Abstract
Mesenchymal stem cells (MSCs) have a very low survival rate after in vivo delivery, which limits their great promise for treating human diseases. Various strategies have been studied to overcome this challenge. However, an overlooked but important potential is to apply exogenous signaling molecules as biochemical cues to promote MSC survival, presumably because it is well-known that MSCs themselves can release a variety of potent signaling molecules. Thus, the purpose of this work was to examine and understand whether the release of exogenous signaling molecules from hydrogels can promote the survival of MSC spheroids. Our data show that more vascular endothelial growth factor (VEGF) but not platelet-derived growth factor BB (PDGF-BB) were released from MSC spheroids in comparison with 2D cultured MSCs. Aptamer-functionalized fibrin hydrogel (aFn) could release exogenous VEGF and PDGF-BB in a sustained manner. PDGF-BB-loaded aFn promoted MSC survival by ~70% more than VEGF-loaded aFn under the hypoxic condition in vitro. Importantly, PDGF-BB-loaded aFn could double the survival rate of MSC spheroids in comparison with VEGF-loaded aFn during the one-week test in vivo. Therefore, this work demonstrated that defined exogenous signaling molecules (e.g., PDGF-BB) can function as biochemical cues for promoting the survival of MSC spheroids in vivo.
Keywords: hydrogel, growth factor, cell delivery, stem cell, spheroid
Graphical Abstract

1. INTRODUCTION
A growing body of evidence has shown that mesenchymal stem cells (MSCs) can release a variety of signaling molecules (e.g., vascular endothelial growth factor (VEGF)).1–3 These signaling molecules are highly potent with the ability to stimulate and regulate neighboring cells to grow or differentiate through paracrine signaling, which may be the most important reason accounting for the in vivo therapeutic effects of MSCs.4,5 However, as MSCs are the source of signaling molecules, these molecules will disappear with the death of MSCs. Therefore, while MSCs have been widely studied for biomedical applications such as regenerative medicine and cancer therapy,6–9 the ultimate success of these MSC-based applications really depends on the survival of MSCs.
Both preclinical and clinical studies have shown that MSCs have a very low survival rate after in vivo delivery.10–13 For instance, previous studies have shown that <1% of MSCs survived in the myocardium at day 4 after the in vivo injection,10 and <5% of MSCs survived at day 5 after delivered to the skin wound.14 Multiple possible reasons account for this low cell survival, including hostile survival environment in tissue defects, mechanical stress with injection, low cell retention in the injection sites, and so forth. To improve the survival of MSCs, numerous methods have been studied, including genetic engineering, cell surface functionalization, codelivery with other agents such as hydrogels, and alternation of in vitro cell culture conditions before in vivo delivery.15–19 While these methods have shown some promise, low MSC survival remains a challenging hurdle.20,21
In most MSC studies, MSCs are expanded in 2D, trypsinized from cell-culture plates, and finally delivered as dissociated cells. As cells usually exist in a three-dimensional space with close cell–cell interactions, recent attention has been paid to the development and delivery of MSC spheroids that allow close cell–cell interactions.22–24 Numerous methods can be applied to develop MSC spheroids, including centrifugation, hanging-drop aggregation, suspension in spinner flasks, and spontaneous aggregation on ultralow attachment surfaces.23,25
Several studies have already suggested that MSC spheroids are superior to dissociated MSCs in maintaining cell survival.25–27 For instance, Bhang et al. found that human cord blood MSCs had a higher in vivo survival rate in the ischemic hindlimb of mice when transplanted as spheroids than single MSCs without aggregation.27 Thus, MSC spheroids would be a better option than dissociated MSCs for in vivo MSC delivery.
Although studies have shown that MSCs themselves can release signaling molecules and they can survive better in the form of spheroids, there is a fundamental question remaining elusive, that is, whether exogenous signaling molecules can promote the survival of MSC spheroids. Presumably because MSCs can release potent signaling molecules, this question has caught very little attention. The purpose of this study was to address this question by codelivering MSC spheroids and exogenous signaling molecules in hydrogels. We hypothesized that a sustained supply of defined exogenous signaling molecules would promote the in vivo survival of MSC spheroids. To test this hypothesis, we synthesized aptamer-functionalized fibrin hydrogels and examined MSC viability in these hydrogels. We further examined MSC survival by subcutaneously implanting the hydrogels loaded with MSC spheroids and exogenous signaling molecules into mice.
2. MATERIALS AND METHODS
2.1. Materials.
Acrylic acid N-hydroxysuccinimide ester (AANHS), sodium bicarbonate (NaHCO3), calcium chloride (CaCl2), tris(2-carboxy ethyl) phosphine hydrochloride (TCEP), and 4% paraformaldehyde solution were purchased from Sigma-Aldrich (St. Louis, MO). Phosphate buffered saline (PBS), Dulbecco’s phosphate buffered saline (DPBS), calcein AM, Live/Dead cell viability assay, bovine serum albumin (BSA), fetal bovine serum (FBS), 0.05% trypsin—EDTA, Dulbecco’s Modified Eagle Medium with GlutaMAX (DMEM), xylene substitute mountant, SlowFade Diamond Antifade Mountant with DAPI, goat antirabbit IgG-Alexa Fluor 546 antibody, α-smooth muscle actin antibody-Alexa Fluor 488 antibody (α-SMA), goat anti-mouse IgG- Alexa Fluor 647 antibody, mouse antihuman STRO1 antibody, and mouse antihuman CD90-FITC were obtained from Thermo-Fisher Scientific (Waltham, MA). Aptamers and complementary sequences were obtained from Integrated DNA Technologies (Coralville, IA). Human thrombin and fibrinogen (Fg) were obtained from Millipore (Billerica, MA). Recombinant human vascular endothelial growth factor-165 (VEGF), VEGF enzyme-linked immunosorbent assay (ELISA) kit, recombinant human platelet-derived growth Factor-BB (PDGF-BB), PDGF-BB ELISA kit, recombinant human basic fibroblast growth factor (bFGF), and bFGF ELISA kit were obtained from PeproTech (Rocky Hill, NJ). Rabbit antimouse CD31 primary antibody was obtained from Cell Signaling Technology (Beverly, MA). CellTiter 96 aqueous one solution cell proliferation assay kit (MTS) was purchased from Promega (Madison, WI). H&E stain kit was obtained from Leica Biosystems (Buffalo Grove, IL). Human bone marrow MSCs were obtained from Thermo-Fisher Scientific (Waltham, MA). Red fluorescent protein (RFP) expressing human bone marrow mesenchymal stem cells (RFP-MSCs) and mesenchymal stem cell growth medium (MSCGM) were obtained from Angio-Proteomie (Boston, MA).
2.2. Methods.
2.2.1. Cell Culture.
Human MSCs and RFP-MSCs were expanded in growth media (DMEM supplemented with 5% FBS for MSCs and MSCGM for RFP-MSCs) in a humidified atmosphere at 37 °C with 5% CO2. For both cell types, the medium was changed every other day and the cells were harvested once they reached 80% confluency. MSCs or RFP-MSCs of passage 3 to 8 were used.
2.2.2. Formation of Cell Spheroids.
Cells were detached using 0.05% Trypsin-EDTA, pelleted by centrifugation at 200 g for 5 min at 37 °C and resuspended in growth media. The density of cells was adjusted to 5 × 105 cells per mL. The cell suspension of 2 mL was added to each well of the 6-well ultralow attachment cell culture plate and incubated overnight in a cell culture incubator. After the formation of spheroids, the growth media were removed and the spheroids were washed two times with DPBS to remove the dead cells. Then the spheroids were either cultured in the growth medium or used for subsequent experiments. Images of the cells before and after formation of spheroids were taken with an optical microscope (Olympus IX73, Center Valley, PA).
2.2.3. Quantification of the Cells.
Spheroids in 500 μL of growth medium were pipetted up and down gently. The spheroid solution (100 μL) was added to a cover glass and the density of the spheroid was quantified under an optical microscope (Olympus IX73, Center Valley, PA). The remaining 400 μL of spheroids was pelleted and the spheroids were washed with DPBS two times. Then the spheroids were treated with 1 mg/mL collagenase solution at 37 °C for 15 min. After the dissociation of spheroids, the cells were stained with Trypan blue and the number of cells was quantified with a hemocytometer. The average cell number per spheroid was quantified by dividing the total cells with the total number of spheroids. Spheroids synthesized from three different batches were quantified to check the rigor in the formation of MSC spheroids.
2.2.4. Staining of Spheroids.
Spheroids were frozen in O.C.T. compound after being washed with DPBS two times. The frozen spheroids were cryo-sectioned into slices with a thickness of 7 μm. The hematoxylin and eosin (H&E) staining was performed with a Leica autostainer (Buffalo Grove, IL). For the immunostaining of CD90 and STRO1, sectioned spheroids were blocked with 2% BSA in PBS at room temperature for 2 h. The blocked samples were incubated with the primary antibody (1:200 dilution) at 4 °C overnight followed by incubation with the corresponding secondary antibody (1:200 dilution) for 2 h at room temperature. After being washed with DPBS three times, the samples were mounted into SlowFade Diamond Antifade Mountant with DAPI and imaged with a fluorescence microscope (Olympus IX73, Center Valley, PA).
2.2.5. Examination of Growth Factor Release from Spheroids.
MSC spheroids were cultured in an ultralow attachment plate with a density of 400 spheroids per well. The same number of dispersed cells were cultured on a normal six-well cell culture plate. After the spheroids and dispersed cells were incubated with cell growth media for 2 days, 1 mL of the cell growth medium was collected and stored at −20 °C for ELISA analysis to examine the levels of VEGF, PDGF-BB, and bFGF according to the protocol provided by the manufacturer.
2.2.6. Culture of MSCs with Exogenous Growth Factors.
MSCs were seeded into a 24-well cell culture plate at the density of 104 cells per mL in the growth medium (DMEM with 5% FBS). After 12 h, the growth medium was replaced by the low serum medium (DMEM supplemented with 0.5% FBS). In another 12 h, the low serum medium was changed into treatment medium (DMEM supplemented with 0.5% FBS and different concentrations of growth factors). The optical images of the cells were taken at different stages. MTS assay was also performed 5 days after cells were cultured in the treatment medium according to the protocol provided by the manufacturer.
2.2.7. Synthesis of Aptamers-Functionalized Fibrin Hydrogel (aFn).
Aptamer-functionalized Fg (aFg) was synthesized as previously reported.28 Briefly, acrylate modified Fg (acrylate-Fg) was synthesized by reacting native Fg with NHS-acrylate. Thiol-modified anti-VEGF aptamer or thiol-modified anti-PDGF-BB aptamer was treated with 50 mM of TCEP and purified with a 100 kDa filter. The treated aptamer was reacted with acrylate-Fg to form aFg that was further purified by filtering with a 100 kDa molecular filter. For the synthesis of aFn, native Fg was first mixed with aFg. This mixture was induced to form aFn in the presence of an equal volume of thrombin solution (20 U/mL). The final concentration of Fg was 10 mg/mL. The final concentrations of anti-VEGF aptamers and anti-PDGF-BB aptamers were 0.5 μM and 0.4 μM, respectively, unless otherwise specified. Native Fn with 10 mg/mL of Fg and 1 U/mL thrombin were used as a control. For the synthesis of growth factors and/or cells loaded with aFn, growth factors and/or cells were premixed with the native Fg solution before the formation of aFn.
2.2.8. Fluorescence Imaging of aFn.
aFn was fixed in a 4% paraformaldehyde solution, washed with PBS, stained with the fluorophore-labeled complementary sequence of the aptamers, and washed with 0.1 M NaHCO3 solution. The stained hydrogels were imaged with the Maestro In Vivo Imaging System (CRI, Woburn, MA)
2.2.9. Scanning Electron Microscopy.
Native Fn and aFn hydrogels were fixed in 4% paraformaldehyde solution and lyophilized in a freeze-dryer (Labconco, Kansas City, MO). After lyophilization, samples were coated with yttrium and imaged with a scanning electron microscope (Zeiss Sigma, U.S.A.).
2.2.10. Measurement of Dextran Retention.
Fn and aFn were soaked into a FITC-dextran solution (20 μg/mL) for 48 h to allow uniform loading. The FITC-dextran loaded hydrogels were incubated with PBS supplemented with 1% BSA under 37 °C. After 24 h, the fluorescence of the solution was measured with a nanodrop (2000C, Thermal Fisher Scientific, Waltham, MA) and the amount of released dextran was quantified by comparing with a standard FITC-dextran solution.
2.2.11. Measurement of Growth Factor Retention.
To synthesize aFn loaded with growth factors, native Fg was mixed with aFg together with 100 ng of VEGF and 100 ng of PDGF-BB. The mixture was combined with an equal volume of thrombin solution (20 U/mL) at 37 °C for 1 h to induce the formation of aFn. The growth factor loaded hydrogels were then incubated in 1 mL of release medium (DMEM supplemented with 0.1% BSA). After 24 h, the release medium was collected and the amount of growth factors in the release medium was quantified with ELISA. The retention of growth factors in aFn was calculated by subtracting the amount of released growth factors from the total amount of loading growth factors.
2.2.12. Evaluation of Sustained Release of Growth Factors.
aFn loaded with growth factors was incubated in the release media at 37 °C. At predetermined time points, the release medium was collected and replenished with 1 mL of new release medium. The collected release media were stored at −80 °C freezer. The concentrations of growth factors in the release media were measured using ELISA kits.
2.2.13. Cell Culture in aFn.
MSC spheroids (100 spheroids) or dispersed MSCs (2 × 105 cells) were mixed with aFg before the synthesis of aFn. The total volume of aFn was 100 μL. After allowing aFn to solidify at 37 °C for 1 h, 0.5 mL of growth medium was added to each well. Dispersed MSCs and MSC spheroids were cultured for 1 day and 5 day in a standard cell culture incubator. Then the dispersed MSCs and the spheroids were stained with Live/Dead assay for 30 min. The stained samples were imaged with a fluorescence microscope using a z-stack protocol (Olympus IX73, Center Valley, PA). Images were projected on the xy-plane over the same z-height.
To test cell survival under hypoxic stress (no oxygen perfusion), 0.5 mL of DMEM supplemented with 0.5% FBS was added to each sample after embedding dispersed MSCs and spheroids into aFn hydrogels. The plate was sealed with a nonpermeable acrylic tape to stop air perfusion. After 5 days, the cells and spheroids were stained using the Live/Dead assays. To quantify the number of live cells, hydrogels were lysed with 0.25% Trypsin-EDTA supplemented with 1 mg/mL collagenase. The lysed samples were centrifuged at 200 g to collect the cells. The collected cells were stained with Calcein AM. Then 20 μL of the stained cells was loaded to a hemocytometer. Both optical and fluorescence images were taken. The images were merged in ImageJ and the number of live cells was quantified.
2.2.14. Examination of MSC Survival in the Spheroids Treated with Exogenous Growth Factors.
MSC spheroids were embedded into aFn hydrogels (0.25 μM anti-VEGF aptamers and 0.2 μM anti-PDGF-BB aptamers) loaded with 50 ng VEGF and 50 ng PDGF-BB. Then the spheroids were cultured in the plate that was sealed with a nonpermeable sterile tape. After 5 days, cells and spheroids were stained with Live/Dead assays and the number of live cells were quantified using a hemocytometer.
2.2.15. Examination of in vivo MSC survival.
The animal experiment was approved by the Pennsylvania State University Institutional Animal Care and Use Committee. The dorsal hair of Male NOD scid mice (12 weeks old, The Jackson Laboratory) was shaved with an electronic razor, treated with Veet gel hair removal cream and cleaned with the DPBS solution. RFP-MSC spheroids (400 spheroids) were subcutaneously delivered into mice with Fn or aFn as a carrier. Four experimental groups included (1) native Fn loaded with 100 ng VEGF and 100 ng PDGF-BB (Fn+VP), (2) aFn loaded with 100 ng VEGF (aFn+V), (3) aFn loaded with 100 ng of PDGF-BB (aFn+P), and (4) aFn loaded with 100 ng of VEGF and 100 ng of PDGF-BB (aFn+VP). At predetermined time points, mice were anaesthetized with isoflurane (5% in oxygen) and imaged with the Maestro In Vivo Imaging System (CRI, Woburn, MA) for examining the cell survival. Mice were sacrificed with CO2 asphyxia 1 week post hydrogel delivery for tissue collection.
2.2.16. H&E Staining.
Collected tissue samples were fixed in 4% paraformaldehyde solution overnight at 4 °C. The fixed samples were blocked in paraffin, sectioned into 7 μm, and stained with an autostainer (Leica, Buffalo Grove, IL). The stained tissues were mounted into xylene substitute mountant and imaged with a BZ-X700 microscope (Keyence, Itasca, IL).
2.2.17. Immunostaining.
Tissues were fixed in 4% paraformaldehyde solution at 4 °C overnight, blocked with paraffin, sectioned into slices of 7 μm, blocked in 2% BSA solution at room temperature for 2 h, and immunostained. The primary antibody was diluted (1:200) into a 2% BSA solution and used to treat the blocked tissues at 4 °C overnight. Then the tissues were washed with PBS four times and incubated with the diluted secondary antibody (1:200) at room temperature for 2 h. After washing with PBS four times, the tissues were mounted in SlowFade Diamond Antifade Mountant with DAPI and imaged with a fluorescence microscope.
2.2.18. Statistics.
All data were presented as mean ± standard deviation (SD) unless otherwise specified. Prisma 5.0 (GraphPad Software Inc., La Jolla) was used for all the statistical analyses. Student’s t test was used to compare two groups. The result was considered statistically different if p < 0.05.
3. RESULTS AND DISCUSSIONS
3.1. Characterization of MSC Spheroids.
MSC-based applications require a large number of cells ranging from 5 × 107 to 109.29 Therefore, we prepared MSC spheroids using the cell suspension culture on an ultralow adherent plate because this method is simple and allows the quick production of a large number of spheroids. MSCs started to aggregate after 1 h and the aggregates formed spheroids within 24 h (Figure 1a). On average, one spheroid was composed of approximately 2000 cells and the variation of the average cell density was small from batch to batch (Figure 1b). This small variation would allow us to produce massive spheroids with similar quality and eliminate the bias caused by the generation of spheroids.
Figure 1.

Characterization of mesenchymal stem cell (MSC) spheroids. (a) Images of MSC spheroids. MSCs were imaged 1 h (left) and 24 h (right) after placed in the ultralow attachment plate, respectively. (b) Average number of MSCs in one spheroid prepared at different batches. (c) H&E staining of MSC spheroid after being cultured for 5 days. (d) Immunostaining of MSC markers. (e) Release of VEGF, PDGF-BB (PDGF), and bFGF from 2D cultured MSCs (i.e., monolayer) and 3D cultured MSC spheroids. n = 3; *, p < 0.05; ***, p < 0.001.
Different from dissociated cells, spheroids have a large mass and may experience the problem of nutrient/waste transport that can cause the death or necrosis of the spheroids. Spheroids with radii greater than 200 μm have been shown to be vulnerable to hypoxia and cell death.30 Thus, to examine whether MSCs could survive in the spheroids under our working conditions, we sectioned the spheroids and performed H&E staining after the spheroids were cultured for 5 days. The images show that the spheroids formed a compact structure without necrosis (Figure 1c), likely because they had a size of smaller than 200 μm. MSCs can differentiate into different cell types such as adipocytes, osteocytes, and chondrocytes.31 The state of their differentiation may affect the in vivo application. Thus, while our focus was on MSC survival, we stained MSCs with two important biomarkers, STRO1 and CD90. After being cultured for 5 days under normal conditions, MSC spheroids uniformly expressed both STRO1 and CD90 (Figure 1d). This indicates that these MSCs were maintained undifferentiated. The results also show that the spheroids could attach and grow on a regular cell culture plate (Supporting Information, Figure S1).
Recent studies have suggested that the in vivo therapeutic effects of MSCs mainly result from their ability to release signaling molecules that stimulate and regulate surrounding cells via paracrine signaling.4,5 Therefore, we examined the amounts of growth factors in the MSC-conditioned medium. Instead of examining all of the possible growth factors secreted by MSCs, we chose three growth factors including VEGF, bFGF, and PDGF-BB for the examination due to the following considerations. MSCs have been found to secret VEGF and bFGF.1–3 VEGF was reported to promote the proliferation of MSC,32 and the bFGF signaling pathway may be involved in supporting MSCs during stress.33 PDGF-BB is a principle survival factor that inhibits the apoptosis of various cell types.33–40 PDGF-BB was also reported to promote the proliferation of MSCs.41 In addition, these three growth factors can promote angiogenesis,42–44 which is important to tissue regeneration. Our results show that spheroids secreted more VEGF and bFGF compared with 2D cultured MSCs (Figure 1e), which is consistent with the reported results in the literature.23,45 Moreover, the amount of VEGF was much higher than that of bFGF. However, both 2D cultured MSCs and MSC spheroids secreted a very low amount of PDGF-BB, which might result from MSCs’ limited capacity of synthesizing PDGF-BB.
3.2. Examination of MSC Growth in the Presence of Exogenous Growth Factors.
We next examined the growth of MSCs in the presence of VEGF, PDGF-BB, or bFGF. We first performed this examination on a 2D cell culture plate. The data indicate that both VEGF and bFGF could increase MSC growth but there was no statistically significant difference compared with DMEM-treated MSCs across all tested concentrations (Figure 2a,b and Supporting Information, Figure S2). By contrast, our results show that PDGF-BB significantly promoted the growth of MSCs even when its concentration was only 5 ng/mL (Figure 2a,b). The growth of MSCs further increased at 10 ng/mL PDGF-BB but decreased slightly at 50 ng/mL, which indicates that 50 ng/mL exceeds the optimal concentration for MSCs growth. In addition to this study, the data from other research groups suggest that PDGF-BB may play an important role in protecting MSCs from apoptosis.33,40,41,46
Figure 2.

Growth of MSCs exposed to exogenous growth factors. (a) Optical images of MSCs. MSCs were cultured for 5 days before imaging. (b) Examination of MSC proliferation after being treated with exogenous growth factors for 5 days. The student’s t test was performed to compare each treatment group with DMEM group. Error bars, standard error; n = 4; **, p < 0.01; ***, p < 0.001.
As bFGF did not significantly increase MSC growth and MSC spheroids did not secret a large amount of bFGF, our following studies were carried out with only VEGF and PDGF-BB. As there is no study showing if the combination of VEGF and PDGF-BB can synergistically increase the survival of MSCs, we treated 2D cultured MSCs with 50 ng/mL of VEGF and 50 ng/mL of PDGF-BB. The results show that the combination of VEGF and PDGF-BB did not promote MSC survival in vitro (Supporting Information, Figure S3).
3.3. Synthesis and Characterization of aFn Hydrogels.
We next synthesized an aptamer-functionalized fibrin hydrogel (aFn). Fibrin is a natural hydrogel that can provide cells with a biocompatible microenvironment.47 It can be developed into injectable hydrogels under physiological condition, which allows easy cell loading and delivery. However, native fibrin hydrogel (Fn) has one major drawback, that is, low retention of growth factors.48 As the sustained presence of growth factors in the hydrogel can provide cells with necessary biochemical stimulation for cell survival and proliferation,49 it is important to solve this problem. Thus, we functionalized fibrinogen, a precursor molecule of fibrin, with nucleic acid aptamers.
The secondary structures of anti-VEGF and anti-PDGF-BB aptamers are shown in Figure 3a. The aptamer-functionalized fibrinogen formed hydrogels under physiological condition (Figure 3b). Importantly, staining of the aFn hydrogels with the fluorophore-labeled complementary sequences showed that the aptamers were indeed conjugated to Fn (Figure 3b and Supporting Information, Figure S4). SEM images demonstrate that the structure of Fn with the aptamer functionalization did not change (Figure 3c and Supporting Information, Figure S4). To confirm that aFn hydrogels could specifically retain target growth factors, we examined the retention of dextran, VEGF and PDGF-BB. Native Fn and aFn showed a similar capability of retaining dextran (Figure 3d). By contrast, with the aptamer functionalization, the retention of VEGF and PDGF-BB increased to 57.6% and 77.3%, respectively (Figure 3e,f). We then tested the sustained release of the two growth factors from aFn. aFn could sustain the release of both VEGF and PDGF-BB for over 2 weeks (Figure 3g,h and Supporting Information, Figure S5). Between day 4 and 14, the amount of daily release was 2 to 8 ng for VEGF and 2 to 6 ng for PDGF-BB. In general, the daily release rate decreases with time due to the decreasing concentration gradient. By contrast, for both VEGF and PDGF-BB, the daily release started to increase from day 6 to 11. It suggests that aFn started to degrade, which accelerated the release of the growth factors.
Figure 3.
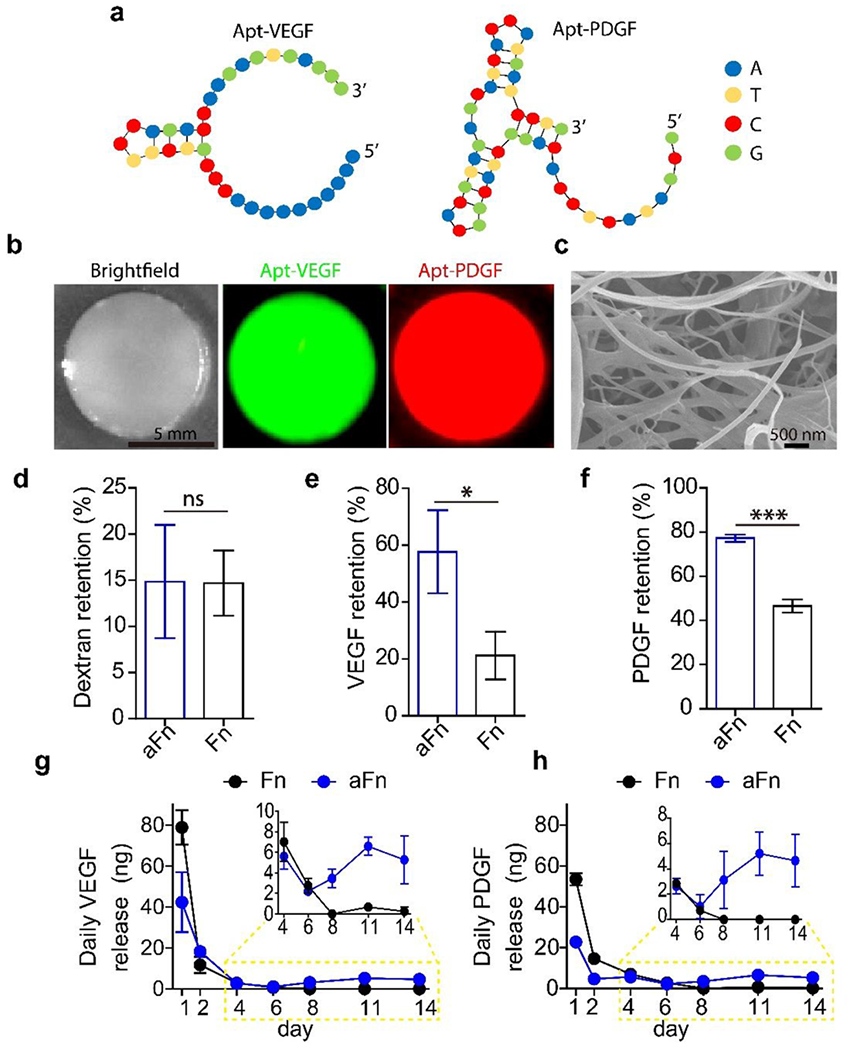
Characterization of aptamer-functionalized fibrin (aFn). (a) Secondary structures of the anti-VEGF aptamer (Apt-VEGF) and the anti-PDGF-BB aptamer (Apt-PDGF). (b) Optical and fluorescence images of aFn. Hydrogels were treated with the complementary sequences of the aptamers and then thoroughly washed. The complementary sequences of Apt-VEGF and Apt-PDGF were labeled with fluorescein amidite and cyanine 5, respectively. (c) SEM image of lyophilized aFn. (d) Dextran retention in Fn and aFn. (e) VEGF retention in Fn and aFn. (f) PDGF-BB retention in Fn and aFn. (g) Daily release of VEGF. (h) Daily release of PDGF-BB. n = 3; ns, no significant difference; *, p < 0.05; ***, p < 0.001.
3.4. Examination of In Vitro Survival of MSC Spheroids in aFn.
Before examining whether exogenous growth factors can promote the survival of MSC spheroids, we studied the survival of the spheroids in aFn without the growth factors. Under a normal cell culture condition, both dissociated MSCs and MSC spheroids could maintain high viability at day 1 and 5 as indicated by the Live/Dead staining (Figure 4a,b). At day 5, dissociated MSCs exhibited an elongation shape, which is the typical morphology of healthy MSCs. In the spheroid group, MSCs formed long branches sprouting out of the spheroids (Figure 4b,c). It suggests that aFn can provide MSC spheroids with a matrix for attachment, sprouting and growth. Lack of interaction of stem cells with host tissues might cause safety issues.50 Fn and aFn hydrogels are biodegradable.49 Thus, aFn provides MSC spheroids with a temporary matrix. With its degradation, the loaded spheroids would interact with the host tissues. This is one of the advantages of using Fn.
Figure 4.

The survival of MSC spheroids in aFn hydrogels. (a,b) Images of MSC and MSC spheroids in aFn hydrogels after cultured at normoxia for 1 day (a) and 5 days (b). Cells were stained using the Live/Dead cell viability assay. Green color, live cells; red color, dead cells. (c) Confocal imaging of MSC spheroids after cultured under normoxia for 5 days in aFn hydrogel. Cells were stained with Calcein AM. (d) Hypoxic culture of MSC and MSC spheroids in aFn hydrogel for 5 days. Cells were stained with Live/Dead assay. (e) Quantification of live cells after being cultured under hypoxia for 5 days. n = 8; *, p < 0.05.
When MSCs are delivered in vivo for biomedical applications, the disease sites are usually a hostile environment that may have a very low level of oxygen. To mimic such an in vivo environment and expose MSCs to stress, we cultured the MSCs and MSC spheroids in an airproof plate for 5 days. Dead cells were observed in both experimental groups (Figure 4d). However, the quantification of the cell number showed that the spheroid group had a significantly higher number of live cells after being cultured for 5 days under hypoxia in comparison with the control group (Figure 4e). It demonstrates that the aggregation state of MSCs in spheroids can improve the survival rate by 55% in aFn hydrogels under stress.
Next, we examined the effects of exogenous growth factors on the survival of spheroids under stress. Spheroids were embedded into aFn loaded with growth factors and cultured in the airproof plate. The images show that MSCs could migrate from the spheroids to the matrix of hydrogels and that some MSCs in the spheroids died in all four groups (Figure 5a). The quantification of live MSCs showed that the aFn+P group had live MSCs 73% more than the aFn+V groups and that the aFn+VP group had live MSCs 80% more than the Fn+VP group (Figure 5b). There was no difference between the aFn+V group and the Fn+VP group or between the aFn+P group and the aFn+VP group. It suggests that PDGF-BB is more potent in promoting the survival of MSC spheroids than VEGF, that aFn is more effective than Fn in promoting the survival of MSC spheroids, and that the combination of VEGF and PDGF-BB has no synergistic effect on the survival of MSC spheroids. The cell analysis using MTS is consistent with the quantification of live cells (Figure 5c). Taken together, the data indicate that the stable sequestration and sustained release of PDGF-BB in aFn hydrogels can promote the survival of MSC spheroids under hypoxic stress.
Figure 5.

The survival of MSC spheroids in aFn hydrogels loaded with exogenous growth factors. (a) Live/Dead staining of MSC spheroids cultured under a hypoxic condition for 5 days. Fn+VP, native Fn hydrogels loaded with VEGF and PDGF-BB; aFn+V, aFn hydrogels loaded with VEGF; aFn+P, aFn hydrogels loaded with PDGF-BB; aFn+VP, aFn hydrogels loaded with both VEGF and PDGF-BB. (b) Quantification of the number of live MSCs. (c) Examination of cell viability using the MTS cell proliferation assay. n = 8 for (b) and n = 3 for (c); ns, no significant difference; **, p < 0.01.
Therapeutic cells are usually delivered into target tissues as dissociated cells with or without polymeric matrices. However, most cells naturally exist in a 3D space with close cell–cell attachment. Moreover, MSCs are anchorage-dependent cells, requiring cell attachment. These intrinsic characteristics suggest that MSC spheroids may be superior to dissociated MSCs in maintaining MSC survival.26,27,51,52 For these considerations, the delivery of MSC spheroids has recently received more and more attention for the improvement of MSC survival.26,53–55 However, while efforts have been recently been made in studying the delivery of MSC spheroids, whether exogenous growth factors can function as biochemical cues in promoting the survival of MSC spheroids has not been explored. Notably, to promote MSC survival and/or therapeutic potential, several studies have shown that MSCs can be functionalized through genetic engineering and the genetically engineered MSCs can release growth factors.56–58 However, genetic engineering requires gene delivery, which may cause the risks of insertional mutagenesis and inflammatory responses.59 Moreover, those studies focused on dissociated MSCs but not MSC spheroids. Other methods were also studied to promote MSC survival, including the use of cell-instructive hydrogels, cell delivery with oxygen-producing hydrogels, and alternation of in vitro culture conditions.60–62 Different from these elegant studies, this work shows that the state of cell aggregation and the supply of exogenous signaling molecules using aFn can promote the survival of MSCs, which may open a new avenue for the delivery of MSCs with a high survival rate.
3.5. Examination of In Vivo Survival of MSC Spheroids.
Most studies for promoting the survival of MSC spheroids were conducted in vitro.51,53,63–65 In this work, after showing that PDGF-BB can promote the in vitro survival of MSC spheroids, we moved forward to examine their in vivo survival. Our time window for examination was 1 week as the literature has shown that the majority of the delivered cells die in vivo within the first week.10,12,16 For example, Toma et al. found that only 0.44% of MSCs survived in the left ventricle 4 days after the delivery,10 McGinley et al. demonstrated that less than 2% of the transplanted MSCs in infarcted heart survived after the first week,12 and Araña et al. reported that virtually all of delivered cells disappeared within 1 week after the delivery to the heart with myocardial infarction.66
aFn hydrogels loaded with growth factors and MSC spheroids were subcutaneously delivered to the dorsal back of mice as illustrated in Figure 6a. Cells expressing fluorescent proteins (e.g., RFP or GFP) have been widely used for in vivo live cell imaging.67,68 Thus, to directly measure the viability of the delivered cells in vivo, we used RFP-MSC spheroids. The strong fluorescence detected at day 1 in all four groups indicates that MSCs in the spheroids were viable right after in vivo delivery (Figure 6b). At day 4, the fluorescence signals from the aFn+P and aFn+VP groups were significantly higher compared with the Fn+VP and aFn+V groups. At day 7, RFP signals in all of the experimental groups significantly decreased in comparison with that at day 4. However, the RFP intensity of the aFn+P group (38%) and that of the aFn+VP group (42%) was approximately 4-fold more than that of the Fn+VP (10%). We further collected the tissue samples and examined the tissue slices using fluorescence imaging. Clusters of RFP-expressing cells could be observed in the sectioned tissues (Figure 6d). The images also indicate that the aFn+P and aFn+VP groups had more RFP-expressing cells than the other two groups. Thus, consistent with the in vitro observation (Figure 5), the in vivo animal study showed that aFn loaded with PDGF-BB or both PDGF-BB and VEGF can promote the survival of MSC spheroids (Figure 6c,d).
Figure 6.

Examination of the in vivo survival of red fluorescent protein-expressing MSC (RFP-MSC) spheroids. (a) Positions of implanted hydrogels with RFP-MSC spheroids. (b) In vivo imaging of spheroids after subcutaneously implanted in NOD scid mice. Color-coding of RFP intensity is equally scaled for all the images in arbitrary units (a.u.). (c) Quantification of the RFP intensity. The fluorescence intensity of each treatment group was normalized to their corresponding average fluorescence intensity at day 1. (d) Fluorescence imaging of tissue slices for the examination of RFP-MSCs in different hydrogels. DAPI was used as a nuclear counterstain. n = 5; ns, no significant difference; **, p < 0.01.
Because MSCs have the potential to differentiate into different types of cells, we also examined whether the survived cells differentiated into other cell types in vivo by staining two important biomarkers including STRO1 and CD90 (Figure 7). CD90 and STRO1 signals were observed to colocalize with the RFP signal. It shows that PDGF-BB in aFn hydrogels promoted the survival of MSCs but did not induce the differentiation of MSCs in vivo. For cell-based therapies, the delivered cells remedy target tissues mainly by two mechanisms including the stimulation of the host cells via paracrine signaling and the engraftment of cells into the surrounding tissues.9,69 According to state-of-the-art knowledge,4,5 the former mechanism mainly accounts for the therapeutic effects of MSCs. Thus, the ability to maintain undifferentiated MSCs after in vivo delivery would benefit MSC therapy.
Figure 7.

Immunostaining of in vivo implanted spheroids. RFP-MSC spheroids with surrounding tissues were collected at day 7 post-transplantation in NOD scid mice. STRO1 and CD90 are two typical MSC markers.
3.6. Examination of Angiogenesis.
To understand whether the angiogenic effect from VEGF and/or PDGF-BB played an important role in promoting the survival of MSC spheroids as shown in Figure 6, we examined angiogenesis.
All of the hydrogels were visible at day 7 after implantation (Figure 8a) and located right above the connective tissue layer (Figure 8b). Except for the aFn+P group, hydrogels in the other three groups were infiltrated with blood vessels that could be observed with naked eyes (Figure 8a). We stained the blood vessels in the hydrogels with CD31 and α-SMA antibodies, two important biomarkers for endothelial and smooth muscle cells of blood vessels. The aFn+V group exhibited a significantly higher number of CD31-positive blood vessels than the Fn+VP while aFn+VP and aFn+P groups did not show significantly increased CD31-positive vessels compared with Fn+VP (Figure 8c,d). This is reasonable since sustained VEGF release was found to promote angiogenesis.28 Angiogenesis can be promoted by the release of growth factors from hydrogels. It can also be promoted by the differentiation of stem cells into endothelial cells.70 In this study, the stained CD31+ cells were mouse endothelial cells since the antibody used for staining is specifically for mouse CD31 antigen. Thus, the observed enhancement of angiogenesis would result from the release of VEGF from the hydrogels. Notably, the α-SMA staining did not show any difference among the four groups (Figure 8e), which suggests that the growth of smooth muscle cells among all of the groups was similar. Although PDGF-BB has been found to promote the growth of smooth muscle cells, this biological response may not be observed within 1 week. Angiogenesis is supposed to promote cell survival. However, we did not observe enhanced MSC survival in the aFn+V group while this group had enhanced CD31-positive staining. One potential reason might be the immaturity of blood vessels formed during the first week, which is partly supported by the α-SMA staining result. This could also explain why the aFn+VP group did not show increased spheroids survival compared with aFn+P group.
Figure 8.

Examination of angiogenesis stimulated by hydrogels loaded with RFP-MSC spheroids. (a) Optical images of the bulk hydrogels and surrounding tissues after implanted in NOD scid mice for 7 days. Yellow arrows indicate the location of hydrogel materials. (b) H&E staining of hydrogels with cells and surrounding tissues. Yellow lines indicate the boundary between hydrogel and host tissue. F, fibrin hydrogels. (c) Immunostaining of CD31 and α-SMA. (d) Quantification of CD31 positive blood vessels. (e) Quantification of α-SMA positive blood vessels. The student’s t test was performed to compare Fn+VP with the other three groups. n = 4; ns, no significant difference; ***, p < 0.001.
Taken together, these results suggest that in the current experimental setting, the observed enhancement of MSC survival (Figure 6) resulted from the stimulation of PDGF-BB but not the aid of angiogenesis. However, as this experiment was conducted in 1 week, we should not rule out the possibility that angiogenesis beyond the first week may improve the survival of MSCs. The aid of angiogenesis and the stimulation of exogenous signaling molecules may promote synergistic effects on the survival of MSC spheroids in the long run, which is not the focus of this work but will be studied in the future. Future studies can also be carried out to explore the potential of using spheroid-loaded aFn hydrogels to treat diseases such as skin wounds and ischemia.
CONCLUSIONS
In summary, we have examined the survival of MSC spheroids with or without the stimulation of exogenous signaling molecules. The aggregation state of MSCs benefits their survival in comparison with the dissociation state in a hydrogel. PDGF-BB can promote the in vitro survival of MSC spheroids in the hydrogel under stress. Importantly, we have for the first time shown that PDGF-BB can significantly promote the in vivo survival of transplanted MSC spheroids independent of angiogenesis. Thus, this work has successfully demonstrated that defined exogenous signaling molecules (e.g., PDGF-BB) can function as biochemical cues for the enhanced survival of transplanted MSC spheroids.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge financial support from the National Institutes of Health (HL122311; AR073364).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.0c05681.
Additional figures and tables (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acsami.0c05681
The authors declare no competing financial interest.
Contributor Information
Nan Zhao, Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States.
James Coyne, Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States.
Lidya Abune, Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States.
Peng Shi, Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States.
Xiaojun Lance Lian, Department of Biomedical Engineering and Department of Biology, The Pennsylvania State University, University Park, Pennsylvania 16802, United States.
Ge Zhang, Department of Biomedical Engineering, The University of Akron, Akron, Ohio 44325, United States.
Yong Wang, Department of Biomedical Engineering, The Pennsylvania State University, University Park, Pennsylvania 16802, United States.
REFERENCES
- (1).Amable PR; Teixeira MVT; Carias RBV; Granjeiro JM; Borojevic R Protein Synthesis and Secretion in Human Mesenchymal Cells Derived from Bone Marrow, Adipose Tissue and Wharton’s Jelly. Stem Cell Res. Ther. 2014, 5, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Schinköthe T; Bloch W; Schmidt A In Vitro Secreting Profile of Human Mesenchymal Stem Cells. Stem Cells Dev. 2008, 17, 199–206. [DOI] [PubMed] [Google Scholar]
- (3).Kinnaird T; Stabile E; Burnett M; Lee C; Barr S; Fuchs S; Epstein S Marrow-Derived Stromal Cells Express Genes Encoding a Broad Spectrum of Arteriogenic Cytokines and Promote in Vitro and in Vivo Arteriogenesis through Paracrine Mechanisms. Circ. Res 2004, 94, 678–685. [DOI] [PubMed] [Google Scholar]
- (4).Wobma HM; Liu D; Vunjak-Novakovic G Paracrine Effects of Mesenchymal Stromal Cells Cultured in Three-Dimensional Settings on Tissue Repair. ACS Biomater. Sci. Eng 2018, 4, 1162– 1175. [DOI] [PubMed] [Google Scholar]
- (5).Kinnaird T; Stabile E; Burnett M; Shou M; Lee C; Barr S; Fuchs S; Epstein S Local Delivery of Marrow-Derived Stromal Cells Augments Collateral Perfusion through Paracrine Mechanisms. Circulation 2004, 109, 1543–1549. [DOI] [PubMed] [Google Scholar]
- (6).Gnecchi M; Zhang Z; Ni A; Dzau VJ Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ. Res 2008, 103, 1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Bhang SH; Cho S-W; La W-G; Lee T-J; Yang HS; Sun A-Y; Baek S-H; Rhie J-W; Kim B-S Angiogenesis in Ischemic Tissue Produced by Spheroid Grafting of Human Adipose-Derived Stromal Cells. Biomaterials 2011, 32, 2734–2747. [DOI] [PubMed] [Google Scholar]
- (8).Sasportas LS; Kasmieh R; Wakimoto H; Hingtgen S; van de Water JA; Mohapatra G; Figueiredo JL; Martuza RL; Weissleder R; Shah K Assessment of Therapeutic Efficacy and Fate of Engineered Human Mesenchymal Stem Cells for Cancer Therapy. Proc. Natl. Acad. Sci. U. S. A 2009, 106, 4822–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Segers VF; Lee RT Stem-Cell Therapy for Cardiac Disease. Nature 2008, 451, 937. [DOI] [PubMed] [Google Scholar]
- (10).Toma C; Pittenger MF; Cahill KS; Byrne BJ; Kessler PD Human Mesenchymal Stem Cells Differentiate to a Cardiomyocyte Phenotype in the Adult Murine Heart. Circulation 2002, 105, 93–98. [DOI] [PubMed] [Google Scholar]
- (11).Gyongyosi M; Blanco J; Marian T; Tron L; Petnehazy O; Petrasi Z; Hemetsberger R; Rodriguez J; Font G; Pavo IJ; Kertesz I; Balkay L; Pavo N; Posa A; Emri M; Galuska L; Kraitchman DL; Wojta J; Huber K; Glogar D Serial Noninvasive in Vivo Positron Emission Tomographic Tracking of Percutaneously Intramyocardially Injected Autologous Porcine Mesenchymal Stem Cells Modified for Transgene Reporter Gene Expression. Circulation: Cardiovascular Imaging 2008, 1, 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).McGinley LM; McMahon J; Stocca A; Duffy A; Flynn A; O’Toole D; O’Brien T Mesenchymal Stem Cell Survival in the Infarcted Heart Is Enhanced by Lentivirus Vector-Mediated Heat Shock Protein 27 Expression. Hum. Gene Ther. 2013, 24, 840–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hofmann M; Wollert KC; Meyer GP; Menke A; Arseniev L; Hertenstein B; Ganser A; Knapp WH; Drexler H Monitoring of Bone Marrow Cell Homing into the Infarcted Human Myocardium. Circulation 2005, 111, 2198–2202. [DOI] [PubMed] [Google Scholar]
- (14).Rustad KC; Wong VW; Sorkin M; Glotzbach JP; Major MR; Rajadas J; Longaker MT; Gurtner GC Enhancement of Mesenchymal Stem Cell Angiogenic Capacity and Stemness by a Biomimetic Hydrogel Scaffold. Biomaterials 2012, 33, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Pons J; Huang Y; Arakawa-Hoyt J; Washko D; Takagawa J; Ye J; Grossman W; Su H Vegf Improves Survival of Mesenchymal Stem Cells in Infarcted Hearts. Biochem. Biophys. Res. Commun. 2008, 376, 419–422. [DOI] [PubMed] [Google Scholar]
- (16).Shi P; Zhao N; Coyne J; Wang Y DNA-Templated Synthesis of Biomimetic Cell Wall for Nanoencapsulation and Protection of Mammalian Cells. Nat. Commun. 2019, 10, 2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Miyahara Y; Nagaya N; Kataoka M; Yanagawa B; Tanaka K; Hao H; Ishino K; Ishida H; Shimizu T; Kangawa K; et al. Monolayered Mesenchymal Stem Cells Repair Scarred Myocardium after Myocardial Infarction. Nat. Med. 2006, 12, 459. [DOI] [PubMed] [Google Scholar]
- (18).Yu J; Du KT; Fang Q; Gu Y; Mihardja SS; Sievers RE; Wu JC; Lee RJ The Use of Human Mesenchymal Stem Cells Encapsulated in Rgd Modified Alginate Microspheres in the Repair of Myocardial Infarction in the Rat. Biomaterials 2010, 31, 7012–7020. [DOI] [PubMed] [Google Scholar]
- (19).Yang F; Cho S-W; Son SM; Bogatyrev SR; Singh D; Green JJ; Mei Y; Park S; Bhang SH; Kim B-S; Langer R; Anderson DG Genetic Engineering of Human Stem Cells for Enhanced Angiogenesis Using Biodegradable Polymeric Nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 3317–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).van der Bogt KE; Schrepfer S; Yu J; Sheikh AY; Hoyt G; Govaert JA; Velotta JB; Contag CH; Robbins RC; Wu JC Comparison of Transplantation of Adipose Tissue-and Bone Marrow-Derived Mesenchymal Stem Cells in the Infarcted Heart. Transplantation 2009, 87, 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Russo V; Young S; Hamilton A; Amsden BG; Flynn LE Mesenchymal Stem Cell Delivery Strategies to Promote Cardiac Regeneration Following Ischemic Injury. Biomaterials 2014, 35, 3956–3974. [DOI] [PubMed] [Google Scholar]
- (22).Yamaguchi Y; Ohno J; Sato A; Kido H; Fukushima T Mesenchymal Stem Cell Spheroids Exhibit Enhanced in-Vitro and in-Vivo Osteoregenerative Potential. BMC Biotechnol. 2014, 14, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Cesarz Z; Tamama K Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Jiang B; Yan L; Miao Z; Li E; Wong KH; Xu R-H Spheroidal Formation Preserves Human Stem Cells for Prolonged Time under Ambient Conditions for Facile Storage and Transportation. Biomaterials 2017, 133, 275–286. [DOI] [PubMed] [Google Scholar]
- (25).Frith JE; Thomson B; Genever PG Dynamic Three-Dimensional Culture Methods Enhance Mesenchymal Stem Cell Properties and Increase Therapeutic Potential. Tissue Eng., Part C 2010, 16, 735–749. [DOI] [PubMed] [Google Scholar]
- (26).Cheng N-C; Chen S-Y; Li J-R; Young T-H Short-Term Spheroid Formation Enhances the Regenerative Capacity of Adipose-Derived Stem Cells by Promoting Stemness, Angiogenesis, and Chemotaxis. Stem Cells Transl Med. 2013, 2, 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Bhang SH; Lee S; Shin J-Y; Lee T-J; Kim B-S Transplantation of Cord Blood Mesenchymal Stem Cells as Spheroids Enhances Vascularization. Tissue Eng. Part A 2012, 18, 2138–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Zhao N; Coyne J; Xu M; Zhang X; Suzuki A; Shi P; Lai J; Fong G-H; Xiong N; Wang Y Assembly of Bifunctional Aptamer-Fibrinogen Macromer for Vegf Delivery and Skin Wound Healing. Chem. Mater. 2019, 31, 1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wollert KC; Drexler H Clinical Applications of Stem Cells for the Heart. Circ. Res. 2005, 96, 151–163. [DOI] [PubMed] [Google Scholar]
- (30).Curcio E; Salerno S; Barbieri G; De Bartolo L; Drioli E; Bader A Mass Transfer and Metabolic Reactions in Hepatocyte Spheroids Cultured in Rotating Wall Gas-Permeable Membrane System. Biomaterials 2007, 28, 5487–5497. [DOI] [PubMed] [Google Scholar]
- (31).Short B; Brouard N; Occhiodoro-Scott T; Ramakrishnan A; Simmons PJ Mesenchymal Stem Cells. Arch. Med. Res. 2003, 34, 565–571. [DOI] [PubMed] [Google Scholar]
- (32).Kong X; Zheng F; Guo L; Yang J; Zhang L; Tang J; Huang Y; Wang J Vegf Promotes the Proliferation of Bone Marrow Derived Mesenchymal Stem Cells through Erk1/2 Signal Pathway. Zhongguo shi yan xue ye xue za zhi 2010, 18, 1292–1296. [PubMed] [Google Scholar]
- (33).Yin X; Hu L; Zhang Y; Zhu C; Cheng H; Xie X; Shi M; Zhu P; Zhao X; Chen W; Zhang L; Arakaki C; Hao S; Wang M; Cao W; Ma S; Zhang X-B; Cheng T Pdgfb-Expressing Mesenchymal Stem Cells Improve Human Hematopoietic Stem Cell Engraftment in Immunodeficient Mice. Bone Marrow Transplant. 2019, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Cabezas R; Avila MF; González J; El-Bachá RS; Barreto GE Pdgf-Bb Protects Mitochondria from Rotenone in T98g Cells. Neurotoxic. Res. 2015, 27, 355–367. [DOI] [PubMed] [Google Scholar]
- (35).Liu Z; Liu H; Jiang J; Tan S; Yang Y; Zhan Y; Wu B Pdgf-Bb and Bfgf Ameliorate Radiation-Induced Intestinal Progenitor/Stem Cell Apoptosis Via Akt/P53 Signaling in Mice. American Journal of Physiology-Gastrointestinal and Liver Physiology 2014, 307, G1033–G1043. [DOI] [PubMed] [Google Scholar]
- (36).Presciutti SM; Paglia DN; Karukonda T; Soung DY; Guzzo R; Drissi H; Moss IL Pdgf-Bb Inhibits Intervertebral Disc Cell Apoptosis in Vitro. J. Orthop. Res. 2014, 32, 1181–1188. [DOI] [PubMed] [Google Scholar]
- (37).Romashkova JA; Makarov SS Nf-Kb Is a Target of Akt in Anti-Apoptotic Pdgf Signalling. Nature 1999, 401, 86–90. [DOI] [PubMed] [Google Scholar]
- (38).Staiger H; Loffler G The Role of Pdgf-Dependent Suppression of Apoptosis in Differentiating 3t3-L1 Preadipocytes. Eur. J. Cell Biol. 1998, 77, 220–227. [DOI] [PubMed] [Google Scholar]
- (39).Vantler M; Karikkineth BC; Naito H; Tiburcy M; Didié M; Nose M; Rosenkranz S; Zimmermann W-H Pdgf-Bb Protects Cardiomyocytes from Apoptosis and Improves Contractile Function of Engineered Heart Tissue. J. Mol. Cell. Cardiol. 2010, 48, 1316–1323. [DOI] [PubMed] [Google Scholar]
- (40).Zhang J-M; Feng F-E; Wang Q-M; Zhu X-L; Fu H-X; Xu L-P; Liu K-Y; Huang X-J; Zhang X-H Platelet-Derived Growth Factor-Bb Protects Mesenchymal Stem Cells (Mscs) Derived from Immune Thrombocytopenia Patients against Apoptosis and Senescence and Maintains Msc-Mediated Immunosuppression. Stem Cells Transl. Med. 2016, 5, 1631–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Chen W; Baylink DJ; Brier-Jones J; Neises A; Kiroyan JB; Rundle CH; Lau K-HW; Zhang X-B Pdgfb-Based Stem Cell Gene Therapy Increases Bone Strength in the Mouse. Proc. Natl. Acad. Sci. U. S. A 2015, 112, E3893–E3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Gerhardt H; Golding M; Fruttiger M; Ruhrberg C; Lundkvist A; Abramsson A; Jeltsch M; Mitchell C; Alitalo K; Shima D; Betsholtz C Vegf Guides Angiogenic Sprouting Utilizing Endothelial Tip Cell Filopodia. J. Cell Biol. 2003, 161, 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Moncion A; Lin M; O’Neill EG; Franceschi RT; Kripfgans OD; Putnam AJ; Fabiilli ML Controlled Release of Basic Fibroblast Growth Factor for Angiogenesis Using Acoustically-Responsive Scaffolds. Biomaterials 2017, 140, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Carmeliet P Angiogenesis in Health and Disease. Nat. Med. 2003, 9, 653. [DOI] [PubMed] [Google Scholar]
- (45).Potapova IA; Gaudette GR; Brink PR; Robinson RB; Rosen MR; Cohen IS; Doronin SV Mesenchymal Stem Cells Support Migration, Extracellular Matrix Invasion, Proliferation, and Survival of Endothelial Cells in Vitro. Stem Cells 2007, 25, 1761–1768. [DOI] [PubMed] [Google Scholar]
- (46).Kang YJ; Jeon ES; Song HY; Woo JS; Jung JS; Kim YK; Kim JH Role of C-Jun N-Terminal Kinase in the Pdgf-Induced Proliferation and Migration of Human Adipose Tissue-Derived Mesenchymal Stem Cells. J. Cell. Biochem 2005, 95, 1135– 1145. [DOI] [PubMed] [Google Scholar]
- (47).Mosesson M Fibrinogen and Fibrin Structure and Functions. J. Thromb. Haemostasis 2005, 3, 1894–1904. [DOI] [PubMed] [Google Scholar]
- (48).Hoare TR; Kohane DS Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar]
- (49).Zhao N; Suzuki A; Zhang X; Shi P; Abune L; Coyne J; Jia H; Xiong N; Zhang G; Wang Y Dual Aptamer-Functionalized in Situ Injectable Fibrin Hydrogel for Promotion of Angiogenesis Via Codelivery of Vascular Endothelial Growth Factor and Platelet-Derived Growth Factor-Bb. ACS Appl. Mater. Interfaces 2019, 11, 18123–18132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Herberts CA; Kwa MS; Hermsen HP Risk Factors in the Development of Stem Cell Therapy. J. Transl. Med. 2011, 9, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Tsai A-C; Liu Y; Yuan X; Ma T Compaction, Fusion, and Functional Activation of Three-Dimensional Human Mesenchymal Stem Cell Aggregate. Tissue Eng. Part A 2015, 21, 1705–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Frith JE; Thomson B; Genever PG Dynamic Three-Dimensional Culture Methods Enhance Mesenchymal Stem Cell Properties and Increase Therapeutic Potential. Tissue Eng., Part C 2010, 16, 735–749. [DOI] [PubMed] [Google Scholar]
- (53).Ho SS; Murphy KC; Binder BY; Vissers CB; Leach JK Increased Survival and Function of Mesenchymal Stem Cell Spheroids Entrapped in Instructive Alginate Hydrogels. Stem Cells Transl. Med. 2016, 5, 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Suryaprakash S; Lao Y-H; Cho H-Y; Li M; Ji HY; Shao D; Hu H; Quek CH; Huang D; Mintz RL; Bago JR; Hingtgen SD; Lee K-B; Leong KW Engineered Mesenchymal Stem Cell/Nanomedicine Spheroid as an Active Drug Delivery Platform for Combinational Glioblastoma Therapy. Nano Lett. 2019, 19, 1701–1705. [DOI] [PubMed] [Google Scholar]
- (55).Ong CS; Zhou X; Han J; Huang CY; Nashed A; Khatri S; Mattson G; Fukunishi T; Zhang H; Hibino N In Vivo Therapeutic Applications of Cell Spheroids. Biotechnol. Adv. 2018, 36, 494–505. [DOI] [PubMed] [Google Scholar]
- (56).Cho H-M; Kim P-H; Chang H-K; Shen Y.-m.; Bonsra K; Kang B-J; Yum S-Y; Kim J-H; Lee S-Y; Choi M.-c.; Kim HH; Jang G; Cho J-Y Targeted Genome Engineering to Control Vegf Expression in Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells: Potential Implications for the Treatment of Myocardial Infarction. Stem Cells Transl. Med. 2017, 6, 1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Deuse T; Peter C; Fedak PWM; Doyle T; Reichenspurner H; Zimmermann WH; Eschenhagen T; Stein W; Wu JC; Robbins RC; Schrepfer S Hepatocyte Growth Factor or Vascular Endothelial Growth Factor Gene Transfer Maximizes Mesenchymal Stem Cell-Based Myocardial Salvage after Acute Myocardial Infarction. Circulation 2009, 120, S247–S254. [DOI] [PubMed] [Google Scholar]
- (58).Matsumoto R; Omura T; Yoshiyama M; Hayashi T; Inamoto S; Koh K-R; Ohta K; Izumi Y; Nakamura Y; Akioka K; Kitaura Y; Takeuchi K; Yoshikawa J Vascular Endothelial Growth Factor-Expressing Mesenchymal Stem Cell Transplantation for the Treatment of Acute Myocardial Infarction. Arterioscler., Thromb, Vasc. Biol. 2005, 25, 1168–1173. [DOI] [PubMed] [Google Scholar]
- (59).Gonçalves GAR; Paiva R. d. M. A. Gene Therapy: Advances, Challenges and Perspectives. Einstein (Sao Paulo) 2017, 15, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Park H; Temenoff JS; Tabata Y; Caplan AI; Mikos AG Injectable Biodegradable Hydrogel Composites for Rabbit Marrow Mesenchymal Stem Cell and Growth Factor Delivery for Cartilage Tissue Engineering. Biomaterials 2007, 28, 3217–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Lennon DP; Edmison JM; Caplan AI Cultivation of Rat Marrow-Derived Mesenchymal Stem Cells in Reduced Oxygen Tension: Effects on in Vitro and in Vivo Osteochondrogenesis. J. Cell. Physiol. 2001, 187, 345–355. [DOI] [PubMed] [Google Scholar]
- (62).Lu Z; Jiang X; Chen M; Feng L; Kang YJ An Oxygen-Releasing Device to Improve the Survival of Mesenchymal Stem Cells in Tissue Engineering. Biofabrication 2019, 11, 045012. [DOI] [PubMed] [Google Scholar]
- (63).Murphy KC; Fang SY; Leach JK Human Mesenchymal Stem Cell Spheroids in Fibrin Hydrogels Exhibit Improved Cell Survival and Potential for Bone Healing. Cell Tissue Res. 2014, 357, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Kim J; Ma T Endogenous Extracellular Matrices Enhance Human Mesenchymal Stem Cell Aggregate Formation and Survival. Biotechnology progress 2013, 29, 441–451. [DOI] [PubMed] [Google Scholar]
- (65).Wang W; Itaka K; Ohba S; Nishiyama N; Chung U-I; Yamasaki Y; Kataoka K 3d Spheroid Culture System on Micropatterned Substrates for Improved Differentiation Efficiency of Multipotent Mesenchymal Stem Cells. Biomaterials 2009, 30, 2705–2715. [DOI] [PubMed] [Google Scholar]
- (66).Arana M; Gavira JJ; Pena E; Gonzalez A; Abizanda G; Cilla M; Perez MM; Albiasu E; Aguado N; Casado M; Lopez B; Gonzalez S; Soriano M; Moreno C; Merino J; Garcia-Verdugo JM; Diez J; Doblare M; Pelacho B; Prosper F Epicardial Delivery of Collagen Patches with Adipose-Derived Stem Cells in Rat and Minipig Models of Chronic Myocardial Infarction. Biomaterials 2014, 35, 143–151. [DOI] [PubMed] [Google Scholar]
- (67).M Hoffman R Cellular and Subcellular Imaging in Live Mice Using Fluorescent Proteins. Curr. Pharm. Biotechnol 2012, 13, 537–544. [DOI] [PubMed] [Google Scholar]
- (68).Mehta SR; Huang R; Yang M; Zhang X-Q; Kolli B; Chang K-P; Hoffman RM; Goto Y; Badaro R; Schooley RT Real-Time in Vivo Green Fluorescent Protein Imaging of a Murine Leishmaniasis Model as a New Tool for Leishmania Vaccine and Drug Discovery. Clin. Vaccine Immunol. 2008, 15, 1764–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Passier R; van Laake LW; Mummery CL Stem-Cell-Based Therapy and Lessons from the Heart. Nature 2008, 453, 322. [DOI] [PubMed] [Google Scholar]
- (70).Wang H; Agarwal P; Xiao Y; Peng H; Zhao S; Liu X; Zhou S; Li J; Liu Z; He X A Nano-in-Micro System for Enhanced Stem Cell Therapy of Ischemic Diseases. ACS Cent. Sci 2017, 3, 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


