Abstract
Exhaled breath test is a typical disease monitoring method for replacing blood and urine samples that may create discomfort for patients. To monitor exhaled breath markers, gas biomedical sensors have undergone rapid progress for non‐invasive and point‐of‐care diagnostic devices. Among gas sensors, metal oxide‐based biomedical gas sensors have received remarkable attention owing to their unique properties, such as high sensitivity, simple fabrication, miniaturization, portability and real‐time monitoring. Herein, we reviewed the recent advances in chemoresistive metal oxide‐based gas sensors with ZnO, SnO2 and In2O3 as sensing materials for monitoring a range of exhaled breath markers (i.e., NO, H2, H2S, acetone, isoprene and formaldehyde). We focused on the strategies that improve the sensitivity and selectivity of metal oxide‐based gas sensors. The challenges to fabricate a functional gas sensor with high sensing performance along with suggestions are outlined.
Keywords: exhaled breath marker, gas medical sensor, metal oxide, nanomaterial, non‐invasive disease diagnosis, point‐of‐care device, selective detection
1. INTRODUCTION
Breath analysis is a promising approach for monitoring diseases and their progression as it provides a non‐invasive, very safe and simple way for all patients as many times as needed. The exhaled breath markers (EBMs) compose of inorganic compounds (e.g. carbon dioxide, carbon monoxide and nitric oxide), voltaic organic compounds (e.g. isoprene, formaldehyde, and acetone) and non‐voltaic compounds (e.g. cytokines and hydrogen peroxide; Cazzola & Novelli, 2010; Kim, Jahan, et al., 2012; Lu et al., 2018; Wang & Sahay, 2009). The abnormal level of EBM carries some information about the disease status and treatment efficiency (Das & Pal, 2020). Many clinical trials focused on EBM for the diagnosis of disease ranging from respiratory, diabetes to cancer (Brinkman et al., 2020; Chang et al., 2018; Gregis et al., 2018; Kabir et al., 2019; Rahman et al., 2020).
To monitor EBM, various techniques have been utilized, such as chromatography, selected ion flow tube–mass spectrometry (SIFT‐MS), proton‐transfer reaction–mass spectrometry (PTR‐MS) and laser spectroscopy technique (Das & Pal, 2020; Kharitonov & Barnes, 2002; Kim, Jahan, et al., 2012). Among them, chromatography–mass spectroscopy (GC‐Mass) is a well‐established method in clinical trials for monitoring EBM, owing to its outstanding precision and selectivity (Wang & Sahay, 2009). However, GC‐Mass application often is limited to laboratory usage due to requiring sophisticated, bulky and high‐cost instruments, and experts (Glockler et al., 2020). To date, the advances in portable gas sensors with the miniaturized size hold great potential to overcome such shortcomings and pave the way for point‐of‐care EBM monitoring.
Chemoresistive metal oxide‐based gas sensors (MGSs) identify the target gas by using a change in electrical resistance that translates to the gas concentration. Recently, MGS has received much attention given for their great potential in breath analysis owing to the advantages such as straightforward integration in a chip, simple manufacturing, high stability and reusability (Cho et al., 2017; Das & Pal, 2020; Guntner et al., 2019; Tai et al., 2020). The metal oxides are categorized into n‐type and p‐type. In the n‐type MGS, adsorbed air oxygen onto the metal oxide receives electrons from metal oxide via conduction bands. This electron transformation causes a reduction in carrier charges in metal oxide surface and formation of the depletion layer and also the formation of oxygen ions (O−2, O− or O2−) (Moseley, 2017). The reducing target gas takes electrons from oxygen ions and transport electrons to the conduction band of metal oxides resulting in an increasing conductivity and reducing resistance. In the case of exposure to the oxidizing target gas, the overall result reduces the conductivity. In the p‐type metal oxide, the adsorbed oxygen traps the electron through the valance band of metal oxide that results in generation holes. The effect of exposure to the reducing or oxidizing target gases on the conductivity is the reverse of n‐type cases. Beyond n‐type or p‐type metal oxide as a sensing material, combining metal oxides with other metal oxides, noble metals and carbon‐based materials might generate p‐p, n‐p and n‐n type heterojunctions and potentially further enhance the electron mobility (Amiri et al., 2020).
Herein, we focus on MGS for disease diagnosis by EBM. Subsequent sections indicate the current state of gas sensors based on the three most common metal oxides including ZnO, SnO2 and In2O3 with paying attention to the role of nanomaterials in enhancing the analytical performance of the sensors. We also describe recent progress in gas sensors along with the strategies to improve their sensing performance.
2. EXHALE BREATH MARKERS
Exhaled breath composes of more than a thousand gases of which some of these gases are known as EBM that any variations in their concentrations initiate disorders and diseases. The most common EBM and their corresponding disease are briefed in Table 1. As shown in some cases, the abnormality in EBM renders several diseases, while several EBMs might be attributed to particular diseases. Still, the exploration of breath analysis in the clinics is very immature. There are great challenges for the standardization of EBM assessments that include but not limited to inter‐individual variability stemming from genetics, human activities or air pollutions. Despite such challenges, there is remarkable progress in revealing the connection between EBM and disorders (Guntner et al., 2019). The identification of acetone as EBM for diagnosis of diabetic people backs to 1857 (Crofford et al., 1977) resuming with continuous efforts on the investigation of EBM to accurately diagnose diseases.
TABLE 1.
Exhale breath markers and corresponding diseases
| Biomarker | Disease | Reference |
|---|---|---|
| Acetone (OC(CH3)2) | Diabetes, lung cancer, congestive heart failure | Hanh et al. (2020), Ruzsanyi and Peter Kalapos (2017), Wang and Sahay (2009) |
| Isoprene | Blood cholesterol level, lipid disorder, fibrosis and cirrhosis | Alkhouri et al. (2015), Salerno‐Kennedy and Cashman (2005) |
| Nitrogen monoxide (NO) | Asthma, chronic obstructive pulmonary disease (COPD), lung disease, airway inflammation, hypertension, rhinitis, cystic fibrosis | Barnes et al. (2006), Birrell et al. (2006), Brindicci et al. (2005), Cazzola and Novelli (2010), Guntner et al. (2019), Kharitonov and Barnes (1996, 2002), Lu et al. (2018), McCluskie et al. (2004), Wang and Sahay (2009) |
| Formaldehyde (HCHO) | Lung cancer | Wehinger et al. (2007) |
| Hydrogen (H2) | Intestinal upset, indigestion in infants | Wang and Sahay (2009) |
| Hydrogen sulphide (H2S) | Asthma, chronic obstructive pulmonary disease (COPD) | Bulemo et al. (2017), Chung (2014), Zhang et al. (2015) |
In a recent study, by taking benefits from data analysis methods, seven biomarkers for the discrimination of lung cancer patients from healthy ones were named as acetone, methyl acetate, isoprene, methyl vinyl ketone, cyclohexane, 2‐methylheptane and cyclohexanone (Rudnicka et al., 2019). To date, by utilizing nanomaterials into sensors, the exhaled breath analysis has become more achievable for rapid and painless disease diagnostic.
3. METAL OXIDE‐BASED GAS SENSORS FOR THE DETECTION OF EXHALED BREATH MARKERS
The analytical performance of gas sensors at the operating temperature and relative humidity (RH) is determined with the following characteristics. (i) Selectivity: the main challenge in MGS is selectivity for the accurate detection of EBM that often is modulated by the type and amount of dopants, grain size, morphologies and preparation protocols (Kim, Kim, et al., 2012; Kim, Choi, et al., 2016). The selectivity of metal oxide‐based gas sensors is often reduced in the interferences of water vapours (Liu et al., 2020). It is of note that the RH of human breath is about 89%–97% (Ferrus et al., 1980). (ii) Limit of detection (LOD): the EBM concentration is in the range of ppb‐ppt, and the MGS should have high sensitivity for detection of trace level of EBM (Das & Pal, 2020). (iii) Stability: MGS should be stable to generate reliable and reproducible results. In the following, we will discuss recent MGS with the focus on ZnO, SnO2 and In2O3. These metal oxides are n‐type semiconductors, meaning that they have a similar mechanism in response to the oxidizing/reducing target gas (Zhang, Liu, et al., 2016). Due to taking benefit from the high electron mobility, they use as sensitive platforms for the detection of gases (Ho et al., 2011; Zhang, Zhou, et al., 2016). Among these three metal oxides, ZnO has been extensively studied in medical gas sensors due to low toxicity, ease of preparation and cost‐effectiveness (Wei et al., 2011). Compared to ZnO, SnO2 and In2O3 standing out for their high stability (Bulemo et al., 2017) The operational temperature of these metal oxides often is high (150–400°C) (Zhang, Liu, et al., 2016). Integration of these metal oxides with noble metal elements and carbon nanostructures is importance as it might lower their operational temperature to room temperature. Table 2 summarizes recent MGS with their key characteristics for EBM detection.
TABLE 2.
ZnO, SnO2 and In2O3‐based gas sensors
| Sensitive material | Target gas | Operational temperature | Detection range (ppm) | LOD (ppm) | RH% | Reference |
|---|---|---|---|---|---|---|
|
3D inverse opal ZnO‐CuO |
Acetone | 310°C | 0.2–50 | 0.1 | 93 | Xie et al. (2015) |
| Ti‐ZnO | Isoprene | 325°C | 20–500 | 9.3 | 90 | Guntner et al. (2016) |
| Ce‐ZnO | Acetone ethanolamine |
RT RT |
1–100 1–500 |
1 – |
54 54 |
Kulandaisamy et al. (2016) |
|
Pt‐ZnO Cu‐ZnO La‐ZnO |
Acetone Acetone NO |
450°C 450°C 400°C |
1–5 1–5 1–5 |
– – – |
95 95 95 |
Cho et al. (2017) |
| ZnO hierarchical | Acetone | RT and 200°C | 1–5 | 1 | 5 | Chen et al. (2017) |
|
3D inverse opal ZnO‐Fe3O4 |
Acetone | 485°C | – | 0.1 | 20 | Zhang et al. (2017) |
| Ag nanowire‐ZnO nanorods | NO | RT | 0.01–0.1 | 0.01 | 10 | Singh et al. (2019) |
| Nanospiral ZnO film | NO | 150C | 10–100 | 10 | 40 | Luo et al. (2020) |
| Pt‐SnO2 nanotubes | H2S | 300°C | 0.1–0.6 | 0.1 | 95 | Bulemo et al. (2017) |
| Pd‐SnO2 nanowires | H2 | 150°C | 10–100 | – | – | Nguyen et al. (2017) |
| RGO‐SnO2 | Ethanol | 300°C | 43–100 | – | 98 | Zito et al. (2017) |
| SnO2 nanoparticles | H2 | 450°C | 100–500 | 1 | 80 | Vasiliev et al. (2018) |
| PdAu‐SnO2 |
Formaldehyde Acetone |
110°C 250°C |
– – |
45 30 |
94 94 |
Li et al. (2019) |
| Au@WO3SnO2 nanofibres | Acetone | 150°C | 0.2–10 | – | 90 | Shao et al. (2019) |
| SnO2/rGO/PANI | H2S | RT | 0.05–10 | 0.05 | 97 | Zhang et al. (2019) |
| Pt@In2O3 core–shell nanowires | Acetone | 320°C | – | 0.01 | 100 | Liu et al. (2018) |
| Pt‐In2O3 mesoporous nanofibres | Acetone | 180°C | 0.01–50 | 0.01 | 85 | Liu et al. (2019) |
| PA/Gr/nanoribbon/In2O3 | NH3 | RT | 0.65–1.69 | 0.65 | – | Xu and Wu (2020) |
Abbreviations: Gr, graphene; PANI, polyaniline; RGO, reduced graphene oxide; RT, room temperature.
3.1. ZnO‐based gas sensors
ZnO is a very active semiconductor metal oxide for disease monitoring due to its excellent biocompatibility, low cost and environmental friendliness. ZnO morphology adjustment is important to maximize the interaction between the adsorbed oxygen and the target gas and thereby the sensitivity of MGS. Sensitivity and selectivity of ZnO‐based metal oxides are often tailored via integration with other metal oxides (e.g. CuO) and noble metal elements that might tune the Schottky barrier modulation and provide multiple p‐n heterojunctions (Kim, Jahan, et al., 2012; Li et al., 2019).
In recent years, the modification of ZnO with CuO for MGS has received remarkable attentions. Various morphologies of ZnO‐CuO nanocomposites were explored for MGSs such as flower‐like (ethanol), nanorods (H2S) (Kim, Jahan, et al., 2012) and three‐dimensional inverse opal (3D IO) structures (acetone) (Xie et al., 2015). ZnO‐CuO nanocomposites provide n‐p type heterojunction that stems from a combination of ZnO (n‐type) and CuO (p‐type) where the fabrication of such heterojunction can lead to increased resistance as an output signal when compared to pure ZnO and CuO (Xie et al., 2015). 3D IO ZnO‐CuO nanocomposite with well‐ordered pores was evaluated for sensing acetone at LOD = 0.1 ppm in breath. The acetone concentration in the exhaled breath of healthy people is approximately 0.3 to 0.9 ppm and that in diabetic patients, type 1 and type 2, is 2.2 ppm and 1.7 ppm, respectively; therefore, the latter sensor can meet the requirement of the proper dynamic linear range and LOD for diabetic diagnosis (Table 2) (da Silva et al., 2016). The interaction between the metal oxide and gases occurs near the surface of metal oxide. However, porous structures not only have active sites near the surface but also have channels at the inner sites that give rise to the number of reaction sites and thereby the sensitivity (Kim, Choi, et al., 2016). 3D IO ZnO‐Fe3O4 nanocomposite also indicated high sensitivity for the detection of acetone, while the overall analytical performance of MGS based on 3D IO ZnO‐CuO nanocomposite is superior to 3D IO ZnO‐Fe3O4 nanocomposite (Table 2) (Zhang et al., 2017). The role of ZnO morphologies in the detection of the target gas was studied, and as a result, 3D IO ZnO response towards acetone was 2.2 times more than ZnO nanoparticles due to the large surface‐to‐volume ratio of 3D IO morphology that boosted the sensitivity by providing more active sites. The high operating temperature in these two works limits their practical applications for breath analysis as per their high‐energy consumption and difficult operation. Importantly, high operating temperature reduces the discrimination capability of MGS in actual breath since voltaic organic compounds might be unstable and decomposed to other compounds.
Metal doping enhances the selectivity of gas sensors and amplifies the response towards the target gas (Kim, Ahn, et al., 2016). Additionally, metal doping leads to rapid response and recovery time. Ti doping on ZnO by flame spray reactor led to good isoprene sensing abilities. The preparation method for Ti‐ZnO particles is illustrated in Figure 1A where Zn and Ti metal ions mixed before spray (Guntner et al., 2016). The incorporation of Ti4+ reduced the particle and crystal size of ZnO by which could further enhance the sensitivity. Isoprene gas is employed for monitoring the high level of cholesterol (Guntner et al., 2016). Treatment with atorvastatin led to the reduction in the exhaled isoprene level suggesting isoprene as a predicting biomarker for evaluating the efficiency of treatment in a rapid manner (Karl et al., 2001). Ce‐doped ZnO amplified the response to acetone at room temperature (RT) owing to the generation of various cerium specious (Ce2+, Ce3+ and Ce4+) that produced many oxygen vacancies for electron transportation with metal oxides and the target gas (Kulandaisamy et al., 2016). However, at RH equal to 54 humidity of breath is not mimicked. In addition to Ce, Pt and La doping on ZnO presented great sensitive materials for acetone detection, as discussed in the following.
FIGURE 1.
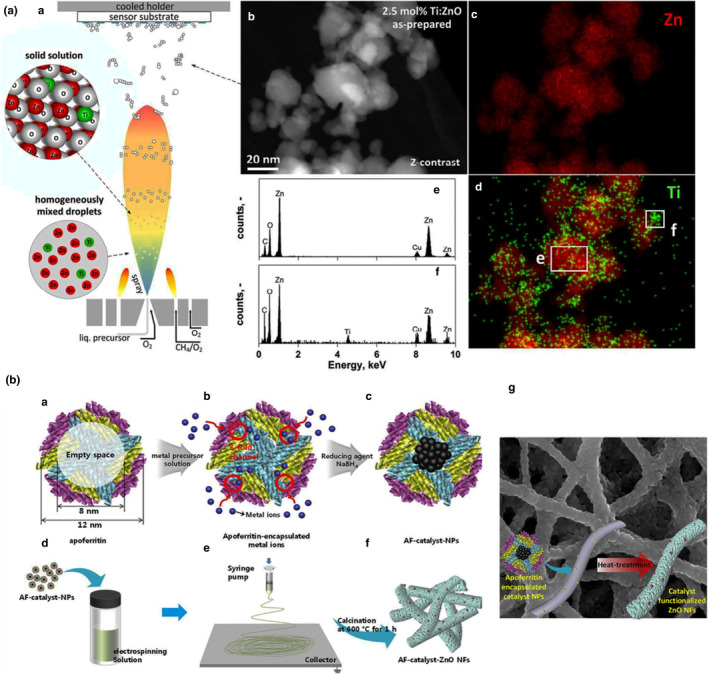
ZnO metal oxide preparation approach for gas sensors. A. (a) Schematic of the preparation process of Ti‐doped ZnO by the flame spray in which the products were directly deposited on the sensing platform. (b) STEM morphology image of Ti‐doped ZnO. Elemental mapping of Zn distribution (red) in (c) and Ti distribution (green) in (d). EDX (e, f) of the selected area in (d); reproduced from Guntner et al. (2016) with the permission from The Royal Society of Chemistry. B. Schematic of the preparation process of nanomaterials with a protein template named apoferritin. (g) The SEM image of Pt‐ZnO nanofibres after calcination (Cho et al., 2017)
The integration of Pt nanomaterials into a sensing platform has been widely reported owing to the high catalytic activity of these nanoparticles in the redox process that enhances sensing characteristics (Cho et al., 2017; Haghshenas et al., 2020). Notably, the methodology for the incorporation of nanomaterial into ZnO plays a significant role in ensuring its catalytic activity in the absence of agglomeration. To provide functional Pt, La and Cu nanoparticles for the modification of ZnO nanofibres, Cho et al. applied the protein template termed apoferritin followed by calcination as shown in Figure 1B (Cho et al., 2017). With the use of apoferritin, the repulsive forces between protein shells not only inhibited the aggregation of nanoparticles but also significantly improved the size distribution of Pt through the encapsulation in apoferritin nanocages. Pt‐functionalized ZnO nanofibre implied the response (Rair/Rgas = 13.07) towards acetone compared with the response (Rair/Rgas = 2.05) of ZnO nanofibres. The catalytic activity of Cu‐ZnO nanofibres resulted in (Rair/Rga = 6.04) and that of La‐ZnO nanofibres led to the responses (Rair/Rgas = 10.6) towards acetone. Similar gas sensors were reported somewhere else where WO3 nanofibres were modified with Pt nanoparticles using apoferritin (Kim et al., 2017; Kim, Choi, et al., 2016).
Operational temperature plays a critical role in the sensing efficiency of MGS. The response of hierarchical ZnO gas sensor towards acetone, as a function of the operating temperature, was studied; the unique U‐shape response attributed to the oxidizing and reducing behaviour of acetone at 25°C and 200°C, respectively (Singh et al., 2019). This study further highlights the challenges associated with MGS rely on high operational temperature regardless of the behaviour of exhaled breath compounds. It is of note that high operational temperature may change the selectivity of MGS (Nguyen et al., 2017). Therefore, developing MGS that enabled to work at room temperature is desirable.
3.2. SnO2‐based gas sensors
SnO2 (n‐type semiconductor) is one of the most explored metal oxides in MGS fields owing to its outstanding stability, wide bandgap and excellent electron mobility. SnO2 with various morphologies, such as powder (Cirera et al., 1999), nanowire (Hwang et al., 2011; Wang et al., 2008), nanotube (Bulemo et al., 2017), SnO2‐ZnO core–shell nanofibre (Choi et al., 2009) and SnO2‐ZnO core–shell nanospheres (Zhang, Zhou, et al., 2016), was studied in gas sensors. In an interesting study, the preparation of porous SnO2 nanotubes was reported from SiO2‐SnO2 composites after SiO2 etching followed by the decoration of exteriors and interiors walls with Pt nanoparticles (Figure 2) (Bulemo et al., 2017). This sensing platform revealed a remarkable response (R air/R gas = 89.3) towards 1 ppm H2S. Excellent surface area, nanosize crystals and the exceptional electrocatalytic property of Pt nanoparticles led to remarkable sensing performance. The high RH (95%) superior sensing performance of this gas sensor is attributed to residual SiO2 that is responsible for humidity adsorption, while it did not contribute to H2S sensing because of dielectric characterization.
FIGURE 2.

Gas sensor based on Pt‐decorated SnO2 nanotubes. (A) Schematic of the preparation process of Pt‐decorated SnO2 nanotubes. (B) Electrospinning nanofibres. (C) SiO2‐cored SnO2 nanofibres. (D) SnO2 nanotubes, (E) TEM image of Pt‐SnO2 nanotubes, EDS mapping of Pt‐SnO2 (F); reprinted with the permission from Bulemo et al. (2017), Copyright (2017) American Chemical Society
SnO2‐based metal oxide sensors often need high operational temperature for gas sensing, and this increases the power consumption and shortens the lifetime of a sensor. To solve this issue, the Pd layer was deposited on SnO2 nanowires prepared through on‐chip growth approach. As a result, it lowered the operational temperature for the detection of H2 to 150°C, while bare SnO2 nanowires were comparably able to detect H2 at a temperature of higher 350°C (Nguyen et al., 2017). Additionally, the selectivity of the gas sensor chip was evaluated in the presence of CO2 and ethanol as interferences. Although the senor did not indicate enough selectivity in ethanol, the response was remarkable for the detection of H2 in the presence of CO2. High operational temperature (150°C in this case) might be the reason for the low selectivity of this sensor as the behaviour of gases at high temperature is complex. Some images from this gas sensor chip are shown in Figure 3.
FIGURE 3.

Metal oxide‐based gas sensor. (a) Fabrication of the sensor chip on the glass substrate. (b) SEM images of SnO2 nanowires as sensing material prepared through growing on a chip. (c, d) Sensor packaging. (e) Schematic of the setup of sensor chip (Nguyen et al., 2017)
The low sensitivity of MGS particularly at high RH of the exhaled breath is one of the greatest challenges of gas sensors. This is due to the presence of superficial hydroxyl groups on metal oxides that conclude in undesirable reactions with false results. To reduce the number of hydroxyl groups on metal oxide surfaces, dry synthesis of nanomaterials and high‐temperature annealing is suggested (Vasiliev et al., 2018). In a study, a spark discharge approach was applied for the dry synthesis of SnO2 to generate airborne SnO2 nanoparticles, which are separated with air gap for the purpose of H2 detection (Vasiliev et al., 2018). Additionally, surface saturation of metal oxide with hydroxyl groups could provide highly sensitive MGS at high RH since they do not adsorb more hydroxyl groups.
With a synergistic effect, the decoration of metal oxides with bimetallic nanoparticles is superior to their individuals for enhancing the sensitivity of MGS. Bimetallic nanoparticle decoration tailors the surface electronic structure of metal oxide and reduces the activation surface energy, and as a result, facilitates the electron transport for sensitive target detection. Generally, to ensure the high catalytic performance of bimetallic nanoparticles when integrated into MGS the size, morphology, dispersibility and also compatibility of bimetallic nanoparticles with metal oxide substrate should be taken into consideration (Kim et al., 2017). In a study, SnO2 nanosheets with flower‐like morphology decorated with PdAu bimetallic nanoparticles showed an excellent sensing platform for the detection of acetone at 250°C with features of reusability and reliability at high RH (Li et al., 2019). PdAu‐SnO2 had the sensitivity towards formaldehyde at the temperature of 110°C. This study highlighted the concern about the cross‐sensitivity in an actual breath when the temperature is high.
The majority of current MGS suffered from low selectivity. Many researchers focus on the optimization of the experimental conditions to overcome this problem. However, developing MGS that relies on EBM separation with a filter and membrane holds a great promise for improving the selectivity of MGS, even in the cases that the materials do not have enough selectivity towards the target gas (Gregis et al., 2018).
Reduced graphene oxide, as a two‐dimensional nanostructure, with excellent surface‐to‐volume ratio, low toxicity and outstanding electron mobility (200,000 cm2 V−1S−1), has been widely utilized for disease monitoring (Feng et al., 2017; Lee et al., 2018; Vajhadin et al., 2020; Zhang et al., 2018). In addition to graphene, other two‐dimensional materials such as 2D‐SnSe2 and Ti3C2MXene have employed for producing flexible MGS owing to lightweight, outstanding flexibility and low cost (Sun et al., 2020; Tannarana et al., 2020). Recently, Lee et al. reviewed carbon‐based materials such as graphene oxide as a sensing substrate for the detection of NO2 gas (Lee et al., 2018). The nanocomposite of reduced graphene oxide and SnO2 indicated the enhanced sensitivity for ethanol detection compared with hollow SnO2 nanostructures in humid and dry conditions (Figure 4) (Zito et al., 2017).
FIGURE 4.
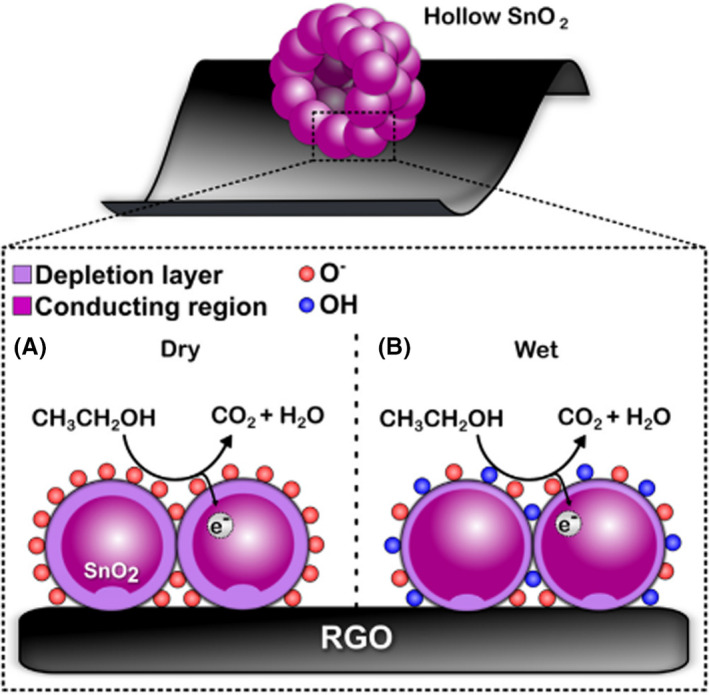
Schematic illustration of ethanol monitoring using reduced graphene oxide‐SnO2 nanocomposites substrate in dry and humid conditions (Zito et al., 2017). Electron transfers from SnO2 to reduced graphene oxide that results in increasing depletion layer in SnO2. In humid conditions, the formation of Sn‐OH led to a reduction in the depletion layer of SnO2 hallow nanospheres and thereby reduction in the resistance resulting in the decrease in ethanol responses
The combination of reduced graphene oxide (p‐type), polyaniline (p‐type) and SnO2 (n‐type) served as a flexible sensing platform for the detection of very low concentration of H2S (0.05 ppm) (Figure 5) (Zhang et al., 2019). Data processing for the recognition of H2S through principal component analysis (PCA) was beneficial to improve the quality and reliability of their sensor. Generally, data analysis methods, including PCA, partial least squares (PLS), neural networks and Gaussian mixture models (GMMs), hold the potential to differentiate the target EBM among thousand EBM in exhaled breath (Rahman et al., 2020).
FIGURE 5.
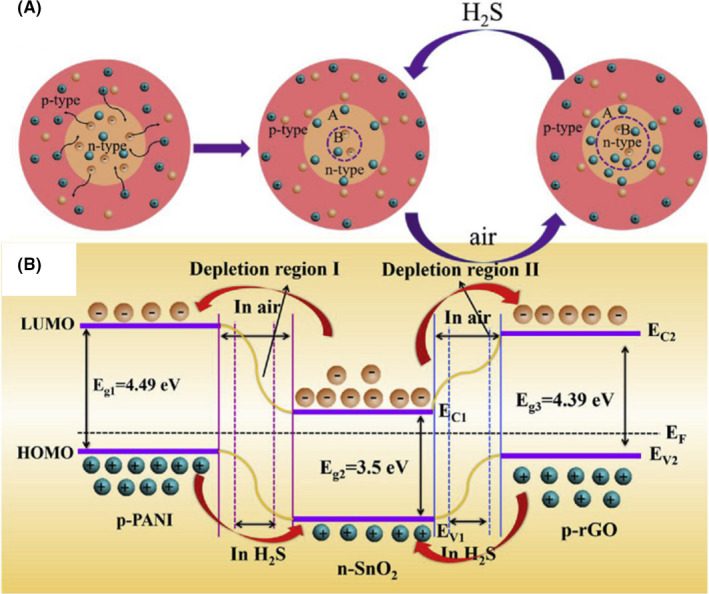
(A) Schematic illustration of the interaction between p‐type and n‐type materials to H2S. (B) P‐n heterojunction in the SnO2/rGO/PANI nanocomposite (Zhang et al., 2019). In the presence of the H2S, the thickness of the electron depletion layers is decreased resulting in a decrease in the sensor resistance
3.3. In2O3‐based gas sensors
In2O3 metal oxide has recently emerged as a promising metal oxide for MGS; however, its potential as a sensing material has been less exploited compared with ZnO and SnO2. In an interesting study, a portable gas sensor was fabricated using Pt@In2O3 core–shell nanowires for real‐time acetone measurement in exhaled breath (Figure 6) (Liu et al., 2018). Pt@In2O3 core–shell structures, prepared by the co‐electrospinning approach, lowered the measured LOD to 0.01 ppm at the operational temperature 320°C. Additionally, the parallel moisture resistance layer (mesoporous silica molecular sieve) that covered the sensing materials led to the functionality of the gas sensor at RH ~100. Liu et al also reported another gas sensor for the detection of acetone by Pt‐decorated In2O3 mesoporous structures at a lower operational temperature (180°C) with very rapid response and recovery time of 6 s and 9 s, respectively (W. Liu et al., 2019). Besides, the sensor indicated high stability for 150 days that can be attributed to SnO2 and the doping effect.
FIGURE 6.

A portable gas sensor based on Pt@In2O3 nanowire for real‐time acetone monitoring (Liu et al., 2018). (A) Schematic of the gas sensor performance. The senor response for measuring acetone in exhaled breath of healthy (B) and diabetic (C) volunteers
4. CONCLUSIONS AND PERSPECTIVES
Rapid advancement in MGS leads to non‐invasive and rapid monitoring of diseases on the basis of EBM. In this study, we reviewed the latest advances in metal oxide‐based gas sensors for the detection of EBM with a focus on ZnO, SnO2 and In2O3 owing to their unique sensing properties, satisfying stability and high compatibility for embedding into miniaturized chips. Despite progress in MGS, it is not profitable to use the majority of MGSs out of laboratories on a large scale. In fact, they are in their infancy and much more efforts needed to produce portable MGS to accurately characterize EBM in the actual exhaled breath. Generally, the bottlenecks of current MGS are as follows: (i) poor selectivity due to the difficulty of recognition of EBM among various chemically similar molecules; (ii) unreliable responses at high RH (~100%) that mimics the amount of moisture in the exhaled breath; and (iii) high operational temperature that restrains the practical application of MGS for breath analysis. Coupling MGS with separation columns or membranes would significantly reduce the concern about the selectivity of the current MGS. In addition, statistically processing MGS responses elevates the selective detection by MGS. Utilizing new humidity‐resistance composites in MGS leads to functional MGS in the humidity of breath.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ACKNOWLEDGEMENTS
The authors thank Yazd University for the funding support.
REFERENCES
- Alkhouri, N. , Singh, T. , Alsabbagh, E. , Guirguis, J. , Chami, T. , Hanouneh, I. , Grove, D. , Lopez, R. , & Dweik, R. (2015). Isoprene in the exhaled breath is a novel biomarker for advanced fibrosis in patients with chronic liver disease: A pilot study. Clinical and Translational Gastroenterology, 6, e112. 10.1038/ctg.2015.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri, V. , Roshan, H. , Mirzaei, A. , Neri, G. , & Ayesh, A. I. (2020). Nanostructured metal oxide‐based acetone gas sensors. A review. Sensors (Basel), 20(11), 3096. 10.3390/s20113096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, P. J. , Chowdhury, B. , Kharitonov, S. A. , Magnussen, H. , Page, C. P. , Postma, D. , & Saetta, M. (2006). Pulmonary biomarkers in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 174(1), 6–14. 10.1164/rccm.200510-1659PP [DOI] [PubMed] [Google Scholar]
- Birrell, M. , McCluskie, K. , Hardaker, E. , Knowles, R. , & Belvisi, M. (2006). Utility of exhaled nitric oxide as a noninvasive biomarker of lung inflammation in a disease model. European Respiratory Journal, 28(6), 1236–1244. [DOI] [PubMed] [Google Scholar]
- Brindicci, C. , Ito, K. , Resta, O. , Pride, N. B. , Barnes, P. J. , & Kharitonov, S. A. (2005). Exhaled nitric oxide from lung periphery is increased in COPD. European Respiratory Journal, 26(1), 52–59. 10.1183/09031936.04.00125304 [DOI] [PubMed] [Google Scholar]
- Brinkman, P. , Ahmed, W. M. , Gómez, C. , Knobel, H. H. , Weda, H. , Vink, T. J. , Nijsen, T. M. , Wheelock, C. E. , Dahlen, S.‐E. , Montuschi, P. , Knowles, R. G. , Vijverberg, S. J. , Maitland‐van der Zee, A. H. , Sterk, P. J. , & Fowler, S. J. (2020). Exhaled volatile organic compounds as markers for medication use in asthma. European Respiratory Journal, 55(2), 1900544. 10.1183/13993003.00544-2019 [DOI] [PubMed] [Google Scholar]
- Bulemo, P. M. , Cho, H. J. , Kim, N. H. , & Kim, I. D. (2017). Mesoporous SnO2 nanotubes via electrospinning‐etching route: highly sensitive and selective detection of H2S molecule. ACS Applied Materials & Interfaces, 9(31), 26304–26313. 10.1021/acsami.7b05241 [DOI] [PubMed] [Google Scholar]
- Cazzola, M. , & Novelli, G. (2010). Biomarkers in COPD. Pulmonary Pharmacology & Therapeutics, 23(6), 493–500. 10.1016/j.pupt.2010.05.001 [DOI] [PubMed] [Google Scholar]
- Chang, J.‐E. , Lee, D.‐S. , Ban, S.‐W. , Oh, J. , Jung, M. Y. , Kim, S.‐H. , Park, S. J. , Persaud, K. , & Jheon, S. (2018). Analysis of volatile organic compounds in exhaled breath for lung cancer diagnosis using a sensor system. Sensors and Actuators B: Chemical, 255, 800–807. 10.1016/j.snb.2017.08.057 [DOI] [Google Scholar]
- Chen, J. , Pan, X. , Boussaid, F. , McKinley, A. , Fan, Z. , & Bermak, A. (2017). Breath level acetone discrimination through temperature modulation of a hierarchical ZnO gas sensor. IEEE Sensors Letters, 1(5), 1–4. 10.1109/lsens.2017.2740222 [DOI] [Google Scholar]
- Cho, H.‐J. , Kim, S.‐J. , Choi, S.‐J. , Jang, J.‐S. , & Kim, I.‐D. (2017). Facile synthetic method of catalyst‐loaded ZnO nanofibers composite sensor arrays using bio‐inspired protein cages for pattern recognition of exhaled breath. Sensors and Actuators B: Chemical, 243, 166–175. 10.1016/j.snb.2016.11.137 [DOI] [Google Scholar]
- Choi, S.‐W. , Park, J. Y. , & Kim, S. S. (2009). Synthesis of SnO2–ZnO core–shell nanofibers via a novel two‐step process and their gas sensing properties. Nanotechnology, 20(46), 465603. [DOI] [PubMed] [Google Scholar]
- Chung, K. F. (2014). Hydrogen sulfide as a potential biomarker of asthma. Expert Review of Respiratory Medicine, 8(1), 5–13. [DOI] [PubMed] [Google Scholar]
- Cirera, A. , Dieguez, A. , Diaz, R. , Cornet, A. , & Morante, J. (1999). New method to obtain stable small‐sized SnO2 powders for gas sensors. Sensors and Actuators B: Chemical, 58(1–3), 360–364. [Google Scholar]
- Crofford, O. B. , Mallard, R. E. , Winton, R. E. , Rogers, N. L. , Jackson, J. C. , & Keller, U. (1977). Acetone in breath and blood. Transactions of the American Clinical and Climatological Association, 88, 128–139. [PMC free article] [PubMed] [Google Scholar]
- da Silva, L. F. , Catto, A. C. , Avansi, W. , Cavalcante, L. S. , Mastelaro, V. R. , Andrés, J. , Aguir, K. , & Longo, E. (2016). Acetone gas sensor based on α‐Ag2WO4 nanorods obtained via a microwave‐assisted hydrothermal route. Journal of Alloys and Compounds, 683, 186–190. 10.1016/j.jallcom.2016.05.078 [DOI] [Google Scholar]
- Das, S. , & Pal, M. (2020). Review—Non‐invasive monitoring of human health by exhaled breath analysis: A comprehensive review. Journal of the Electrochemical Society, 167(3), 37562. 10.1149/1945-7111/ab67a6 [DOI] [Google Scholar]
- Feng, Q. , Li, X. , & Wang, J. (2017). Percolation effect of reduced graphene oxide (rGO) on ammonia sensing of rGO‐SnO2 composite based sensor. Sensors and Actuators B: Chemical, 243, 1115–1126. 10.1016/j.snb.2016.12.075 [DOI] [Google Scholar]
- Ferrus, L. , Guenard, H. , Vardon, G. , & Varene, P. (1980). Respiratory water loss. Respiration Physiology, 39(3), 367–381. 10.1016/0034-5687(80)90067-5 [DOI] [PubMed] [Google Scholar]
- Glockler, J. , Jaeschke, C. , Kocaoz, Y. , Kokoric, V. , Tutuncu, E. , Mitrovics, J. , & Mizaikoff, B. (2020). iHWG‐MOX: A hybrid breath analysis system via the combination of substrate‐integrated hollow waveguide infrared spectroscopy with metal oxide gas sensors. ACS Sensors, 5(4), 1033–1039. 10.1021/acssensors.9b02554 [DOI] [PubMed] [Google Scholar]
- Gregis, G. , Sanchez, J.‐B. , Bezverkhyy, I. , Guy, W. , Berger, F. , Fierro, V. , Bellat, J.‐P. , & Celzard, A. (2018). Detection and quantification of lung cancer biomarkers by a micro‐analytical device using a single metal oxide‐based gas sensor. Sensors and Actuators B: Chemical, 255, 391–400. 10.1016/j.snb.2017.08.056 [DOI] [Google Scholar]
- Guntner, A. T. , Abegg, S. , Konigstein, K. , Gerber, P. A. , Schmidt‐Trucksass, A. , & Pratsinis, S. E. (2019). Breath sensors for health monitoring. ACS Sensors, 4(2), 268–280. 10.1021/acssensors.8b00937 [DOI] [PubMed] [Google Scholar]
- Guntner, A. T. , Pineau, N. J. , Chie, D. , Krumeich, F. , & Pratsinis, S. E. (2016). Selective sensing of isoprene by Ti‐doped ZnO for breath diagnostics. Journal of Materials Chemistry B, 4(32), 5358–5366. 10.1039/c6tb01335j [DOI] [PubMed] [Google Scholar]
- Haghshenas, M. , Mazloum‐Ardakani, M. , Alizadeh, Z. , Vajhadin, F. , & Naeimi, H. (2020). A sensing platform using Ag/Pt core‐shell nanostructures supported on multiwalled carbon nanotubes to detect hydroxyurea. Electroanalysis, 32, 1–10. 10.1002/elan.202060020 [DOI] [Google Scholar]
- Hanh, N. H. , Van Duy, L. , Hung, C. M. , Van Duy, N. , Heo, Y.‐W. , Van Hieu, N. , & Hoa, N. D. (2020). VOC gas sensor based on hollow cubic assembled nanocrystal Zn2SnO4 for breath analysis. Sensors and Actuators A: Physical, 302, 111834. 10.1016/j.sna.2020.111834 [DOI] [Google Scholar]
- Ho, C.‐H. , Chan, C.‐H. , Tien, L.‐C. , & Huang, Y.‐S. (2011). Direct optical observation of band‐edge excitons, band gap, and Fermi level in degenerate semiconducting oxide nanowires In2O3. The Journal of Physical Chemistry C, 115(50), 25088–25096. [Google Scholar]
- Hwang, I.‐S. , Choi, J.‐K. , Woo, H.‐S. , Kim, S.‐J. , Jung, S.‐Y. , Seong, T.‐Y. , & Lee, J.‐H. (2011). Facile control of C2H5OH sensing characteristics by decorating discrete Ag nanoclusters on SnO2 nanowire networks. ACS Applied Materials & Interfaces, 3(8), 3140–3145. [DOI] [PubMed] [Google Scholar]
- Kabir, E. , Raza, N. , Kumar, V. , Singh, J. , Tsang, Y. F. , Lim, D. K. , Szulejko, J. E. , & Kim, K.‐H. (2019). Recent advances in nanomaterial‐based human breath analytical technology for clinical diagnosis and the way forward. Chemistry, 5(12), 3020–3057. 10.1016/j.chempr.2019.08.004 [DOI] [Google Scholar]
- Karl, T. , Prazeller, P. , Mayr, D. , Jordan, A. , Rieder, J. , Fall, R. , & Lindinger, W. (2001). Human breath isoprene and its relation to blood cholesterol levels: new measurements and modeling. Journal of Applied Physiology, 91(2), 762–770. 10.1152/jappl.2001.91.2.762 [DOI] [PubMed] [Google Scholar]
- Kharitonov, S. , & Barnes, P. (1996). Nitric oxide in exhaled air is a new marker of airway inflammation. Monaldi Archives for Chest Disease. Archivio Monaldi per Le Malattie Del Torace, 51(6), 533. [PubMed] [Google Scholar]
- Kharitonov, S. A. , & Barnes, P. J. (2002). Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers, 7(1), 1–32. 10.1080/13547500110104233 [DOI] [PubMed] [Google Scholar]
- Kim, B. Y. , Ahn, J. H. , Yoon, J. W. , Lee, C. S. , Kang, Y. C. , Abdel‐Hady, F. , & Lee, J. H. (2016). Highly selective xylene sensor based on NiO/NiMoO4 nanocomposite hierarchical spheres for indoor air monitoring. ACS Applied Materials & Interfaces, 8(50), 34603–34611. 10.1021/acsami.6b13930 [DOI] [PubMed] [Google Scholar]
- Kim, J. , Kim, W. , & Yong, K. (2012). CuO/ZnO Heterostructured Nanorods: Photochemical synthesis and the mechanism of H2S gas sensing. The Journal of Physical Chemistry C, 116(29), 15682–15691. 10.1021/jp302129j [DOI] [Google Scholar]
- Kim, K. H. , Jahan, S. A. , & Kabir, E. (2012). A review of breath analysis for diagnosis of human health. TrAC Trends in Analytical Chemistry, 33, 1–8. 10.1016/j.trac.2011.09.013 [DOI] [Google Scholar]
- Kim, S. J. , Choi, S. J. , Jang, J. S. , Cho, H. J. , Koo, W. T. , Tuller, H. L. , & Kim, I. D. (2017). Exceptional high‐performance of Pt‐based bimetallic catalysts for exclusive detection of exhaled biomarkers. Advanced Materials, 29(36), 1700737. 10.1002/adma.201700737 [DOI] [PubMed] [Google Scholar]
- Kim, S. J. , Choi, S. J. , Jang, J. S. , Kim, N. H. , Hakim, M. , Tuller, H. L. , & Kim, I. D. (2016). Mesoporous WO3 nanofibers with protein‐templated nanoscale catalysts for detection of trace biomarkers in exhaled breath. ACS Nano, 10(6), 5891–5899. 10.1021/acsnano.6b01196 [DOI] [PubMed] [Google Scholar]
- Kulandaisamy, A. J. , Elavalagan, V. , Shankar, P. , Mani, G. K. , Babu, K. J. , & Rayappan, J. B. B. (2016). Nanostructured Cerium‐doped ZnO thin film – A breath sensor. Ceramics International, 42(16), 18289–18295. 10.1016/j.ceramint.2016.08.156 [DOI] [Google Scholar]
- Lee, S. W. , Lee, W. , Hong, Y. , Lee, G. , & Yoon, D. S. (2018). Recent advances in carbon material‐based NO2 gas sensors. Sensors and Actuators B: Chemical, 255, 1788–1804. 10.1016/j.snb.2017.08.203 [DOI] [Google Scholar]
- Li, G. , Cheng, Z. , Xiang, Q. , Yan, L. , Wang, X. , & Xu, J. (2019). Bimetal PdAu decorated SnO2 nanosheets based gas sensor with temperature‐dependent dual selectivity for detecting formaldehyde and acetone. Sensors and Actuators B: Chemical, 283, 590–601. 10.1016/j.snb.2018.09.117 [DOI] [Google Scholar]
- Liu, L. , Fei, T. , Guan, X. , Lin, X. , Zhao, H. , & Zhang, T. (2020). Room temperature ammonia gas sensor based on ionic conductive biomass hydrogels. Sensors and Actuators B: Chemical, 320, 128318. 10.1016/j.snb.2020.128318 [DOI] [Google Scholar]
- Liu, W. , Xie, Y. , Chen, T. , Lu, Q. , Ur Rehman, S. , & Zhu, L. (2019). Rationally designed mesoporous In2O3 nanofibers functionalized Pt catalysts for high‐performance acetone gas sensors. Sensors and Actuators B: Chemical, 298, 126871. 10.1016/j.snb.2019.126871 [DOI] [Google Scholar]
- Liu, W. , Xu, L. , Sheng, K. , Zhou, X. , Dong, B. , Lu, G. , & Song, H. (2018). A highly sensitive and moisture‐resistant gas sensor for diabetes diagnosis with Pt@In2O3 nanowires and a molecular sieve for protection. NPG Asia Materials, 10(4), 293–308. 10.1038/s41427-018-0029-2 [DOI] [Google Scholar]
- Lu, Z. , Huang, W. , Wang, L. , Xu, N. , Ding, Q. , & Cao, C. (2018). Exhaled nitric oxide in patients with chronic obstructive pulmonary disease: a systematic review and meta‐analysis. International Journal of Chronic Obstructive Pulmonary Disease, 13, 2695–2705. 10.2147/COPD.S165780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, P. , Xie, M. , Luo, J. , Kan, H. , & Wei, Q. (2020). Nitric oxide sensors using nanospiral ZnO thin film deposited by GLAD for application to exhaled human breath. RSC Advances, 10(25), 14877–14884. 10.1039/d0ra00488j [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskie, K. , Birrell, M. A. , Wong, S. , & Belvisi, M. G. (2004). Nitric oxide as a noninvasive biomarker of lipopolysaccharide‐induced airway inflammation: possible role in lung neutrophilia. Journal of Pharmacology and Experimental Therapeutics, 311(2), 625–633. [DOI] [PubMed] [Google Scholar]
- Moseley, P. T. (2017). Progress in the development of semiconducting metal oxide gas sensors: a review. Measurement Science and Technology, 28(8), 82001. 10.1088/1361-6501/aa7443 [DOI] [Google Scholar]
- Nguyen, K. , Hung, C. M. , Ngoc, T. M. , Thanh Le, D. T. , Nguyen, D. H. , Nguyen Van, D. , & Nguyen Van, H. (2017). Low‐temperature prototype hydrogen sensors using Pd‐decorated SnO2 nanowires for exhaled breath applications. Sensors and Actuators B: Chemical, 253, 156–163. 10.1016/j.snb.2017.06.141 [DOI] [Google Scholar]
- Rahman, S. , Alwadie, A. S. , Irfan, M. , Nawaz, R. , Raza, M. , Javed, E. , & Awais, M. (2020). Wireless E‐nose sensors to detect volatile organic gases through multivariate analysis. Micromachines (Basel), 11(6), 10.3390/mi11060597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicka, J. , Kowalkowski, T. , & Buszewski, B. (2019). Searching for selected VOCs in human breath samples as potential markers of lung cancer. Lung Cancer, 135, 123–129. 10.1016/j.lungcan.2019.02.012 [DOI] [PubMed] [Google Scholar]
- Ruzsanyi, V. , & Peter Kalapos, M. (2017). Breath acetone as a potential marker in clinical practice. Journal of Breath Research, 11(2), 24002. 10.1088/1752-7163/aa66d3 [DOI] [PubMed] [Google Scholar]
- Salerno‐Kennedy, R. , & Cashman, K. D. (2005). Potential applications of breath isoprene as a biomarker in modern medicine: a concise overview. Wiener Klinische Wochenschrift, 117(5–6), 180–186. 10.1007/s00508-005-0336-9 [DOI] [PubMed] [Google Scholar]
- Shao, S. , Chen, X. , Chen, Y. , Lai, M. , & Che, L. (2019). Ultrasensitive and highly selective detection of acetone based on Au@WO3‐SnO2 corrugated nanofibers. Applied Surface Science, 473, 902–911. 10.1016/j.apsusc.2018.12.208 [DOI] [Google Scholar]
- Singh, P. , Hu, L. L. , Zan, H. W. , & Tseng, T. Y. (2019). Highly sensitive nitric oxide gas sensor based on ZnO‐nanorods vertical resistor operated at room temperature. Nanotechnology, 30(9), 95501. 10.1088/1361-6528/aaf7cb [DOI] [PubMed] [Google Scholar]
- Sun, S. , Wang, M. , Chang, X. , Jiang, Y. , Zhang, D. , Wang, D. , Zhang, Y. , & Lei, Y. (2020). W18O49/Ti3C2Tx Mxene nanocomposites for highly sensitive acetone gas sensor with low detection limit. Sensors and Actuators B: Chemical, 304, 127274. 10.1016/j.snb.2019.127274 [DOI] [Google Scholar]
- Tai, H. , Wang, S. , Duan, Z. , & Jiang, Y. (2020). Evolution of breath analysis based on humidity and gas sensors: Potential and challenges. Sensors and Actuators B: Chemical, 318, 10.1016/j.snb.2020.128104 [DOI] [Google Scholar]
- Tannarana, M. , Solanki, G. K. , Bhakhar, S. A. , Patel, K. D. , Pathak, V. M. , & Pataniya, P. M. (2020). 2D‐SnSe2 Nanosheet functionalized Piezo‐resistive flexible sensor for pressure and human breath monitoring. ACS Sustainable Chemistry & Engineering, 8(20), 7741–7749. 10.1021/acssuschemeng.0c01827 [DOI] [Google Scholar]
- Vajhadin, F. , Ahadian, S. , Travas‐Sejdic, J. , Lee, J. , Mazloum‐Ardakani, M. , Salvador, J. , Aninwene, G. E. , Bandaru, P. , Sun, W. , & Khademhossieni, A. (2020). Electrochemical cytosensors for detection of breast cancer cells. Biosensors and Bioelectronics, 151, 111984. 10.1016/j.bios.2019.111984 [DOI] [PubMed] [Google Scholar]
- Vasiliev, A. , Varfolomeev, A. , Volkov, I. , Simonenko, N. , Arsenov, P. , Vlasov, I. , Ivanov, V. , Pislyakov, A. , Lagutin, A. , Jahatspanian, I. , & Maeder, T. (2018). Reducing humidity response of gas sensors for medical applications: Use of spark discharge synthesis of metal oxide nanoparticles. Sensors (Basel), 18(8), 2600. 10.3390/s18082600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B. , Zhu, L. , Yang, Y. , Xu, N. , & Yang, G. (2008). Fabrication of a SnO2 nanowire gas sensor and sensor performance for hydrogen. The Journal of Physical Chemistry C, 112(17), 6643–6647. [Google Scholar]
- Wang, C. , & Sahay, P. (2009). Breath analysis using laser spectroscopic techniques: breath biomarkers, spectral fingerprints, and detection limits. Sensors (Basel), 9(10), 8230–8262. 10.3390/s91008230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehinger, A. , Schmid, A. , Mechtcheriakov, S. , Ledochowski, M. , Grabmer, C. , Gastl, G. A. , & Amann, A. (2007). Lung cancer detection by proton transfer reaction mass‐spectrometric analysis of human breath gas. International Journal of Mass Spectrometry, 265(1), 49–59. 10.1016/j.ijms.2007.05.012 [DOI] [Google Scholar]
- Wei, A. , Pan, L. , & Huang, W. (2011). Recent progress in the ZnO nanostructure‐based sensors. Materials Science and Engineering: B, 176(18), 1409–1421. 10.1016/j.mseb.2011.09.005 [DOI] [Google Scholar]
- Xie, Y. , Xing, R. , Li, Q. , Xu, L. , & Song, H. (2015). Three‐dimensional ordered ZnO–CuO inverse opals toward low concentration acetone detection for exhaled breath sensing. Sensors and Actuators B: Chemical, 211, 255–262. 10.1016/j.snb.2015.01.086 [DOI] [Google Scholar]
- Xu, L.‐H. , & Wu, T.‐M. (2020). Synthesis of highly sensitive ammonia gas sensor of polyaniline/graphene nanoribbon/indium oxide composite at room temperature. Journal of Materials Science: Materials in Electronics, 31(9), 7276–7283. 10.1007/s10854-020-03299-6 [DOI] [Google Scholar]
- Zhang, D. , Wu, Z. , & Zong, X. (2019). Flexible and highly sensitive H2S gas sensor based on in‐situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sensors and Actuators B: Chemical, 289, 32–41. 10.1016/j.snb.2019.03.055 [DOI] [Google Scholar]
- Zhang, J. , Liu, X. , Neri, G. , & Pinna, N. (2016). Nanostructured materials for room‐temperature gas sensors. Advanced Materials, 28(5), 795–831. 10.1002/adma.201503825 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Lu, H. , Yan, C. , Yang, Z. , Zhu, G. , Gao, J. , Yin, F. , & Wang, C. (2018). Fabrication of conductive graphene oxide‐WO3 composite nanofibers by electrospinning and their enhanced acetone gas sensing properties. Sensors and Actuators B: Chemical, 264, 128–138. 10.1016/j.snb.2018.02.026 [DOI] [Google Scholar]
- Zhang, J. , Wang, X. , Chen, Y. , & Yao, W. (2015). Exhaled hydrogen sulfide predicts airway inflammation phenotype in COPD. Respiratory Care, 60(2), 251–258. 10.4187/respcare.03519 [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Dong, B. , Xu, L. , Zhang, X. , Chen, J. , Sun, X. , Xu, H. , Zhang, T. , Bai, X. , Zhang, S. , & Song, H. (2017). Three‐dimensional ordered ZnO–Fe3O4 inverse opal gas sensor toward trace concentration acetone detection. Sensors and Actuators B: Chemical, 252, 367–374. 10.1016/j.snb.2017.05.167 [DOI] [Google Scholar]
- Zhang, R. , Zhou, T. , Wang, L. , Lou, Z. , Deng, J. , & Zhang, T. (2016). The synthesis and fast ethanol sensing properties of core–shell SnO2@ZnO composite nanospheres using carbon spheres as templates. New Journal of Chemistry, 40(8), 6796–6802. 10.1039/c6nj00365f [DOI] [Google Scholar]
- Zito, C. A. , Perfecto, T. M. , & Volanti, D. P. (2017). Impact of reduced graphene oxide on the ethanol sensing performance of hollow SnO2 nanoparticles under humid atmosphere. Sensors and Actuators B: Chemical, 244, 466–474. 10.1016/j.snb.2017.01.015 [DOI] [Google Scholar]


