coronavirinae is further divided into four genera: Alpha‐, Beta‐, Gamma‐ and Deltacoronavirus. 15 Phylogenic analysis revealed that SARS‐CoV‐2 is closely related to the beta‐coronaviruses. Similar to other coronaviruses, the genome of SARS‐CoV‐2 is positive‐sense single‐stranded RNA [(+) ssRNA] with a 5′‐cap, 3'‐UTR poly(A) tail. The length of the SARS‐CoV‐2 genome is less than 30 kb, in which there are 14 open reading frames (ORFs), encoding non‐structural proteins (NSPs) for virus replication and assembly processes, structural proteins including spike (S), envelope (E), membrane/matrix (M) and nucleocapsid (N), and accessory proteins. 16 , 17 The first ORF contains approximately 65% of the viral genome and translates into either polyprotein pp1a (nsp1–11) or pp1ab (nsp1–16). Among them, six nsps (NSP3, NSP9, NSP10, NSP12, NSP15 and NSP16) play critical roles in viral replication. Other ORFs encode structural and accessory proteins. 18 , 19 The S protein is a transmembrane protein that facilitates the binding of viral envelop to angiotensin‐converting enzyme 2 (ACE2) receptors expressed on host cell surfaces. Functionally, the spike protein is composed of receptor binding (S1) and cell membrane fusion (S2) subunits. 20 The N protein attaches to the viral genome and is involved in RNA replication, virion formation and immune evasion. The nucleocapsid protein also interacts with the nsp3 and M proteins. 21 The M protein is one of the most abundant and well‐conserved proteins in the virion structure. This protein promotes the assembly and budding of viral particles through interaction with N and accessory proteins 3a and 7a. 22 , 23 The E protein is the smallest component in the SARS‐CoV‐2 structure that facilitates the production, maturation and release of virions. 18
The most complex component of the coronaviruses genome is the receptor‐binding domain (RBD) in the spike protein. 24 , 25 Six RBD amino acids are necessary for attaching to the ACE2 receptor and hosting SARS‐CoV‐like coronaviruses. According to multiple sequence alignment, they are Y442, L472, N479, D480, T487 and Y4911 in SARS‐CoV, and L455, F486, Q493, S494, N501 and Y505 in SARS‐CoV‐2. 26 Therefore, SARS‐CoV‐2 and SARS‐CoV vary with respect to five of these six residues. The SARS‐CoV strain genome sequences derived from humans were very close to those in bats. Even so, several differences have been identified between the gene sequences of the S gene and the ORF3 and ORF8 gene sequences that encode the attachment and fusion proteins and replication proteins, respectively. 27 Specific MERS‐CoV strains derived from camels were shown to be identical to those extracted from humans, with the exception of differences between the genomic regions S, ORF4b and ORF3. 28 In addition, genome sequencing‐based experiments have shown that human MERS‐CoV strains are phylogenetically linked to those of bats. However, for the S proteins, the species have a similar genome and protein structures. 29 Based on the recombination studies of orf1ab and S encoding genes, the MERS‐CoV was derived from the interchange of genetic elements between coronaviruses in camels and bats. In comparison, with a 96% overall identification, the primary protease is strongly protected among SARS‐CoV‐2 and SARS‐CoV. 29 , 30 , 31
The ACE2 protein is found in many mammalian body tissues, primarily in the lungs, kidneys, gastrointestinal tract, heart, liver and blood vessels. 32 ACE2 receptors are vital elements in regulating the renin‐angiotensin‐aldosterone system pathway. Based on structural experiments and biochemical studies, SARS‐CoV‐2 appears to have an RBD that strongly binds to ACE2 receptors of humans, cats, ferrets and other organisms with the homologous receptors. 33
The genome sequencing of SARS‐CoV‐2 in January 2020 was shown to be 96% identical to the bat coronavirus (BatCoV) RaTG13 genome and 80% identical to the SARS‐CoV genome. 34 However, significant differences exist. For example, the protein 8a sequence in the SARS‐CoV genome is absent in the 2019‐nCoV, and the protein 8b sequence of SARS‐CoV‐2 is 37 amino acids longer than that in SARS‐CoV. 35
Alignment sequence analysis of the CoV genome indicates non‐structural and structural proteins being 60% and 45% identical, respectively, among various types of CoVs. 36 These data show that nsps are more conservative than structural proteins. RNA viruses have a higher mutational load as a result of shorter replication times (Figure 1 ). 36 Based on comparative genomic studies between SARS‐CoV‐2 and SARS‐like coronaviruses, there are 380 amino acid substitutions in the nsps genes and 27 mutations in genes encoding the spike protein S of SARS‐CoV‐2. These variations might explain the different behavioral patterns of SARS‐CoV‐2 compared to SARS‐CoVs. 8 For example, the primary N501 T mutation in the Spike protein of SARS‐CoV‐2 could have significantly increased its binding affinity to ACE2. 37
FIGURE 1.
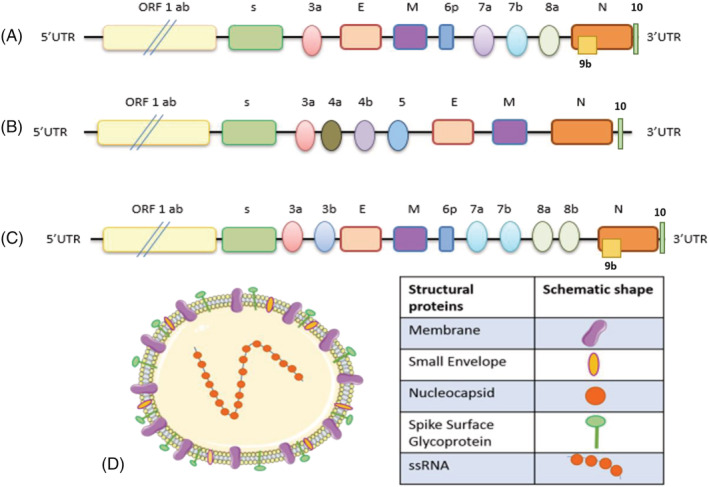
The schematic genomic structure of coronavirus. (a) COVID‐19. (b) MERS‐CoV. (c) SARS‐CoV. The typical coronavirus genome is a single‐stranded, which is approximately 25–32 kb. It contains 5' caps and 3'‐UTR tails. (d) Coronavirusencoding structural proteins four structural genes, including spike, envelope, membrane and nucleocapsid genes, as well as accessory proteins (3a, 3b, 6, 7a, 7b, 8b, 9b and ORFs)
2.1. Pathogenesis of SARS‐CoV‐2
The entry of the SARS‐CoV‐2 into host cells and release their genomes into target cells is dependent on a sequence of steps. The virus uses the protein spike, which is important for assessing tropism and virus transmissibility. Additionally, SARS‐CoV‐2 even targets human respiratory epithelial cells with ACE2 receptors, indicating a structure of RBD similar to SARS‐CoV. 38 After attachment of the S1‐RBD to the ACE2 receptor, host cell‐surface proteases such as TMPRSS2 (transmembrane serine protease 2) act on a critical cleavage site on S2. 38 This results in membrane fusion and viral infection. Following virus entry, the uncoated genomic RNA is translated into polyproteins (pp1a and pp1ab) and then assembled into replication/transcription complexes with virus‐induced double‐membrane vesicles (DMVs). Subsequently, this complex replicates and synthesizes a nested set of subgenomic RNA by genome transcription, encoding structural proteins and some accessory proteins. Newly formed virus particles are assembled by mediating the endoplasmic reticulum and the Golgi complex. Finally, virus particles are budded and released into the extracellular milieu compartment. Thus, both the viral replication cycle and progression begin. 10
Inside the host cells, survival of SARS CoVs is maintained by multiple strategies to evade the host immune mechanism, which can also be generalized to SARS‐CoV‐2. 39 , 40 As a result of the lack of pathogen‐associated molecular patterns on DMVs originating from the first step of SARS‐CoVs infection, they are not recognized by pattern recognition receptors of the host immune system. 25 Nsp1 can impede the interferon (IFN)‐I responses through several mechanisms, such as a silencing of the host translational system, the induction of host mRNA degradation and the repression of transcription factor signal transducer and activator of transcription (STAT)1 phosphorylation. Nsp3 antagonizes interferon and cytokine production by blocking the phosphorylation of interferon regulation factor 3 (IRF3) and interrupting the nuclear factor‐kappa B (NF‐ΚB) signaling pathway. NSPs 14 and 16 cooperate to form a viral 5′ cap similar to that of the host. Thus, the viral RNA genome is not recognized by immune system cells. 41 The accessory proteins ORF3b and ORF6 can disrupt the IFN signaling pathway by inhibiting IRF3 and NF‐KB‐dependent IFNβ expression and blocking the JAK‐STAT signaling pathway, respectively. Also, IFN signaling is flattened by structural proteins M and N, which result in a disturbance in TANK‐binding kinase 1 (TBK1)/IKB kinase ε and TRAF3/6‐TBK1‐IRF3/NF‐ΚB/AP1 signals. 22 , 39 Because the D614 G mutation is found in the outer spike protein of the virus, this attracts a huge amount of attention from the human immune system and may therefore impair the ability of SARS‐CoV‐2 to avoid vaccine‐induced immunity. D614 G is not in the RBD, although it is involved in the interaction between individual spike protomers that regulate their mature trimeric form on the surface of the virion by hydrogen bonding. 42 Korber et al. reported that the SARS‐CoV‐2 variant in the D614 G spike protein has become influential across the globe. Although clinical and in vitro evidence indicate that D614 G alters the phenotype of the virus, the effect of the mutation on replication, pathogenesis, vaccine and therapy development is relatively unknown. 43 From in vitro and clinical evidence, it is apparent that D614 G has a distinct phenotype, although it is not clear whether this is the outcome of verified adaptation to human ACE2, as well as whether it enhances transmissibility, or will have a significant impact. 43
2.2. Diagnosis of COVID‐19
Early diagnosis and isolation of suspected patients play a vital role in controlling this outbreak. 44 The specificity and sensitivity of different diagnostic techniques differs between populations and the types of equipment employed. 45 Several proceedures have been recommended for the diagnosis of COVID‐19:
-
A
Clinical presentation
COVID‐19 symptoms are observed approximately 5 days after incubation. 46 The median time of symptom onset from COVID‐19 incubation is 5.1 days, and those infected display symptoms for 11.5 days. 47 This duration was shown to have a close link with the patient's immune system and age. Gastrointestinal symptoms include diarrhea, vomiting and anorexia, recorded in almost 40% of patients. 48 , 49 Up to 10% of patients with gastrointestinal symptoms show no signs of fever or respiratory tract infections. 50 COVID‐19 has also been linked to hypercoagulable disease, elevating the risk of venous thrombosis. 51 There are also records of neurological symptoms (such as fatigue, dizziness and disturbed awareness), ischemic and hemorrhagic strokes, and muscle damage. 52 Many extrapulmonary symptoms comprise skin and eye manifestations. Italian researchers have identified skin manifestations in 20% of patients. 53 The clinical outlook for children can progressively worsen as a result of respiratory failure, which could not be corrected within 1–3 days by traditional oxygen (i.e. nasal catheter 54 ) in severe cases; the hallmarks are septic shock, sepsis, extreme and continuum bleeding as a result of coagulation abnormalities, and metabolic acidosis. 55
Septic shock could cause severe damage and impair several organs, in addition to a severe pulmonary infection. When extrapulmonary system dysfunction occurs, including the circulatory and the digestive systems, septic shock is probable, and the mortality rate increases substantially. 55 Premature delivery and intrauterine hypoxia occur when the prenate is deprived of an adequate environment of oxygen. Insidious symptoms require specific care in some newborn and preterm infants. Reports have indicated a good prognosis for children within 1 or 2 weeks. 55 Children are prone to a hyperinflammatory response to COVID‐19 similar to Kawasaki disease, which responds well to management, for which a new term is being coined. 56
Also, considerable research has revealed the age distribution of adolescent patients between the ages of 25 to 89 years. Many elderly patients were between 35 and 55 years, and fewer cases among newborns and infants were found. An analysis of the initial transmission dynamics of the virus showed that the median age of patients was 59 years, varying from 15 to 89 years; most (59%) were male. 48
-
B
Nonspecific screening tests for COVID‐19 in exposed patients
The findings of most blood tests are usually nonspecific but could help determine the causes of the disease. A complete blood count typically shows a normal or low count of white blood cells and lymphopenia. C‐reactive protein (CRP) and erythrocyte sedimentation rate were generally increased, which would optimally be rechecked on days 3, 5 and 7 after admission. 1 , 57 , 58 Creatine kinase plus myoglobin, aspartate aminotransferase and alanine aminotransferase, lactate dehydrogenase, D‐dimer, and creatine phosphokinase levels could be increased in severe forms of COVID‐19 disease. During viral‐bacterial co‐infections, procalcitonin levels may be elevated. 59 , 60 In a systematic review and meta‐analysis study, Pormohammad et al. 61 investigated the accessible laboratory results obtained among 2361 SARS‐CoV2 patients, with the results demonstrating 26% leukopenia, 13.3% leukocytosis and 62.5% lymphopenia. Also, among 2200 patients, 91% and 81% revealed elevated platelets (thrombocytosis) and CRP, respectively. 61 Additionally, a review of case studies identified clinical diagnosis and clinical parameter modification in a 47‐year‐old man diagnosed with the disease from Wuweian. 62
To investigate the effect of the coronavirus during the acute phase of the disease, plasma cytokines/chemokines tumor necrosis factor (TNF)‐α and interleukin (IL)‐1β, IL1RA, IL2, IL4, IL5, IL‐6, IL‐10, IL13, IL15 and IL17A were measured. 1 , 63 One study showed that macrophages and dendritic cells play crucial roles in an adaptive immune system. These cells contain inflammatory cytokines and chemokines, such as IL‐6, IL‐8, IL‐12, TNF‐α, monocyte chemoattractant protein‐1, granulocyte‐macrophage colony‐stimulating factor and granulocyte colony‐stimulating factor. These inflammatory reactions could cause a systemic inflammation. 64
Therefore, fecal and urine tests have been recommended for patients and health staff to detect possible alternate transmission. Consequently, the advancement of tools for determining the different transmission modes, including fecal and urine samples, is urgently warranted to develop strategies for inhibiting and minimizing transmission, as well as develop therapies to control the disease. 65
-
C
Radiological findings
Chest X‐ray examination may display diverse imaging characteristics or patterns in COVID‐19 patients with a different disease severity and duration. Imaging results differ based on patient age, disease stage during screening, immune competency and drug therapy protocols. 66 On the other hand, computed tomagraphy (CT) imaging is essential for monitoring disease progression and assessing therapeutic effectiveness. It can be re‐examined 1 to 2 days after admission, based on the Diagnostic and Treatment Protocols Regulation (DTPR). 67
The cardinal hallmark of COVID‐19 was multiple, bilateral, posterior and peripheral ground‐glass opacities with or without pulmonary consolidation and, in severe cases, infiltrating shadows. 68 Autopsy analysis of a COVID‐19 patient displayed fluid accumulation and hyaline membrane formation in alveolar walls, which may be the primary pathological driver of the ground‐glass opacity. 69
However, further studies indicated that small patchy shadows, pleural changes, a subpleural curvilinear line and reversed halo signs are generally observed in COVID‐19 patients. 70 , 71 The intralobular lines and thickened interlobular septa were shown in a crazy‐paving pattern on the ground‐glass opacity background. 67 Also, several lobar lesions can be found in the respiratory system in children with a severe infection. Evidence showed that chest CT manifestations (pulmonary edema) reported for COVID‐19 are generally close to SARS and MERS. 69
Evidence has indicated that an initial chest CT has a higher detection rate (approximately 98%) compared to reverse transcriptase‐polymerase chain reaction (RT‐PCR) (approximately 70%) in infected patients. Xie et al. 72 demonstrated that about 3% of patients have no primary positive RT‐PCR but have a positive chest CT; therefore, both tests are recommended for COVID‐19 patients. CT of the chest comprises an urgent and simple method for detecting initial COVID‐19 infection with a high sensitivity for prompt diagnosis and disease progression monitoring in patients. Particular notice should be paid to the role of radiologists in finding novel infectious diseases.
-
D
Molecular diagnosis
The clinical diagnosis of COVID‐19 is focused primarily on epidemiological data, clinical symptoms and some adjuvant technologies, such as nucleic acid detection and immunological assays. In addition, the isolation of SARS‐CoV‐2 requires high‐throughput equipment (biosafety level‐3) to ensure personnel safety. Moreover, serological tests have not yet been validated. In the field of molecular diagnosis, there are three main issues: (i) decreasing the number of false negatives by detecting minimal amounts of viral RNA; (ii) avoiding the number of false positives through the correct differentiation of positive signals between different pathogens; and (iii) a high capacity for fast and accurate testing of a large number of samples in a short time. 73
2.3. Nucleic acid detection
Two widely used technologies for SARS‐CoV‐2 nucleic acid detection are the real‐time RT‐PCR (rRT‐PCR) and high‐throughput sequencing. Nevertheless, as a result of a reliance on equipment and high costs, high‐throughput sequencing in clinical diagnosis is restricted. Access to the whole genome structure of SARS‐CoV‐2 has helped the design of specific primers and has introduced the best diagnostic protocols. 47 , 74 In the first published reports on applying the rRT‐PCR in COVID‐19 diagnosis, targeting the spike gene region (S) of SARS‐COV‐2 has shown remarkable specificity and limited sensitivity. 68 Later, the sensitivity of this technique was greatly improved by the use of specific probes for the other viral‐specific genes, including RNA‐dependent RNA polymerase (RdRp) in the ORF1ab region, Nucleocapsid (N) and Envelop (E). To avoid cross‐reaction with other human coronaviruses and prevent the potential genetic drift of SARS‐CoV‐2, two molecular targets should be involved in this assay: one nonspecific target to detect other CoVs, and one specific target for SARS‐CoV‐2. The comparison of the results obtained from targeting all studied genes exhibited that the RdRp gene is the most appropriate target with the highest sensitivity. The RdRp assays were validated in approximately 30 European laboratories using synthetic nucleic acid technology. 73 Currently, Chan et al. 75 have proposed a novel RT‐PCR assay targeting a sequence of the RdRp/Hel that could detect low SARS‐CoV‐2 load in the upper respiratory tract, plasma and saliva samples without any cross‐reactivity with other common respiratory viruses. Although the CDC‐recommended assays in the USA rely on two nucleocapsid proteins N1 and N2, the WHO recommends the E gene assay as a first‐line screening, followed by the RdRp gene assay as a confirmatory test. Based on the most recent evidence, the QIAstat‐Dx SARS‐CoV‐2 panel, a multiplex RT‐real time PCR system targeting genes RdRp and E, remains highly sensitive despite the nucleotide variations affecting the annealing of the PCR assay. 76
Generally, quantitative (RT‐PCR) RT‐qPCR has high specificity as a gold standard assay for the final diagnosis of COVID‐19. However, its sensitivity could be variable based on viral load, RNA extraction technique, sampling source and disease stage during the time of sampling. Indeed, the RT‐PCR false‐positive results are related to the cross‐contamination of samples and handling errors. By contrast, inaccuracies during any stage of the collection, storage and processing of samples may lead to false‐negative results. Some studies have revealed that samples from the upper respiratory tract (bottom of the nostrils and the oropharynx) are more desirable for the RT‐PCR assay as a result of many viral copies. 77 Moreover, other shortcomings of RT‐qPCR assays include biological safety hazards arising from maintaining and working on patient samples, as well as time‐consuming and cumbersome nucleic acid detection process. 66 , 68
To improve the molecular diagnostic techniques for COVID‐19, isothermal amplification‐based methods are currently in development. The loop‐mediated isothermal amplification (LAMP) utilizes the DNA polymerase and 4 to 6 different primers binding to the distinct sequences on the target genome. 78 In the LAMP reactions, the amplified DNA is indicated by turbidity arising from a by‐product of the reaction, a detectable color generated by a pH‐sensitive dye, or fluorescence produced by a fluorescent dye. 79 The approach occurs at a single temperature, in less than 1 hour, and with minimal background signals. The LAMP diagnostic testing for COVID‐19 is more specific and sensitive compared to the conventional RT‐PCR assays and does not dependent on specialized laboratory equipment such as a thermocycler. However, as a result of the multiplicity of primers used in this method, optimizing the reaction conditions presents a major challenge. 80 , 81
2.4. Microarray‐based technique
Microarray is a rapid and high‐throughput method for the COVID‐19 assay. 82 As a brief summary of the protocol, the coronavirus RNA will first produce cDNA labeled with specific probes via reverse transcription. Complementary DNA is produced by coronavirus RNA templates and then through reverse transcription labeling with particular probes. The labeled targets are hybridized to the probe microarray. Free DNAs are removed by washing the solution. Finally, particular probes identify COVID‐19 RNA. 82 Shi et al. 83 successfully performed SARS‐CoV detection in samples from patients. In their study, Xu et al. 84 investigated a wide range of spike gene polymorphisms with great accuracy. Also, other studies have designed fluorescence and nonfluorescence methods to detect the entire coronavirus genus with promising efficacy. 85 , 86 Jiang et al. 87 constructed a SARS‐CoV‐2 proteome microarray consisting of 18 out of 28 expected proteins and administered it to 29 convalescent cases to characterize the immunoglobulin (Ig)G and IgM reactions in the sera. It was revealed that all of these patients had IgM and IgG antibodies, which recognize and bind SARS‐CoV‐2 proteins, especially S1and N proteins. In addition to these proteins, important antibody responses to NSP5 and ORF9b are also recognized. The S1‐specific IgG signal relates strongly to age and lactate dehydrogenase lactate dehydrogenase levels and negatively relates to the lymphocyte ratio. Shen et al. progressed the RT‐LAMP experiment to show signals using a quenching probe with the same efficiency as the standard RT‐PCR test with respect to MERS‐CoV identification. 80
-
E
Immunological diagnosis
Antigen detection and immunological techniques can be used for a rapid and cost‐effective diagnosis at the same time as providing an alternative to molecular methods. Immunological techniques including the immunofluorescence assay, direct fluorescence antibody test, nucleocapsid protein detection assay, protein chip, semiconductor quantum dots and the microneutralization assay define a binding between a viral antigen and a specific antibody. 88 , 89 , 90 , 91 These immunological methods are simple to operate but have low specificity/sensitivity. In the case of COVID‐19, virus morphology can be observed by electron microscopy according to traditional Koch’s postulates. 92 , 93 Serological tests can improve coronavirus detection such that associated antigens and monoclonal antibodies can represent a new diagnostic approach for future development (Figure 2). 94 , 95
FIGURE 2.
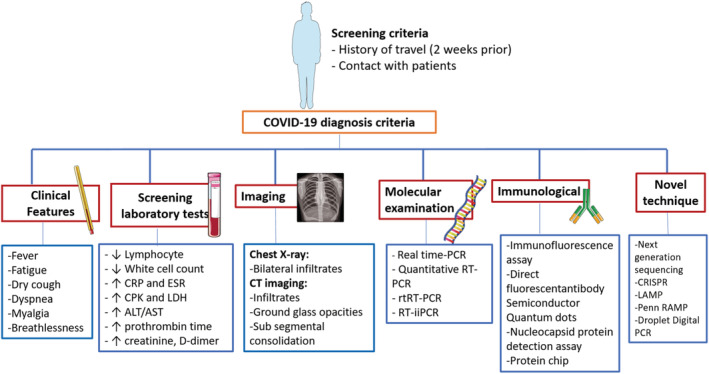
Diagnostic protocol recommended for COVID‐19
Serological tests could be specific to one type of immunoglobulin, they can concurrently measure IgM and IgG antibodies, or they may be absolute antibody examinations, which often measure IgA antibodies. 96 Based on the specific procedure and device, these experiments will usually be carried out within 1–2 hours after a sample arrives in the laboratory and is loaded onto the appropriate platform. 97 Guo et al. 98 indicated that IgA and IgM antibodies have positive rates of 93.0% and 85.5% after 3–6 days, respectively. Also, 78.0% of positive IgG antibodies were detected during 10–18 days. The efficiency of detection by an IgM enzyme‐linked immunposorbent assay (ELISA) is higher than that of qPCR after 5.5 days of symptom onset. After 5 days, IgM ELISA detection is more efficient than a qPCR.
Moreover, the combination of PCR and IgM ELISA increased the detection rate by 98.5%. 98 Xiang et al. 99 tested 63 infected patients of SARS‐CoV‐2 admitted to Jinyintan Hospital in Wuhan, Hubei, China. Patient serum samples were evaluated using an ELISA and indirect ELISA IgG capture. The study results indicate that IgM was positive with an accuracy of 64.3%, a sensitivity of 44.4% and a specificity of 100% in 28 of 63 samples. The sample identification of 52 cases also showed a positive IgG test with a sensitivity of 82.54%, a specificity of 100% and an accuracy of 88.8%. In addition, a sensitivity of 87.3% was achieved using IgM and IgG combination analysis. 99
Liu et al. evaluated the anti‐IgM and anti‐IgG produced against recombinant spike protein and nucleocapsid protein of SARS‐CoV‐2 in 397 PCR confirmed COVID‐19 patients and 128 negative cases at eight distinct clinical sites. The average sensitivity and specificity of the examination was 88.5% and 90.5%, respectively. The findings showed considerable detection consistency among the different types of venous and fingerstick blood samples. Compared to a single IgM or IgG test, the IgM‐IgG combination analysis has a higher effectiveness and sensitivity. 37 , 100 Therefore, it is important and urgent to improve several sensitive and specific supplementary approaches for COVID‐19 diagnosis.
-
F
Novel techniques
2.5. CRISPR technique
Nucleic acid detection with CRISPR‐Cas13a/C2c2 is a highly rapid, sensitive and specific molecular detection platform, which may aid in the epidemiology, diagnosis and control of the disease. In addition, Cas13a/C2c2 can detect the expression of transcripts in live cells and different diseases. 101 , 102 Zhang et al. presented a protocol for the detection of COVID‐19 using the CRISPR diagnostics‐based SHERLOCK (Specific High Sensitivity Enzymatic Reporter UnLOCKing) technique. RNA fragments of the SARS‐CoV‐2 virus help detect target sequences of approximately 100 copies. The experiment is performed by isothermal amplification of the extracted nucleic acid of samples from patients and then amplification of the viral RNA sequence via Cas13 and is finally read out by a paper dipstick in less than 1 hour. 103 , 104 Huang et al. 105 established a CRISPR‐based assay by a custom CRISPR Cas12a/gRNA complex. They used a fluorescent probe to identify target amplicons produced by standard RT‐PCR or isothermal recombinase polymerase amplification. This method showed specific detection at places not equipped with the PCR systems needed for qPCR diagnostic tests in real time. The analysis allows the identification of SARS‐CoV‐2 positive samples with a test‐to‐response time of approximately 50 minutes and a detection limit of two copies of each sample to be detected. The findings of the CRISPR test on nasal samples collected from persons with COVID‐19 were comparable with matched data achieved from the CDC‐approved RT‐qPCR test. 105
Broughton et al. 106 described the development of a fast (< 40 min), simple‐to‐implement and precise CRISPR‐Cas12‐based lateral flow test to diagnose SARS‐CoV‐2 from RNA extract from a nasal swab. Using artificial reference samples and clinical specimens from patients, comprising patients diagnosed with COVID‐19 disease and 42 patients with other respiratory illnesses, they confirmed their process. This CRISPR‐based approach has provided a visual and quicker alternative option to the SARS‐CoV‐2 real‐time RT‐PCR method used in the US Centers for Disease Control and Prevention, with approximately 100% negative predictive agreement and 95% positive predictive agreement. 106
2.6. LAMP‐based technique
Loop‐mediated isothermal amplification (LAMP) is a new isothermal nucleic acid amplification method with great efficiency. This is used to amplify RNAs and DNAs with high specificity and sensitivity as a result of its exponential amplification feature and six particular target sequences diagnosed by four separate primers. 107 The LAMP assay is rapid and does not need high‐priced reagents or equipment. Furthermore, the gel electrophoresis method is widely utilized for investigation of the amplified items to detect endpoints. Hence, the LAMP test will help to decrease the cost of coronavirus detection. Several strategies for the detection of coronavirus based on LAMP are defined here, as developed and performed in clinical diagnosis. 108
Poon et al. 109 have reported a simple LAMP test in the SARS study and demonstrated the feasibility of this method for SARS‐CoV detection. The SARS‐CoV ORF1b site was selected for SARS detection and amplified in the presence of six primers via the LAMP reaction, and then the amplified products were assessed by gel electrophoresis. The sensitivity and detection levels in LAMP test for SARS are close to those of traditional PCR‐based techniques. Pyrc et al. 110 effectively applied LAMP to HCoV‐NL63 detection with a desirable sensitivity and specificity in mobile cell cultures and clinical specimens. Particularly, one replica of RNA template was found to be responsible for the detection restriction. Amplification is observed as fluorescent dye or magnesium pyrophosphate precipitation. These techniques can be achieved in real time by monitoring the turbidity of the pyrophosphate or fluorescence, which correctly overcome the restriction of endpoint detection. 110
Shirato et al. 111 developed a beneficial RT‐LAMP assay for the diagnosis and epidemiological monitoring of human MERSCoV. This method was highly specific, without any cross‐reaction with other specific respiratory viruses, and detected as few as 3.4 copies of RNA. 111 Subsequently, they developed the RT‐LAMP assay by revealing a sign using a quenching probe (QProbe), which has the same efficiency as the usual real‐time RT‐PCR test with respect to MERSCoV detection. 112
Based on other evidence, a nucleic acid visualization method was developed that combines RT‐LAMP and a vertical flow visualization strip for MERS detection. 113
2.7. Penn RAMP technology
Based on the effectiveness reported by Zhang et al. 104 using the comparatively less sensitive LAMP, the improved sensitivity of the Penn‐RAMP technique achieved by Huang et al. 114 , which is attributable to an updated two‐step LAMP protocol, can prove to be substantially effective as a diagnostic. To amplify specific targets by recombinase polymerase amplification, in which all targets are simultaneously amplified, the Penn‐RAMP requires a preliminary reaction with outer LAMP primers. A next highly precise LAMP reaction is then triggered. Especially, the first stage uses F3 and B3 outer LAMP primers, whereas the other four RAMP primers are further mixed in the stage 2. Compared to normal LAMP, this ‘nested’ concept considerably improved the sensitivity of LAMP by approximately 10–100 times, especially when working with distilled and crude samples. 115 Additionally, when extended to mock trials, the Penn‐RAMP methodology was given a 100% approval rating at 7–10 copies of viral RNA per reaction, compared to a 100% approval rating at the 700 viral RNA copies needed for PCR analysis. 114 , 115
2.8. Droplet digital PCR
For the direct identification and quantification of DNA and RNA targets, droplet digital PCR (ddPCR) comprises an extremely sensitive technique. 116 It has been widely used for infectious disease conditions, particularly because of its ability to identify a few copies of viral genomes accurately and efficiently. 117 If low‐level and/or residual viral existence identification is appropriate, ddPCR quantitative data are much more insightful than those provided by regular RT‐PCR tests. In view of the need to restrict (as far as possible) false‐negative results in COVID‐19 diagnosis, use of the ddPCR can provide a vital support. Even so, the ddPCR assay is still very rarely studied in clinical settings and there is currently no available evidence for European cases. 118
2.9. Next‐generation sequencing (NGS)‐based technique
RNA viruses come in great assortment of varieties, and they are the etiological specialists of numerous significant human and animal infectious diseases. 119
RNA viruses comprise the major variety and are the etiologic agents of very infectious diseases in humans and animals such as SARS, hepatitis, influenza and IB (avian infectious bronchitis). High‐throughput NGS technology has a vital role in primary and accurate diagnosis. 120 In addition, the NGS method can detect whether or not various types of virus comprise a pathogen. The fast novel technique of viruses by NGS, including DNA‐sequencing and RNA‐sequencing has developed the identification of viral diversity. 121 The identification of a huge range of pathogen using NGS technologies is also significant for controlling viral infection caused by a new pathogen. 122 In recent years, the advancement of the NGS method via RNA‐sequencing has enabled us to make great progress in the fast recognition of new RNA viruses. RNA‐sequencing detects millions of reversely transcribed DNA fragments from complex RNA samples at the same time using random primers. 123 Chen et al. 122 reported a new duck coronavirus using the RNA‐sequencing method, which differed from that of chicken IBV (infectious bronchitis virus). 122 The new duck‐specific CoV was a possible new species within the genus Gamma‐coronavirus, as shown by sequences of the viral 1b gene from three regions.
In conclusion, the outbreak of a novel virus emerged at the end of December 2019. COVID‐19 spread immediately and challenged medicine, economics and public health worldwide. Numerous evidence proposed that the ACE2 receptors comprise crucial structural proteins for virus budding and entry into host cells. Both transmission from unidentified intermediate hosts to cross‐species and human to human transmission have been recognized. Hence, early detection and isolation of suspected patients can play an essential role in controlling this outbreak. Currently, diagnostic methods for COVID‐19 are numerous; hence, it is imperative to choose a suitable detection protocol. Each of the described techniques has its specific disadvantages and advantages. Both chest CT imaging and RT‐PCR tests are recommended for COVID‐19 patients. However, the use of PCR requires various equipment and a well‐established laboratory. LAMP can be detected with low numbers of DNA or RNA templates within 1 hour. Microarray is an expensive method for COVID‐19 diagnosis, and other newly developed methods also require additional investigation to achieve rapid development and detection in the future. Given that the number of infected cases is rapidly increasing, future studies should reveal the secrets of the molecular pathways of the virus with respect to developing targeted vaccines and antiviral treatments.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
MM and HC drafted the article. MM and HC provided critical revision of the article. SB carried out native editing. NP and AE developed the theory to investigate a specific aspect and supervised the findings of the work, as well as conceived the presented idea. MM, HC, AS, SB, NP and AE discussed the results and contributed to the final manuscript submitted for publication.
Mohamadian M, Chiti H, Shoghli A, Biglari S, Parsamanesh N, Esmaeilzadeh A. COVID‐19: Virology, biology and novel laboratory diagnosis. J Gene Med. 2021;23:e3303 10.1002/jgm.3303
Malihe Mohamadian and Hossein Chiti contributed equally to this work.
Contributor Information
Negin Parsamanesh, Email: neginparsa.684@gmail.com.
Abdolreza Esmaeilzadeh, Email: a46reza@yahoo.com.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this review because no datasets were generated or analyzed during the present study.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodríguez‐Morales AJ, MacGregor K, Kanagarajah S, Patel D, Schlagenhauf P. Going global–travel and the 2019 novel coronavirus. Travel Med Infect Dis. 2020;33:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. New England J Med. 2003;348:1953‐1966. [DOI] [PubMed] [Google Scholar]
- 4. de Groot RJ, Baker SC, Baric RS, et al. Commentary: Middle East respiratory syndrome coronavirus (MERS‐CoV): announcement of the coronavirus study group. J Virol. 2013;87:7790‐7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. New England J Med. 2003;348:1967‐1976. [DOI] [PubMed] [Google Scholar]
- 6. Zhu N, Zhang D, Wang W. et al. A novel coronavirus from patients with pneumonia in China, 2019. New England J Med. 2019;2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asghari A, Naseri M, Safari H, Saboory E, Parsamanesh N. The novel insight of SARS‐CoV‐2 molecular biology and pathogenesis and therapeutic options. DNA Cell Biol. 2020;39:1741‐1753. [DOI] [PubMed] [Google Scholar]
- 8. Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID‐19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26:729‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. WHO site . https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 10. Ashour HM, Elkhatib WF, Rahman M, Elshabrawy HA. Insights into the recent 2019 novel coronavirus (SARS‐CoV‐2) in light of past human coronavirus outbreaks. Pathogens. 2020;9:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peeri NC, Shrestha N, Rahman MS, et al. The SARS, MERS and novel coronavirus (COVID‐19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020;49:717‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tahmasebi S, Khosh E, Esmaeilzadeh A. The outlook for diagnostic purposes of the 2019‐novel coronavirus disease. J Cell Physiol. 2020;235:9211‐9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Paraskevis D, Kostaki EG, Magiorkinis G, Panayiotakopoulos G, Sourvinos G, Tsiodras S. Full‐genome evolutionary analysis of the novel corona virus (2019‐nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infection, genetics and. Evolution. 2020;79:104212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fahmi M, Kubota Y, Ito M. Nonstructural proteins NS7b and NS8 are likely to be phylogenetically associated with evolution of 2019‐nCoV. Infect Genet Evol. 2020;104272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phan MVT, Ngo Tri T, Hong Anh P, Baker S, Kellam P, Cotten M. Identification and characterization of Coronaviridae genomes from Vietnamese bats and rats based on conserved protein domains. Virus Evol. 2018;4(2):vey035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abduljali J, Abduljali B. Epidemiology, genome and clinical features of the pandemic SARS‐CoV‐2: a recent view. New Micr New Infect. 2020;35:100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parsamanesh N, Pezeshgi A, Hemmati M, Jameshorani M, Saboory E. Neurological manifestations of coronavirus infections: role of angiotensin‐converting enzyme 2 in COVID‐19. Int J Neurosci. 2020. [DOI] [PubMed] [Google Scholar]
- 18. Naqvi AAT, Fatima K, Mohammad T, et al. Insights into SARS‐CoV‐2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krichel B, Falke S, Hilgenfeld R, Redecke L, Uetrecht C. Processing of the SARS‐CoV pp1a/ab nsp7‐10 region. Biochem J. 2020;477:1009‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khailany RA, Safdar M, Ozaslan M. Genomic characterization of a novel SARS‐CoV‐2. Gene Rep. 2020;19:100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mu J, Xu J, Zhang L, et al. SARS‐CoV‐2‐encoded nucleocapsid protein acts as a viral suppressor of RNA interference in cells. Sci China Life Sci. 2020;63:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Astuti I. Ysrafil. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): an overview of viral structure and host response. Diab Metab Syndr. 2020;14:407‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Voss D, Pfefferle S, Drosten C, et al. Studies on membrane topology. N‐Glycosylation and Functionality of SARS‐CoV Membrane Protein Virology Journal. 2009;6:79‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou P, Yang X, Wang X, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kang S, Yang M, Hong Z, et al. Crystal structure of SARS‐CoV‐2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm Sin B. 2020;10:1228‐1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wan Y, Graham R, Baric R, Li F. An analysis based on decade‐long structural studies of SARS 3, JVI accepted manuscript posted online 29 January 2020. J Virol. 2020;94(7):e00127‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chu DK, Hui KP, Perera RA, et al. MERS coronaviruses from camels in Africa exhibit region‐dependent genetic diversity. Proc Natl Acad Sci. 2018;115:3144‐3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lau SK, Li KS, Tsang AK, et al. Genetic characterization of Betacoronavirus lineage C viruses in bats reveals marked sequence divergence in the spike protein of pipistrellus bat coronavirus HKU5 in Japanese pipistrelle: implications for the origin of the novel Middle East respiratory syndrome coronavirus. J Virol. 2013;87:8638‐8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lau SK, Zhang L, Luk HK, et al. Receptor usage of a novel bat lineage C betacoronavirus reveals evolution of Middle East respiratory syndrome‐related coronavirus spike proteins for human dipeptidyl peptidase 4 binding. J Infect Dis. 2018;218:197‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khan S, Siddique R, Shereen MA, et al. Emergence of a novel coronavirus, severe acute respiratory syndrome coronavirus 2: biology and therapeutic options. J Clin Microbiol. 2020;58(5):e00187‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Devaux CA, Rolain JM, Raoult D. ACE2 receptor polymorphism: susceptibility to SARS‐CoV‐2, hypertension, multi‐organ failure, and COVID‐19 disease outcome. J Microbiol Immun Infect = Wei Mian Yu Gan Ran Za Zhi. 2020;53:425‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS‐CoV‐2. Nat Med. 2020;26:450‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pillay TS. Gene of the month: the 2019‐nCoV/SARS‐CoV‐2 novel coronavirus spike protein. J Clin Pathol. 2020;73:366‐369. [DOI] [PubMed] [Google Scholar]
- 35. Malik YS, Kumar N, Sircar S, et al. Coronavirus disease pandemic (COVID‐19): challenges and a global perspective. Pathogens. 2020;9(7):519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020;92(9):1518‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Voto C, Berkner P, Brenner C. Overview of the pathogenesis and treatment of SARS‐CoV‐2 for clinicians: a comprehensive literature review. Cureus. 2020;12(9):e10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Domingo P, Mur I, Pomar V, Corominas H, Casademont J, de Benito N. The four horsemen of a viral apocalypse: the pathogenesis of SARS‐CoV‐2 infection (COVID‐19). EBioMedicine. 2020;58:102887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shang J, Wan Y, Luo C, et al. Cell entry mechanisms of SARS‐CoV‐2. Proc Natl Acad Sci. 2020;117:11727‐11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Totura AL, Baric RS. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr Opin Virol. 2012;2:264‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grubaugh ND, Hanage WP, Rasmussen AL. Making sense of mutation: what D614 G means for the COVID‐19 pandemic remains unclear. Cell. 2020;182:794‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Korber B, Fischer WM, Gnanakaran S, et al. Tracking changes in SARS‐CoV‐2 spike: evidence that D614 G increases infectivity of the COVID‐19 virus. Cell. 2020;182:812‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Novel CPERE . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi. 2020;41(2):145‐151. [DOI] [PubMed] [Google Scholar]
- 45. Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM. Variation of a test's sensitivity and specificity with disease prevalence. Cmaj. 2013;185:E537‐E544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID‐19 infection: origin, transmission, and characteristics of human coronaviruses. Journal of Advanced Research. 2020;24:91‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adhikari SP, Meng S, Wu Y‐J, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. J‐J Z, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730‐1741. [DOI] [PubMed] [Google Scholar]
- 50. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan. China Jama. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID‐19 pneumonia: a random association? Eur Heart J. 2020;41:1858‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol. 2020;77:683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5):e212‐e213. [DOI] [PubMed] [Google Scholar]
- 54. Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID‐19): a systematic review of imaging findings in 919 patients. Am J Roentgenol. 2020;215(1):87‐93. [DOI] [PubMed] [Google Scholar]
- 55. Chen Z‐M, Fu J‐F, Shu Q, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020;16:240‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Loomba RS, Villarreal E, Flores S. Covid‐19 and Kawasaki syndrome: should we really be surprised? Cardiol Young. 2020;30(7):1059‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA. 2020;323:1406‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roumen R, Van Meurs P, Kuypers H, Kraak W, Sauerwein R. Serum interleukin‐6 and C reactive protein responses in patients after laparoscopic or conventional cholecystectomy. The European journal of surgery=. Acta Chirurg. 1992;158:541‐544. [PubMed] [Google Scholar]
- 59. Bangash MN, Patel J, Parekh D. COVID‐19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pormohammad A, Ghorbani S, Baradaran B, et al. Clinical Characteristics, laboratory findings, radiographic signs and outcomes of 52,251 patients with confirmed covid‐19 infection: a systematic review and meta‐analysis; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Han W, Quan B, Guo Y, et al. The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J Med Virol. 2020;92:461‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS‐CoV infection in humans is associated with a pro‐inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hossein‐Khannazer N, Shokoohian B, Shpichka A, Aghdaei HA, Timashev P, Vosough M. Novel therapeutic approaches for treatment of COVID‐19. J Mol Med (Berl). 2020;98:789‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreakhe epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID‐19: comparison to RT‐PCR. Radiology. 2020;296(2):200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vyakaranam AR, Crona J, Norlén O, Hellman P, Sundin A. 11C‐hydroxy‐ephedrine‐PET/CT in the diagnosis of Pheochromocytoma and Paraganglioma. Cancer. 2019;11:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Eurosurveillance. 2020;25(3):2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID‐19) pneumonia. Radiology. 2020;295:715‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology. 2020;296:E32‐E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li X, Zeng X, Liu B, Yu Y. COVID‐19 infection presenting with CT halo sign. Radiol: Cardioth Imag. 2020;2(1):e200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019‐nCoV pneumonia: relationship to negative RT‐PCR testing. Radiology. 2020;296:E41‐E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Caruana G, Croxatto A, Coste AT, et al. Diagnostic strategies for SARS‐CoV‐2 infection and interpretation of microbiological results. Clin Microbiol Infect. 2020;26:1178‐1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chan JF‐W, Yip CC‐Y, To KK, et al. Improved molecular diagnosis of COVID‐19 by the novel, highly sensitive and specific COVID‐19‐RdRp/Hel real‐time reverse transcription‐polymerase chain reaction assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58(5):e00310‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Peñarrubia L, Ruiz M, Porco R, et al. Multiple assays in a real‐time RT‐PCR SARS‐CoV‐2 panel can mitigate the risk of loss of sensitivity by new genomic variants during the COVID‐19 outbreak. Int J Infect Dis. 2020;97:225‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Oliveira BA, Oliveira LC, Sabino EC, Okay TS. SARS‐CoV‐2 and the COVID‐19 disease: a mini review on diagnostic methods. Rev Inst Med Trop Sao Paulo. 2020;62:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease‐19 (COVID‐19): relationship to duration of infection. Radiology. 2020;119(5):1000‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Udugama B, Kadhiresan P, Kozlowski HN, et al. Diagnosing COVID‐19: the disease and tools for detection. ACS Nano. 2020;14:3822‐3835. [DOI] [PubMed] [Google Scholar]
- 80. Shen M, Zhou Y, Ye J, et al. Recent advances and perspectives of nucleic acid detection for coronavirus. J Pharm Anal. 2020;10(2):97‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yang W, Dang X, Wang Q, et al. Rapid detection of SARS‐CoV‐2 using reverse transcription RT‐LAMP method. medRxiv. 2020. [Google Scholar]
- 82. Chen Q, Li J, Deng Z, Xiong W, Wang Q. Hu Y‐q. comprehensive detection and identification of seven animal coronaviruses and human respiratory coronavirus 229E with a microarray hybridization assay. Intervirology. 2010;53:95‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shi R, Ma W, Wu Q, et al. Design and application of 60mer oligonucleotide microarray in SARS coronavirus detection. Chin Sci Bull. 2003;48:1165‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xu Y, He B, Pan Y, et al. Systematic review and meta‐analysis on vitamin D receptor polymorphisms and cancer risk. Tumor Biology. 2014;35:4153‐4169. [DOI] [PubMed] [Google Scholar]
- 85. de Souza Luna LK, Heiser V, Regamey N, et al. Generic detection of coronaviruses and differentiation at the prototype strain level by reverse transcription‐PCR and nonfluorescent low‐density microarray. J Clin Microbiol. 2007;45:1049‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hardick J, Metzgar D, Risen L, et al. Initial performance evaluation of a spotted array Mobile analysis platform (MAP) for the detection of influenza a/B, RSV, and MERS coronavirus. Diagn Microbiol Infect Dis. 2018;91:245‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jiang HW, Li Y, Zhang HN, et al. SARS‐CoV‐2 proteome microarray for global profiling of COVID‐19 specific IgG and IgM responses. Nat Commun. 2020;11:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chan K‐H, Chan JF‐W, Tse H, et al. Cross‐reactive antibodies in convalescent SARS patients' sera against the emerging novel human coronavirus EMC (2012) by both immunofluorescent and neutralizing antibody tests. J Infect. 2013;67:130‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hoey J. Updated SARS case definition using laboratory criteria. Cmaj. 2003;168:1566‐1567. [PMC free article] [PubMed] [Google Scholar]
- 90. Roh C, Jo SK. Quantitative and sensitive detection of SARS coronavirus nucleocapsid protein using quantum dots‐conjugated RNA aptamer on chip. Journal of Chemical Technology & Biotechnology. 2011;86:1475‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Valizadeh H, Abdolmohammadi‐Vahid S, Danshina S, et al. Nano‐curcumin therapy, a promising method in modulating inflammatory cytokines in COVID‐19 patients. Int Immunopharmacol. 2020;89(Part B):107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guarner J. Three emerging coronaviruses in two decades: The story of SARS, MERS, and now COVID‐19. Am J Clin Pathol. 2020;153(4):420‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hageman JR. The coronavirus disease 2019 (COVID‐19). Pediatr Ann. 2020;49:e99‐e100. [DOI] [PubMed] [Google Scholar]
- 94. Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID‐19 coronavirus (SARS‐CoV‐2) based on SARS‐CoV immunological studies. Viruses. 2020;12:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Casadevall A, Pirofski L. The convalescent sera option for containing COVID‐19. J Clin Invest. 2020. 10.1172/JCI138003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Abdolreza Esmaeilzadeh RE. Immunobiology and immunotherapy of COVID‐19: a clinically updated overview. J Cell Phys. 2020;1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Smithgall MC, Dowlatshahi M, Spitalnik SL, Hod EA, Rai AJ. Types of assays for SARS‐CoV‐2 testing: a review. Lab Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID‐19). Clin Infect Dis. 2020;71:778‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Xiang J, Yan M, Li H, et al. Evaluation of enzyme‐linked immunoassay and colloidal gold‐Immunochromatographic assay kit for detection of novel coronavirus (SARS‐Cov‐2) causing an outbreak of pneumonia (COVID‐19). MedRxiv. 2020. [Google Scholar]
- 100. Chlamydas S, Papavassiliou AG, Piperi C. Epigenetic mechanisms regulating COVID‐19 infection. Epigenetics. 2020;30:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gootenberg JS, Abudayyeh OO, Lee JW, et al. Nucleic acid detection with CRISPR‐Cas13a/C2c2. Science. 2017;356:438‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wright AV, Nuñez JK, Doudna JA. Biology and applications of CRISPR systems: harnessing nature's toolbox for genome engineering. Cell. 2016;164:29‐44. [DOI] [PubMed] [Google Scholar]
- 103. https://broad.io/sherlockprotocol
- 104. Huang Z, Tian D, Liu Y, et al. Ultra‐sensitive and high‐throughput CRISPR‐powered COVID‐19 diagnosis. Biosens Bioelectron. 2020;164:112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Broughton JP, Deng X, Yu G, et al. CRISPR–Cas12‐based detection of SARS‐CoV‐2. Nat Biotechnol. 2020;38:870‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Notomi T, Okayama H, Masubuchi H, et al. Loop‐mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:e63‐e63, 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Enosawa M, Kageyama S, Sawai K, et al. Use of loop‐mediated isothermal amplification of the IS900 sequence for rapid detection of cultured Mycobacterium avium subsp. paratuberculosis. J Clin Microbiol. 2003;41:4359‐4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Poon LL, Leung CS, Tashiro M, et al. Rapid detection of the severe acute respiratory syndrome (SARS) coronavirus by a loop‐mediated isothermal amplification assay. Clin Chem. 2004;50:1050‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pyrc K, Milewska A, Potempa J. Development of loop‐mediated isothermal amplification assay for detection of human coronavirus‐NL63. J Virol Methods. 2011;175:133‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Shirato K, Yano T, Senba S, et al. Detection of Middle East respiratory syndrome coronavirus using reverse transcription loop‐mediated isothermal amplification (RT‐LAMP). Virol J. 2014;11:139‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Shirato K, Semba S, El‐Kafrawy SA, et al. Development of fluorescent reverse transcription loop‐mediated isothermal amplification (RT‐LAMP) using quenching probes for the detection of the Middle East respiratory syndrome coronavirus. J Virol Methods. 2018;258:41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Huang P, Wang H, Cao Z, et al. A rapid and specific assay for the detection of MERS‐CoV. Front Microbiol. 2018;9:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang Y, Odiwuor N, Xiong J, et al. Rapid molecular detection of SARS‐CoV‐2 (COVID‐19) virus RNA using colorimetric LAMP. MedRxiv. 2020. [Google Scholar]
- 114. Kashir J, Yaqinuddin A. Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID‐19. Med Hypotheses. 2020;141:109786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Song J, Liu C, Mauk MG, et al. Two‐stage isothermal enzymatic amplification for concurrent multiplex molecular detection. Clin Chem. 2017;63:714‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mu D, Yan L, Tang H, Liao Y. A sensitive and accurate quantification method for the detection of hepatitis B virus covalently closed circular DNA by the application of a droplet digital polymerase chain reaction amplification system. Biotechnol Lett. 2015;37:2063‐2073. [DOI] [PubMed] [Google Scholar]
- 117. Alteri C, Scutari R, Stingone C, et al. Quantification of HIV‐DNA and residual viremia in patients starting ART by droplet digital PCR: their dynamic decay and correlations with immunological parameters and virological success. J Clin Virol. 2019;117:61‐67. [DOI] [PubMed] [Google Scholar]
- 118. Alteri C, Cento V, Antonello M, et al. Detection and quantification of SARS‐CoV‐2 by droplet digital PCR in real‐time PCR negative nasopharyngeal swabs from suspected COVID‐19 patients. PloS One. 2020;15(9):e0236311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bordería AV, Stapleford KA, Vignuzzi M. RNA virus population diversity: implications for inter‐species transmission. Curr Opin Virol. 2011;1:643‐648. [DOI] [PubMed] [Google Scholar]
- 120. Orílio AF, Lucinda N, Dusi AN, Nagata T, Inoue‐Nagata AK. Complete genome sequence of arracacha mottle virus. Arch Virol. 2013;158:291‐295. [DOI] [PubMed] [Google Scholar]
- 121. Gaudin M, Desnues C. Hybrid capture‐based next generation sequencing and its application to human infectious diseases. Front Microbiol. 2018;9:2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chen G‐Q, Zhuang Q‐Y, Wang K‐C, et al. Identification and survey of a novel avian coronavirus in ducks. PLoS One. 2013;8(8):e72918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wang K‐C, Chen G‐Q, Jiang W‐M, et al. Complete genome sequence of a hemagglutination‐negative avian paramyxovirus type 4 isolated from China. Genome Announc. 2013;1:e00045‐e00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this review because no datasets were generated or analyzed during the present study.


