Abstract
Introduction
Despite being widely used as a screening tool, a rigorous scientific evaluation of infrared thermography for the diagnosis of minimally symptomatic patients suspected of having COVID‐19 infection has not been performed.
Methods
A consecutive sample of 60 adult individuals with a history of close contact with COVID‐19 infected individuals and mild respiratory symptoms for less than 7 days and 20 confirmed COVID‐19 negative healthy volunteers were enrolled in the study. Infrared thermograms of the face were obtained with a mobile camera, and RT‐PCR was used as the reference standard test to diagnose COVID‐19 infection. Temperature values and distribution of the face of healthy volunteers and patients with and without COVID‐19 infection were then compared.
Results
Thirty‐four patients had an RT‐PCR confirmed diagnosis of COVID‐19 and 26 had negative test results. The temperature asymmetry between the lacrimal caruncles and the forehead was significantly higher in COVID‐19 positive individuals. Through a random forest analysis, a cut‐off value of 0.55°C was found to discriminate with an 82% accuracy between patients with and without COVID‐19 confirmed infection.
Conclusions
Among adults with a history of COVID‐19 exposure and mild respiratory symptoms, a temperature asymmetry of ≥ 0.55°C between the lacrimal caruncle and the forehead is highly suggestive of COVID‐19 infection. This finding questions the widespread use of the measurement of absolute temperature values of the forehead as a COVID‐19 screening tool.
Keywords: COVID‐19, diagnosis, machine learning, screening, thermography
1. INTRODUCTION
The ongoing COVID‐19 pandemic caused by coronavirus SARS‐CoV‐2 has created significant challenges for the healthcare systems such as identifying patients in the early days of the disease to offer adequate support measures, monitor disease progression, and prevent further transmission. 1 The latter is important from a public health perspective, as it has been reported that up to 70% of patients with a COVID‐19 diagnosis confirmed by RT‐PCR are either asymptomatic or present with minimal symptoms, posing a significant threat of contagion if not identified. 2 Infrared thermography (IRT) is a technique that creates a visible image from the invisible infrared radiation emitted by an object. 3 In the skin, while several factors contribute to its thermal pattern, arguably, the most important is blood flow. Vasodilation of skin capillaries due to hyperaemia or inflammation is associated with increases in temperature, and conversely, vasoconstriction or devascularisation of the skin, with temperature drops. 4 IRT is very sensitive for detecting small changes in the temperature distribution of the face, particularly around the medial lower eyelid and the lacrimal caruncle, which are areas that respond to autonomic changes in blood flow. 5 Moreover, this technology has been used with moderate success as a screening tool to detect illness during past respiratory virus epidemics. 6 , 7 Despite temperature readings with infrared thermometers and IRT being widely used as a screening tool in the ongoing COVID‐19 pandemic, there are shockingly no reports about its diagnostic performance. 8 Therefore, the objective of this study was to conduct a pilot study to determine the diagnostic accuracy of IRT to discriminate between minimally asymptomatic individuals with and without RT‐PCR confirmed diagnosis of COVID‐19. Based on our previous research on acute inflammatory conditions, 9 we hypothesized that while absolute forehead temperature readings would probably have limited diagnostic value, temperature measurements of the difference between forehead and eye caruncle would offer a higher diagnostic yield.
2. MATERIALS AND METHODS
This was a prospective observational study of patients with mild respiratory symptoms suggestive of COVID‐19 infection treated during summer 2020 in an emergency department of Hospital Central “Dr Ignacio Morones Prieto” in San Luis Potosi, Mexico. The study was approved by the Research and Ethics Committee of the Hospital (registration number 22‐20) and conducted following the Declaration of Helsinki. All participants provided written informed consent before any study procedures. Reporting of the study conforms to broad EQUATOR guidelines. 10
Inclusion criteria were onset of symptoms for less than 7 days with a previous history of close contact with a COVID‐19 infected person. Patients were excluded if they presented any previous vascular or skin disorder in the face, heart failure history, pneumonia, sepsis, or shock. Clinical information recorded included demographic data, smoking history, previous chronic illness history, signs, symptoms and constants present at the ED, a complete blood count, coagulation panel, serum glucose, creatinine, aspartate and alanine transaminases, electrolytes, and high‐sensitivity C‐reactive protein (hs‐CRP).
The index test consisted of a digital infrared thermogram of the face obtained with a mobile FLIR ONE Pro camera (FLIR Systems) attached to an iPad mini. The camera has a 160 × 120‐pixel size thermal resolution, a spectral range of 8 to 14 µm, and a thermal sensitivity of 70 mK at 30°C. The camera has a scene temperature ranging from −20°C to 400°C and can detect temperature differences as small as 0.1°C. For image acquisition, the camera was left on for 3 minutes before acquiring the images to allow the stabilization of the sensor. Afterwards, auto‐calibration of the instrument was done following the manufacturer's instructions. All the temperature measurements were taken following the Thermographic Imaging in Sports and Exercise Medicine (TISEM) checklist, 11 at a distance of 0.3 m and an angle of 90° relative to the forehead, in a closed room under controlled conditions of light and external radiation exposure, controlled room temperature (23°C) and atmospheric humidity of 40% after allowing the subject an acclimatization period of 15 minutes, and before any invasive medical procedure. To avoid contaminating the devices, they were placed inside of a disposable zip bag. All imaging was done when patients were not febrile. In the case of patients with a history of fever, the imaging was done after at least 4 hours of the last febrile episode.
Thermographic analysis of the images was performed using the FLIR Tools Quick‐Report v.1.2 software (FLIR Systems, version 5.70, 2016) by a researcher blinded to the clinical data and final diagnosis. The skin emissivity was set at 0.98 for all acquired images. The researcher delineated a region of interest (ROI) corresponding to each lacrimal caruncle, as this area has previously been shown to be a site of dynamic temperature changes in response to inflammation and autonomic stimuli, as well as in the middle patient's forehead (Figure 1). Both mean temperatures in degrees Celsius were recorded, as well as the thermal asymmetry between them.
FIGURE 1.
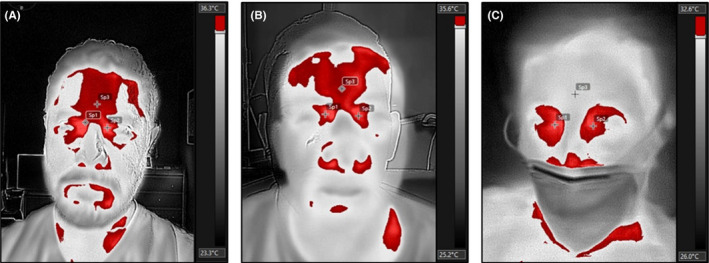
Infrared thermograms. Digital infrared thermograms of a healthy control (A), a confirmed COVID‐19 negative patient (B) and a confirmed positive COVID‐19 patient (C) are shown. The images were converted into greyscale values, and the regions of maximum temperature values selected in red. Non‐COVID‐19 subjects show similar maximum temperature distribution in the lacrimal caruncles and forehead (crosses). COVID‐19 positive individuals show significantly higher temperature values on the lacrimal caruncles than on the forehead, thereby the forehead is not selected as a “hotspot” when thresholding the image
Final diagnosis of COVID‐19 was done by real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) from nasopharyngeal swabs. The test was performed by an independent party (Statal Public Health Laboratory). Patients under investigation who had a first negative test required a second negative test 72 hours apart to be confirmed as COVID‐19 negative. Finally, a sample of 20 confirmed COVID‐19 negative hospital staff (healthy volunteers) was recruited as a negative control.
2.1. Statistical analysis
Data are presented as mean and standard deviation or proportions for continuous or categorical data, respectively. Analysis was performed using the statistical software R v.4.0.1 (The R Core Team, 2020) at the 95% confidence interval. Differences between healthy volunteers and patients with and without confirmed COVID‐19 infection were analysed by two‐sided ANOVA followed by Tukey post hoc tests or Chi‐squared tests. Afterwards, a machine learning random forest model was used to perform multivariable analysis of all variables and categorize the patients as COVID‐19 positive or negative. The data were split into a train and a test set (70/30%), and the trained model was validated using fivefold cross‐validation before testing it in the test set. Reported diagnostic accuracy is on the test set data.
3. RESULTS
A total of 60 patients were included in the study. From these, 34 had an RT‐PCR confirmed diagnosis of COVID‐19 and 26 had negative test results. A STARD flowchart of the study design can be accessed in the supplementary material. The demographic characteristics of the patients and healthy controls are shown in Table 1. Except for elevated prothrombin time (13.5 ± 2.3 vs 12.2 ± 0.9 seconds), lower serum glucose (5.8 ± 1.6 vs 7.1 ± 2.9 mmol/L), and higher hs‐CRP levels (8.9 ± 0.7 vs 6.6 ± 3.1 mg/L), no significant differences were found in the symptoms, signs, clinical constants or laboratory results of COVID‐19 positive patients compared to negative patients (Table 2 and 3).
TABLE 1.
Sociodemographic and temperature characteristics
| Control (n = 20) | COVID‐19 negative (n = 26) | COVID‐19 positive (n = 34) | P‐value | |
|---|---|---|---|---|
| Age (y) | 48.9 ± 12.5 | 55.8 ± 16.5 | 53.4 ± 17.8 | .36 |
| Gender | Female = 10 (50%); Male = 10 (50%) | Female = 10 (38%); Male = 16 (62%) | Female = 16 (47%); Male = 18 (53%) | .70 |
| BMI | 26.8 ± 2.6 | 27.1 ± 2.8 | 27.9 ± 3.2 | .29 |
| Chronic disease | 6 (30%) | 15 (58%) | 19 (56%) | .11 |
| Current smoker | 2 (10%) | 6 (23%) | 5 (15%) | .47 |
| Forehead temperature (°C) | 31.1 ± 1.7 | 32.2 ± 1.9 | 32.4 ± 1.9 | .06 |
| Lacrimal caruncle temperature (°C) | 31.2 ± 1.7 | 32.5 ± 1.9 | 33.5 ± 1.8* | <.001 |
| Temperature asymmetry (°C) | 0.08 ± 0.2 | 0.35 ± 0.2* | 1.2 ± 0.9 * , a | <.001 |
P < 0.05 vs COVID‐19 negative.
P < .05 vs Control.
TABLE 2.
Patient clinical characteristics
| COVID‐19 negative (n = 26) | COVID‐19 positive (n = 34) | P‐value | |
|---|---|---|---|
| Onset of symptoms (days) | 5 IQR 3 | 5 IQR 4 | .43 |
| Cough | 18 (69%) | 28 (82%) | .39 |
| Dyspnea | 10 (38%) | 17 (50%) | .50 |
| Fever | 15 (58%) | 19 (56%) | .98 |
| Rhinorrhea | 4 (15%) | 2 (6%) | .41 |
| Myalgia | 10 (39%) | 14 (41%) | .96 |
| Cephalea | 6 (23%) | 10 (29%) | .84 |
| Diarrhoea | 8 (23%) | 3 (11%) | .44 |
| Heart rate (bpm) | 83 ± 10 | 82 ± 11 | .75 |
| Respiratory rate (rpm) | 18 ± 3 | 19 ± 3 | .43 |
| Mean arterial pressure (mm Hg) | 82.4 ± 11.6 | 80.2 ± 11.1 | .47 |
| Temperature (°C) | 36.1 ± 0.5 | 36.4 ± 0.4 | .09 |
| SaO2 (%) | 93.5 ± 3.2 | 92.4 ± 2.4 | .38 |
TABLE 3.
Patient laboratory characteristics
| COVID‐19 negative (n = 26) | COVID‐19 positive (n = 34) | P‐value | |
|---|---|---|---|
| Leukocytes (103) | 10.2 ± 2.7 | 9.3 ± 3.5 | .26 |
| Neutrophils (%) | 74.4 ± 12.9 | 78.3 ± 10.6 | .20 |
| Lymphocytes (%) | 11.3 ± 5.7 | 14.2 ± 6.9 | .08 |
| Haemoglobin (g/dL) | 12.6 ± 2.2 | 12.9 ± 2.1 | .61 |
| Haematocrit (%) | 40.1 ± 7.8 | 41.2 ± 7.9 | .60 |
| Platelets (103) | 272 ± 110 | 231 ± 98 | .14 |
| Prothrombin time (s) | 12.2 ± 0.9 | 13.5 ± 2.3 | .012 |
| Activated partial thromboplastin time (s) | 30.7 ± 5.7 | 28.5 ± 3.8 | .07 |
| Glucose (mmol/L) | 7.1 ± 2.9 | 5.8 ± 1.6 | .039 |
| Creatinine (µmol/L) | 101.5 ± 36.4 | 98.8 ± 46.9 | .81 |
| Aspartate transaminase (IU/L) | 43.8 ± 16.7 | 51.3 ± 22.9 | .16 |
| Alanine transaminase (IU/L) | 54.4 ± 31.3 | 45.1 ± 21.9 | .18 |
| Na (mEq/L) | 141.1 ± 2.7 | 139.5 ± 3.5 | .21 |
| K (mEq/L) | 4.0 ± 0.5 | 3.8 ± 0.6 | .31 |
| Cl (mEq/L) | 105.9 ± 5.8 | 99.3 ± 24.1 | .35 |
| Ca (mEq/L) | 4.2 ± 0.2 | 4.0 ± 0.2 | .76 |
| Mg (mEq/L) | 1.7 ± 0.4 | 1.8 ± 0.3 | .54 |
| PO4 (mEq/L) | 1.4 ± 0.3 | 1.3 ± 0.2 | .55 |
| High‐sensitivity CRP (mg/L) | 6.6 ± 3.1 | 8.9 ± 0.8 | <.001 |
Absolute temperature readings exhibited a wide degree of variation between subjects (range 27.6 to 36.9°C); therefore, as hypothesized, no significant differences in the forehead temperature were detected between groups. However, COVID‐19 positive patients showed a significantly higher temperature in the lacrimal caruncles compared to the other groups. Significant differences were found between the three groups for the temperature asymmetry of the lacrimal caruncles and forehead (Table 1, Figure 1, and Figure 2). A statistically significant positive association was found between hs‐CRP levels and temperature asymmetry values (r 2 = .165, P < .001). For each increase in 1 unit of the hs‐CRP values, there was a 0.09°C (95%CI 0.04 to 0.14) increase in the temperature asymmetry ([Link], [Link], [Link], [Link], [Link]). When all clinical, laboratory and thermographic data from people suspected of having COVID‐19 infection were analysed in a random forest model (60 samples, 49 predictors, 2 classes: positive or negative), the temperature asymmetry was found to be independently associated with the final diagnosis. A cut‐off value ≥ 0.55°C discriminates with 82% (95%CI 71% to 94%) accuracy between patients with and without COVID‐19 infection (Sn = 0.90, Sp = 0.71, PPV = 0.82, NPV = 0.83, AUC = 0.964) ([Link], [Link], [Link], [Link], [Link]).
FIGURE 2.
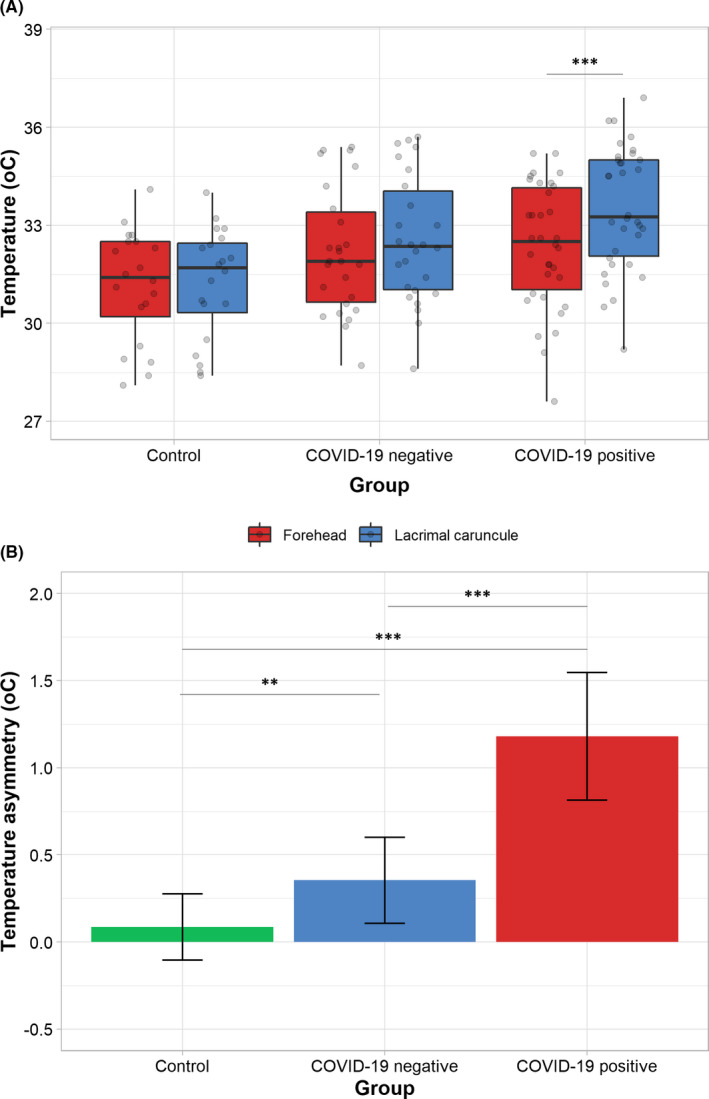
Infrared thermogram analysis. The average temperature of the forehead and lacrimal caruncles was measured and analysed. No significant differences in forehead absolute temperature were found across groups. However, the average lacrimal caruncle temperature in controls was found to be lower than those in COVID‐19 negative and positive patients, although the differences were significant only between controls and the latter group (A). When the temperature distribution was analysed as the temperature asymmetry between the forehead and caruncle, significant differences between groups were found (B). COVID‐19 individuals showed the largest temperature asymmetry, followed by COVID‐19 negative individuals and controls. ** P < .01, *** P < .001
To assess if sex, age, or BMI were correlated with temperature asymmetry, we analysed them through a multivariable linear model. Our results show that none of the variables affect temperature asymmetry except for the disease status ([Link], [Link], [Link], [Link], [Link]).
An a posteriori statistical power calculation was performed. For a mean temperature asymmetry difference of 0.82 ± 0.51°C between COVID‐19 positive and negative individuals, the statistical power calculated at an Alpha level of 0.05 was 98%.
4. DISCUSSION
COVID‐19 infection is characterized by a cytokine‐driven inflammatory response with systemic vascular repercussion. 12 , 13 Our previous research has demonstrated that the temperature patterns of the skin highly correlate with acute inflammatory changes in deep tissues. 9 Moreover, because the face is highly vascularized, some of its regions dramatically respond to endothelial and autonomic changes, making them an ideal region to map inflammation. On contrast to other areas of the face, such as the forehead, that possess a relatively stable temperature, previous research has demonstrated the lacrimal caruncle to be subject to dynamic temperature changes in response to a number of stimuli, including pain and inflammation. 5 , 14 , 15 For this reason, we hypothesized that the systemic vascular and inflammatory response to COVID‐19 infection could be detected in that area, relative to that of more temperature stable regions.
Our results show that temperature asymmetry of the lacrimal caruncle and forehead has a powerful discriminatory capacity to detect COVID‐19 infection. As shown in Figure 1, both the forehead and lacrimal caruncles show “hotspots” that can effectively be used to discriminate between healthy and diseased individuals as they are one of the most salient features of a face's thermogram. Moreover, the increased temperature asymmetry observed in COVID‐19 infected individuals is in line with higher hs‐CRP levels and a modest correlation for both measurements, further supporting the hypothesis that temperature asymmetry of the face is related to inflammation. Taken together, the results suggest that even paucisymptomatic COVID‐19 infection is associated with a more robust inflammatory response than other mild respiratory illnesses and that this response can be captured using infrared thermography.
IRT measurements and forehead temperature readings are being used as screening tools where a large gathering of people occurs, such as shelters, airports, and supermarkets. However, the current tools are limited to identifying people with a fever and mostly only take into account the forehead temperature. 16 , 17 However, the presence of fever is non‐specific and identification of paucisymptomatic patients, who may not present with fever, is a need for the ongoing COVID‐19 pandemic. In contrast to the imaging strategies previously used, we designed our study not to identify patients with fever but to determine if the thermal pattern of the face could be used as a diagnostic marker of COVID‐19 infection. Because fever may alter these patterns, for example by increasing the absolute temperature of the lacrimal caruncle, 18 thereby, introducing potential confounding factors to the analysis, we imaged the study's subjects while they were afebrile. Based on our findings, the absolute temperature recordings cannot discriminate between people with and without COVID‐19 infection, thereby questioning the validity of this approach. To adequately screen people who are either asymptomatic or with minimal symptoms, relative temperature measurements that factor thermal asymmetry need to be taken into account. This feature can be engineered into AI algorithms to increase the sensitivity of automated screening tools intended for settings such as workplaces and schools. 19 , 20
Infrared thermography captures the heat emitted by the skin and not the human body's central temperature; therefore, it is subjected to wider degrees of intersubject variation, as well as variations due to age and sex differences, environmental factors, and the circadian rhythm. 14 , 21 , 22 For these reasons, measurement of the thermal asymmetry between a target and a control region is required to control for these factors. Temperature asymmetry has previously been used to identify skin inflammatory conditions, 23 breast cancer, 24 systemic inflammatory diseases, 25 septic shock, 26 and the healing potential of wounds. 27 , 28 , 29 Our past research demonstrates, for example, that temperature asymmetries as low as 0.35°C can be used to discriminate between patients with and without appendicitis with the same level of accuracy as ultrasound evaluations. 9 Thermography's principles and medical applications have been revised in‐depth by Ring and Ammer. 30
Another possibility that can explain the differences in the temperature asymmetry observed is the fact that respiratory virus have a well‐described ocular tropism. 31 Previous reports have demonstrated that coronavirus can be isolated from tears 32 and can cause conjunctivitis in humans. 33 In the latter case, it is hypothesized that because the conjunctiva is a mucous membrane, it can serve as a site for the direct inoculation of infected droplets and that the nasolacrimal system can act as a conduit for viral migration, thus enabling the transmission of infected tears into the respiratory tract via the nasolacrimal duct and vice versa. 34 Furthermore, the eye possesses an independent ocular renin‐angiotensin‐aldosterone system with abundant expression of ACE receptors in the conjunctiva and cornea. 35 Additionally, SARS‐CoV‐2 viral invasion can also be mediated through attachment to the CD147 receptor, which is expressed in tears and different ocular tissues, including conjunctiva, corneal epithelium and endothelium, stromal keratocytes, and retinal pigment epithelium. 34 , 36 Taken together, these facts strongly suggest that the eye's tissues are actively involved in COVID‐19 infection and progression, and that thermal imaging of these structures offers an insight into the infectious process.
A potential limitation of the study is the fact that we did not control for the intake of medications that could affect the characteristics of the vascular supply to the face, such as NSAIDs or antihypertensives. However, because distribution of chronic illnesses was similar between groups and we used a relative temperature measurement (temperature asymmetry) rather than an absolute reading, we believe the effect of this potential confounder to be partly controlled. Nonetheless, future studies should take this into account and control as much as possible the effect of medication on temperature recordings.
Despite the very promissory results of our pilot study, caution for emitting a final recommendation is warranted. The predictive value of infrared thermography will likely vary depending on the natural course of the illness and a variety of environmental factors. Therefore, more extensive trials are required to confirm our findings and validate them in different settings. Future studies should focus on determining whether the thermal patterns we identified are maintained or even increased during febrile episodes and if they can still be used to discriminate between people with and without COVID‐19 infection.
CONFLICT OF INTEREST
None declared. MMJ receives doctoral support from the Mexican National Council for Science and Technology (CONACYT). JLRG holds a Mitacs Elevate Postdoctoral Fellowship. The funding agencies were not involved in the study design, data collection, analysis, or decision of where to publish.
AUTHOR CONTRIBUTION
This work was conducted in San Luis Potosi, Mexico. MMJ and JRG contributed to the conceptualization of the study and its supervision. MMJ and VLG contributed to data collection. SKM and MYL contributed to data curation and analysis. ASRG and JRG contributed to statistical analysis of the data. All authors contributed to writing of the manuscript. The authors would like to thank Octavio L. Yanes‐Lane for his contributions to the design of the study.
Funding information
No specific funding was used for this research project.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Table S1‐S3
Martinez‐Jimenez MA, Loza‐Gonzalez VM, Kolosovas‐Machuca SE, Yanes‐Lane ME, Ramirez‐GarciaLuna AS, Ramirez‐GarciaLuna JL. Diagnostic accuracy of infrared thermal imaging for detecting COVID‐19 infection in minimally symptomatic patients. Eur J Clin Invest.2021;51:e13474. 10.1111/eci.13474
Martinez‐Jimenez and Loza‐Gonzalez authors contributed equally to this study.
Contributor Information
Mario A. Martinez‐Jimenez, Email: jose.ramirezgarcialuna@mail.mcgill.ca.
Jose L. Ramirez‐GarciaLuna, Email: jose.ramirezgarcialuna@mail.mcgill.ca.
REFERENCES
- 1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239‐1242. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 2. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vo‐Dinh T. Thermal Imaging for Biological and Medical Diagnostics. Biomedical Photonics Handbook. 10.1201/9780203008997-25. [DOI] [Google Scholar]
- 4. Sagaidachnyi AA, Fomin AV, Usanov DA, Skripal AV. Thermography‐based blood flow imaging in human skin of the hands and feet: a spectral filtering approach. Physiol Meas. 2017;38(2):272‐288. 10.1088/1361-6579/aa4eaf [DOI] [PubMed] [Google Scholar]
- 5. Kolosovas‐Machuca ES, Martínez‐Jiménez MA, Ramírez‐GarcíaLuna JL, et al. Pain measurement through temperature changes in children undergoing dental extractions. Pain Res Manage. 2016;2016:5. 10.1155/2016/4372617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Priest PC, Duncan AR, Jennings LC, Baker MG. Thermal image scanning for influenza border screening: results of an airport screening study. PLoS One. 2011;6(1):e14490. 10.1371/journal.pone.0014490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cho KS, Yoon J. Fever screening and detection of febrile arrivals at an international airport in Korea: association among self‐reported fever, infrared thermal camera scanning, and tympanic temperature. Epidemiol Health. 2014;36:e2014004. 10.4178/epih/e2014004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Udugama B, Kadhiresan P, Kozlowski HN, et al. Diagnosing COVID‐19: the disease and tools for detection. ACS Nano. 2020;14(4):3822‐3835. 10.1021/acsnano.0c02624 [DOI] [PubMed] [Google Scholar]
- 9. Ramirez‐GarciaLuna JL, Vera‐Bañuelos LR, Guevara‐Torres L, et al. Infrared thermography of abdominal wall in acute appendicitis: Proof of concept study. Infrared Phys Technol. 2020;105:103165. 10.1016/j.infrared.2019.103165 [DOI] [Google Scholar]
- 10. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35‐53. 10.1111/j.1365-2362.2009.02234.x [DOI] [PubMed] [Google Scholar]
- 11. Moreira DG, Costello JT, Brito CJ, et al. Thermographic imaging in sports and exercise medicine: A Delphi study and consensus statement on the measurement of human skin temperature. J Therm Biol. 2017;69:155‐162. 10.1016/j.jtherbio.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 12. Costela‐Ruiz VJ, Illescas‐Montes R, Puerta‐Puerta JM, Ruiz C, Melguizo‐Rodríguez L. SARS‐CoV‐2 infection: The role of cytokines in COVID‐19 disease. Cytokine Growth Factor Rev. 2020;54:62‐75. doi:10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID‐19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198‐209. 10.1111/his.14134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haddad DS, Brioschi ML, Baladi MG, Arita ES. A new evaluation of heat distribution on facial skin surface by infrared thermography. Dentomaxillofac Radiol. 2016;45(4):20150264. 10.1259/dmfr.20150264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rustemeyer J, Radtke J, Bremerich A. Thermography and thermoregulation of the face. Head Face Med. 2007;3:17. 10.1186/1746-160X-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. CDC . Communities, Schools Workplaces, Events. Centers for Disease Control and Prevention. Published April 30, 2020. Accessed August 28, 2020. https://www.cdc.gov/coronavirus/2019‐ncov/community/homeless‐shelters/screening‐clients‐respiratory‐infection‐symptoms.html.
- 17. Canada T. Temperature Screening for Air Travel. gcnws. Published June 12, 2020. Accessed August 28, 2020. https://www.canada.ca/en/transport‐canada/news/2020/06/temperature‐screening‐for‐air‐travel.html.
- 18. Zhou Y, Ghassemi P, Chen M, et al. Clinical evaluation of fever‐screening thermography: impact of consensus guidelines and facial measurement location. J Biomed Opt. 2020;25(9): 10.1117/1.JBO.25.9.097002 097002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alwashmi MF. The use of digital health in the detection and management of COVID‐19. Int J Environ Res Public Health. 2020;17(8): 10.3390/ijerph17082906 2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heymann DL, Shindo N. WHO scientific and technical advisory group for infectious hazards. COVID‐19: what is next for public health? Lancet. 2020;395(10224):542‐545. 10.1016/S0140-6736(20)30374-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kolosovas‐Machuca ES, González FJ. Distribution of skin temperature in Mexican children. Skin Res Technol. 2011;17(3):326‐331. 10.1111/j.1600-0846.2011.00501.x [DOI] [PubMed] [Google Scholar]
- 22. Christensen J, Vaeth M, Wenzel A. Thermographic imaging of facial skin—gender differences and temperature changes over time in healthy subjects. Dentomaxillofac Radiol. 2012;41(8):662‐667. 10.1259/dmfr/55922484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zouboulis CC, Nogueira da Costa A, Jemec GBE, Trebing D. Long‐wave medical infrared thermography: a clinical biomarker of inflammation in hidradenitis suppurativa/acne inversa. Dermatology. 2019;235(2):144‐149. 10.1159/000495982 [DOI] [PubMed] [Google Scholar]
- 24. Morales‐Cervantes A, Kolosovas‐Machuca ES, Guevara E, et al. An automated method for the evaluation of breast cancer using infrared thermography. EXCLI J. 2018;17:989‐998. 10.17179/excli2018-1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spalding SJ, Kwoh CK, Boudreau R, et al. Three‐dimensional and thermal surface imaging produces reliable measures of joint shape and temperature: a potential tool for quantifying arthritis. Arthritis Res Ther. 2008;10(1):R10. 10.1186/ar2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ortiz‐Dosal A, Kolosovas‐Machuca ES, Rivera‐Vega R, Simón J, González FJ. Use of infrared thermography in children with shock: a case series. SAGE Open Med Case Rep. 2014;2: 10.1177/2050313X14561779.2050313X14561779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martínez‐Jiménez MA, Ramirez‐GarciaLuna JL, Kolosovas‐Machuca ES, Drager J, González FJ. Development and validation of an algorithm to predict the treatment modality of burn wounds using thermographic scans: Prospective cohort study. PLoS One. 2018;13(11):e0206477. 10.1371/journal.pone.0206477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martínez‐Jiménez MA, Aguilar‐García J, Valdés‐Rodríguez R, et al. Local use of insulin in wounds of diabetic patients: higher temperature, fibrosis, and angiogenesis. Plast Reconstr Surg. 2013;132(6):1015e‐1019e. 10.1097/PRS.0b013e3182a806f0 [DOI] [PubMed] [Google Scholar]
- 29. Martínez‐Jiménez MA, Valadez‐Castillo FJ, Aguilar‐García J, et al. Effects of local use of insulin on wound healing in non‐diabetic patients. Plast Surg (Oakv). 2018;26(2):75‐79. 10.1177/2292550317740688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ring EFJ, Ammer K. Infrared thermal imaging in medicine. Physiol Meas. 2012;33(3):R33‐R46. 10.1088/0967-3334/33/3/R33 [DOI] [PubMed] [Google Scholar]
- 31. Belser JA, Rota PA, Tumpey TM. Ocular tropism of respiratory viruses. Microbiol Mol Biol Rev. 2013;77(1):144‐156. 10.1128/MMBR.00058-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loon S‐C, Teoh SCB, Oon LLE, et al. The severe acute respiratory syndrome coronavirus in tears. Br J Ophthalmol. 2004;88(7):861‐863. 10.1136/bjo.2003.035931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vabret A, Mourez T, Dina J, et al. Human coronavirus NL63, France. Emerg Infect Dis. 2005;11(8):1225‐1229. 10.3201/eid1108.050110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID‐19) affect the eyes? a review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28(3):391‐395. 10.1080/09273948.2020.1738501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holappa M, Vapaatalo H, Vaajanen A. Many faces of renin‐angiotensin system ‐ focus on eye. Open Ophthalmol J. 2017;11:122‐142. 10.2174/1874364101711010122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. La Distia NR, Putera I, Khalisha DF, Septiana I, Ridwan AS, Sitompul R. Are eyes the windows to COVID‐19? Systematic review and meta‐analysis. BMJ Open Ophthalmol. 2020;5(1):e000563. 10.1136/bmjophth-2020-000563 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Table S1‐S3


