Abstract
The COVID‐19 pandemic has ushered in a new era of food safety. To date, there is no evidence to suggest that consuming food is associated with COVID‐19. Nevertheless, COVID‐19's impact on food safety and security has been grave. The world is currently experiencing several supply chain issues as a direct result of extensive lockdowns and impacts on essential workers' safety. However, disruption in the food supply, while catastrophic in nature, has created opportunities for the advancement of medical science, data processing, security monitoring, foodborne pathogen detection, and food safety technology. This article will discuss the key components for food safety during the COVID‐19 pandemic. The discussion will draw from lessons learned early in the outbreak and will analyze the etiology of the disease through a food safety perspective. From there, we will discuss personal protective equipment, detection of SARS‐CoV‐2, useful surrogates to study SARS‐CoV‐2, and the expanding field of data science, from the food safety point of view. In the future, scientists can apply the knowledge to the containment of COVID‐19 and eventually to future pandemics.
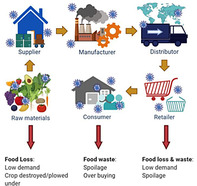
Impact of COVID‐19 on food safety and security of the food supply chain. Viral icons represent the aggregate accumulation of viral particles in discreet links in the supply chain. The red arrows indicate parts of the food supply chain experience food loss and waste, which ultimately impact food and economic security.
1. INTRODUCTION
The COVID‐19 pandemic has upended the daily life of people all across the globe and has threatened the security of the world's food supply. Currently, the COVID‐19 pandemic is impacting the nation's food and agricultural system, and food producers are facing many challenges. The aggressive spread of COVID‐19 has raised many questions about the safety of essential food workers and the safety and security of our food supply. To date, there is no evidence to suggest that handling food or consuming food is associated with COVID‐19 (Center for Disease Control (CDC), 2020a). However, the impact of COVID‐19 on food security has been grave, and traditional food safety approaches may not be sufficient for combating the problem.
One major question pertains to the survival of SARS‐CoV‐2, the causative agent of COVID‐19, on food and food contact surfaces, and the potential risk to consumers and essential workers. Essential workers are at risk for exposure to SARS‐CoV‐2, depending on the duties they perform. In particular, food workers are at high risk because their duties often require long hours indoors and close interaction with other people (CDC, 2020b). If infected, essential workers could transmit COVID‐19 by touching food or food packaging, harboring viable virus particles and then touching their own mouth, nose, or possibly their eyes (CDC, 2020b). In order to keep these essential employees and the public safe, many food‐processing facilities have suspended their operations until worker hygiene, environmental sanitation, and food supply safety can be assured (Kang et al., 2020). However, facility closures have severe, perhaps disastrous repercussions for many in the supply chain, and therefore transmission mechanisms need investigation.
The disruption in the food supply, while catastrophic in nature, has created opportunities for the advancement of medical science, data processing, security monitoring, foodborne pathogen detection, and food safety technology (Beckman et al., 2013; Shahidi, 2020). The food production sector, in particular, is shifting away from traditional safety approaches toward more modern and sophisticated controls in processing, testing, traceability, and distribution. Data science and artificial intelligence (AI) networks offer powerful tools that can process the multidimensional information needed for enhanced food security and safety (Jribi, Ben Ismail, Doggui, & Debbabi, 2020). New guidance from the U.S. Federal Drug Administration (FDA, 2020), combined with rapid technological advances in real‐time data acquisition, AI networks, and mechanization, has ushered in paradigm shifts with respect to the global food economy and food safety (Jribi et al., 2020). While many of these tools already exist, there is still an immediate need for a centralized, curated, and comprehensive platform for food safety data that is readily assessable. These technological advances often trickle down to consumers in the form of a web‐based and mobile application (Chen, Jiang, & Hu, 2020; Shahidi, 2020; Wilder‐Smith, Chiew, & Lee, 2020). Food producers, processors, handlers, and consumers can use these platforms to making data‐informed decisions to safeguard food.
The forced quarantine of millions of global citizens created impacts on consumer behavior, many of which will have continued and lasting consequences. What has emerged is an opportunity for new data science tools to augment existing strategies for decision‐making about work health and food safety as a situation rapidly occurs. However, the prerequisites for these advancements are strong Good Agriculture Practices, Good Manufacturing Practices, COVID‐19 testing and contact tracing, and essential worker protections. This article will review the food safety lessons learned and discuss the technological advances that have occurred since the COVID‐19 outbreak and their impacts on the food supply and safety.
2. VIRAL BEHAVIOR IN THE FOOD SUPPLY
Viruses are obligate intracellular parasites that require susceptible host cells for propagation and host infection (Bosch et al., 2018). Foodborne viruses (unlike foodborne bacteria) are unable to replicate in food, but most of these viruses are incredibly stable in the environment (Cook, 2001). Many foodborne viruses can survive in foods, on hands, feces, and food contact surfaces and floors for extended periods (Cook, 2001). A large number of different viruses may be found in the human gastrointestinal tract causing a wide variety of diseases. Although any virus able to cause disease after ingestion could be potentially considered foodborne and/or waterborne, most reported viral foodborne illnesses are caused by human norovirus or hepatitis (Miranda & Schaffner, 2019).
SARS‐CoV‐2 is primarily transmitted through person‐to‐person contact and not specifically through foods; however, it is still a major threat to food security (Figure 1). The spread of SARS‐CoV‐2 has caused major supply chain issues, not seen since the Great Depression of the 1930's (Shahidi, 2020). The abrupt closures of high volume food industries like restaurants, schools, hotels, and amusement parks caused a sharp downturn in demand for food (Jribi et al., 2020). Simultaneously, consumers rushed to grocery stores resulting in empty shelves, shortages, and price spikes (Miranda & Schaffner, 2019). According to the United Foods and Commercial Worker union, 13 meat packing working died from COVID‐19 cause massive shutdowns of processing plants (CDC, 2020a). Many plants have since reopened with the implementation of physical barriers and personal protective equipment (PPE). In order to protect essential workers and consumers, we need a better understanding of the physiology of the virus, how it can survive on surfaces and food, how it is detected, and how PPE works.
FIGURE 1.
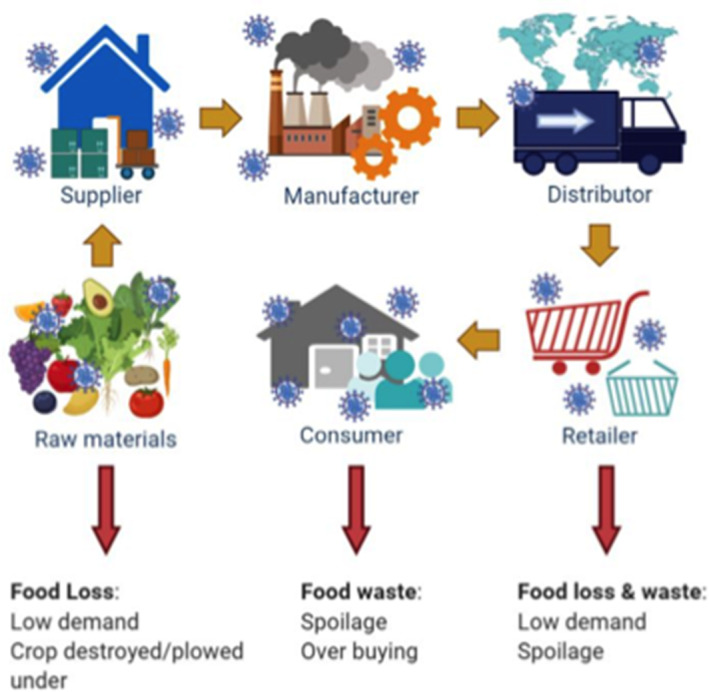
Impact of COVID‐19 on food safety and security of the food supply chain. Viral icons represent the aggregate accumulation of viral particles in discreet links in the supply chain. The red arrows indicate parts of the food supply chain experience food loss and waste, which ultimately impact food and economic security
2.1. Enveloped versus nonenveloped virus
Overall, the physiology of the virus particle and the presence or absence of an envelope tells us little about what disease the virus may cause or what species it might infect (Chen, Jiang, & Hu, 2020); however, it is still critical information for determining its persistence in the environment (Figure 2). Virus particles without an envelope tend to possess a more robust viral capsid that is more resistant to environmental pressures than enveloped viruses (Miranda & Schaffner, 2019). The viral envelope is a modified form of the host's cell membranes, either the outer membrane surrounding an infected host cell or internal membranes, such as a nuclear membrane or endoplasmic reticulum (Chen, Jiang, & Hu, 2020). A lot of communicable viruses of clinical importance, such as hepatitis B virus, hepatitis C virus, human immunodeficiency virus, and influenza viruses, are tail‐less and have a lipid bilayer that is also known as enveloped viruses (Spriggs, Harwood, & Tsai, 2019). These enveloped viruses contain a number of envelope proteins on the lipid layer that are able to identify receptors on a host's membrane for the binding purpose (Spriggs et al., 2019). Enveloped viruses are more susceptible to denaturation by adverse pH, temperature, desiccation, or the presence of chemicals (Firquet et al., 2015; Wood & Payne, 1998). Therefore, nonenveloped viruses are commonly used as surrogate viruses for enveloped viruses, which require a higher biosafety level of the laboratory to determine the stress stability or antiviral effects of disinfectants.
FIGURE 2.
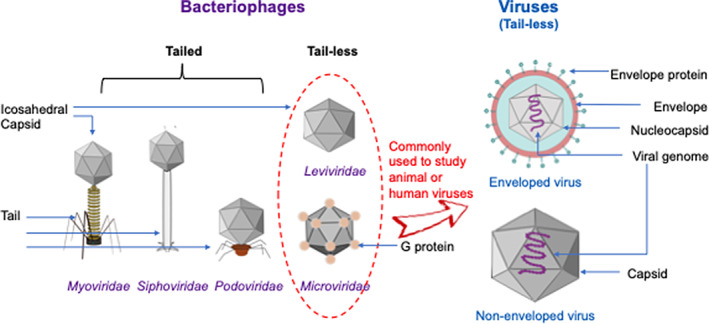
Structural similarity (tail‐less) and the difference between bacteriophages and animal (and human) viruses. Two types of tail‐less viruses that can infect animal or human: enveloped and nonenveloped viruses. Different morphologies of bacteriophages belong to families Myoviridae, Siphoviridae, Podoviridae, Microviridae (such as phiX174 phage), and Leviviridae (such as MS2 phage)
Nonenveloped viruses, such as adenoviruses (Norovirus), rhinovirus, coxsackieviruses, rotavirus, and poliovirus, do not contain a lipid envelope and have the protein‐based capsid exposed to the surrounding environment (Chen et al., 2020). Without the presence of receptor proteins on the lipid envelop, these viruses must directly penetrate the plasma membrane or through various mechanisms of endocytosis to enter the host cells (Spriggs et al., 2019).
SARS‐COV‐2 is an enveloped virus classified under the family Coronaviridae, and its structure can help to predict it's potential behavior in the food supply. There are four main sub‐groupings of Coronaviridae, known as alpha, beta, gamma, and delta (Chen, Rui, et al., 2020). The alpha and beta coronaviruses typically affect mammal, while gamma and delta typically affect birds (Chen, Rui, et al., 2020). The beta‐type coronavirus represents the viral linage that causes severe acute respiratory syndrome (SARS), possibly due to the unique spike protein that binds to the ACE2 receptors of epithelial cells (Chen, Rui, et al., 2020)
2.2. Surrogates for SAR‐CoV‐2 studies on food
The emergence of SARS‐CoV‐2 has intensified the need for laboratories that can safely handle and process highly infectious viruses. Since SARS‐CoV‐2 is novel, both CDC and WHO released interim guidelines for collecting, handling, and testing presumptive SARS‐CoV‐2 samples (Iwen, Stiles, & Pentella, 2020). The CDC has suggested the use of both biosafety level 3 (BSL3) facilities and practices for virus isolation in cell culture and characterization of viral agents that are recovered in cultures of SARS‐CoV‐2 samples (Iwen et al., 2020). One of the high exposure risks associated with SARS‐CoV‐2 is airborne transmission; therefore, containment by BSL‐3 facilities is necessary to protect laboratory workers (Iwen et al., 2020).
The requirement of a BSL‐3 lab is a major hurdle for the study of COVID‐19's transmission though the food supply because the engineering controls necessary to keep investigator safe often hinders protocols for real‐world application (Iwen et al., 2020). In order to safely study SARS‐CoV‐2 in the food‐processing environment, investigators have identified several possible surrogates to mimic the viral response to potential interventions. It is necessary that the results from surrogate experiments take into consideration the limitations of working with a surrogate. In order for the efficacy of the intervention to be confirmed, validation studies are required with SARS‐CoV‐2 at the BSL‐3 level. However, these validation studies often provide limitations in sample size and real‐world considerations.
The use of surrogates for studying the environmental survival of SARS‐CoV‐2 can increase our understanding of the survival and persistence of this virus in the environment, food contact surfaces, and in foods. There are two pathogenic beta human coronaviruses strains (229E and OC43) that have served as BSL‐2 surrogates for SARS‐CoV‐2 in survival studies (Tan et al., 2020). However, previous studies suggested that the survival of 229E and OC43 on surfaces might be shorter than that of SARS‐CoV‐2 (Yépiz‐Gómez, Gerba, & Bright, 2013). Therefore, additional potential surrogates representing other coronaviruses should be evaluated. Transmissible gastroenteritis virus (TGEV) is an alpha coronavirus that causes diarrhea in swine (Dellanno, Vega, & Boesenberg, 2009a; Gretebeck & Subbarao, 2015). While similar in structure, it attacks porcine aminopeptidase N, a cellular receptor, to aide in its entry (Gretebeck & Subbarao, 2015). Previous studies on TGEV survival in aerosols demonstrate greater survival at low RH, a finding reflected in the results of previous studies of coronaviruses and other enveloped viruses in aerosols (Gretebeck & Subbarao, 2015). Murine hepatitis virus (MHV) is a beta coronavirus that is a common respiratory and enteric pathogen of laboratory (Dellanno, Vega, & Boesenberg, 2009b). The advantages of using these two viruses as surrogates are the fact that they can be readily propagated and assayed in cell culture systems and the fact that there is no human infection risk.
Bacteriophage (or phage) is a type of virus that can infect certain bacteria, also known as their hosts. Phages are attractive surrogates because of the low potential impact on human health and easy propagation. Phages are commonly used as surrogates to study highly pathogenic viruses in which the quantification of the viruses is hard to approach or the lack of equipment with sufficient biosafety level to conduct the experiment (Sommer et al., 2019). However, phages and human pathogenic viruses have fundamentally different structures. The majority of eukaryotic viruses are tail‐less, and thus the members of the phages belong to the Caudovirales order, such as coliphages T4 and T7, might not be the most suitable models. Therefore, tail‐less phages like MS2, Φ6, and ΦX174 are commonly explored as model viruses frequently used for the disinfection studies in either field or laboratory settings (Turgeon, Toulouse, Martel, Moineau, & Duchaine, 2014). Phage MS2 belongs to a group I F‐specific phage from the family of Leviviridae and is a nonenveloped single‐stranded RNA virus with an icosahedral virion (Sommer et al., 2019). Based on the structural similarities, MS2 is often used as a model for human enteric viruses such as Hepatitis E virus and norovirus. MS2 has also been used as a model for viral aerosol study, even though it is more resistant to a coronavirus in terms of aerosolization, sampling, and UV light stress factors (Turgeon et al., 2014).
Viral behavior in suspension is often dictated by the charge of the protein coat, which is characterized by the isoelectric point (pI). The pI and the hydrophobicity of the surface can influence viral behavior within a droplet. For example, MS2 is a nonenveloped virus with a relatively low pI of pH 3.9 and is ionically repulsed from soil surfaces and exhibit less adsorption than viruses with higher pIs (Dowd, Pillai, Wang, & Corapcioglu, 1998; Mathieu, Yu, Zuo, Da Silva, & Alvarez, 2019). SAR‐CoV‐2 is enveloped and has a predicted pI of pH 5.95, which is possible to absorb faster into porous surfaces reducing the transfer efficiency (Beckman et al., 2013). However, little is known about the hydrophobic nature of the SARS‐COV‐2 envelope, which could be the determining factor of its behavior.
2.3. Emerging pathogens requirements and the EPA N‐list
The EPA List N consists of EPA‐registered disinfectant products that meet the requirements for use against COVID‐19 under the Emerging Viral Pathogens guidance. The guidance stipulates that an antimicrobial product capable of inactivating a nonenveloped virus should be able to inactivate any enveloped virus. Many sanitizer companies are relying on this assumption due to the lack of testing laboratories with BSL 3 access to work with SARS‐CoV‐2. A previous study evaluated the effects of a number of antiviral interventions commonly used in the food‐associated environment and found that sodium hypochlorite was the most effective against norovirus, a nonenveloped virus (Kamarasu, Hsu, & Moore, 2018). Another study used human coronavirus (HCoV) 229E to test the viral environmental resistance by use of various disinfectants under different conditions (temperature and reactivation time), and found that 70% ethanol and 0.1% sodium hypochlorite could result in approximately 3 log reductions of HCoV 229E within 30 s to 1 min (Geller, Varbanov, & Duval, 2012). However, the authors found some antiseptic discrepancies from the chlorine‐based compounds between two different HCoVs (229E vs. OC43) (Geller et al., 2012). Therefore, it is critical to bridge the information gap concerning the effects of common disinfectants on the EPA N list on the mitigation of SARS‐CoV‐2 to prevent the spread of COVID‐19 disease in a food‐processing environment (Kampf, Todt, Pfaender, & Steinmann, 2020).
3. DETECTION OF SARS‐COV‐2
As the COVID‐19 impact global communities and the food system, detection and testing have become critical to measure its spread and determine the most effective containment approach to protect the food supply and workers. However, there are no official guidelines and protocols for monitoring SARS‐CoV‐2 in public spaces and surfaces. For clinical and epidemiological purposes, the U.S. Center for Disease Control and Prevention (CDC) recommends two types of detection methods: diagnostic and serology tests (Kang et al., 2020). Under the diagnostic method, nucleic acid‐based and antigen‐based tests are employed, while directly measuring virus protein‐specific antibodies falls under the serology method (Nalla et al., 2020). The principle of the nucleic acid‐based detection approach for SARS‐CoV‐2 is shown in Figures 3 and 4. It is feasible that this approach can be extended onto inanimate surface samples in combination with the standard protocol, International Standardization Organization (ISO) 15216 for norovirus and hepatitis A, wherein cotton swabs are used to collect samples from food preparation surfaces and fomites (Park et al., 2015). Under the emergency use authorization (EUA)‐approved assay by the U.S. CDC, laboratories have developed quantitative RT‐PCR (qRT‐PCR) for detecting SARS‐CoV‐2 within 4–6 hr (Broughton et al., 2020). The current CDC 2019‐Novel Coronavirus (2019‐CoV) rtRT‐PCR is intended for the qualitative detection of viral RNA from respiratory specimens only wherein primers and probes were selected from the nucleocapsid (N) gene, with additional primers/probes for human RNase P gene (RP) in control samples (Broughton et al., 2020).
FIGURE 3.
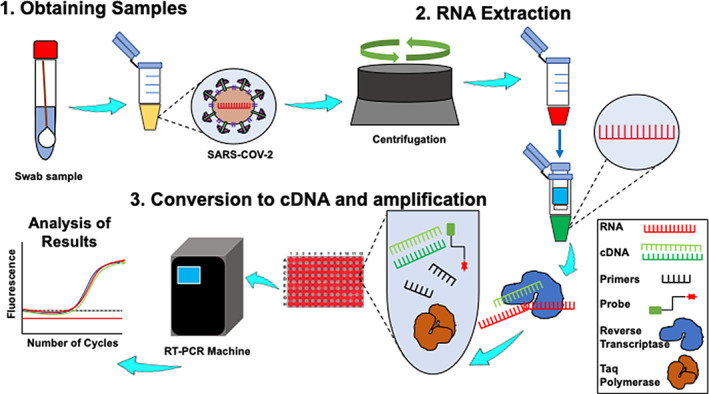
The general principle and workflow of nucleic acid‐based detection approach for SARS‐COV‐2. (1) Swabbing is the most common technique to collect samples from suspected areas, that is, nasopharyngeal area, and inanimate surfaces. Resuspended buffer containing SARS‐COV‐2 is centrifuged for concentration and purification prior to extraction. (2) RNA is extracted using spin columns, which are, then (3) converted to cDNA and amplification using RT‐PCR for analysis and quantification
FIGURE 4.
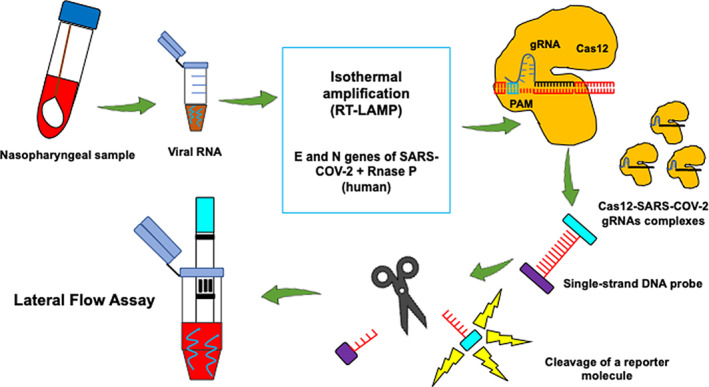
CRISPR–Cas12‐based lateral flow assay for detecting SARS‐CoV‐2. Nasopharyngeal swab samples are used to extract viral RNA, which are then utilized into SARS‐CoV‐2 DNA Endonuclease‐Targeted CRISPR Trans Reporter (DETECTR) and lateral flow strip assay. Extracted RNA undergoes RT‐LAMP and Cas12 detection of predefined coronavirus sequences. A reporter molecule is then cleaved to confirm the detection of the virus. The DETECTR assay can be run in approximately 30–40 min and visualized on a lateral flow strip. The Cas12 fluorescent signal is detectable in <1 min, while the visual signal by lateral flow is within 5 min (Broughton et al., 2020)
The performance of various SARS‐CoV‐2 molecular‐based detection assays was previously compared, and it showed that among the assays included, E‐gene primer‐probe set described by Corman et al. (2020) and the N2 set of the CDC were the most sensitive with no‐cross reactivity (Nalla et al., 2020). CRISPR–Cas12‐based lateral flow assay has also been developed, which featured a visual readout, shorter turnaround time with superior positive (95%), and negative (100%) predictive agreement with the U.S. CDC's rtRT‐PCR assay (Broughton et al., 2020)(Figure 3). However, although nucleic acid amplification is the gold standard for detecting enteric viral pathogens (Bloomfield, Balm, & Blackmore, 2015), it presents disadvantages as well. Reverse transcriptase‐PCR can detect RNA but not the infectious virus (Bullard et al., 2020). Viral RNA is also unstable, rapidly degrading over time, and more importantly, the virus itself is evolving; thus, periodic genetic sequencing is necessary to ensure optimum performance of primers and probes (Patel et al., 2020).
Virus spread and transmission between food producers, retailers, and consumers is a great concern during the pandemic. In order to accurately detect SARS‐CoV‐2 in the food supply, thorough recovery from foods and food contact surfaces is critical. The concentration of infectious viral particles in contaminated food items and surfaces is relatively low partly due to its inability to replicate outside host cells and susceptibility to harsh conditions in free environments. Therefore, researchers incorporate separation, concentration, and purification steps in order to achieve efficient recovery and limit background noise.
There is no standardized or universal recovery method, and the application of the most appropriate method is determined based on the food matrix (i.e., presence of PCR inhibitors such as organic compounds, fats, Ca2+, and sugars) and the sensitivity required. A comparative study on four recovery methods for noroviruses showed that among ultrafiltration, immunomagnetic separation, ultracentrifugation, and polyethylene glycol (PEG) precipitation techniques, ultracentrifugation had the highest recovery in ham and lettuce, while PEG precipitation had the best recovery yield in raspberries (Summa, von Bonsdorff, & Maunula, 2012). However, the recovery efficiency of SARS‐CoV‐2 may not be the same from nonenveloped enteric viruses due to structural differences (Ahmed et al., 2020). By using murine hepatitis virus (MHV) as a surrogate to SARS‐COV‐2 in wastewater, it was found that the adsorption‐extraction method (pre‐acidification then filtration using 0.45‐μm pore size electronegative membranes) with MgCl2 was the most efficient concentration technique among seven RT‐qPCR reaction‐based recovery methods (Ahmed et al., 2020). More importantly, future optimization of virus elution steps while reducing the release of prominent PCR inhibitors can also immensely improve recovery and achieve more accurate test results of virus detection in the food supply.
4. TRANSMISSION OF COVID‐19 THROUGH THE FOOD SUPPLY
Transmission of COVID‐19 is occurring through respiratory droplets generated by coughing, sneezing, or talking (Bourouiba, 2020). The persistence and inactivation of pathogen‐laden droplets and aerosols in complex biological fluids are poorly understood (Tang et al., 2013). When these respiratory droplets settle on inanimate objects, they become potential reservoirs for infection (Bourouiba, 2020; Lopez et al., 2013). Understanding the transmission of SARS‐COV‐2 in fomites is needed to predict the potential for its transmission. This information can be used to understand the spread of disease in food processing environments and the potential for sanitizing surfaces to reduce transfer efficiency (Tang et al., 2013). However, calculating the risk of an individual contracting the disease is extremely difficult in a pandemic situation because many essential factors needed for the model are often missing (Bourouiba, 2020). Furthermore, a recent study published in a medical journal found that the aerosols containing SARS‐CoV‐2 could persist on inanimate surfaces, such as propylene plastic surfaces and stainless steel, up to 72 hr and the virus was more resilient than the closely‐related SARS‐CoV‐1 coronavirus (Van Doremalen et al., 2020). Although significant efforts have been focusing on the effectiveness of various disinfectants on HCoV, including SARS‐CoV‐1 and SARS‐CoV‐2, similar information conducted associated with the food‐processing environment is significantly lacking and needed.
Food processing takes place at a wide variety of conditions depending on the product being produced. For example, most fruits and vegetables are kept at refrigerated temperatures and/or modified atmospheres during transport, storage, and processing. However, these conditions can change drastically when the product enters the market and is purchased by the consumer. Assessment of the risk posed by SARS‐CoV‐2 for foods and food contact surfaces requires data on the survival within the environmental variables, such as ambient air temperature and relative humidity. Current research suggests that lower air temperatures enhanced coronavirus virus survival, possibly slowing reaction rates. In addition, viral inactivation may take place when viral capsids accumulate at the air–water interface of a solution, causing structural damage (Chen, Rui, et al., 2020; Prussin et al., 2018; Yépiz‐Gómez et al., 2013). Desiccation during food storage may also be an important contributor to inactivation on surfaces (Chen, Jiang, & Hu, 2020; Yeap et al., 2016), as loss of water molecules triggers oxygen radical formation (Oliveiros, Caramelo, Ferreira, & Caramelo, 2020).
4.1. Respiratory droplets
A respiratory droplet is a particle produced during expiration that consists of bacteria and viruses encapsulated in water, proteins, and mucus. Respiratory droplets generated by coughing, sneezing, or talking are common forms of disease transmission for respiratory infections. Infection containment strategies are developed based on whether a respiratory disease is going to be mostly present on surfaces or could travel through air handling systems. The ability of SARS‐CoV‐2 to persist in droplets possibly contributes to the observed infectivity and spread of the disease (Bourouiba, 2020). The rate at which droplets or aerosol settle in a surface or evaporate also depends on properties of the ambient environment (temperature, humidity, and airflow) (Bourouiba, 2020). The evaporation and inactivation of pathogen‐laden droplets and aerosols in complex biological fluids are poorly understood (Bourouiba, 2020). The degree and rate of evaporation depend strongly on ambient temperature, humidity conditions, and the trajectory of expiration.
4.2. OSHA guidance and PPE
The Occupational Safety and Health Administration (OSHA) developed COVID‐19 guidance based on traditional infection prevention and industrial hygiene practices (U.S. Department of Labor: OSHA, 2020). The guidance is advisory in nature and focuses on the need for employers to implement engineering, administrative, and work practice controls and PPE. Engineering and administrative controls are considered more effective in minimizing exposure to SARS‐CoV‐2; however, the critical role of food processing and preparation has forced many workers to forgo these measures (U.S. Department of Labor: OSHA, 2020). Instead, workers are relying heavily on PPE to prevent exposure to SARS‐CoV‐2. PPE, especially face coverings, are needed to protect essential workers from exposures to respiratory droplets that could transmit COVID‐19.
The use of surgical masks or respirators is one practice that may reduce the risk of infectious disease transmission between infected and non‐infected persons (Bałazy et al., 2006). The efficiency of filtration in respirators and masks depends on the filter characteristics, including fiber diameter, charge of fibers, packing density, filter thickness, as well as particle properties, such as diameter, density, and velocity (Bałazy et al., 2006). Respirators are more effective than surgical masks and rely upon a tight seal to the individual face in order to filter out particles (Bałazy et al., 2006). The N95 filtering facepiece respirators and surgical masks are commonly used to protect the human respiratory system against fine airborne particles that are known to be associated with various respiratory diseases (Bałazy et al., 2006). Virus particles can potentially penetrate through both respirators and surgical masks and enter the human respiratory system because of their small size. The protection against the airborne viral agents provided by some N95 respirators may fall below 95%, especially at higher inhalation flow rates. A surgical mask can only provide a physical barrier and protect the user from splashes of large droplets being expelled (Beckman et al., 2013). The efficacy of the surgical masks is significantly lower than that of the N95 respirators with a maximum efficiency of 80% under ideal conditions (Bałazy et al., 2006). Therefore, most information is needed to determine which face covering is appropriate for the designated task in food production environments.
Microdroplets are often used to model the possible infection routes and are practical tools for demonstrating the proper use of PPE (Bourouiba, 2020). These experiments are especially helpful in communicating the visual effect of viral spread and reinforce sanitary measures. Also, the data collected can be used to uncover new modes of transmission. For example, from visualizing the trajectory of a sneeze, we know that respiratory droplets can travel up to 28 ft, and the aerosol generated can remain in the air over 30 min (Bourouiba, 2020). Furthermore, these experiments revealed that microdroplets created through speaking could easily be a vehicle for virus transmission (Bourouiba, 2020) and reinforced guidance on the use of masks. Currently, what is missing is information that can protect workers in the food sectors and reduce the probability of the food they handing being vectors for COVID‐19.
4.3. Impact on essential workers
For most employers, protecting workers will depend on emphasizing basic infection prevention measures. However, many social distancing measuring may be impractical in food processing and preparation. In addition, recommendations for separations of 3–6 ft (1–2 m) may underestimate the distance, timescale, and persistence over which the expiratory cloud and its pathogenic payload travel and underestimate the potential exposure range for essential workers (Beckman et al., 2013). Previous coronaviruses outbreaks (MERS‐COV and SARS‐COV) did not implicate food as transmission routes (Gretebeck & Subbarao, 2015; Oliveiros et al., 2020; Prussin et al., 2018). However, with the knowledge on the persistence of SARS‐CoV‐2 on surfaces, it is very likely that both symptomatic and asymptomatic food handlers can transfer respiratory droplets on food items and food contact materials, by touching eyes and mucosal membranes. Therefore, wearing of appropriate PPE and social distancing is vitally important for essential workers handling food. It is important to consider identified hazards for any employee safety program to successfully perform tasks safely with low to negligible risks of infection.
5. ECONOMIC IMPACT AND DATA SCIENCE SOLUTIONS
Data science and analytics is changing the food industry by allowing organizations to protect essential workers, prevent cross‐contamination, and create new distribution platforms (Park et al., 2019). In addition, data science is a powerful marketing tool for food companies to reach their target audiences (Weidner, Yang, & Hamm, 2019). Analytical platforms can combine data on temperature, humidity, and duration in the food chain to predict pathogen infection and take action before a contamination event. For example, food inspectors can use data analytics to analyze historical data on 13 crucial variables to pinpoint the riskiest establishments and make better use of resources (Weidner et al., 2019). The emergence of COVID‐19 has accelerated the development of new analytics platforms that are adapted to the changing landscape of food safety (Shahidi, 2020). High quality curated data sets are transformed into engaging visual tools delivered to the food processors and consumers so they can make evidence‐based decisions and inform both parties on best practices while commerce is taking place.
5.1. Data‐driven alternative markets for produce and other food products
Many web‐based services that handle food delivery are providing an economic necessity in the food supply, allowing consumers to avoid the grocery store during the COVID‐19 outbreak. The current market projection of application‐based food delivery services and meal kits is 16.3 billion dollars and has seen huge increases from 2019, which is mostly attributed to COVID‐19 (Jribi et al., 2020; Weidner et al., 2019). With the dynamics of food acquisition for consumers changing during the pandemic, there is a need to reevaluate food safety. Improving food safety is a vital issue for public health, and food supply transparency and traceability can be a significant asset. There are several mobile applications available for download that provide consumers and processors with direct information critical in maintaining food security (Jribi et al., 2020; Weidner et al., 2019). In particular, these applications provide information on food storage conditions, contact tracing, health inspections, safe handling, and avoidance of food waste (Shahidi, 2020). There is a need to monitor the conditions (i.e., temperature and humidity) of food purchased from mobile apps through the various distribution mechanisms because certain high‐risk foods are inherently vulnerable. For example, the distribution changes could lead to an increase in the incidences of temperature abuse in transit, especially when the transition to the new distribution mechanism is rapid (Shahidi, 2020). Mobile applications powered by data analytics can track the various exposures of food during transportation, evaluate risk, and allow for intervention to be immediately deployed.
Recently, U.S. Food and Drug Administration (FDA) launched the New Era of Smarter Food Initiative and Blueprint to leverage data science technology to enhance food safety (FDA, 2020). Existing technology such as blockchain can be adapted to current traceability efforts along for faster response times in outbreak situations. These systems would create new data pipelines, allowing the FDA and other regulators to explore new tools and approaches to analyze the risks. Through the implementation of AI and machine learning, the review of imported foods at the point of entry and insurance of compliance with U.S. food safety standards can potentially be strengthened and expedited. This included improvements in traceability and adherence to HACPP standards through increase monitoring of critical control points. In addition, consumers who have turned to online portals during the pandemic for food delivery would benefit from enhanced monitoring. In the future, smart labels could provide monitoring of ambient conditions and information on potential temperature abuse and impacts on shelf‐life.
5.2. Food security and waste
Food waste is a growing global issue and poses a challenge to food security, food safety, the economy, and environmental sustainability. Approximately a third (1.3 tons) is wasted every year (Jribi et al., 2020). In the United States alone, food losses and waste amounts reached roughly 310 billion dollars (Jribi et al., 2020). The carbon footprint of food waste has been estimated at 3.3 billion tons of CO2 equivalent per year. This is attributed to fossil fuels need to grow transport and food, and through food waste decomposition itself (Jribi et al., 2020). In the era of COVID‐19, the food supply chain has been immune to disruption, as consumer buying habits have adjusted to sheltering in place. Recent estimates placed the increase of food waste and lose around 12% in individual households (Aldaco et al., 2020). This increase has had a downstream effect on municipal waste management, in many cases, combined with the shortage of essential work has overwhelmed the system (Aldaco et al., 2020).
6. CONCLUSIONS
The era of COVID‐19 has resulted in a paradigm shift for safe food practices and reinforced the safety habits for essential workers and consumers. In the coming years, the food science and technology community will be in a position to strategically plan and contribute to the recovery of the food sector. This will require collaborations with other allied disciplines and stakeholders to shape the policies of governments in order to ensure the readiness of the food supply chain in responding to any future pandemic. As the outbreak unfolds and data is collected, there will more information about the efficacy of isolation measures, quarantines, and potential medical interventions for infected individuals. While the development of widespread vaccination programs and development of herd immunity will take time, this information will guide policies on best practices in the interim. In summary, the COVID‐19 pandemic has posed unprecedented challenges to the security and safety of the food sector, but with the help of scientists and technologists, we can overcome these challenges and succeed in providing safe, nutritious, and sufficient food to the global population.
ACKNOWLEDGMENTS
The authors would like to acknowledge the United States Department of Agriculture (USDA NIFA AFRI) food safety grant under award number 2015‐69003‐23410.
Lacombe A, Quintela I, Liao Y, Wu VCH. Food safety lessons learned from the COVID‐19 pandemic. J Food Saf. 2021;41:e12878. 10.1111/jfs.12878
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- Ahmed, W. , Bertsch, P. M. , Bivins, A. , Bibby, K. , Farkas, K. , Gathercole, A. , … Kitajima, M. (2020). Comparison of virus concentration methods for the RT‐qPCR‐based recovery of murine hepatitis virus, a surrogate for SARS‐CoV‐2 from untreated wastewater. Science of the Total Environment, 739, 139960. 10.1016/j.scitotenv.2020.139960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldaco, R. , Hoehn, D. , Laso, J. , Margallo, M. , Ruiz‐Salmon, J. , Cristobal, J. , … Vazquez‐Rowe, I. (2020). Food waste management during the COVID‐19 outbreak: A holistic climate, economic and nutritional approach. Science of the Total Environment, 742, 140524. 10.1016/j.scitotenv.2020.140524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bałazy, A. , Toivola, M. , Adhikari, A. , Sivasubramani, S. K. , Reponen, T. , & Grinshpun, S. A. (2006). Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? American Journal of Infection Control, 34(2), 51–57. 10.1016/j.ajic.2005.08.018 [DOI] [PubMed] [Google Scholar]
- Beckman, S. , Materna, B. , Goldmacher, S. , Zipprich, J. , D'Alessandro, M. , Novak, D. , & Harrison, R. (2013). Evaluation of respiratory protection programs and practices in California hospitals during the 2009‐2010 H1N1 influenza pandemic. American Journal of Infection Control, 41(11), 1024–1031. 10.1016/j.ajic.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield, M. G. , Balm, M. N. D. , & Blackmore, T. K. (2015). Molecular testing for viral and bacterial enteric pathogens: Gold standard for viruses, but don't let culture go just yet? Pathology, 47(3), 227–233. 10.1097/PAT.0000000000000233 [DOI] [PubMed] [Google Scholar]
- Bosch, A. , Gkogka, E. , Le Guyader, F. S. , Loisy‐Hamon, F. , Lee, A. , van Lieshout, L. , … Phister, T. (2018). Foodborne viruses: Detection, risk assessment, and control options in food processing. International Journal of Food Microbiology, Vol. 285, pp. 110–128. 10.1016/j.ijfoodmicro.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouiba, L. (2020). Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID‐19. JAMA—Journal of the American Medical Association, 323(18), E1–E2. 10.1001/jama.2020.4756 [DOI] [PubMed] [Google Scholar]
- Broughton, J. P. , Deng, X. , Yu, G. , Fasching, C. L. , Servellita, V. , Singh, J. , … Chiu, C. Y. (2020). CRISPR–Cas12‐based detection of SARS‐CoV‐2. Nature Biotechnology, 38(7), 870–874. 10.1038/s41587-020-0513-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard, J. , Dust, K. , Funk, D. , Strong, J. E. , Alexander, D. , Garnett, L. , … Poliquin, G. (2020). Predicting infectious SARS‐CoV‐2 from diagnostic samples. Clinical Infectious Diseases, ciaa638. 10.1093/cid/ciaa638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention . (2020a). What food and grocery pick‐up and delivery drivers need to know about COVID‐19. Atlanta: CDC; Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/food-grocery-drivers.html [Google Scholar]
- Center for Disease Control and Prevention (2020b). Meat and poultry processing workers and employers, Atlanta: CDC. Retrieved from https://www.cdc.gov/coronavirus/2019‐ncov/community/organizations/meat‐poultry‐processing‐workers‐employers.html [Google Scholar]
- Chen, K. , Jiang, S. , & Hu, A. (2020). Advances in the etiology of COVID‐19. Chinese Journal of Microbiology and Immunology (China), 40, 256–261. 10.3760/cma.j.cn112309-20200312-00115 [DOI] [Google Scholar]
- Chen, T. M. , Rui, J. , Wang, Q. P. , Zhao, Z. Y. , Cui, J. A. , & Yin, L. (2020). A mathematical model for simulating the phase‐based transmissibility of a novel coronavirus. Infectious Diseases of Poverty, 9(1), 1–8. 10.1186/s40249-020-00640-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, N. (2001). Viruses in food. CPD Infection, 2, 98–101. [Google Scholar]
- Corman, V. M. , Landt, O. , Kaiser, M. , Molenkamp, R. , Meijer, A. , Chu, D. K. , & Mulders, D. G. (2020). Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Eurosurveillance, 25(3), 2000045. 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellanno, C. , Vega, Q. , & Boesenberg, D. (2009a). The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. American Journal of Infection Control, 37(8), 649–652. 10.1016/j.ajic.2009.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd, S. E. , Pillai, S. D. , Wang, S. , & Corapcioglu, M. Y. (1998). Delineating the specific influence of virus isoelectric point and size on virus adsorption and transport through sandy soils. Applied and Environmental Microbiology, 64(2), 405–410. 10.1128/aem.64.2.405-410.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firquet, S. , Beaujard, S. , Lobert, P. E. , Sané, F. , Caloone, D. , Izard, D. , & Hober, D. (2015). Survival of enveloped and nonenveloped viruses on inanimate surfaces. Microbes and Environments, 30(2), 140–144. 10.1264/jsme2.ME14145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller, C. , Varbanov, M. , & Duval, R. E. (2012). Human coronaviruses: Insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses, 4(11), 3044–3068. 10.3390/v4113044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretebeck, L. M. , & Subbarao, K. (2015). Animal models for SARS and MERS coronaviruses. Current Opinion in Virology, 13, 123–129. 10.1016/j.coviro.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwen, P. C. , Stiles, K. L. , & Pentella, M. A. (2020). Safety considerations in the laboratory testing of specimens suspected or known to contain the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). American Journal of Clinical Pathology, 153(5), 567–570. 10.1093/ajcp/aqaa047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jribi, S. , Ben Ismail, H. , Doggui, D. , & Debbabi, H. (2020). COVID‐19 virus outbreak lockdown: What impacts on household food wastage? Environment, Development and Sustainability, 22(5), 3939–3955. 10.1007/s10668-020-00740-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarasu, P. , Hsu, H. Y. , & Moore, M. D. (2018). Research progress in viral inactivation utilizing human norovirus surrogates. Frontiers in Sustainable Food Systems, 2(21), 1–4. 10.3389/fsufs.2018.00089 [DOI] [Google Scholar]
- Kampf, G. , Todt, D. , Pfaender, S. , & Steinmann, E. (2020). Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. Journal of Hospital Infection, 104(3), 246–251. 10.1016/j.jhin.2020.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, S. , Peng, W. , Zhu, Y. , Lu, S. , Zhou, M. , Lin, W. , … Deng, M. (2020). Recent progress in understanding 2019 novel coronavirus (SARS‐CoV‐2) associated with human respiratory disease: Detection, mechanisms and treatment. International Journal of Antimicrobial Agents, 55(5), 105950. 10.1016/j.ijantimicag.2020.105950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, G. U. , Gerba, C. P. , Tamimi, A. H. , Kitajima, M. , Maxwell, S. L. , & Rose, J. B. (2013). Transfer efficiency of bacteria and viruses from porous and nonporous fomites to fingers under different relative humidity conditions. Applied and Environmental Microbiology, 79(18), 5728–5734. 10.1128/AEM.01030-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu, J. , Yu, P. , Zuo, P. , Da Silva, M. L. B. , & Alvarez, P. J. J. (2019). Going viral: Emerging opportunities for phage‐based bacterial control in water treatment and reuse. Accounts of Chemical Research, 52(4), 849–857. 10.1021/acs.accounts.8b00576 [DOI] [PubMed] [Google Scholar]
- Miranda, R. C. , & Schaffner, D. W. (2019). Virus risk in the food supply chain. Current Opinion in Food Science, 30(1), 43–48. 10.1016/j.cofs.2018.12.002 [DOI] [Google Scholar]
- Nalla, A. K. , Casto, A. M. , Casto, A. M. , Huang, M. L. W. , Perchetti, G. A. , Sampoleo, R. , … Greninger, A. L. (2020). Comparative performance of SARS‐CoV‐2 detection assays using seven different primer‐probe sets and one assay kit. Journal of Clinical Microbiology, 58(6), e00557–e00520. 10.1128/JCM.00557-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveiros, B. , Caramelo, L , Ferreira, N C , & Caramelo, F. (2020). Role of temperature and humidity in the modulation of the doubling time of COVID‐19 cases . medRxiv. 10.1101/2020.03.05.20031872 [DOI]
- Park, C.‐Y. , Kim, K. , Roth, S. , Beck, S. , Kang, J. W. , Tayag, M. C. , & Griffin, M. (2019). Global shortage of personal protective equipment amid COVID‐19: Supply chains, bottlenecks, and policy implications. ADB Briefs, 108, 1–10. 10.22617/BRF200128-2 [DOI] [Google Scholar]
- Park, G. W. , Lee, D. , Treffiletti, A. , Hrsak, M. , Shugart, J. , & Vinjé, J. (2015). Evaluation of a new environmental sampling protocol for detection of human norovirus on inanimate surfaces. Applied and Environmental Microbiology, 81(17), 5987–5992. 10.1128/AEM.01657-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, R. , Babady, E. , Theel, E. S. , Storch, G. A. , Pinsky, B. A. , George, K. S. , … Bertuzzi, S. (2020). Report from the american society for microbiology covid‐19 international summit, 23 March 2020: Value of diagnostic testing for sars–cov‐2/covid‐19. MBio, 11(2), e00722–e00720. 10.1128/mBio.00722-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin, A. J. , Schwake, D. O. , Lin, K. , Gallagher, D. L. , Buttling, L. , & Marr, L. C. (2018). Survival of the enveloped virus Phi6 in droplets as a function of relative humidity, absolute humidity, and temperature. Applied and Environmental Microbiology, 84(12), e00551–e00518. 10.1128/AEM.00551-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi, F. (2020). Does COVID‐19 affect food safety and security? Journal of Food Bioactives, 9, 1–3. 10.31665/jfb.2020.9212 [DOI] [Google Scholar]
- Sommer, J. , Trautner, C. , Witte, A. K. , Fister, S. , Schoder, D. , Rossmanith, P. , & Mester, P. J. (2019). Don't shut the stable door after the phage has bolted—The importance of bacteriophage inactivation in food environments. Viruses, 11(5), 468. 10.3390/v11050468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs, C. C. , Harwood, M. C. , & Tsai, B. (2019). How nonenveloped viruses hijack host machineries to cause infection. Advances in Virus Research, 104, 97–122. 10.1016/bs.aivir.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summa, M. , von Bonsdorff, C. H. , & Maunula, L. (2012). Evaluation of four virus recovery methods for detecting noroviruses on fresh lettuce, sliced ham, and frozen raspberries. Journal of Virological Methods, 183(2), 154–160. 10.1016/j.jviromet.2012.04.006 [DOI] [PubMed] [Google Scholar]
- Tan, C. W. , Chia, W. N. , Chen, M. I.‐C. , Hu, Z. , Young, B. E. , Tan, Y.‐J. , … Want, L.‐F. (2020). A SARS‐CoV‐2 surrogate virus neutralization test (sVNT) based on antibody‐mediated blockage of ACE2‐spike (RBD) protein‐protein interaction. Nature Research, 1–5. 10.21203/rs.3.rs-24574/v1 [DOI] [PubMed] [Google Scholar]
- Tang, J. W. , Nicolle, A. D. , Klettner, C. A. , Pantelic, J. , Wang, L. , Suhaimi, A. B. , … Tham, K. W. (2013). Airflow dynamics of human jets: Sneezing and breathing ‐ potential sources of infectious aerosols. PLoS One, 8(4), 1–7. 10.1371/journal.pone.0059970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon, N. , Toulouse, M. J. , Martel, B. , Moineau, S. , & Duchaine, C. (2014). Comparison of five bacteriophages as models for viral aerosol studies. Applied and Environmental Microbiology, 80(14), 4242–4250. 10.1128/AEM.00767-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Labor: Occupational Safety and Health Administration . (2020). Guidance on preparing workplaces for COVID‐19. OSHA, 35, 1–35. https://www.osha.gov/Publications/OSHA3990.pdf [Google Scholar]
- U.S. Food and Drug Administration (2020), New era of smarter food. Retrieved from https://www.fda.gov/food/new-era-smarter-food-safety
- Van Doremalen, N. , Bushmaker, T. , Morris, D. H. , Holbrook, M. G. , Gamble, A. , Williamson, B. N. , … Munster, V. J. (2020). Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. New England Journal of Medicine, 382(16), 1564–1567. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner, T. , Yang, A. , & Hamm, M. W. (2019). Consolidating the current knowledge on urban agriculture in productive urban food systems: Learnings, gaps and outlook. Journal of Cleaner Production, 209, 1637–1655. 10.1016/j.jclepro.2018.11.004 [DOI] [Google Scholar]
- Wilder‐Smith, A. , Chiew, C. J. , & Lee, V. J. (2020). Can we contain the COVID‐19 outbreak with the same measures as for SARS? The Lancet Infectious Diseases, 20(5), e102–e107. 10.1016/S1473-3099(20)30129-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, A. , & Payne, D. (1998). The action of three antiseptics/disinfectants against enveloped and nonenveloped viruses. Journal of Hospital Infection, 38(4), 283–295. 10.1016/S0195-6701(98)90077-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap, J. W. , Kaur, S. , Lou, F. , DiCaprio, E. , Morgan, M. , Linton, R. , & Li, J. (2016). Inactivation kinetics and mechanism of a human norovirus surrogate on stainless steel coupons via chlorine dioxide gas. Applied and Environmental Microbiology, 82(1), 116–123. 10.1128/AEM.02489-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yépiz‐Gómez, M. S. , Gerba, C. P. , & Bright, K. R. (2013). Survival of respiratory viruses on fresh produce. Food and Environmental Virology, 5(3), 150–156. 10.1007/s12560-013-9114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


