Abstract
Angiotensin II (AngII), the effector peptide of the renin angiotensin system and has an important role in regulating cardiovascular hemodynamics and structure. AngII is an important biomarker for certain diseases that are associated with cardiovascular disorders, i.e., influenza, SARS‐CoV‐2, tumors, hypertension, etc. However, AngII presents in blood in very low concentrations and they are not stable due to their reactivity, therefore spontaneous detection of AngII is a big challenge. In this study, AngII‐imprinted spongy columns (AngII‐misc) synthesized for AngII detection from human serum, and characterized by surface area measurements (BET), swelling tests, scanning electron microscopy (SEM), FTIR studies. AngII binding studies were achieved from aqueous environment and maximum binding capacity was found as 0.667 mg/g. It was calculated that the AngII‐miscs recognized AngII 8.27 and 14.25 times more selectively than competitor Angiotensin I and Vasopressin molecules. Newly produced AngII‐misc binds 60.5 pg/g AngII from crude human serum selectively. It has a great potential for spontaneous detection of AngII from human serum for direct and critical measurements in serious diseases, that is, heart attacks, SARS‐CoV‐2, etc.
Keywords: angiotensin(II), cardiovascular disorders, Cryogels, molecular imprinting
1. INTRODUCTION
AngiotensinII (AngII) is an active hormone of the Renin angiotensin System (RAS) and has an important role within the complex network of other endocrine, paracrine systems, where RAS affects many tissue and organ systems. 1 , 2 , 3 AngII effects include vasoconstriction, aldosterone release, antidiuretic hormone synthesis, sympathetic activation, and salt absorption from kidney tubules. 4 However, when the RAS system disorders occur, all these effects could lead to the development of hypertension. The most important effect in hypertension is the direct contraction effect of AngII on arterial smooth muscles. It also causes irregularity in endothelial cells, medial hypertrophy and increase in connective tissue, causing atherosclerosis, growth of myositis in the heart muscle, development of left ventricular hypertrophy and development of heart failure. 3 , 5 , 6 Moreover, current studies showed that AngII levels increase, due to the Severe Acute Syndrome Coronavirus‐2 (SARS‐CoV‐2) triggers the down regulation of angiotensin converting enzyme 2 (ACE‐2). 7 , 8 That is why AngII is an important biomarker for certain diseases, i.e., heart attacks, SARS‐CoV‐2, influenza infections, tumors, etc., that are associated with hypertension, hypotension. 9 However, AngII presents in blood in very low concentrations and they are not stable due to their reactivity, therefore spontaneous detection of AngII is a big challenge. 10 , 11
AngII is mostly detected in biological fluids using HPLC‐radioimmunoassay (RIA), 12 enzyme‐linked immunosorbent assay (ELISA) 13 and LC–MS/MS 14 methods. Although these methods detect AngII for diagnosis, they have important drawbacks, i.e., time consuming, laborious, includes hazardous radioactive materials (for RIA), limited storage stability (for ELISA). That is why there is still a vital need to develop alternative methods for direct detection of AngII from body fluids. 10 , 11
Molecular imprinting is a technique, which is used for recognizing a target molecule (e.g., ion, protein, peptides, etc.) from a complex media, in a single step with high selectivity. Basically molecularly imprinted polymers designed in three steps; An interaction occurs between the functional monomers and the template molecule. The functional monomer‐template complex is polymerized in presence of crosslinkers and monomers. And the target molecule is removed from the polymer to get target specific cavities with the shape and chemical structure memory towards target. 15 , 16 , 17 The fact that they can be stored for several years without any change in their performance, they are reusable materials. Their advantages enabled them to be used as synthetic recognition elements in many application fields, i.e., diagnosis, separation, purification, in health, food, environment, etc. 18 , 19 , 20 Although imprinting of many small molecules is successfully applied in MIPs, there are still difficulties in the printing of large molecules such as protein. So far, polymerization methods such as bulk (3D), surface (2D) or partial (epitope) printing has been used for proteins. 21 , 22
Cryogels are soft materials which are synthesized at subzero temperatures (between 0 and −20), with three‐dimensional sponge‐like elastic morphology and pore sizes from a few microns to several hundred microns. 23 , 24 Interconnected macrospores provide low flow resistance property to the structure and thus the spongy structure allows to work without difficulty even with viscous liquids. 25 , 26 When these properties of spongy columns are combined with the molecular printing technique, it is possible to obtain advantageous materials with high selectivity that can provide ease of working with viscous liquids such as blood. 27 , 28
In this study we offer an alternative method for direct AngII detection from Human serum in a single step with high selectivity. For this purpose we prepared PHEMA based molecularly AngII imprinted spongy columns. The selectivity studies were performed against AngI and VASP, and AngII detection was achieved from human serum as well.
2. EXPERIMENTAL
2.1. Materials
Angiotensin II, Angiotensin I Human Acetate, Arg8‐Vasopressin Acetate, 1‐Vinylimidazole (VIM), Angiotensin II EIA Kit (Cat No: RAB0010), 2‐hydroxyethyl methacrylate (HEMA), N,N, N′,N′‐tetramethyl ethylene diamine (TEMED), ammonium persulfate (APS), methylene‐bis acrylamide (MBAA), Human Serum (H4522‐20ML) and other chemicals supplied from Sigma‐Aldrich, St. Louis, MO, USA. All water used in the experiments was purified using a Millipore Direct‐Q 3 UV Ultrapure (Type 1) water purification system (MerckAG, Darmstadt, Germany).
2.2. Preparation of AngII‐misc and non‐imprinted polymer (Nisc) columns
AngII‐molecularly imprinted spongy column (AngII‐misc) was prepared as follows, AngII‐VIM complex was prepared by dissolving AngII and functional monomer VIM (31 mg) in 1/10 n/n mol ratios in 1 ml of water and stored at +4°C for 12 hr to complete complexation. One milliliter of HEMA and 0.25 g MBAA was dissolved in 5 and 6 ml of water, respectively, to obtain 10% monomer concentrations. Then prepared pre‐complex was added into that mixture. Finally 150 μl of APS solution (10% w/w) and 10 μl of TEMED were added to the polymerization mixture as initiator and mixed in the ice bath. Then the prepared mixture was equally divided into 3 ml syringes and frozen in a cryostat at −16°C for 10 hr to complete the polymerization process. Polymers taken from the cryostate and left to room temperature for melting ice crystals in frozen polymeric columns and let them form interconnected macropores to get spongy columns. Non‐imprinted spongy columns (Nisc) were prepared in the same way without using AngII, these columns functioned as a control group. The prepared columns were washed with ethanol (30:70, v/v) for 30 min and water for 10 min, respectively, at room temperature to remove unreacted monomers and impurities.
2.3. Template removal from the AngII‐misc
AngII molecules removed from the AngII‐misc to get AngII specific cavities. Twenty milliliters of desorption buffer PBS buffer (10 mM, pH 7.4) was passed through the column for 2 hr continuously. At the end of this cycle a washing step was applied by passing 20 ml of water through the column. All measurements were done at 283 nm, using UV‐spectrophotometer (Genesys 150, Thermo Fisher Scientific). These steps repeated until no AngII detected in elution solution, spectrophometrically. The template removal amount was calculated using;
| (1) |
In here, Q TR (mg/g) is removal amount of template from unit mass of dry polymer, C i and C f are AngII concentrations before and after treatment of desorption agent with AngII‐misc, V (ml) is volume of desorption agent and m (g) is mass of dry polymer. Removal ratio is calculated using:
| (2) |
Template‐removed AngII‐misc were washed with water using a peristaltic pump for 30 min at room temperature and stored in water at +4°C until use.
2.4. Characterization studies
Water uptake ratio of AngII‐misc and Nisc were determined by swelling tests. Briefly; the AngII‐misc and Nisc were dried in lyophilizer and weighed with an accuracy of ±0.0001 and placed in a beaker containing 20 ml of water for 2 hr at 25 ± 0.5°C at constant temperature. Then it was removed from the beaker and weighed by removing excess water from the surface. Dry and wet weights are averaged of three repeated operations, the water uptake ratio of the material is determined with the following equation:
| (3) |
W 0 and W s are the weights of the AngII‐misc/Nisc before and after swelling in g, respectively.
In order to determine the macropore amount of AngII‐misc/Nisc, they were immersed in water and swollen samples weighed (W 1) in g. Then, swollen cryogel sample was squeezed delicately to remove water in macropores of the cryogels and weighed (W 2) in g. The macropore amount of AngII‐misc and Nisc was calculated using the equation below.
| (4) |
The polymerization efficiency of the prepared cryogels is calculated using the dry weight of produced cryogels (W p) and the weight of total reactant monomers (W m) used for polymerization. Polymerization yield calculated as follows:
| (5) |
Synthesized AngII‐misc and Nisc morphologies were characterized by Scanning Electron Microscope SEM (JEOL JSM 5600, Jeol Co., Tokyo, Japan). Specific surface area of the AngII‐misc and Nisc using Brunauer–Emmett–Teller (BET) analysis (Flowsorb II 2300, Micromeritics Instrument Corporation, Norcross, GA) via multipoint method. FTIR analysis was used to identify the chemical structure of the AngII, AngII‐VIM precomplex, AngII‐misc by FTIR Spectrometer (Nicolet iS10, Thermo Fisher Scientific) between 4000 and 600 cm−1.
2.5. AngII binding studies from aqueous solutions
AngII binding onto AngII‐misc and Nisc from aqueous solutions was performed by a continuous system using a peristaltic pump, and all experiments were repeated three times and average of obtained results were reported. The effects of the equilibrium AngII concentration onto AngII binding was investigated in the range of 0.01–0.2 mg/ml AngII concentrations. The effects of binding time onto AngII binding capacity was investigated in the range of 0–120 min and the effect of flow rate was studied 0.5–4 ml/min. In each step, columns were equilibrated with PBS buffer by applying 5 ml of PBS to the column. All AngII solutions were prepared in PBS buffer (10 mM, pH 7.4). All parameters were evaluated using UV‐spectrophotometer at 283 nm and the binding capacities were calculated using:
| (6) |
Here, Q is binding capacity (mg/g), C o and C f are AngII concentrations of the AngII solutions before and after the interaction with AngII‐misc and Nisc columns (mg/ml), V is volume of the solution (ml), m is the dried mass of the column (g).
2.6. Selectivity studies
The selectivity of AngII‐misc against AngII molecules was examined by selectivity tests. In this study, the binding behavior of the AngII molecule was compared against competitor AngI and VASP molecules. These molecules are chosen as competitors because they have similar chemical structure and size with AngII molecules. (AngII, 1046 Da; AngI, 1296.5 Da; VASP, 1084.23 Da). For this purpose the 5 ml of 0.05 mg/ml of Ang II, AngI and VASP was prepared in PBS at pH 7.4 buffers, separately and applied to AngII‐misc and Nisc one by one. Binding amounts were calculated by taking 100 μl of samples before and after treatments and evaluated using UV‐spectrophotometer.
The selective AngII binding from human serum was also performed, in 1/10 and 1/20 fold diluted human serum with PBS buffer (10 mM, pH 7.4). Human serum was spiked with AngII to get 0.05 mg/ml and then it was diluted in the ratios of 1/10, 1/20. Then the 3 ml of diluted serum samples were applied to AngII‐misc for 2 hr at room temperature by a peristaltic pump. After 2 hr the final serum sample was taken and diluted to 500 pg/ml using 10 mM PBS buffer (pH 7.4), due to sigma elisa kit working in the range of 1–1000 pg/ml. Another 3 ml of crude human serum spiked with AngII to get 5 pg/ml and directly applied to the AngII‐misc. Five hundred microliters of serum samples was taken before and after treatment with AngII‐misc. All collected experimental samples were then analyzed using commercial Angiotensin II EIA Kit by sandwich ELISA method. All AngII binding amounts were calculated according to ELISA kit technical report by ELISA (Awareness Technology Inc.). 11
2.7. Reusability studies
Reusability of AngII‐miscs and Nisc were tested by applying AngII to the same column 10 times. 0.5 mg/ml of AngII was prepared freshly for each cycle in PBS pH 7.4 and after each binding step, AngII molecules desorbed from AngII‐misc, by circulating 1 M NaCl in PBS (10 mM, pH 7.4) through AngII‐misc for 2 hr at room temperature. AngII concentration in desorption medium was measured spectrophotometrically at 283 nm. The AngII desorption ratio was calculated using:
| (7) |
After each desorption step, excess of water was pumped through the AngII‐misc and Nisc for washing and columns were equilibrated with PBS buffer at pH 7.4 for 10 min before reuse.
3. RESULTS
3.1. Characterization studies
Swelling tests were performed for analyzing water uptake ratio, and macropore amount. Table 1 summarized the swelling tests results both for AngII‐misc and Nisc. Water uptake ratios were calculated as 94.3 and 92% and the macroporosity amount was calculated as 82 and 80% for AngII‐misc and Nisc, respectively. Polymerization yields were found as 88 and 84% for AngII‐misc and Nisc, respectively. Table 1 also shows the BET analysis results and the specific surface areas of AngII‐misc and Nisc were found to be 52 and 47 m2/g, which leads sufficient interaction areas for selective recognition studies.
TABLE 1.
Swelling ratio, macroporosity, surface area and polymerization yield of AngII‐misc and Nisc
| Water uptake ratio (%) | Macroporosity amount (%) | Surface area (m2/g) | Polymerization yield (%) | |
|---|---|---|---|---|
| AngII‐misc | 94.3 | 82 | 52 | 88 |
| Nisc | 92 | 80 | 47 | 84 |
The surface morphology of the prepared Ang‐misc and Nisc samples were examined by scanning electron microscopy (SEM) that provides high magnification. In this study, Figure 1a,b show the interior structures of Ang‐misc and 1C and D Nisc, respectively. As can be clearly seen, all columns have interconnected pores and the pores have 50–100 μM in diameter which allows the easy fluid flow through the column. SEM photos also showed rough walls, which lead to high surface area for effective interaction with AngII and AngII specific cavities on Ang‐misc.
FIGURE 1.

SEM photos of AngII‐misc (a and b); Nisc (c and d)
Figure 2 shows the FTIR spectrum of AngII, VIM, AngII‐VIM complex, and AngII‐misc. As can be seen from the spectrum; AngII has C=C stretching peak around 1600 cm−1 and aromatic C—H bending peak around 1500 cm−1 also a broad band was observed in 3600–3400 cm−1 due to O—H stretching vibration. 29 AngII has C—H stretching band at around 2964 cm−1, C=C stretching and N—H bending at around 1623 cm−1. C=N—C aromatic ring stretching observed at around 1514 cm−1 in ANGII and 1502 cm−1 in VIM, respectively. C—H ring bending at around 1111 cm−1, C—N— ring bending at around 1025 cm−1 observed at around both in Ang II and VIM, respectively, in different intensities. C—H stretching vibration was observed at 2951 cm−1 and C—O stretching at around 1250 cm−1 for both Ang II and VIM. N—H stretching peak was observed clearly at around 2800–3000 cm−1 and a carboxylic acid OH stretching band was seen at 3110.62 cm−1 band for VIM. 30 After complexation the O—H stretching around 3400 cm−1 was observed as a broad peak and the intensity of this peak was increased and shifted higher frequencies due to the H bonding of complexation. C=N stretching band was also shifted left after complexation and C=N—C aromatic ring stretching peak shifted higher frequencies and intensity of that peak increased. These changes showed that the precomplex was formed between AngII and VIM. According to FTIR results of AngII‐MIP; common bands from HEMA monomers are seen at around 3400 cm−1 (—OH stretch band) and 1700 cm−1 (—C=O stretch band) clearly. When the spectra examined, characteristic imidazole ring stretching vibration band at around 1530 cm‐1, ring vibration at around 1249, 1153, and 1076 cm−1 C—H bending in plane ring can be seen clearly. These data confirmed the presence of VIM in AngII‐misc.
FIGURE 2.

FTIR spectrum of (a) AngII, (b) VIM, (c) AngII‐VIM complex, and (d) AngII‐misc
3.2. Template removal studies
Template removal ratio was calculated as 85% AngII from the AngII‐misc and removal amount found as 680 μg AngII/g AngII‐misc.
3.3. Equilibrium AngII concentration effect on AngII binding amount
The effect of equilibrium AngII solution concentration on maximum AngII binding was determined by applying aqueous solutions of AngII molecules in concentrations ranging from 0.01 to 0.2 mg/ml to the column under the same conditions. AngII binding amount increased at the beginning, then the maximum binding capacity of the column remained unchanged after the concentration of 0.05 mg/ml. This behavior can be explained by the AngII concentration difference (ΔC AngII). ΔC AngII is the driving force for AngII binding on AngII‐misc and it increases with increasing AngII concentration so an increase in binding capacity observed with increasing driving force and binding capacity, as well. Saturation of the recognition zones then prohibits the binding. Maximum AngII binding capacity was calculated as 0.667 mg/g AngII‐misc and 0.222 mg AngII/g Nisc (Figure 3).
FIGURE 3.

Effect of equilibrium initial AngII concentration on AngII binding amount. m dry: 0.1027 g, V: 3 ml, time: 120 min, pH 7.4, flow rate: 0.5 ml/min, T: 25°C
Adsorption isotherms describe the relationship between the amount of adsorbate (q e) adsorbed by the adsorbent at a constant temperature and the adsorbate concentration (C e) remaining in the solution at equilibrium. The parameters obtained from the adsorption isotherms provide useful information about the surface properties, the adsorption mechanism and the interaction between the adsorbent and the adsorbate. The Langmuir and Freundlich adsorption isotherms were used for understanding adsorption behavior of the synthesized AngII‐misc and Nisc towards AngII molecules. This isotherm model assumes that the adsorption is monolayer and the surface is homogeneous. 31 According to the Langmuir isotherm, all binding sites in the adsorbent are equally energized and suitable for binding of at most one adsorbate molecule. 32 Linearized Langmuir adsorption isotherm equation is given below:
| (8) |
Q is the capacity of molecules that bind to the material (mg/ml), C e is the concentration of the given molecule in the solution (mg/L), b is constant of Langmuir (ml/mg) and Q max is the highest adsorption capacity (mg/g). 1/Q max is calculated by the point where 1/Q graph crosses the y‐axis against 1/C e and the slope gives the value of 1/Q max × b. 32
The Freundlich isotherm assumes that adsorption occurs physically and reversibly on heterogeneous surfaces. According to this isotherm model, adsorption is multi‐layer adsorption, the surface is heterogeneous and binding sites are not equal energetically. The Freundlich isotherm model is an exponential equation and assumes that adsorption occurs through multiple layers instead of a single layer. 31 , 33 Linearized Freundlich equation can be given as:
| (9) |
In this equation, Q eq is the amount of adsorption (mg/g) and C e is the equilibrium concentration in the solution (mg/L). K f and 1/n are Freundlich constants, which are indicating adsorption capacity and adsorption intensity. Experimental data were adapted to the Freundlich model and lnC eq was plotted against lnQ eq. Adsorption constants were calculated from the cut‐off point and slope. 33
Table 2. summarizes the calculated Langmuir and Freundlich adsorption isotherm constants. The calculated Q L, Q F, and the R 2 values showed that the adsorption data is more compatible with Langmuir isotherm, and it can be concluded that the monolayer adsorption is favorable for AngII‐misc. These results also in agreement with Figure 4, in this figure the plot of experimental binding amount well fitted with calculated Langmuir binding amount rather than Freundlich.
TABLE 2.
Langmuir and Freundlich adsorption isotherm constants for AngII
| Experimental | Langmuir constants | Freundlich constants | ||||
|---|---|---|---|---|---|---|
| Q ex (mg/g) | Q L (mg/g) | b (ml/mg) | R 2 | Q F (mg/g) | n | R 2 |
| 0.667 | 0.772 | 57.24 | 0.9766 | 2.37 | 2.00 | 0.8406 |
FIGURE 4.

Comparison of experimental AngII binding amount vs Langmuir and Freundlich AngII binding amounts
3.4. Effects of interaction time on AngII binding
In order to determine the effect of interaction time on maximum AngII binding amount of the column, 3 ml of 0.05 mg/ml AngII solution was interacted with the column with a flow rate of 0.5 ml/min at pH 7.4. Samples were taken for 2 hr with certain time intervals and the maximum binding time interval was determined. It was observed that the maximum binding was reached at 60th min. and binding time was taken as a basis in all subsequent experiments (Figure 5).
FIGURE 5.

Effects of interaction time on AngII binding amount and kinetic binding models. AngII conc: 0.05 mg/ml, m dry: 0.1027 g, V: 3 ml, pH 7.4, flow rate: 0.5 ml/min, T: 25°C
Time‐binding relationship used in kinetic model calculations to understand adsorption controlling mechanisms. In here pseudo‐first order kinetic and pseudo second‐order kinetic equations were used for the adsorption of an analyte from its aqueous solution. The pseudo first‐order kinetic model calculated using Lagergren's equation; Δq t/dt = k 1(q eq − q t ) and linearized as log(q eq − q t ) = log(q eq) – (k 1 t)/2.303. In here, k 1 is first order adsorption rate constant (min−1); q eq and q t are AngII adsorption amounts at equilibrium and at time t (mg/g), respectively. The pseudo‐second order equation based on adsorption equilibrium capacity expressed as; Δq t/dt = k 2 (q eq − q t ) and can be linearized as (t/q t ) = (1/k 2 q eq 2) + (1/q eq) t. Here k 2 is pseudo‐second order adsorption rate constant (g mg−1/min). The rate constants k 1, k 2, and equilibrium adsorption amounts q eq obtained intercepts and slopes of log(q eq − q t ) vs t plot for first order (Figure 5b) and (t/q t ) vs t plots for second order (Figure 5c), respectively and summarized in Table 3.
TABLE 3.
Adsorption kinetic model constants and adsorption amounts
| Exp. | Pseudo‐first‐order‐kinetic | Pseudo‐second‐order‐kinetic | |||||
|---|---|---|---|---|---|---|---|
| Initial conc. (mg/ml) | q eq (mg/g) | k 1 (1/min) | q eq (mg/g) | R 2 | k 2 (1/min) | q eq (mg/g) | R 2 |
| 0.05 | 0.667 | 0.059 | 2.024 | 0.98 | 23.8 | 0.688 | 0.99 |
According to results shown in Table 3 the adsorption process can be expressed via second order mechanism. It is obvious that the R 2 of pseudo second order is 0.99 greater than that of pseudo first order; moreover the theoretical pseudo second order q e (0.688 mg/g) is closer to experimental q e (0.667 mg/g) than that of pseudo first order (2.024 mg/g). As a result, pseudo‐second order mechanisms control the adsorption process via chemisorption rather than diffusion, obtained results support the specific interactions occur between AngII and AngII‐misc via size and chemical structure. 31 , 34
3.5. Effects of flow rate on AngII Binding
The effect of flow rate on AngII binding was investigated. 0.05 mg/ml AngII in PBS (10 mM, pH 7.4) solution was applied to the column at different flow rates; 0.1, 0.5, 1.0, and 2 ml/min. As shown in Figure 6, adsorbed AngII amount was decreased by increasing flow rate, due to limited interaction between column and AngII molecules. The maximum AngII binding capacity of the appropriate flow rate was determined as 0.5 ml/min, and the rest of the study was performed at this point.
FIGURE 6.
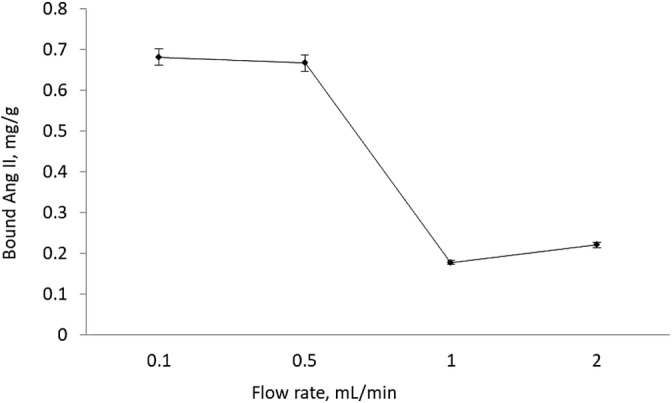
Effects of flow rate on AngII binding. AngII conc: 0.05 mg/ml, m dry: 0.1027 g, V: 3 ml, pH 7.4, time: 120 min, T: 25°C
3.6. Selectivity studies
Selectivity experiments were done for demonstrating the selectivity of AngII‐misc towards Ang II and competitors; AngI and VASP. Figure 7 summarized the selective binding amounts of all molecules onto AngII‐misc and Nisc. This figure showed that the AngII binding amount is higher for AngII‐misc than competitors. This is an evidence for selective binding and obtained data also used for calculation of distribution coefficient K d, imprinting factor If and selectivity coefficients k, for evaluating selectivity in detail. 34 , 35
IGURE 7.

FSelectivity studies. Concentrations of AngII, Ang I, VASP: 0.05 mg/ml, m dry: 0.1027 g, V: 3 ml, pH 7.4, flow rate: 0.5 ml/min, time: 120 min, T: 25°C
The distribution coefficient (K d, ml/g) calculated according to following equation:
K d = [(C i − C f)/C f] × V/m (10)
Here C i is initial solution concentration (mg/ml); C f is concentration of the solution after interaction with the column (mg/ml); V is volume of solution (ml) and m is dry column weight (g).
The recognition characteristic of AngII‐misc columns is determined by the imprinting factor (IF). IF value allowed us to compare the selective binding behavior of imprinted and non‐imprinted spongy columns.
| (11) |
In this equation, K d Misc is the distribution coefficient of AngII‐misc columns and K d Nisc is the distribution coefficient of Nisc columns.
The selectivity coefficient (k) is an indicator of the selectivity of the column for the template molecule. Selectivity coefficients were calculated by comparing the selective binding behavior of AngII‐misc towards AngII versus AngI and VASP. k is calculated with the equation given below:
| (12) |
K d Misc template is distribution coefficient of AngII‐misc towards AngII and K d Misc competitor is distribution coefficient of AngII‐misc towards competitors.
Table 4 shows that the AngII‐misc was 8.27 times more selective against the AngI molecule and 14.25 times more selective against VASP. It was concluded that the AngII‐misc can recognize AngII specifically. All molecules have similar chemical structures and molecular weights, but results showed that AngII‐misc can recognize AngII more specifically. The specific recognition occurred because, AngII molecule surrounded with VIM functional monomers via secondary interactions and molded well, not only based on the size, but chemical structure as well. That is why slight differences in chemical structure and size of the molecules can be recognized by newly synthesized AngII‐misc.
TABLE 4.
Summary of selectivity studies; distribution coefficient, imprinting factor, and selectivity coefficients
| AngII‐misc | NIP | |||
|---|---|---|---|---|
| K d (ml/g) | K d (ml/g) | IF | k | |
| AngII | 54.6 | 4.9 | 11,1 | |
| AngI | 6.6 | 4.14 | 1.6 | 8.27 |
| VASP | 3.8 | 2.7 | 1.4 | 14.25 |
3.7. The selective AngII binding from human serum
The selective binding AngII from human serum was achieved using 1:10, 1:20 diluted human serum, which have 0.005 and 0.0025 mg/ml AngII concentrations, respectively. The ELISA results were evaluated and the AngII binding amounts were found as 0.054 mg/g and 0.028 mg/g with 87% and 92% recovery. When 5 pg Np/ml crude (undiluted) serum applied to column; selective binding capacity was calculated as 60.5 pg/g, it is important to note that 96% of the AngII can successfully rebound from crude human serum. 11
3.8. Reusability studies
In order to examine the reusability of the produced columns, the AngII solution in a certain concentration was applied to the same column 10 times and the results obtained after 10 binding desorption cycles are shown in Figure 8. The decrease in capacity of AngII binding of the columns is negligible (3%) and obtained results showed that the synthesized AngII‐misc can be used several times without any capacity decrease or any structural change.
FIGURE 8.

Reusability of AngII‐misc columns. AngII conc: 0.05 mg/ml, mdry: 0.1027 g, V: 3 ml, pH 7.4, flow rate: 0.5 ml/min, time: 120 min, T: 25°C
4. CONCLUSION
AngII, an important biomarker in human blood for cardiovascular diseases, SARS‐CoV‐2, influenza infections, tumors, etc. AngII‐misc was synthesized for the detection of AngII from human serum for the purpose of early diagnosis, prognosis and treatment of related diseases. AngII binding capacity was found as 0.667 mg/g from aqueous solution and AngII‐misc can recognize AngII 8.27 and 14.25 times more selective than AngI and VASP, respectively. The AngII‐misc can bind AngII from human serum as 60.5 pg/g with 96% recovery. This study showed that the column synthesized can be used as an alternative method for the diagnosis of AngII from blood and has a potential for further studies.
AUTHOR CONTRIBUTIONS
Mehtap Yıldırım: Investigation; writing‐original draft; writing‐review and editing. Gözde Baydemir Peşint: Investigation; methodology; resources; supervision; writing‐original draft; writing‐review and editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/btpr.3112.
ACKNOWLEDGMENTS
This work was supported by the Scientific Research Projects Coordination Unit of Adana Alparslan Türkeş Science and Technology University (grant number:19332002) and conducted in Nanotechnology Research and Application Laboratory in Adana Alparslan Türkeş Science and Technology University/Çukurova Development Agengy/Turkey (TR62/18ÜRET/0032).
Yıldırım M, Baydemir Peşint G. Molecularly imprinted spongy columns for Angiotensin(II) recognition from human serum. Biotechnol Progress. 2021;37:e3112. 10.1002/btpr.3112
Funding information Çukurova Development Agengy/Adana Alparslan Türkeş Science and Technology University/Nanotechnology Application and Research Laboratory, Grant/Award Number: TR62/18ÜRET/0032; Scientific Research Projects Coordination Unit of Adana Alparslan Türkeş Science and Technology University, Grant/Award Number: 19332002
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Unger T. Neurohormonal modulation in cardiovascular disease. Am Heart J. 2000;139:2‐8. [DOI] [PubMed] [Google Scholar]
- 2. Brunner HR, Nussberger J, Waeber B. Inhibitors of the renin‐angiotensin system. Arzneimittelforschung. 1993;43:274‐288. [PubMed] [Google Scholar]
- 3. Lan TH, Huang XQ, Tan HM. Vascular fibrosis in atherosclerosis. Cardiovasc Pathol. 2013;5:401‐407. [DOI] [PubMed] [Google Scholar]
- 4. Levenson B, Herrera C, Wilson BH. New ACC global heart attack treatment initiative (improving STEMI care worldwide). J Am Coll Cardiol. 2020;75:1605‐1613. 10.1016/j.jacc.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 5. Bhatnagar D, Kaur I, Kumar A. Ultrasensitive cardiac troponin I antibody based nanohybrid sensor for rapid detection of human heart attack. Int J Biol Macromol. 2015;95 505‐510. [DOI] [PubMed] [Google Scholar]
- 6. Mitro P, Mudrakova K, Mığkova H, Dudas J, Kırsch P, Valoğık G. Hemodynamic parameters and heart rate variability during a tilt test in relation to gene polymorphism of renin‐angiotensin and serotonin system. J Compilation. 2008;31:1571‐1581. [DOI] [PubMed] [Google Scholar]
- 7. Bautista‐Vargas M, Bonilla‐Abadial FA, Cañas C. Potential role for tissue factor in the pathogenesis of hypercoagulability associated with in COVID‐19. J Thromb Thrombolysis. 2020;50:479‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silva ACS, Lanza K, Palmeira VA, Costa LB, Flynn JT. Update on the renin–angiotensin–aldosterone system in pediatric kidney disease and its interactions with coronavirus. Pediatr Nephrol. 2020. 10.1007/s00467-020-04759-1.29 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swanoski MT, Lutfiyya MN, Amaro ML, Akers MF, Huot KL. Knowledge of heart attack and stroke symptomology: a cross‐sectional comparison of rural and non‐rural US adults. BMC Public Health. 2012;12:283‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Navar GL, Mitchell KD, Harrison‐Bernard LM, Kobori H, Nisbiyama A. Intrarenal angiotensin II levels in Normal and hypertensive states. J Renin‐Aldosterone Syst. 2001;2:176‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montezano AC, Cat AN, Rios FJ, Touyz RM. Angiotensin II and vascular injury. Curr Hypertens Rep. 2014;6:431‐444. [DOI] [PubMed] [Google Scholar]
- 12. Brosnihan KB, Chappell MC. Measurement of angiotensin peptides: HPLC‐RIA methods. Mol Biol. 2017;1527:81‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chappell MC. Biochemical evaluation of the renin‐angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol. 2016;310:137‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cui L, Nithipatikom K, Campbell WB. Simultaneous analysis of angiotensin peptides by LC‐MS and LCMS/MS: metabolism by bovine adrenal endothelial cells. Anal Biochem. 2007;369:27‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Polyakov MV. Adsorption properties and structure of silica gel adsorption properties on the character of its porosity. Zhurnal Fizieskoj Khimii. 1931;2:799‐805. [Google Scholar]
- 16. Mosbach K, Ramström O. The emerging technique of molecular imprinting and its future impact on biotechnology. Nat Biotechnol. 1996;14:163‐170. [Google Scholar]
- 17. Wullf G. Molecular imprinting in cross‐linked materials with the aid of molecular templates‐a way towards artificial antibodies. Angew Chem Int Ed. 1995;34:1812‐1832. [Google Scholar]
- 18. Mosbach K, Arshady R. Synthesis of substrate‐selective polymers by host–guest polymerization. Macromol Chem Phys. 1981;182:687‐692. [Google Scholar]
- 19. Sellergren B. Sellergren B, Molecularly Imprinted Polymers: Man‐Made Mimics of Antibodies and Their Applications in Analytical Chemistry. 1st ed. New York: Elsevier; 2001. https://www.elsevier.com/books/molecularly-imprinted-polymers/sellergren/978-0-444-82837-8. [Google Scholar]
- 20. Martin‐Esteban A. Molecularly‐imprinted polymers as a versatile, highly selective tool in sample preparation. TrAC Trends Anal Chem. 2013;45:169‐181. [Google Scholar]
- 21. Haupt K. Molecularly imprinted polymers in analytical chemistry. R Soc Chem/The Analyst. 2001;126:747‐756. [DOI] [PubMed] [Google Scholar]
- 22. Haupt K, Mosbach K. Molecularly imprinted polymers and their use in biomimetic sensors. J Am Chem Soc. 2000;100:2495‐2504. [DOI] [PubMed] [Google Scholar]
- 23. Hajizadeh S, Kirsebom H, Leistner A, Mattiasson B. Composite cryogel with immobilized concanavalin a for affinity chromatography of glycoproteins. J Sep Sci. 2012;35:2978‐2985. [DOI] [PubMed] [Google Scholar]
- 24. Kirsebom H, Topgaard D, Galaev IY, Mattiasson B. Modulating the porosity of cryogels by influencing the non‐frozen liquid phase through the addition of inert solutes. Langmuir. 2010;26:16129‐16133. [DOI] [PubMed] [Google Scholar]
- 25. Lozinsky VI, Galaev IY, Plieva FM, Savina IN, Jungvid H, Mattiasson B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003;10:445‐451. [DOI] [PubMed] [Google Scholar]
- 26. Rabieizadeh M, Kashefimofrad SM, Naeimpoor F. Monolithic molecularly imprinted cryogel for lysozyme recognition. J Sep Sci. 2014;37:2983‐2990. [DOI] [PubMed] [Google Scholar]
- 27. Baydemir G, Bereli N, Andaç M, Say R, Galaev IY, Denizli A. Supermacroporous poly(hydroxyethyl methacrylate) based cryogel with embedded bilirubin imprinted particles. React Funct Polym. 2009;69:36‐42. [Google Scholar]
- 28. Andaç M, Denizli A. Affinity‐recognition‐based polymeric cryogels for protein depletion studies. R Soc Chem. 2014;59:31130‐31141. [Google Scholar]
- 29. Tekin K, Uzun L, Şahin ÇA, Bektaş S, Denizli A. Preparation and characterization of composite cryogels containing imidazole group and use in heavy metal removal. React Funct Polym. 2011;71:985‐993. [Google Scholar]
- 30. Daoud‐Attieh M, Chaib H, Armutçu C, Uzun L, Elkak A, Denizli A. Immunoglobulin G purification from bovine serum with pseudo‐specific supermacroporous cryogels. Sep Purif Technol. 2013;118:816‐822. [Google Scholar]
- 31. Göktürk I, Tamahkar E, Yılmaz F, Denizli A. Protein depletion with bacterial cellulose nanofibers. J.Chromatogr B. 2018;1099:1‐9. [DOI] [PubMed] [Google Scholar]
- 32. Aksu Z, Calik A, Dursun AY, Demircan Z. Biosorption of iron (III)–cyanide complex anions to Rhizopus arrhizus: application of adsorption isotherms. Process Biochem. 1999;34:483‐491. [Google Scholar]
- 33. Aslıyüce S, Matiasson B, Denizli A. Combined protein A imprinting and cryogelation for production of spherical affinity material. Biomed Chromatogr. 2019;33:e4605. [DOI] [PubMed] [Google Scholar]
- 34. Baydemir G, Denizli A. Heparin removal from human plasma using molecular imprinted cryogels. Artif Cells Nanomed Biotechnol. 2015;43:403‐412. [DOI] [PubMed] [Google Scholar]
- 35. Zhang W, She X, Wang L, et al. Preparation, characterization and application of a molecularly imprinted polymer for selective recognition of Sulpiride. Materials. 2017;10:475‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


