Calcineurin inhibitors (CNIs) are narrow therapeutic index medications, with drug concentrations altered by factors such as drug‐drug interactions, clinical symptoms including diarrhea, and hepatic dysfunction. Supratherapeutic CNI concentrations can produce a myriad of toxicities, the most worrisome of which are renal injury and seizures.
New York State was the epicenter of COVID‐19 infection in the United States. 1 The extent of tacrolimus variability in patients infected with COVID‐19 infection is unknown. The purpose of this study was to determine whether COVID‐19 infection affects the trough concentration of tacrolimus in solid‐organ transplant recipients by evaluating the changes in dose‐corrected trough concentrations before and during infection.
In this IRB‐approved, multicenter retrospective study, we identified all solid‐organ transplant recipients with a SARS‐CoV‐2 PCR‐positive nasopharyngeal swab from March 15 to April 9, 2020. The primary outcome was the difference in dose‐corrected trough concentration at baseline and upon presentation for COVID‐19 infection. The baseline value was the last known tacrolimus trough concentration and dose within 1 year prior to COVID‐19 infection presentation. The value upon presentation was defined as an appropriately timed trough concentration and dose within 7 days of a SARS‐CoV‐2 PCR‐positive nasopharyngeal swab. Appropriateness of tacrolimus trough concentrations was determined based on pharmacist clinical judgment. Patients were stratified based on diarrhea as a presenting symptom, as this has been shown to increase tacrolimus concentrations. Key safety endpoints include the incidence of acute kidney injury (AKI) as defined by the acute kidney injury network (AKIN) criteria and clinically relevant neurotoxicity (eg, seizures) upon presentation. The Wilcoxon signed rank test was used to compare differences in dose‐corrected trough concentrations and trough concentrations within the same patient comparing before and at presentation or highest trough value for COVID‐19 infection. Statistics were performed using STATA version 14.2 (College Station, TX).
A total of 102 patients were included; baseline characteristics are presented in Table 1. The median dose‐corrected tacrolimus trough level and the tacrolimus trough levels were elevated from baseline to presentation [Figure 1A,C]. No difference was noted in those patients with diarrhea [Figure 1B,D]. No patients were concomitantly receiving hydroxychloroquine, lopinavir/ritonavir, or remdesivir at the time of presentation.
TABLE 1.
Baseline characteristics and laboratory values
| N = 102 | |
| Male sex | 67 (65.7) |
| Age, years | 52.8 (41.2–62.1) |
| Organ | |
| Kidney | 63 (61.8) |
| Liver | 10 (9.8) |
| Heart | 11 (10.8) |
| Lung | 13 (12.8) |
| Liver/kidney | 1 (1) |
| Kidney/heart | 1 (1) |
| Kidney/pancreas | 3 (2.9) |
| Center | |
| Weill Cornell Medical Center | 38 (37.3) |
| Columbia University Irving Medical Center | 64 (62.7) |
| Race | |
| White | 34 (33.3) |
| Black | 25 (24.5) |
| Asian | 7 (6.9) |
| Other | 36 (35.3) |
| Hispanic | 40 (39.2) |
| Diabetes | 42 (41.2) |
| Drug interaction | 18 (17.7) |
| Azithromycin | 8 (44.4) |
| Fluconazole | 2 (11.1) |
| Voriconazole | 1 (5.6) |
| Clotrimazole | 2 (11.1) |
| Posaconazole | 2 (11.1) |
| Isavuconazole | 1 (5.6) |
| Erythromycin | 1 (5.6) |
| Azithromycin + Voriconazole | 1 (5.6) |
| Diarrhea | 41 (40.2) |
| Inpatient | 92 (90.2) |
| Intubated/ICU | 20 (19.6) |
| Mortality | 14 (13.7) |
| Time from transplant to SARS‐CoV‐2 PCR positive, years | 4 (1–9.3) |
| Time from baseline tacrolimus trough concentration to SARS‐CoV‐2 PCR positive, days | 42 (20–95) |
| Time from SARS‐CoV‐2 PCR positive to tacrolimus trough on presentation, days | 1 (0–2) |
| Time from SARS‐CoV‐2 PCR positive to tacrolimus highest trough value, days | 2 (1–5) |
| Scr, mg/dl | |
| Before | 1.5 (1.2–2.3) |
| Presentation | 2 (1.2–4) |
| Highest trough | 2.6 (1.5–4.8) |
| Laboratories at presentation for COVID‐19 infection | |
| AST, U/L | 32 (19–45) |
| ALT, U/L | 21 (15–30) |
| Tbili, mg/dl | 0.4 (0.3–0.6) |
| ESR, mm/hr | 69 (38–96) |
| IL‐6, pg/ml | 30.9 (10.5–80.8) |
| Procalcitonin, ng/ml | 0.3 (0.13–0.62) |
| Ferritin, ng/ml | 1001 (466.5–2092) |
| LDH, U/L | 346 (247–442) |
| CK, U/L | 91 (49–152) |
| CRP | |
| Weill Cornell, mg/dl | 13.3 (5.3–20) |
| Columbia, mg/L | 97.3 (49.9–139.1) |
| D‐dimer | |
| Weill Cornell, ng/ml | 474 (292–1100) |
| Columbia, mcg/ml | 1.22 (0.66–2.2) |
| Troponin | |
| Weill Cornell, ng/ml | 0 (0–0.1) |
| Columbia, ng/L | 24 (12–59) |
All values represented as N (%) or median (IQR).
FIGURE 1.
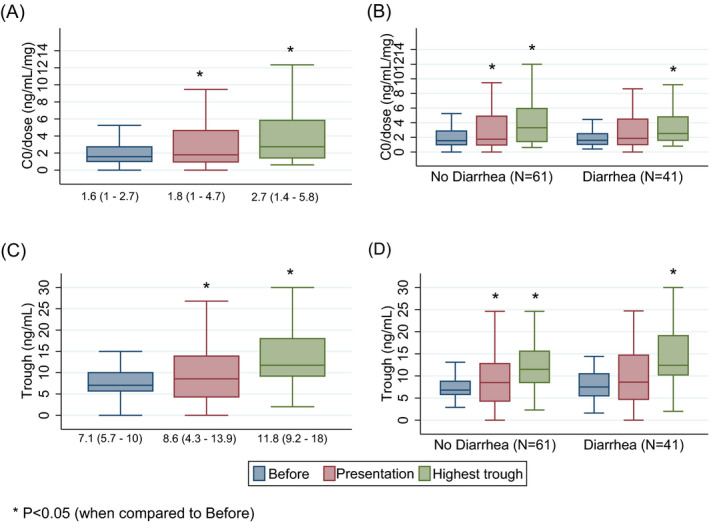
A, Dose‐corrected trough concentration (B) stratified by diarrhea as a presenting symptom. C, Trough concentration (D) stratified by diarrhea as a presenting symptom
Twenty‐one patients (20.6%) presented with a trough concentration >15 ng/ml. Mortality was higher in those patients presenting with a trough above 15 ng/ml 6/21 (28.6%) vs. 8/81 (9.9%) in those below; p = .027. There was no difference in values for AST, ALT, or bilirubin in the patients presenting with a trough value above or below 15 ng/ml. No patients presented with overt seizure activity, while 52 (51%) presented with AKI.
We are the first to report an increase in tacrolimus serum concentrations in solid‐organ transplant recipients with COVID‐19 infection. While the mechanism of this association may be unclear, COVID‐19 infection has been associated with many physiologic alterations including cytokine storm, 2 venous thromboembolism, 3 AKI, 4 neurologic abnormalities, 5 and multisystem inflammatory syndrome (MIS‐C) in children 6 without a yet identified mechanism. The phenotypic plasticity of COVID‐19 infection in organ transplant recipients has been described as a higher incidence of diarrhea and higher disease severity upon presentation. 1 However, diarrhea is unlikely to explain the increased exposure in our cohort because trough concentrations were higher in patients without diarrhea as a presenting symptom.
There are a few hypotheses that may explain the association between COVID‐19 infection and increased tacrolimus concentrations. First, patient factors not easily measured, such as the combination of volume contraction from poor oral intake and fever, along with increased tacrolimus bioavailability in patients with anorexia may increase tacrolimus trough concentrations. Additionally, pro‐inflammatory states including increased expression of IL‐6 have been shown in vitro to decrease cytochrome P450 3A4 activity contributing to phenoconversion. 7 , 8
A notable limitation of this study was the variability in time course of disease when tacrolimus troughs were captured. Another limitation is that the majority of our patients were admitted to hospital, and as such, our results may not pertain to ambulatory patients with less severe illness. 9
Monitoring of COVID‐19‐positive patients poses unique challenges related to increased exposure of healthcare personnel, other non‐infected patients, and the community when using transportation. Our results suggest that a significant number of patients infected with COVID‐19 infection could be overexposed to tacrolimus. Closer monitoring of trough tacrolimus concentration should be considered in all SARS‐CoV‐2‐positive solid‐organ transplant patients to ensure safe drug exposure and minimize complications of immunosuppression.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
David M. Salerno, PharmD, Danielle Kovac, PharmD, Heather Corbo, PharmD, Douglas L. Jennings, PharmD, Jennifer Lee, PharmD, Jason Choe, PharmD, Jenna Scheffert, PharmD, Jessica Hedvat, PharmD, Justin Chen, PharmD, Demetra Tsapepas, PharmD, Russell Rosenblatt, MD, Benjamin Samstein, MD, Karim Halazun, MD, Elizabeth Verna, MD, Marcus Pereira, MD, Syed A. Husain, MD, Sumit Mohan, MD, and Robert S. Brown Jr, MD: Designed research; David M. Salerno, PharmD, Danielle Kovac, PharmD, Russell Rosenblatt, MD, Elizabeth Verna, MD, Syed A. Husain, MD, Sumit Mohan, MD, and Robert S. Brown Jr, MD: Wrote the manuscript; David M. Salerno, PharmD, Danielle Kovac, PharmD, Heather Corbo, PharmD, Jennifer Lee, PharmD, Jason Choe, PharmD, Jenna Scheffert, PharmD, Jessica Hedvat, PharmD, Justin Chen, PharmD, Demetra Tsapepas, PharmD, and Corey Brennan, MPH: Performed the research; David M. Salerno, PharmD, Danielle Kovac, PharmD, Elizabeth Verna, MD, Syed A. Husain, MD, Sumit Mohan, MD, and Robert S. Brown Jr, MD: Analyzed the data; and David M. Salerno, PharmD, Danielle Kovac, PharmD, Heather Corbo, PharmD, Douglas L. Jennings, PharmD, Jennifer Lee, PharmD, Jason Choe, PharmD, Jenna Scheffert, PharmD, Jessica Hedvat, PharmD, Justin Chen, PharmD, Demetra Tsapepas, PharmD, Demetra Tsapepas, PharmD, Benjamin Samstein, MD, Karim Halazun, MD, Elizabeth Verna, MD, Marcus Pereira, MD, Syed A. Husain, MD, Sumit Mohan, MD, and Robert S. Brown Jr, MD: Interpreted the data.
Salerno DM, Kovac D, Corbo H, et al. SARS‐CoV‐2 infection increases tacrolimus concentrations in solid‐organ transplant recipients. Clin Transplant. 2021;35:e14193. 10.1111/ctr.14193
Funding informationNo funding provided support for this research.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800‐1808. 10.1111/ajt.15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jose RJ, Manuel A. COVID‐19 cytokine storm: the interplay between inflammation and coagulation. Lancet. 2020;8(6):e46‐e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID‐19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72‐81. 10.1007/s11239-020-02138-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ronco C, Reis T, Husain‐Syed F. Management of acute kidney injury in patients with COVID‐19. Lancet Respir Med. 2020;8(7):P738‐P742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mao L, Jin H, Wang M. Neurological manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. Jama Neurol. 2020;77(6):683‐690. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riphagen S, Gomez X, Gonzalez‐Martinez C, et al. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet. 2020;395:1607‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simon F, Garcia J, Guyot L, et al. Impact of interleukin‐6 on drug‐metabolizing enzymes and transporters in intestinal cells. The AAPS Journal. 2020;22:16. [DOI] [PubMed] [Google Scholar]
- 8. Shah RR, Smith RL. Addressing phenoconversion: the Achilles' heel of personalized medicine. Br J Clin Pharmacol. 2014;79(2):222‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Husain SA, Dube G, Morris H, et al. Early outcomes of outpatient management of kidney transplant recipients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15(8):1174‐1178. 10.2215/CJN.05170420 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


