Abstract
Background:
Previous studies reported various symptoms of Parkinson’s disease (PD) associated with sex. Some were conflicting or confirmed in only one study.
Objectives:
We examined sex associations to PD phenotypes cross-sectionally and longitudinally in large-scale data.
Methods:
We tested 40 clinical phenotypes, using longitudinal, clinic-based patient cohorts, consisting of 5946 patients, with a median follow-up of 3.1 years. For continuous outcomes, we used linear regressions at baseline to test sex-associated differences in presentation, and linear mixed-effects models to test sex-associated differences in progression. For binomial outcomes, we used logistic regression models at baseline and Cox regression models for survival analyses. We adjusted for age, disease duration, and medication use. In the secondary analyses, data from 17 719 PD patients and 7588 non-PD participants from an online-only, self-assessment PD cohort were cross-sectionally evaluated to determine whether the sex-associated differences identified in the primary analyses were consistent and unique to PD.
Results:
Female PD patients had a higher risk of developing dyskinesia early during the follow-up period, with a slower progression in activities of daily living difficulties, and a lower risk of developing cognitive impairments compared with male patients. The findings in the longitudinal, clinic-based cohorts were mostly consistent with the results of the online-only cohort.
Conclusions:
We observed sex-associated contributions to PD heterogeneity. These results highlight the necessity of future research to determine the underlying mechanisms and importance of personalized clinical management.
Keywords: Parkinson’s disease, gender, sex, dyskinesias, cognitive impairment, activities of daily livings
The prevalence of Parkinson’s disease (PD) is 1.5–2.0 times higher in men than in women. This discrepancy suggests the potential existence of sex-associated factors that modify the disease process. Identifying the interplay between sex and PD has the potential to assist the development of disease-modifying therapy, inform patient management strategies, and allow the planning of more efficient clinical trials. Researchers have previously investigated sex-associated differences in phenotypes among patients with PD.1–3 Male PD patients have been reported to present akinesia/rigid features,4 cognitive impairment,5–7 daytime sleepiness,8 and rapid eye movement (REM) sleep behavioral disorder (RBD) more frequently than female PD patients.9,10 In contrast, anxiety disorder/depression11–14 and dyskinesia11,15–17 were documented to occur more frequently in female PD patients than in male PD patients. However, these studies were generally small in sample size and predominantly performed in a cross-sectional setting.
In this study, we analyzed longitudinal data from 12 PD cohorts, representing 5946 participants, with a median of 3.1 years of follow-up. This study had two objectives: (1) to identify the baseline differences between men and women, in terms of disease presentation, and (2) to identify the influences of sex on longitudinal symptom trajectory. Further, we analyzed the Fox Insight dataset, an online-only, PD research cohort, to assess whether the observations made using the longitudinal datasets were consistent in an independent dataset. Moreover, by analyzing the data from both PD participants and non-PD participants in the Fox Insight dataset, we were able to evaluate differences in the prevalence of self-reported outcomes between participants with and without PD. This analysis further illustrated that some of the identified differences may be influenced by general differences between men and women, whereas others are disease-specific.
Patients and Methods
Participants
Longitudinal Cohorts
We analyzed data from 12 longitudinal PD cohorts, from North America, Europe, and Australia, in this study (Table 1). Among these cohorts, the following four studies enrolled people with early-phase PD who were not being treated at the time of study enrollment (de novo cohorts): Parkinson’s Progression Markers Initiative (PPMI), Parkinson Research Examination of CEP-1347 Trial study and its subsequent prospective study (PreCEPT/PostCEPT), the Norwegian ParkWest study (PARKWEST), and Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism (DATATOP). Other cohorts included Parkinsonism Incidence and Cognitive and Non-motor heterogeneity In Cambridgeshire (PICNICS), National Institutes of Health Exploratory Trials in Parkinson’s Disease Large Simple Study 1 (NET_PD_LS1), Drug Interaction with Genes in Parkinson’s Disease (DIGPD), Parkinson’s Disease Biomarker Program (PDBP), Harvard Biomarkers Study (HBS), ParkFit Study (PARKFIT), Profiling Parkinson’s Disease Study (PROPARK), and Udall Centers Program (UDALL_PENN). Participants’ information was obtained under appropriate written consent and with local institutional and ethical approval. The summary of the designs and inclusion/exclusion criteria applied to these cohorts are documented in the Supplemental Materials. The study protocols were approved at the local institutional review boards, and the participants provided written informed consent.
TABLE 1.
Baseline characteristics of study cohorts
| Cohort | N | f-u, y | European, % | Female, % | Stratum | Age, year old | Duration, y | LD, % | DA, % |
|---|---|---|---|---|---|---|---|---|---|
| PPMI | 408 | 7.0 | 95.1 | 34.6 | Male | 62.15 (9.86) | 0.53 (0.49) | – | – |
| Female | 60.76 (9.60) | 0.60 (0.63) | |||||||
| PreCEPT_PostCEPT | 390 | 6.9 | 97.7 | 33.8 | Male | 59.83 (9.51) | 0.79 (0.80) | – | – |
| Female | 60.96 (9.56) | 0.84 (0.85) | |||||||
| PARKWEST | 181 | 5.0 | 100 | 37.8 | Male | 67.82 (9.21) | 0.16 (0.10) | – | – |
| Female | 68.36 (9.10) | 0.20 (0.14)* | |||||||
| DATATOP | 796 | 1.1 | 97.7 | 33.7 | Male | 61.45 (9.35) | 1.16 (1.14) | – | – |
| Female | 60.34 (9.80) | 1.10 (1.05) | |||||||
| PICNICS | 122 | 3.5 | 98.4 | 35.2 | Male | 67.85 (8.40) | 0.30 (0.49) | 30.4 | 17.7 |
| Female | 67.93 (10.28) | 0.12 (0.50)* | 27.9 | 23.3 | |||||
| NET_PD_LS1 | 1705 | 4.0 | 92.7 | 35.7 | Male | 62.07 (9.32) | 1.55 (1.08) | 57.5 | 60.4 |
| Female | 61.20 (10.06) | 1.54 (1.10) | 55.3 | 63.3 | |||||
| DIGPD | 350 | 3.0 | 85.8 | 39.4 | Male | 61.45 (10.34) | 2.55 (1.52) | 65.6 | 77.8 |
| Female | 62.40 (9.61) | 2.46 (1.59) | 62.3 | 63.0 | |||||
| PDBP | 486 | 3.0 | 93.0 | 39.7 | Male | 65.03 (9.13) | 5.31 (4.74) | 81.9 | 50.2* |
| Female | 64.87 (8.67) | 5.22 (4.78) | 76.9 | 56.8 | |||||
| HBS | 482 | 1.9 | 96.3 | 35.3 | Male | 65.79 (9.67) | 4.28 (4.79) | 73.7 | 39.4 |
| Female | 66.60 (9.40) | 3.97 (4.30) | 70.0 | 42.4 | |||||
| PROPARK | 327 | 5.0 | NA | 33.9 | Male | 59.56 (10.29) | 6.48 (5.00) | 67.1 | 69.9 |
| Female | 59.51 (11.63) | 6.98 (4.18) | 64.0 | 79.3 | |||||
| PARKFIT | 466 | 2.0 | NA | 33.3 | Male | 65.28 (7.41) | 4.97 (4.25) | NA | NA |
| Female | 65.49 (7.60) | 5.38 (4.76) | |||||||
| UDALL_PENN | 233 | 4.0 | 94.4 | 30.9 | Male | 70.53 (7.29) | 5.73 (4.96) | 84.5 | 46.0 |
| Female | 70.14 (8.15) | 6.64 (5.80) | 90.3 | 56.9 | |||||
| Fox Insight (non-PD) | 7588 | – | 95.8 | 78.8 | Male | 63.55 (7.89) | – | – | – |
| Female | 62.51 (7.39)* | ||||||||
| Fox Insight (PD) | 17 719 | – | 96.4 | 45 | Male | 66.72 (7.16) | 4.61 (3.24) | 80.3 | 29.4 |
| Female | 66.00 (7.10)* | 4.50 (3.25)* | 76.8* | 35.0* |
f-u, median follow-up period; European, European descent; Duration, mean disease duration; LD, levedopa use; DA, dopamine agonist use. Age, mean (standard deviation).
P < 0.05 for t test comparing with male versus female.
Abbreviations: DATATOP, Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism; DIGPD, Drug Interaction with Genes in Parkinson’s Disease; HBS, Harvard Biomarkers Study; NET-PD_LS1, NIH Exploratory Trials in Parkinson’s Disease Large Simple Study 1; PARKFIT, ParkFit study; PARKWEST, The Norwegian ParkWest study; PDBP, Parkinson’s Disease Biomarker Program; PICNICS, Parkinsonism Incidence and Cognitive and Non-motor heterogeneity In Cambridgeshire; PPMI, Parkinson’s Progression Markers Initiative; PreCEPT_PostCEPT, Parkinson Research Examination of CEP-1347 Trial and PostCEPT; PROPARK, Profiling Parkinson’s disease study; and UDALL_PENN, Morris K. Udall Centers for Parkinson’s Research.
Fox Insight
To evaluate the consistency of results from the longitudinal dataset, we explored an independent dataset, Fox Insight. Fox Insight is an online-only, PD research cohort.18 The details of the study are available online (https://foxinsight.michaeljfox.org/). Individuals, aged 18 or older, with and without PD, were enrolled through in-person referral or online advertisements. The participants provided online informed consent, and self-reported demographic, characteristics, symptoms, medical history, and PD medication data were collected. Although Fox Insight is a longitudinal study, we analyzed the data cross-sectionally for the present study because the follow-up periods were relatively short (eg, the median follow-up period was 0.4 years for Non-Motor Symptoms Questionnaire). During the analysis step, we adjusted for age and disease duration. To limit the impacts of the extreme data points, we included participants from the middle 80% of the age distribution and the disease duration distribution (only among PD participants), which excluded any participants younger than the lower 10th percentile (< 46.8 years old) or older than the 90th percentile (> 77.4 years old) and PD patients with a disease duration shorter than 1 year (10th percentile) and longer than 13.5 years (90th percentile).
Measurements
Clinical Data Harmonization Among the 12 Cohorts
Twenty-three measurements, 11 binomial and 9 continuous measurements, were analyzed as outcome measures. Binomial outcomes included constipation, mild cognitive impairment, depression, daytime sleepiness, hyposmia, insomnia, wearing off, dyskinesias, RBD, restless-leg syndrome, and modified Schwab and England Activities of Daily Living Scale scores of 70 or lower (SEADL70). Some binomial outcomes had study-specific outcomes, and these criteria are summarized in the Supplemental Materials. For continuous outcomes, we collected the Hoehn and Yahr (HY) stage scale, total and sub-scores for the Unified Parkinson’s Disease Rating Scale (UPDRS) or the Movement Disorder Society–revised version (MDS-UPDRS), Mini-Mental State Examination, Montreal Cognitive Assessment (MoCA), and modified Schwab and England Activities of Daily Living Scale (SEADL). UPDRS scores were normalized to the z-values (UPDRS*_scaled).
Fox Insight
The February 2020 data were downloaded from https://foxden.michaeljfox.org. The demographic and disease status data were obtained from enrollment and registration questionnaires. For clinical outcomes of interest, we obtained the responses from the following questionnaires: Geriatric Depression Scale (GDS) for depression (score of six or higher);19 Non-Motor Symptoms Questionnaire (NMS-QUEST) for constipation, depressed mood (mood depressed) and a proxy for lack of the sense of smell/taste;20 MDS-UPDRS Part II questionnaire; REM Sleep Behavior Disorder Single-Question Screen;21 15-item Penn Parkinson’s Disease Daily Activities Questionnaire (PDAQ-15) for cognition-related instrumental functional abilities;22 and Understanding the Impact of Off and On in Parkinson’s Patients Questionnaire for dyskinesia and wearing off.
Statistical Analysis
Linear and logistic models were used to analyze baseline differences in PD presentation between male and female patients, per cohort. For binomial outcomes, a minimum of 25 outcomes should be observed in the analyzed cohort. Covariates were the linear and square terms of age and disease duration, to adjust for linear and nonlinear effects. We also adjusted for levodopa (L-dopa) and dopamine agonist use. To test differences in the progression rates among continuous outcomes, we used linear mixed-effects models, with the same covariates as the baseline models and random effects on the individual intercept and slope (change per year). We evaluated sex-associated differences in progression rates by testing the interaction between sex and disease duration. Survival analyses were conducted among those who did not have an outcome at baseline. Cox regression models were used, adjusting for the same covariates as those used in the baseline models. Any outcomes with fewer than 20 events over the follow-up period were not analyzed. The R model statements for these analyses are summarized in the Supplemental Materials.
Then, we combined the cohort-level results with an inverse variance-weighted random-effect model. We focused on robust associations throughout the cohorts; therefore, meta-analyses with P-values less than 0.05 for a test of homogeneity were excluded from further evaluations. Any associations with a two-sided P-value of 0.05, after Bonferroni-correction for the number of total analyses, were considered significant.
For the analysis of the Fox Insight dataset, we tested two terms: the mean difference between men and women (main term) and the interaction between sex and disease duration (interaction term). The adjusted covariates were linear and square age, linear and square disease duration, and indicators of L-dopa and dopamine agonist usage. We further analyzed the association between sex and outcomes among non-PD participants, adjusted for linear and square age. Then, we conducted a test of homogeneity between sex-associated differences identified among PD cases and non-PD participants, to evaluate whether the sex differences were PD-specific or reflected differences observed in the non-PD population. In the analyses for this dataset, we used a significance level of 0.05 for the raw P-value because the purpose of these analyses was to evaluate consistency with the longitudinal analyses.
All the statistical analyses and drawings were executed using R version 3.6 and python version 3.7. The analysis scripts are available at https://github.com/neurogenetics/PDpheno_by_sex.
Results
The cohort participants are summarized in Table 1. Participants in these cohorts varied in age and PD stage; however, most participants were in relatively early PD phases. The majority of participants were of European descent. Fox Insight included more female participants than the other cohorts, and the ratio of women to men was especially high among non-PD participants, as previously described.23 Moreover, we did not observe a significant difference in age of diagnosis between the men and the women among each cohort except for Fox Insight, in which the female patients had on average 0.61 (SD: 0.12) years younger age of diagnosis than the male patients. Interestingly, the age of non-PD participants in Fox Insight was also younger than male non-PD participants. The younger age of onset may be reflecting different age distributions of the study population by sex in Fox Insight. In the following analyses, we adjusted for age, disease duration, and medications.
In total, we conducted 40 meta-analyses, using the clinic-based longitudinal data, three of which were rejected following a test of heterogeneity, with a significance level of 0.05. Using the Bonferroni correction of multiple comparisons, we set our P-value (P) threshold to 0.05/37 = 0.00135. Among these associations, nine were significant, and the direction and magnitude of associations linked to being female compared with being male are shown in Table 2 and Figs. 1 and 2. (All meta-analysis results can be found in Supplemental Materials.)
TABLE 2.
Meta-analysis results for significant associations with sex and phenotypes (reference: male)
| Outcome | Beta | SE | P | P-adj | Mean [95%CI] |
|---|---|---|---|---|---|
| Progression analysis | |||||
| Cognitive_Impairment | −0.436 | 0.102 | 2.1E-05 | 7.7E-4 | 0.65 [0.53, 0.79] (HR) |
| Dyskinesia | 0.255 | 0.055 | 4.1E-06 | 1.6E-4 | 1.29 [1.16, 1.44] (HR) |
| UPDRS2_scaled | −0.139 | 0.029 | 1.1E-06 | 4.1E-5 | −0.14 [−0.20, −0.08] |
| UPDRS_scaled | −0.113 | 0.025 | 5.3E-06 | 2.0E-4 | −0.11 [−0.16, −0.06] |
| Baseline analysis | |||||
| Dyskinesia | 0.434 | 0.129 | 7.3E-04 | 0.0277 | 1.54 [1.20, 1.99] (OR) |
| MoCA | 0.634 | 0.186 | 6.8E-04 | 0.0251 | 0.63 [0.27, 1.00] |
| UPDRS2_scaled | −0.124 | 0.031 | 6.5E-05 | 0.0024 | −0.12 [−0.18, −0.06] |
| UPDRS3_scaled | −0.114 | 0.031 | 2.5E-04 | 0.0093 | −0.11 [−0.17, −0.05] |
| UPDRS_scaled | −0.107 | 0.027 | 6.9E-05 | 0.0026 | −0.11 [−0.16, −0.05] |
Progression analyses test the association between incidence rates (binomial) or rates of change per years (continuous) and sex. The models were adjusted for age and disease duration (both linear and square terms), indictors for levodopa and/or agonist usages. “_scaled” scores were normalized (mean 0, standard deviation of 1) to the baseline distributions as the original scores.
SE, standard error; P-adj, Bonferroni adjusted P (raw-P times 37 [the number of multiple comparisons]).
Mean [95%CI], Mean and 95% confidence interval of the difference in each scale. HR, hazard ratio; OR, odds ratio, UPDRS, unified Parkinson’s disease rating scale; MoCA, Montreal Cognitive Assessment.
FIG. 1.

Forest plots depicting sex differences in outcomes in progression analyses. DATATOP, Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism; DIGPD, Drug Interaction with Genes in Parkinson’s Disease; HBS, Harvard Biomarkers Study; NET-PD_LS1, NIH Exploratory Trials in Parkinson’s Disease Large Simple Study 1; PARKFIT, ParkFit study; PARKWEST, The Norwegian ParkWest study; PDBP, Parkinson’s Disease Biomarker Program; PICNICS, Parkinsonism Incidence and Cognitive and Non-motor heterogeneity In Cambridgeshire; PPMI, Parkinson’s Progression Markers Initiative; PreCEPT_PostCEPT, Parkinson Research Examination of CEP-1347 Trial and PostCEPT; PROPARK, Profiling Parkinson’s disease study; and UDALL_PENN, Morris K. Udall Centers for Parkinson’s Research.P, non-adjusted P-values; I_sq, I2 statistic; QEp, test of heterogeneity. “_scaled” scores were normalized (mean 0, standard deviation of 1) to the baseline distributions as the original scores.
FIG. 2.
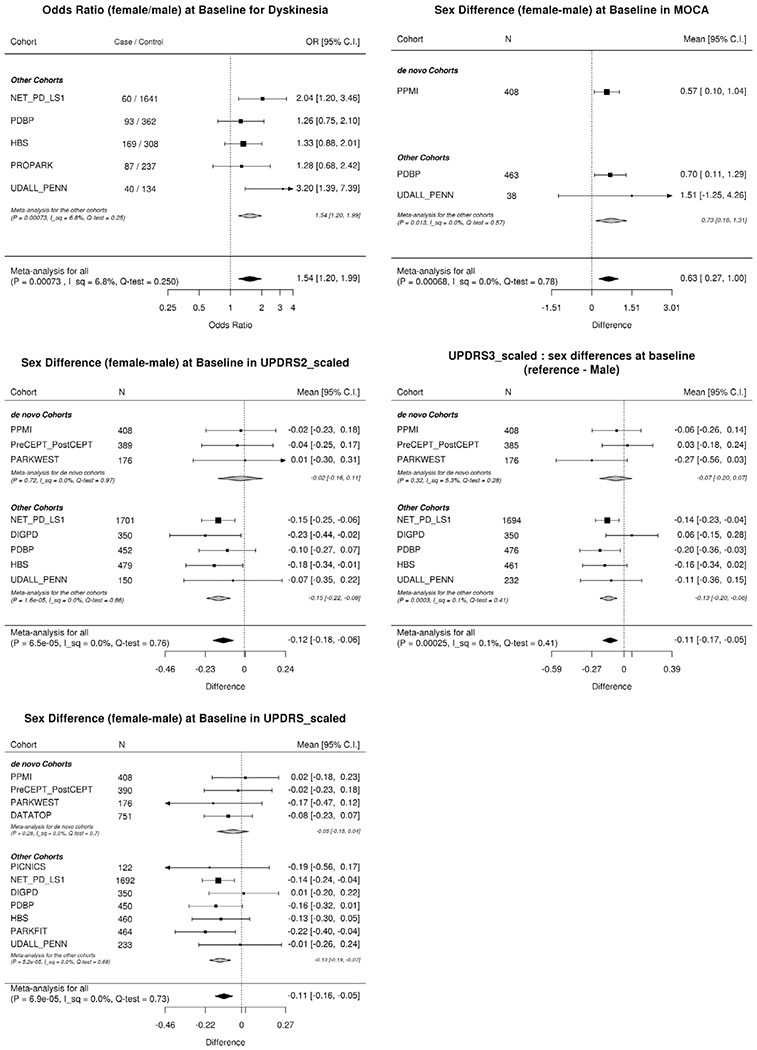
Forest plots depicting sex differences in outcomes in baseline analyses. DATATOP, Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism; DIGPD, Drug Interaction with Genes in Parkinson’s Disease; HBS, Harvard Biomarkers Study; NET-PD_LS1, NIH Exploratory Trials in Parkinson’s Disease Large Simple Study 1; PARKFIT, ParkFit study; PARKWEST, The Norwegian ParkWest study; PDBP, Parkinson’s Disease Biomarker Program; PICNICS, Parkinsonism Incidence and Cognitive and Non-motor heterogeneity In Cambridgeshire; PPMI, Parkinson’s Progression Markers Initiative; PreCEPT_PostCEPT, Parkinson Research Examination of CEP-1347 Trial and PostCEPT; PROPARK, Profiling Parkinson’s disease study; and UDALL_PENN, Morris K. Udall Centers for Parkinson’s Research.P, non-adjusted P-values; I_sq, I2 statistic; QEp, test of heterogeneity. “_scaled” scores were normalized (mean 0, standard deviation of 1) to the baseline distributions as the original scores
Female PD patients were less likely to develop cognitive impairments over time (hazard ratio [HR] 0.65 [0.53, 0.79] [mean {95% confidence interval}], P = 2.1E-5) than male PD patients, and an even stronger association was observed when we adjusted for years of education (HR 0.59 [0.48, 0.73], P = 4.6E-7, Supplemental Material). This association remained significant when we further adjusted for the baseline MoCA score (HR 0.56 [0.37, 0.86], P = 0.007) or the baseline MMSE score (HR 0.67 [0.51, 0.90], P = 0.007, Supplemental Material) at the significance level of 0.05. In addition, the baseline MoCA scores were higher in female patients (0.63 [0.27, 1.00]) than in male patients, whereas the baseline MMSE score was not significantly different between sexes (P = 0.97, Supplemental Materials).
Female patients presented with a higher rate of developing dyskinesia (HR 1.29 [1.16, 1.44]). To assess the impacts of weight, body mass index (BMI), and medication on this association, we conducted ad hoc analyses on a subset of data (PDBP, PPMI, and NET_PD_LS1: 2281 participants) for which height at baseline, weight at baseline, and medication at visits were recorded. We adjusted the analyses for each of these factors. With the “weight” adjustment, the association was no longer significant (P = 0.058), whereas the magnitude of the association became larger when adjusted for L-dopa dosages or L-dopa equivalent dosages. Adjusting for BMI did not substantially change the magnitude of the association (Beta: from 0.284 to 0.249), and the sex difference remained still significant (Supplemental Materials). Consistent with the higher incidence rate of dyskinesia in female patients, female PD patients in non-de novo cohorts also presented more dyskinesia at baseline than male patients.
Activities of daily living (ADL), captured in the UPDRS Part II, were better in female PD patients than in male PD patients in the baseline analysis (−0.12 [−0.18, −0.06], in the z-score), and the progression rate was slower in female patients than in male patients (−0.14 [−0.20, −0.08] in z-score per year). We added post-hoc analyses of UPDRS Part II scores in the different versions separately. The baseline score differences (female–male) were − 0.57 [−1.20, 0.06] (P = 0.07) in MDS-UPDRS and − 0.52 [−0.82, −0.21] (P = 7.9E-4) in the original UPDRS. The differences in the progression rate were − 0.81 [−1.18, −044] (P = 1.4E-5) in MDS-UPDRS and − 0.43 [−0.71, −0.15] (P = 2.5E-3) in the original UPDRS. A more detailed analysis of the forest plots of the UPDRS Part II scores at baseline showed that the associations between sex and UPDRS Part II were not apparent among the de novo cohorts but, rather, were driven by differences observed in the non-de novo cohorts (Fig. 1). Although we did not find significant sex-associated differences in progression rates in the UPDRS Parts I/III/TV, the rate of change for the total UPDRS scores was significantly milder in female patients than in male patients (−0.11 [−0.16, −0.06] per year, in the z-score). In the raw scores, the sex-associated difference (female–male) in rate of change in MDS-UPDRS total score (female–male) was −2.7 [−3.47, −1.95] (P = 2.3E −12) and that of the original UPDRS total score was −0.91 [−1.33, −0.49], (P = 2.66E-05). When only considering the de novo cohorts, similar results were reported for UPDRS Part III, with a slower progression rate in female patients than in male patients (−0.14 [−0.21, −0.07] in z-score per year, P = 2.6E-5, Supplemental Materials). This was corresponding to −1.59 [−2.47, −0.71] (P = 4.6E-4) per year difference (female–male) in the rate of change in MDS-UPDRS Part III or − 1.01 [−1.78, −0.24] (P = 0.01) per year in the original UPDRS Part III.
Finally, female patients also had lower scores on the UPDRS Part III and the UPDRS total score compared with male patients during the baseline analyses.
When analyzing similar phenotypes within the Fox Insight dataset, we generally confirmed the results of the longitudinal dataset analyses (Table 3). In the Fox Insight dataset analysis, the interaction terms between sex and disease duration indicated the average sex-associated differences in the longitudinal trajectories for the outcomes. For example, a positive association for the interaction between disease duration and PDAQ-15 indicated that the PDAQ-15 scores for female patients were higher than those in male patients (ie, better cognition-related instrumental functional abilities) among patients with longer disease durations in the Fox Insight dataset. To illustrate this, we visualized the sex differences, stratified by disease duration (Supplemental Materials). The results are consistent with those for the longitudinal dataset analysis, indicating that female patients had a lower risk of developing cognitive impairments during the disease course. Similarly, the results from the Fox Insight dataset were consistent with the increased rate of dyskinesia development among female patients compared with male patients, and the lower scores and a slower deterioration rate in UPDRS Part II among female patients, as observed in the longitudinal analyses.
TABLE 3.
Analysis results for sex difference in main term and interaction term with sex and disease duration in replication cohort (reference: male)
| PD |
Control |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interaction term |
Main term |
Main term |
||||||||||
| Outcome | Beta | SE | P | Consistency with LT analysis | Beta | SE | P | Consistency with LT analysis | Beta | SE | P | Test of Homogeneity in main effect |
| UPDRS2 total | −0.185 | 0.033 | 3.2E-08 | Consistent | −0.487 | 0.184 | 8.1E-03 | Consistent | ||||
| Cognitive IADL (PDAQ-15) | 0.219 | 0.049 | 6.8E-06 | Consistent | −0.024 | 0.266 | 0.928 | Consistent | −0.668 | 0.297 | 0.025 | 0.106 |
| Dyskinesia | 0.073 | 0.032 | 0.024 | Consistent | −0.311 | 0.205 | 0.129 | |||||
| Wearing off | −0.001 | 0.041 | 0.977 | Consistent | 0.259 | 0.217 | 0.232 | Consistent | ||||
| Depression (GDS total > 5) | −0.013 | 0.010 | 0.203 | Consistent | −0.030 | 0.056 | 0.596 | Consistent | −0.165 | 0.066 | 0.012 | 0.120 |
| pRBD (single question) | −0.003 | 0.011 | 0.767 | Consistent | −0.698 | 0.059 | 1.2E-32 | −0.696 | 0.080 | 4.6E-18 | 0.990 | |
| NMSQ_Awake | 0.001 | 0.012 | 0.963 | Consistent | −0.216 | 0.072 | 2.5E-03 | −0.292 | 0.091 | 1.3E-03 | 0.514 | |
| NMSQ_Sleep | −0.003 | 0.011 | 0.789 | Consistent | 0.406 | 0.058 | 2.4E-12 | 0.492 | 0.064 | 1.4E-14 | 0.317 | |
| NMSQ_Feel | −0.004 | 0.010 | 0.676 | Consistent | 0.384 | 0.055 | 4.3E-12 | 0.342 | 0.065 | 1.6E-07 | 0.628 | |
| NMSQ_Constipation | −0.018 | 0.010 | 0.072 | Consistent | 0.173 | 0.055 | 1.7E-03 | 0.310 | 0.075 | 3.8E-05 | 0.143 | |
| NMSQ_Smell | −0.011 | 0.011 | 0.314 | Consistent | 0.034 | 0.059 | 0.568 | Consistent | −0.326 | 0.111 | 3.3E-03 | 4.2E-03 |
“Main term” is the average difference between the women and the men (reference: males). “Interaction term” is the interaction between disease duration and sex. The adjusted covariates were linear and square age for non-PD participants. For PD participants, linear and square disease duration, and indicators of levodopa and dopamine agonist usage were further adjusted.
SE, standard error; PDAQ-15, the Penn Parkinson’s Daily Activities Questionnaire-15 (15-item measure of cognitive instrumental activities of daily living (IADL) for Parkinson’s disease patients derived from the original 50-item PDAQ), ranging 0–60 (the lower the worse); Depression, Geriatric Depression Scale score more than 5. Consistency with longitudinal dataset analyses was evaluated for outcomes (Consistency with LT analysis).
Abbreviations: NMSQ, Non Motor Symptom Questionnaire; NMSQ_awake: difficult to stay awake; NMSQ_Sleep, difficulty getting sleep at night; NMSQ_Feel, feeling sad, “low” or “blue”; NMSQ_Smell, loss or change in your ability to taste or smell.
In addition, null differences between male and female patients in the presentation and progression of wearing off, depression, and hyposmia were also supported by the Fox Insight dataset. In contrast, the loss of the sense of smell/taste was significantly more frequently reported in men among the control participants. Having PD might diminish the general sex difference associated with this phenotype.
Single-question answers for RBD and some NMSQuest questionnaire questions regarding “difficult to stay awake” (NMSQ_Awake), “difficulty in getting to sleep” (NMSQ_Sleep), “feeling sad, low or blue” (NMSQ_Feel), and NMSQ_Constipation were significantly different according to sex in the Fox Insight dataset. The prevalences of similar outcomes, such as possible RBD, daytime sleepiness, insomnia, depression, and constipation, were not significantly associated with sex in the meta-analyses of 12 longitudinal cohorts. However, the test for these associations gives raw P-values less than 0.05, with the same directions as the Fox Insight results. The primary analyses may not have included large enough sample sizes to detect these associations. All of the sex-phenotype associations among PD participants, not significant in the longitudinal dataset but significant in the Fox Insight dataset, were also significant among non-PD participants. In addition, based on the test of homogeneity between the results from PD and non-PD participants, suggesting that the magnitudes of these sex-associated differences in PD participants did not differ from those in non-PD participants.
Discussion
We analyzed clinic-based, longitudinal data from 5946 participants and meta-analyzed the differences in presentation and progression of phenotypes between men and women with PD. We also used web-based, online cohorts and analyzed data from 17 719 PD patients and 7588 non-PD participants to confirm our results. The results suggested that female PD patients develop dyskinesia early, progress more slowly with respect to ADL restrictions, and are less likely to develop cognitive impairments. For some non-motor symptoms explored in the online questionnaires (eg, possible RBD, daytime sleepiness, insomnia, depressive mood, and constipation), we found significant sex-associated differences among PD participants, only in the Fox Insight dataset. These unconfirmed sex-associated differences may not be specific to PD, as we also observed the same associations in the non-PD participants.
Some studies have previously reported that female patients demonstrated an increased risk of developing earlier and more severe dyskinesia11,15 and a longer duration of dyskinesia.16 These reports are consistent with the faster development of dyskinesia among female patients and the large rate of UPDRS Part IV score increases observed in our study. The reasons for this phenomenon are not fully understood, but the relatively higher L-dopa dosages with respect to body weight in women may be partially responsible.17 For example, the commonly used L-dopa tablet contains 50 mg or 100 mg L-dopa, and this is relatively a larger jump for those with less weight, and that may result in stronger treatment for them compared with those with more weight. Our ad hoc analyses also suggested that body weight plays a role in the association between sex and the early development of dyskinesia.
Contradictory results have been reported previously with regard to sex-associated differences in ADL impacts. Two studies evaluated patients who underwent surgical treatment for PD. One study observed no differences in the UPDRS Part II scores between men and women, whereas the other study reported that women had worse scores than men. In these studies, women had a longer duration of disease, which may have affected the results. Another cross-sectional study also reported worse UPDRS Part II scores among female patients.11 They reported that, among the five categories of overall ADL capacity, the two most-severe categories were more frequent among women than men, based on the results of a chi-squared test, whereas our analyses used UPDRS Part II scores and multivariable regression models. These different outcome measurements and statistical approaches may account for different results.
The slower development of cognitive declines in female patients was reported by some longitudinal studies.5,6,24 The executive and attention features were primarily affected in PD patients. Although Alzheimer’s disease, for which women confer more risk, is emphasized as disability in the memory feature, the executive and attention features are primarily affected in PD patients. MoCA is more sensitive for detecting dysfunctions in these areas than MMSE,25 and this may be one of the reasons that we observed baseline difference in MoCA but not MMSE. In contrast, the longitudinal differences in the rates of decline for either the MoCA or MMSE were not significantly different between the two sexes, in our data. Interestingly, MoCA scores were sometimes reported to be higher in healthy aging women than in men.26–28 The slower development of cognitive impairment observed in female patients may reflect their relatively high baseline abilities in the areas that are susceptible to PD, although neither the baseline MoCA score nor MMSE score was able to completely explain the association between sex and the development of cognitive impairment in the current data.
Several associations that were previously reported were not observed in the current analysis. RBD was reported to be more prevalent in men with PD than in women with PD,9,10 although some studies have disagreed.29,30 We were unable to confirm this association in the current longitudinal dataset. Although the prevalence of possible RBD, as detected by single-question screening was higher in male patients among the Fox Insight cohort, a similarly increased prevalence in possible RBD for non-PD male participants makes the PD-specific nature of this association questionable. Female PD patients were more depressed, according to previous reports.11–14 We were not able to confirm a sex-associated difference in the presentation or progression of depression, in either the longitudinal data or the Fox Insight dataset. However, female PD patients expressed a depressive mood more frequently than male patients, in response to the related NMSQuest question (“feeling sad, ‘low’ or ‘blue’”) from the Fox Insight dataset. However, the magnitude of the association was not different between PD and non-PD participants, indicating that the sex difference associated with this outcome may not be PD-specific. Regarding the NMSQ items evaluated, the similar null results except for NMSQ_Smell were reported previously in a cross-sectional analysis of de novo PD patients.31 Regarding the discrepancy in NMSQ_Smell, it may be possible that the sex difference in reported loss of smell/taste may be detectable only in the de novo PD stage.
The current study has some limitations. Fox Insight is an online-only cohort, which is inherently different from a clinic-based cohort; however, our analyses were mostly consistent across these two different settings. In addition, because the study participants were almost all of European descent, the generalizability of these observations across different ancestrally distinct groups should be verified. In this study, we focused on the overall associations between sex and phenotypes and did not separate the biological mechanisms from the environmental mechanisms. For example, the effect of estrogen on PD has been investigated frequently, and the conflicting results were reported.32 but we did not collect necessary data to rigorously evaluate the impact of estrogen on the differences. Similarly, we did not have enough data to investigate environmental factors such as smoking, alcohol, diet, physical activity levels, and socioeconomic factors. The different distribution of these factors by sex may explain the differences we observed in the current study. Well-designed studies are warranted to dissect the overall differences into each underlying pathway.
Despite some limitations, the current study has some strengths. First, the total number of participants examined in our longitudinal analysis was one of the largest populations studied. Second, although each study had different cohort characteristics, we controlled for heterogeneity and multiple comparisons to detect robust signals. Most of the associations identified between sex and disease presentation and progression were consistent between the longitudinal cohort and analyses performed using the independent Fox Insight dataset. Thus, our results could be generalized to PD patients across various disease stages in different contexts, given the range of studies incorporated. Third, by comparing PD patients with non-PD individuals, we obtained insight into whether sex-associated phenotypes in PD were disease-specific or reflected more general sex differences. Finally, female PD patients have been an underrepresented population in clinical trials.33 The current work emphasizes the importance of recognizing gender biases when developing treatments for PD in the real world.
Supplementary Material
Acknowledgment:
We thank all study participants and their family, investigators, and members of the following studies: Parkinson Study Group: Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism (DATATOP); Drug Interaction with Genes in Parkinson’s Disease (DIGPD); Harvard Biomarkers Study (HBS); NET-PD_LS1, NIH Exploratory Trials in Parkinson’s Disease Large Simple Study 1; The Norwegian ParkWest study (ParkWest); Parkinson’s Disease Biomarker Program (PDBP); Parkinsonism Incidence and Cognitive and Non-motor heterogeneity In Cambridgeshire (PICNICS); Parkinson’s Progression Markers Initiative (PPMI); Parkinson Study Group: Parkinson Research Examination of CEP-1347 Trial (PreCEPT) and its following study (PostCEPT); Profiling Parkinson’s disease study (ProPark); Morris K. Udall Centers for Parkinson’s Research (Udall); and Fox Insight study.
PPMI—a public-private partnership—is funded by The Michael J. Fox Foundation for Parkinson’s Research and funding partners, including AbbVie, Allergan, Avid Radiopharmaceuticals, Biogen, BioLegend, Bristol-Myers Squibb, Celgene, Denali Incorporated, GE Healthcare, Genentech, GlaxoSmithKline, Eli Lilly and Company, Lundbeck, Merck & Co., Meso Scale Discovery, Pfizer, Piramal, Prevail Therapeutics, Roche, Sanofi Genzyme, Servier Laboratories, Takeda, Teva, UCB, Verily, Voyager Therapeutics, and Golub Capital (www.ppmi-info.org/fundingpartners). The Fox Insight study (FI) is funded by The Michael J. Fox Foundation for Parkinson’s Research. We would like to thank the Parkinson’s community for participating in this study to make this research possible.
We also thank the following grants and financial supporters of aforementioned studies; DATATOP was supported by a Public Health Service grant (NS24778) from the National Institute of Neurological Disorders and Stroke (NINDS); by grants from the General Clinical Research Centers Program of the National Institutes of Health at Columbia University (RR00645), the University of Virginia (RR00847), the University of Pennsylvania (RR00040), the University of Iowa (RR00059), Ohio State University (RR00034), Massachusetts General Hospital (RR01066), the University of Rochester (RR00044), Brown University (RR02038), Oregon Health Sciences University (RR00334), Baylor College of Medicine (RR00350), the University of California (RR00827), Johns Hopkins University (RR00035), the University of Michigan (RR00042), and Washington University (RR00036), the Parkinson’s Disease Foundation at Columbia-Presbyterian Medical Center, the National Parkinson Foundation, the Parkinson Foundation of Canada, the United Parkinson Foundation, Chicago, the American Parkinson’s Disease Association, New York, and the University of Rochester; DIGPD is supported by Assistance Publique Hôpitaux de Paris, funded by a grant from the French Ministry of Health (PHRC 2008, AOM08010) and a grant from the Agence Nationale pour la Sécurité des Médicaments (ANSM 2013); HBS is supported by the Harvard NeuroDiscovery Center, The Michael J. Fox Foundation, NINDS U01NS082157, U01NS100603, and the Massachusetts Alzheimer’s Disease Research Center NIA P50AG005134; NET-PD_LS1 was supported by NINDS grants U01NS043128; ParkFit is supported by ZonMw (the Netherlands Organization for Health Research and Development (75020012)) and The Michael J. Fox Foundation for Parkinson’s research, VGZ (health insurance company), GlaxoSmithKline, and the National Parkinson Foundation; ParkWest is supported by the Research Council of Norway, the Western Norway Regional Health Authority, Stavanger University Hospital Research Funds, and the Norwegian Parkinson’s Disease Association; PDBP is a consortium with NINDS initiative; PICNICS has received funding from the Cure Parkinson’s Trust, the Van Geest Foundation and is supported by the National Institute of Health Research Cambridge Biomedical Research Centre; PPMI is supported by The Michael J. Fox Foundation for Parkinson’s research; PreCEPT and PostCEPT were funded by NINDS 5U01NS050095-05, Department of Defense Neurotoxin Exposure Treatment Parkinson’s Research Program. Grant Number: W23RRYX7022N606, The Michael J. Fox Foundation for Parkinson’s research, Parkinson’s Disease Foundation, Lundbeck Pharmaceuticals. Cephalon Inc, Lundbeck Inc, John Blume Foundation, Smart Family Foundation, RJG Foundation, Kinetics Foundation, National Parkinson Foundation, Amarin Neuroscience LTD, CHDI Foundation Inc, National Institutes of Health (NHGRI, NINDS), Columbia Parkinson’s Disease Research Center; ProPARK is funded by the Alkemade-Keuls Foundation, Stichting Parkinson Fonds, Parkinson Vereniging, The Netherlands Organisation for Health Research and Development; Udall is supported by NINDS.
Funding agency: This study was supported by the Intramural Research Program the National Institute on Aging (NIA, Z01-AG000949-02), Biogen Idec, and The Michael J. Fox Foundation for Parkinson’s Research.
Footnotes
Relevant conflicts of interests/financial disclosures: Nothing to report.
Ethical Compliance Statement
The study protocols were approved by the local institutional review boards, and all the participants provided written (longitudinal studies) or online (Fox Insight) informed consent. We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Miller IN, Cronin-Golomb A. Gender differences in Parkinson’s disease: clinical characteristics and cognition. Mov Disord 2010;25:2695–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Picillo M, Nicoletti A, Fetoni V, Garavaglia B, Barone P, Pellecchia MT. The relevance of gender in Parkinson’s disease: a review. J Neurol 2017;264:1583–1607. [DOI] [PubMed] [Google Scholar]
- 3.Georgiev D, Hamberg K, Hariz M, Forsgren L, Hariz G-M. Gender differences in Parkinson’s disease: a clinical perspective. Acta Neurol Scand 2017;136:570–584. [DOI] [PubMed] [Google Scholar]
- 4.Haaxma CA, Bloem BR, Borm GF, et al. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2007;78:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locascio JJ, Corkin S, Growdon JH. Relation between clinical characteristics of Parkinson’s disease and cognitive decline. J Clin Exp Neuropsychol 2003;25:94–109. [DOI] [PubMed] [Google Scholar]
- 6.Cholerton B, Johnson CO, Fish B, et al. Sex differences in progression to mild cognitive impairment and dementia in Parkinson’s disease. Parkinsonism Relat Disord 2018;50:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anang JBM, Gagnon J-F, Bertrand J-A, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 2014; 83:1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez-Martin P, Falup Pecurariu C, Odin P, et al. Gender-related differences in the burden of non-motor symptoms in Parkinson’s disease. J Neurol 2012;259:1639–1647. [DOI] [PubMed] [Google Scholar]
- 9.Özekmekçi S, Apaydin H, Kiliç E. Clinical features of 35 patients with Parkinson’s disease displaying REM behavior disorder. Clin Neurol Neurosurg 2005;107:306–309. [DOI] [PubMed] [Google Scholar]
- 10.Yoritaka A, Ohizumi H, Tanaka S, Hattori N. Parkinson’s disease with and without REM sleep behaviour disorder: are there any clinical differences? Eur Neurol 2009;61:164–170. [DOI] [PubMed] [Google Scholar]
- 11.Baba Y, Putzke JD, Whaley NR, Wszolek ZK, Uitti RJ. Gender and the Parkinson’s disease phenotype. J Neurol 2005;252:1201–1205. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez HH, Lapane KL, Ott BR, Friedman JH. Gender differences in the frequency and treatment of behavior problems in Parkinson’s disease. Mov Disord 2000;15:490–496. [DOI] [PubMed] [Google Scholar]
- 13.Riedel O, Klotsche J, Spottke A, et al. Cognitive impairment in 873 patients with idiopathic Parkinson’s disease. J Neurol 2008; 255:255–264. [DOI] [PubMed] [Google Scholar]
- 14.Scott B, Borgman A, Engler H, Johnels B, Aquilonius SM. Gender differences in Parkinson’s disease symptom profile. Acta Neurol Scand 2000;102:37–43. [DOI] [PubMed] [Google Scholar]
- 15.Accolla E, Caputo E, Cogiamanian F, et al. Gender differences in patients with Parkinson’s disease treated with subthalamic deep brain stimulation. Mov Disord 2007;22:1150–1156. [DOI] [PubMed] [Google Scholar]
- 16.Hariz G-M, Lindberg M, Hariz MI, Tommy Bergenheim A. Gender differences in disability and health-related quality of life in patients with Parkinson’s disease treated with stereotactic surgery. Acta Neurol Scand 2003;108:28–37. [DOI] [PubMed] [Google Scholar]
- 17.Zappia M, Crescibene L, Arabia G, et al. Body weight influences pharmacokinetics of levodopa in Parkinson’s disease. Clin Neuropharmacol 2002;25:79–82. [DOI] [PubMed] [Google Scholar]
- 18.Smolensky L, Amondikar N, Crawford K, et al. Fox insight collects online, longitudinal patient-reported outcomes and genetic data on Parkinson’s disease. Sci Data 2020;7:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17:37–49. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhuri KR, Martinez-Martin P, Schapira AHV, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: the NMSQuest study. Mov Disord 2006;21:916–923. [DOI] [PubMed] [Google Scholar]
- 21.Postuma RB, Arnulf I, Hogl B, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord 2012;27:913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brennan L, Siderowf A, Rubright JD, et al. The Penn Parkinson’s daily activities Questionnaire-15: psychometric properties of a brief assessment of cognitive instrumental activities of daily living in Parkinson’s disease. Parkinsonism Relat Disord 2016;25:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chahine LM, Chin I, Caspell-Garcia C, et al. Comparison of an online-only Parkinson’s disease research cohort to cohorts assessed in person. J Parkinsons Dis 2020;10:677–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pigott K, Rick J, Xie SX, et al. Longitudinal study of normal cognition in Parkinson disease. Neurology 2015;85:1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pendlebury ST, Markwick A, de Jager CA, Zamboni G, Wilcock GK, Rothwell PM. Differences in cognitive profile between TIA, stroke and elderly memory research subjects: a comparison of the MMSE and MoCA. Cerebrovasc Dis 2012;34:48–54. [DOI] [PubMed] [Google Scholar]
- 26.Thomann AE, Goettel N, Monsch RJ, et al. The Montreal cognitive assessment: normative data from a German-speaking cohort and comparison with international normative samples. J Alzheimer’s Dis 2018;64:643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konstantopoulos K, Vogazianos P, Doskas T. Normative data of the Montreal cognitive assessment in the Greek population and parkinsonian dementia. Arch Clin Neuropsychol 2016;31:246–253. [DOI] [PubMed] [Google Scholar]
- 28.Borland E, Nägga K, Nilsson PM, Minthon L, Nilsson ED, Palmqvist S. The Montreal cognitive assessment: normative data from a large Swedish population-based cohort. J Alzheimer’s Dis 2017;59:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bjørnarå KA, Dietrichs E, Toft M. REM sleep behavior disorder in Parkinson’s disease – is there a gender difference? Parkinsonism Relat Disord 2013;19:120–122. [DOI] [PubMed] [Google Scholar]
- 30.Bugalho P, Silva JA, Neto B. Clinical features associated with REM sleep behavior disorder symptoms in the early stages of Parkinson’s disease. J Neurol 2011;258:50–55. [DOI] [PubMed] [Google Scholar]
- 31.Picillo M, Amboni M, Erro R, et al. Gender differences in nonmotor symptoms in early, drug naïve Parkinson’s disease. J Neurol 2013;260:2849–2855. [DOI] [PubMed] [Google Scholar]
- 32.Shulman LM. Is there a connection between estrogen and Parkinson’s disease? Parkinsonism Relat Disord 2002;8:289–295. [DOI] [PubMed] [Google Scholar]
- 33.Tosserams A, Araújo R, Pringsheim T, et al. Underrepresentation of women in Parkinson’s disease trials. Mov Disord 2018;33:1825–1826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


