Abstract
Background
Despite advances in critical care for acute respiratory distress syndrome (ARDS), some survivors in the acute phase are unable to wean from extracorporeal membrane oxygenation (ECMO) or mechanical ventilation. To date, little is known regarding whether lung transplantation confers a survival benefit for irreversible ARDS.
Methods
This retrospective study was conducted using the United Network for Organ Sharing database (May 2005–December 2018). Patients with restrictive lung disease were divided into two groups: patients with and without ARDS. Propensity score matching identified recipients without ARDS for the control group.
Results
A total of 63 patients with ARDS were waitlisted for lung transplantation, while 39 received a lung transplant after a median waitlist duration of 8 days. Seventy‐eight patients were matched as controls. In the ARDS group, the median age was 30 years, and the median lung allocation score was 88.4. Among the 39 recipients, 30 (76.9%) received ECMO support prior to transplantation. Lung transplantation for ARDS and restrictive lung disease showed similar 90‐day (87.2% vs. 88.5%, p = .80), 1‐year (82.1% vs. 85.9%, p = .52), and 3‐year (69.2% vs. 65.4%, p = .94) survival rates.
Conclusions
Lung transplantation provides acceptable outcomes in selected patients with irreversible ARDS.
Keywords: acute respiratory distress syndrome, extracorporeal membrane oxygenation, lung transplantation
1. INTRODUCTION
Acute respiratory distress syndrome (ARDS) is defined as a life‐threatening respiratory condition characterized by a change in compliance of the lungs resulting in hypoxia. Despite advances in clinical care, including lung‐protective ventilation strategies 1 , 2 and the advent of extracorporeal membrane oxygenation (ECMO), 3 , 4 regardless of etiology, the mortality rate of ARDS has remained at 30%–45%. 5 Regardless of successful recovery from a critical condition, patients with ARDS continue to develop long‐term respiratory insufficiency. 6 , 7
Much of the sustained respiratory insufficiency occurs once ARDS progresses to the fibroproliferative and then the fibrotic stage. Recovery from the disease process becomes slow and weaning from the ventilator and/or ECMO becomes challenging. This is evident with a reported increase in mortality rate in patients with ARDS who progress to the fibroproliferative and fibrotic stages, as seen on high‐resolution computed tomography (CT) by the presence of traction bronchiectasis or other signs of fibrotic changes. 8 , 9
As other treatment modalities, such as ECMO or prolonged mechanical ventilatory support, have been successful in the setting of ARDS, the use of lung transplantation remains relatively low. However, there remains a substantial number of patients who may benefit from a lung transplant, such as those with persistent ARDS who are unable to recover while on ECMO or mechanical ventilatory support due to devastating lung injuries, as well as patients with chronic pulmonary symptoms after recovery when bronchiectasis and pulmonary fibrosis persist on chest CT. 6 To date, the literature consists of 10 case reports 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 and 1 single‐center case series 20 primarily focusing on the use of lung transplantation as a life‐saving treatment in the setting of ARDS. The largest single‐center case series report from South Korea found a reasonable long‐term outcome after lung transplantation for ARDS with a 3‐year survival rate of 78%. 20 Due to the rarity of this clinical scenario, lung transplantation as the definitive treatment in patients with ARDS remains ill‐defined and controversial. Nevertheless, lung transplantation for ARDS, especially novel coronavirus 2019 (COVID‐19)‐associated ARDS attracted public attention. Therefore, we performed a retrospective analysis of the United Network for Organ Sharing (UNOS) database to report national outcomes following lung transplantation in patients with a diagnosis of ARDS.
2. PATIENTS AND METHODS
2.1. Data source
We performed a retrospective review of the UNOS database based on Organ Procurement and Transplantation Network (OPTN) data and identified all lung transplants performed between May 2005 and December 2018. The UNOS database contains all the relevant data of the organ donor and the transplant candidate/recipient for scientific and educational purposes. All patients waitlisted for lung transplantation with a diagnosis of ARDS as the primary indication at the time of evaluation (UNOS variable name “DIAG” in dataset) was registered as “ARDS/PNEUMONIA” in the UNOS database. This study conforms to the principles of the Declaration of Helsinki of 1975 and the Ethics Committee for Clinical Research at the University of Pittsburgh approved the study protocol (STUDY20050181). The UNOS dataset is deidentified, and thus informed consent was not required for this study.
2.2. Statistical methods
Univariate analyses were performed using the Mann–Whitney test for continuous variables and chi‐square test for categorical variables. Survival analysis was performed using the Kaplan–Meier method and the differences in survival outcomes between groups were compared using the log‐rank test. Propensity score matching was performed to identify lung transplant recipients without ARDS in group D of the UNOS database (restrictive lung disease) for the control group. Only recipients with complete data on the matching variables (lung allocation score [LAS], sex, age, transplant type) were included, which yielded 14 022 cases. Propensity matching was performed with the “MatchIt” program using nearest neighbor matching at a ratio of 2:1 for controls:cases. The ARDS status (yes/no) was regressed based on sex, lung allocation score at time of transplant, age, and transplant type to create the propensity score. Seventy‐eight control lung transplant recipients with restrictive disease were identified for 39 lung transplant recipients with ARDS. Propensity matching and survival analyses were performed using R (v. 3.6.2) and univariate tests were performed using SPSS (v. 27.0).
3. RESULTS
A total of 63 patients were identified as waitlisted for lung transplantation with a primary diagnosis of ARDS between May 2005 and March 2018. Thirty‐nine of these patients underwent lung transplantation which represents 0.15% (39/25 541) of all lung transplant recipients during the study period. Table 1 compares the baseline characteristics of patients with a primary diagnosis of ARDS who were transplanted compared to those who did not receive a transplant. While all other characteristics were similar between these two groups, patients with ARDS who received a transplant were older at the time of listing (median age 34.0 vs. 23.5, p < .027). Among the 24 patients who did not receive a transplant, 17 were removed from the waiting list due to a change in their clinical condition. Thirteen patients (54.2%) were removed because of their clinical deterioration or death; on the other hand, four patients (16.7%) were removed because of their clinical improvement.
TABLE 1.
Univariable comparison of characteristics between patients with ARDS who received a lung transplant and those who did not
| Characteristics | No transplant (n = 24) | Transplant (n = 39) | p |
|---|---|---|---|
| Age at listing | |||
| Median | 23.5 | 34.0 | .027 |
| Range | 2–62 | 11–67 | |
| Male sex, n (%) | 10 (41.7) | 21 (53.8) | .34 |
| LAS at removal | |||
| Median | 85.78 | 88.38 | .14 |
| Range | 0.00–92.24 | 45.61–91.51 | |
| Waitlist time (days) | |||
| Median | 25.5 | 8.0 | .006 |
| Range | 2–1637 | 1–669 | |
| BMI at removal | |||
| Median | 23.39 | 25.37 | .21 |
| Range | 15.5–32.8 | 14.0–33.4 | |
| Creatinine at removal | |||
| Median | 0.70 | 0.62 | .57 |
| Range | 0.34–1.40 | 0.10–4.24 | |
| ECMO at listing, n (%) | 16 (66.7) | 28 (71.8) | .67 |
| Ventilator at listing, n (%) | 14 (58.3) | 18 (46.2) | .35 |
| History of cigarette use, n (%) | 4 (16.7) | 10 (25.6) | .41 |
Abbreviations: ARDS, acute respiratory distress syndrome; BMI, body mass index; ECMO, extracorporeal membrane oxygenation; LAS, lung allocation score.
Table 2 includes the characteristics of lung transplant recipients in the ARDS and propensity‐matched control groups. The median age of the recipients with ARDS was 35 years (range 11–67 years), and 5 patients were less than 18 years old. Out of 39 patients, 18 were female and 21 were male. The median LAS at the time of an accepted offer in the ARDS group was 88.4 (range 45.6–91.5). The median waiting time from listing to transplantation was 8 days (range 1–669) in this cohort. At the time of transplantation, out of 39 lung transplant recipients, 30 patients with ARDS (76.9%) required ECMO support as a bridge to lung transplantation and 5 patients with ARDS (12.8%) were on mechanical ventilator support without ECMO support. Four patients (10.3%) received supplemental oxygen therapy without mechanical ventilatory support. Out of 30 patients with ARDS on ECMO, nine (30.0%) remained without mechanical ventilator support (ambulatory ECMO).
TABLE 2.
Patient characteristics comparing ARDS lung transplant recipients versus the control group
| Characteristics | Control (n = 78) | ARDS (n = 39) | p |
|---|---|---|---|
| Age (years) | |||
| Median | 33 | 35 | .87 |
| Range | 0–65 | 11–67 | |
| Male sex, n (%) | 36 (46.2) | 21 (53.8) | .43 |
| Median lung allocation score | |||
| Median | 89.4 | 88.4 | .16 |
| Range | 43.6–96.2 | 45.6–91.5 | |
| Waiting list time, days | |||
| Median | 34 | 8 | .00019 |
| Range | 1–4082 | 1–669 | |
| Body mass index (median) | 22.3 | 25.4 | .041 |
| Serum creatinine (median) | 0.70 | 0.64 | .46 |
| Serum total bilirubin (median) | 0.50 | 0.60 | .056 |
| ECMO bridge to transplantation (n, %) | 23 (29.5) | 30 (76.9) | <.0001 |
Data of the analysis comparing the characteristics of the 39 patients with ARDS and the 78 control patients. All p‐values are two‐sided and are based on Mann–Whitney tests (for continuous variables) or chi‐square tests (for categorical variables).
Abbreviations: ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation.
Table 3 includes the donor and surgical characteristics of all lung transplant recipients with a primary diagnosis of ARDS. Among 39 lung transplant recipients for ARDS, 36 (92.3%) received a double lung transplantation and 3 received single lung transplantation (7.7%). The median donor age was 30 years (range 10–63). The ischemic time was 6.3 ± 2.1 h. In‐hospital mortality occurred in four patients (10.3%). The cause of death was primary graft dysfunction (n = 1), ventricular failure (n = 1), multiorgan failure (n = 1), and pulmonary embolism with small bowel ischemia (n = 1). Eight patients (20.5%) required renal replacement therapy after transplantation. The median length of hospitalization after transplantation was 33 days, while the median follow‐up period was 24.4 months. The 90‐day, 1‐, and 3‐year survival rates were 87.2%, 82.1%, and 69.2%, respectively (Figure 1). There were no significant differences in the donor or surgical characteristics or post‐transplant outcome between lung transplant recipients with ARDS who required ECMO support as a bridge to transplantation versus those who did not require ECMO support, with the exception of in‐hospital mortality (Table 4). In‐hospital mortality was significantly higher in the patients who did not receive ECMO support as a bridge to lung transplantation (p = .032). However, the Kaplan–Meier survival analysis did not find any statistical difference between these two groups (Figure 2). Table 5 includes a comparison of characteristics between the patients who survived and those who were deceased at 1 year; patients who were deceased had a significantly higher serum creatinine concentration at transplantation than those who survived (p = .05).
TABLE 3.
Donor and surgical characteristics as well as postoperative outcomes of patients in the acute respiratory distress syndrome and control groups
| Characteristics | Control (n = 78) | ARDS (n = 39) | p |
|---|---|---|---|
| Donor age (years) | |||
| Median | 27 | 30 | .24 |
| Range | 0–61 | 10–63 | |
| Donor cigarette history (n, %) | 5 (6.4) | 3 (7.7) | .59 |
| Donor CDC high risk (n, %) | 11 (14.1) | 7 (17.9) | .59 |
| Ischemic time (hours) (mean, SD) | 5.9 (1.8) | 6.3 (2.1) | .60 |
| Length of stay (days) (median, IQR) | 27 (24) | 33 (36) | .09 |
| Transplant type | |||
| Single (n, %) | 2 (2.6) | 3 (7.7) | .20 |
| Double (n, %) | 76 (97.4) | 36 (92.3) | |
| Dialysis post‐transplant (n, %) | 13 (16.7) | 8 (20.5) | .42 |
| Airway dehiscence (n, %) | 0 | 0 (2.6) | .16 |
| Survival time (days) (median, IQR) | 736 (1195) | 583 (1648) | .50 |
| In‐hospital mortality | 7 (9.0) | 4 (10.3) | 1.00 |
Abbreviations: ARDS, acute respiratory distress syndrome; CDC, The Centers for Disease Control and Prevention; IQR, interquartile range; SD, standard deviation.
FIGURE 1.
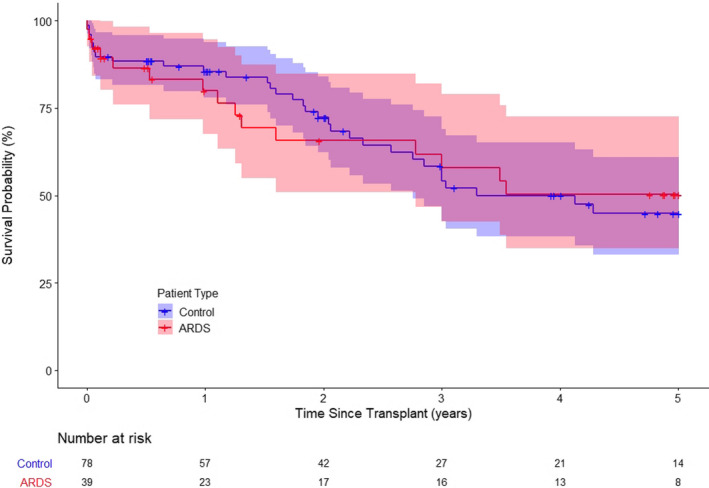
Kaplan–Meier analysis showing no significant difference between the ARDS group and the propensity‐matched control group in terms of survival rate (3‐year survival; χ2(1) = 0.005, p = .94:5‐year survival; χ2(1) = 0.008, p = .93)
TABLE 4.
Extracorporeal membrane oxygenation versus no extracorporeal membrane oxygenation bridge data of patients with acute respiratory distress syndrome
| Characteristics | No‐ECMO, (n = 9) | ECMO, (n = 30) | p |
|---|---|---|---|
| Age (years) (mean, SD) | 48.7 (17.2) | 34.7 (14.7) | .10 |
| Range | 17–67 | 11–63 | |
| Sex (male) (n, %) | 5 (55.6) | 16 (53.3) | .91 |
| LAS at transplant (mean, SD) | 76.2 (20.7) | 86.3 (27.7) | .52 |
| Waitlist time (days) (median, IQR) | 7 (15) | 8 (17) | .73 |
| Body mass index (kg/m2) (mean, SD) | 25.6 (3.4) | 25.1 (5.1) | .81 |
| Serum creatinine (mean, SD) | 1.20 (1.40) | 0.72 (0.54) | .06 |
| Serum total bilirubin (mean, SD) | 0.59 (0.33) | 0.94 (0.96) | .71 |
| Ischemic time (hours) (mean, SD) | 5.6 (0.72) | 6.4 (2.30) | .30 |
| Transplant type | |||
| Single (n, %) | 2 (22.2) | 1 (3.3) | .13 |
| Double (n, %) | 7 (77.8) | 29 (96.7) | |
| Length of stay (days) (median, IQR) | 26.0 (45) | 37.5 (34) | .074 |
| Dialysis post‐transplant (n, %) | 1 (11.1) | 7 (23.3) | .60 |
| Survival time (days) (median, IQR) | 583 (2167) | 597 (1625) | .68 |
| In‐hospital mortality (n, %) | 3 (33.3) | 1 (3.3) | .032 |
Abbreviations: ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; LAS, lung allocation score; SD, standard deviation.
FIGURE 2.
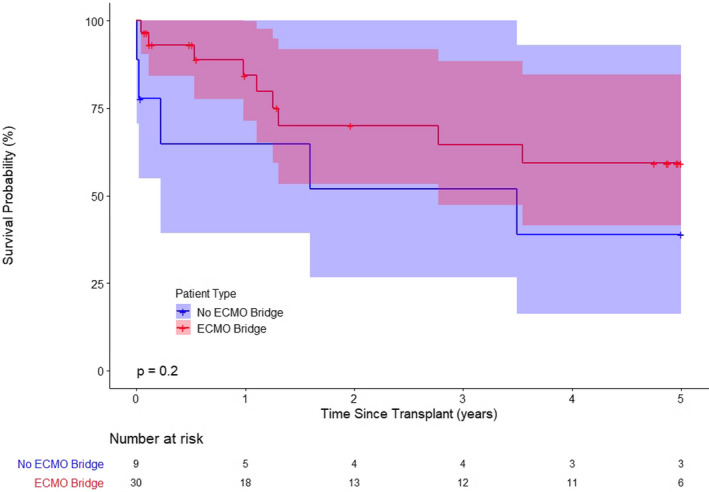
Kaplan–Meier analysis showing no significant differences in survival between ARDS recipients under extracorporeal membrane oxygenation (ECMO) bridge to transplantation and ARDS recipients without ECMO support (1‐year survival: χ2(1) = 2.40, p = .12; 3‐year survival: χ2(1) = 1.02, p = .31; 5‐year survival: χ2(1) = 1.67, p = .20)
TABLE 5.
Comparison of characteristics between patients who survived and those who were deceased at 1 year
| Characteristics | Survived, (n = 23) | Deceased, (n = 7) | p |
|---|---|---|---|
| Age (years) | |||
| Median (IQR) | 30.0 (30.0) | 38.0 (22.5) | .5 |
| Range | 11–60 | 13–67 | |
| Male sex, n (%) | 10 (43.5) | 7 (100.0) | .008 |
| LAS at transplant | |||
| Median (IQR) | 87.13 (8.7) | 88.99 (1.7) | .13 |
| Range | 46.3–90.9 | 84.13–91.0 | |
| Waitlist time (days) | |||
| Median (IQR) | 7 (16) | 11 (35) | .74 |
| Range | 2–249 | 1–73 | |
| BMI at transplant | |||
| Median (IQR) | 25.1 (4.8) | 24.6 (10.2) | .74 |
| Range | 14.0–33.4 | 18.3–30.0 | |
| Creatinine at transplant | |||
| Median (IQR) | 0.6 (0.3) | 0.7 (0.4) | .05 |
| Range | 0.1–2.15 | 0.5–4.24 | |
| ECMO at listing, n (%) | 17 (73.9) | 4 (57.1) | .4 |
| Ventilator at listing, n (%) | 12 (52.3) | 3 (42.9) | .67 |
| History of cigarette use, n (%) | 3 (13.0) | 0 (0.0) | .31 |
Seven patients had not yet reached 1 year of follow‐up and were excluded from the analysis.
Abbreviations: BMI, body mass index; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; LAS, lung allocation score.
When compared to the propensity‐matched control group with restrictive lung disease, the ARDS group had more recipients who required ECMO support as a bridge to transplantation (29.5% vs. 76.9%, p < .0001) even though the control group was matched using the LAS score. The median body mass index was higher in the ARDS group (22.3 vs. 25.4, p = .041), and the median waitlist time was significantly shorter (34 vs. 8 days, p = .00019). There was no difference in the post‐transplant outcome between the groups. Lung transplantation for ARDS and restrictive lung disease had similar 90‐day (87.2% vs. 88.5%, p = .80), 1‐year (82.1% vs. 85.9%, p = .52), and 3‐year (69.2% vs. 65.4%, p = .94) survival rates (Figure 1). The survival outcome of patients who required ECMO as a bridge to lung transplantation was comparable between the ARDS and control group (Figure 3).
FIGURE 3.
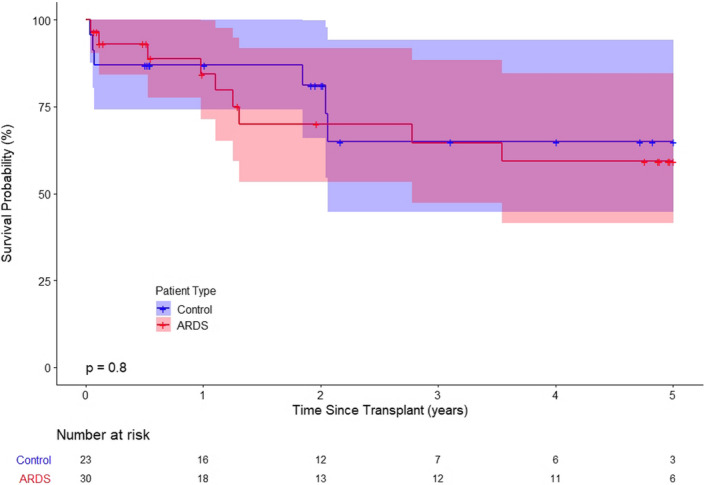
Kaplan–Meier analysis showing no significant difference in post‐transplant survival after extracorporeal membrane oxygenation (ECMO) bridge to lung transplantation between ARDS recipients and propensity‐matched controls (1‐year survival: χ2(1) = 0.00, p = .98; 3‐year survival: χ2(1) = 0.00, p = .93; 5‐year survival: χ2(1) = 0.06, p = .80)
4. DISCUSSION
Lung transplantation can be the definitive treatment option for patients with irreversible ARDS when their disease progresses from the fibroproliferative to the fibrotic stage, with comparable outcomes to other restrictive lung diseases. In our analysis of lung transplantation in patients with ARDS in the United States, even in a group predominately consisting of patients who require ECMO as a bridge to transplantation, outcomes remained acceptable with a 10.3% in‐hospital mortality and 82.1% 1‐year survival rate.
To date, there is no published data that summarizes the outcome of lung transplantation for ARDS using the UNOS database. There are 10 published case reports 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 and 1 case series 20 regarding lung transplantation for patients with ARDS, including 1 case of H1N1 influenza, 12 1 of Paraquat‐induced lung injury, 13 and 5 of inhalation lung injury. 20 Increasing case reports are being published regarding COVID‐19‐associated ARDS. 17 , 18 , 19 Historically, lung transplantation for ARDS has been offered to relatively young recipients before the emergence of COVID‐19. The recipient age of reported cases prior to cases of COVID‐19 were 35.7 ± 13.9 years. 10 , 11 , 12 , 13 , 14 , 15 , 16 As for COVID‐19‐associated ARDS, published cases are older (62.8 ± 9.6 years). 17 , 18 , 19 Similar to our study, previously reported recipients required ECMO support as a bridge to transplantation in 20 out of 23 cases (87.0%). 10 , 11 , 12 , 20 The survival outcome reported by Chang and colleagues was similar to that observed in this study, and the 1‐year survival rate post‐transplantation was reported as 78.0%. 20 In addition to these published case reports and series, lung transplantation procedures for vaping‐related ARDS and COVID‐19‐associated ARDS have been reported by the media. Lung transplantation has been indicated for ARDS as the last resort to manage these current public health emergencies. The main issue related to transplantation in these populations is the lack of information regarding long‐term survival because these reports are anecdotal and mainly focused on immediate surgical outcomes.
According to a previous study analyzing all adult lung transplant recipients in the UNOS registry data between 2005 and 2015, the mean lung transplant recipient age was 53.6 ± 14.1 years. 21 The OPTN/Scientific Registry of Transplant Recipients (SRTR) 2017 Annual Data Report found the median LAS of lung transplant recipients at the time of transplantation to be 40.3. Recipients with ARDS in this study tended to be younger (34.4 ± 17.4 years) and sicker (LAS 84.1 ± 10.0) than recipients with other diagnoses. The proportion of lung recipients who required mechanical ventilation and ECMO was 6.0% and 5.2% in the OPTN/SRTR annual report, 22 whereas in our cohort mechanical ventilation and ECMO were required in 76.9% and 12.8% of patients with ARDS. Although the control group was propensity‐matched using the LAS score, the ARDS group had a significantly larger number of patients on ECMO prior to transplantation (29.5% vs. 76.9%, p < .0001). Additionally, when compared to the propensity‐matched control group, the ARDS group had a significantly shorter waiting time for transplantation. These results suggest that, while the LAS score has improved the allocation of available organs, it does not fully depict the course of disease. These findings highlight the more fulminant nature of ARDS compared to other restrictive lung diseases.
Out of 63 patients with a primary diagnosis of ARDS who were placed on the waiting list for a lung transplantation, 24 (38.1%) were de‐listed after the median waitlist duration of 25.5 days. The decision for de‐listing occurred relatively quick after the initial listing. This is likely because 44 patients (65.7%) were on ECMO at the time of listing. A single‐center experience of ECMO as a bridge to lung transplantation reported the median duration from ECMO to de‐listing as 14 days, while the median duration from ECMO to transplant/death was 12 days. 23 The main challenge with listing patients with ARDS for lung transplantation is the balance between allowing these patients to recover from their insult versus listing them at an appropriate time to allow for good outcomes following transplantation. This balance is very challenging due to the lack of consensus regarding this clinical decision, as well as the limited time in which these patients may become too clinically unstable for transplantation. The fact that 4 (6.3%) among 63 listed patients were de‐listed because of clinical improvement also highlights the difficulty in judging who should be listed for a lung transplantation as a result of ARDS. Most of the recipients of a single lung transplant in this study were patients who were able to wean from ECMO or mechanical ventilation. In these cases, allowing enough time for lung recovery may enable the option of a single lung transplantation.
The survival benefit of lung transplantation for ARDS is currently unknown. Previous case reports do not include long‐term outcomes and this report is the first to summarize long‐term outcome post‐lung transplantation for ARDS using the UNOS database. The survival outcomes of the ARDS group were comparable to that of the propensity‐matched control group, despite the fact that the overwhelming majority of these patients (76.9%) required preoperative ECMO as a bridge to transplantation and 12.8% received mechanical ventilation without ECMO support at the time of transplantation. This is particularly intriguing as the outcomes of ECMO as a bridge to lung transplantation, which is one of the biggest risk factors for early mortality, were reported to be 57%–93% at 12 months 23 , 24 , 25 , 26 , 27 , 28 and 62%–80% at 3 years. 25 , 26 , 28 However, in this study, there were more in‐hospital mortalities post‐lung transplant in patients who did not receive ECMO support. Additionally, a higher creatinine concentration at the time of transplantation was found to be a significant risk factor for 1‐year mortality. While, more evidence is needed to further support lung transplantation in patients with ARDS, this study suggests transplantation is an effective and uncompromising treatment in the setting of ARDS.
This study has several limitations. The first is the small number of patients in the ARDS cohort. While this limits our generalizability, our limited cohort likely represents the national lung transplant ARDS cohort as reported by a widely used national database. Second, the variables we could examine were limited to those included in the UNOS database. The database does not include all the clinically relevant parameters, for example, the etiology of ARDS or severity of fibrosis, which may be helpful to identify the indications and contraindications. Finally, with the UNOS database, the lack of data regarding various key clinical variables such as the date of onset, intubation, or initiation of ECMO limits our ability to offer insight regarding the best timing to consider lung transplantation in patients with ARDS.
In conclusion, the current study demonstrates that the use of lung transplantation in the setting of end‐stage lung disease secondary to ARDS can provide a reasonable survival benefit when compared to alternative therapies. Trends show that national practices favor the use of lung transplantation in younger recipients who are in critical condition requiring ECMO and/or mechanical ventilation support. A more detailed review is required regarding best practices in order to maximize long‐term survival and allograft function.
5. DISCLAIMER
The data reported here have been supplied by UNOS as the contractor for the OPTN. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or an interpretation by the OPTN or the US Government.
AUTHOR CONTRIBUTIONS
TH participated in the research design, writing of the paper, performance of the research, and data analysis; JPR participated in the writing of the paper, performance of the research, and data analysis; EGC participated in the writing of the paper and performance of the research; KN participated in the performance of the research and data analysis; MRM participated in the research design and writing of the paper; JDL participated in the research design; PGS participated in the research design, writing of the paper, and performance of the research.
ACKNOWLEDGEMENT
The authors would like to thank Editage (http://www.editage.com) for language editing and proofreading.
Harano T, Ryan JP, Chan EG, et al. Lung transplantation for the treatment of irreversible acute respiratory distress syndrome. Clin Transplant.2021;35:e14182. 10.1111/ctr.14182
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Organ Procurement and Transplantation Network (OPTN) data. Data are available from the authors with the permission of the United Network for Organ Sharing.
REFERENCES
- 1. Brower RG, Matthay MA, Morris A, et al. Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 2. Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2013; 10.1002/14651858.CD003844.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. [DOI] [PubMed] [Google Scholar]
- 4. Combes A, Hajage D, Capellier G, et al. EOLIA Trial Group, REVA, and ECMONet. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. [DOI] [PubMed] [Google Scholar]
- 5. Maca J, Jor O, Holub M, et al. Past and present ARDS mortality rates: a systematic review. Respir Care. 2017;62:113–122. [DOI] [PubMed] [Google Scholar]
- 6. Herridge MS, Tansey CM, Matté A, et al. Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. [DOI] [PubMed] [Google Scholar]
- 7. Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long‐term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung JH, Kradin RL, Greene RE, Shepard JO, Digumarthy SR. CT predictors of mortality in pathology confirmed ARDS. Eur Radiol. 2011;21:730–737. [DOI] [PubMed] [Google Scholar]
- 9. Ichikado K, Suga M, Muranaka H, et al. Prediction of prognosis for acute respiratory distress syndrome with thin‐section CT: validation in 44 cases. Radiology. 2006;238:321–329. [DOI] [PubMed] [Google Scholar]
- 10. Turner DA, Rehder KJ, Bonadonna D, et al. Ambulatory ECMO as a bridge to lung transplant in a previously well pediatric patient with ARDS. Pediatrics. 2014;134:e583–e585. [DOI] [PubMed] [Google Scholar]
- 11. Brichon PY, Barnoud D, Pison C, Perez I, Guignier M. Double lung transplantation for adult respiratory distress syndrome after recombinant interleukin 2. Chest. 1993;104:609–610. [DOI] [PubMed] [Google Scholar]
- 12. Wang Q, Pan S, Zhang S, Shen G, Huang M, Wu M. Lung transplantation in pulmonary fibrosis secondary to influenza A pneumonia. Ann Thorac Surg. 2019;108:e233–e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Licker M, Schweizer A, Hohn L, Morel DR, Spiliopoulos A. Single lung transplantation for adult respiratory distress syndrome after paraquat poisoning. Thorax. 1998;53:620–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iacono A, Groves S, Garcia J, Griffith B. Lung transplantation following 107 days of extracorporeal membrane oxygenation. Eur J Cardiothorac Surg. 2010;37:969–971. [DOI] [PubMed] [Google Scholar]
- 15. Salam S, Kotloff R, Garcha P, et al. Lung transplantation after 125 days on ECMO for severe refractory hypoxemia with no prior lung disease. ASAIO J. 2017;63:e66–e68. [DOI] [PubMed] [Google Scholar]
- 16. Jurmann MJ, Schaefers HJ, Demertzis S, Haverich A, Wahlers T, Borst HG. Emergency lung transplantation after extracorporeal membrane oxygenation. ASAIO J. 1993;39:M448–M452. [DOI] [PubMed] [Google Scholar]
- 17. Han W, Zhu M, Chen J, et al. Lung transplantation for elderly patients with end‐stage COVID‐19 pneumonia. Ann Surg. 2020;272:e33–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen JY, Qiao K, Liu F, et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019‐related pulmonary fibrosis. Chin Med J (Engl). 2020;133:1390–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lang C, Jaksch P, Hoda MA, et al. Lung transplantation for COVID‐19‐associated acute respiratory distress syndrome in a PCR‐positive patient. Lancet Respir Med. 2020;8:1057–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang Y, Lee SO, Shim TS, et al. Lung transplantation as a therapeutic option in acute respiratory distress syndrome. Transplantation. 2018;102:829–837. [DOI] [PubMed] [Google Scholar]
- 21. Roy BS, Alarcon D, Walia R, Chapple KM, Bremner RM, Smith MA. Is there an age limit to lung transplantation? Ann Thorac Surg. 2015;100:443–451. [DOI] [PubMed] [Google Scholar]
- 22. Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2017 annual data report: lung. Am J Transplant. 2019;19:404–484. [DOI] [PubMed] [Google Scholar]
- 23. Biscotti M, Gannon WD, Agerstrand C, et al. Awake extracorporeal membrane oxygenation as bridge to lung transplantation: a 9‐year experience. Ann Thorac Surg. 2017;104:412–419. [DOI] [PubMed] [Google Scholar]
- 24. Toyoda Y, Bhama JK, Shigemura N, et al. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J Thorac Cardiovasc Surg. 2013;145:1065–1071. [DOI] [PubMed] [Google Scholar]
- 25. Bermudez CA, Rocha RV, Zaldonis D, et al. Extracorporeal membrane oxygenation as a bridge to lung transplant: midterm outcomes. Ann Thorac Surg. 2011;92:1226–1232. [DOI] [PubMed] [Google Scholar]
- 26. Hoopes CW, Kukreja J, Golden J, Davenport DL, Diaz‐Guzman E, Zwischenberger JB. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg. 2013;145:862–868. [DOI] [PubMed] [Google Scholar]
- 27. George TJ, Beaty CA, Kilic A, Shah PD, Merlo CA, Shah AS. Outcomes and temporal trends among high‐risk patients after lung transplantation in the United States. J Heart Lung Transplant. 2012;31:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shafii AE, Mason DP, Brown CR, et al. Growing experience with extracorporeal membrane oxygenation as a bridge to lung transplantation. ASAIO J. 2012;58:526–529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Organ Procurement and Transplantation Network (OPTN) data. Data are available from the authors with the permission of the United Network for Organ Sharing.


