Important Compound Classes
Title
Small Molecule Modulators of SIGMA-1 and SIGMA-2 receptors and uses Thereof
Patent Publication Number
WO 2020/112846 A1
Publication Date
June 04, 2020
Priority Application
US 62/771,826
Priority Date
November 27, 2018
Inventors
Schiltz, G. E.; Iyamu, I. D.; Mishra, R. K.; Lv, W.; Malik, N.
Assignee Company
Northwestern University [US/US]; 633 Clerk Street, Evanston, Illinois 60208, United States
Disease Area
Alzheimer’s disease
Biological Target
SIGMA-1 and SIGMA-2 Receptors
Summary
The sigma receptors were initially identified as opioid receptors but have now been implicated in a number of functions including modulation of ion channels, synaptic plasticity, drug abuse, and gastrointestinal function. In addition, the sigma receptors have been shown to involve in a large number of diverse conditions such as pain, cancer, addiction, depression, neurodegenerative conditions, and amyolateral sclerosis, which suggest that their pharmacological regulation can be useful drugs to treat several of these conditions.
The sigma receptors are classified into two subtypes, known as sigma-1 receptor (S1R) and sigma-2 receptor (S2R). Sigma-1 receptor (S1R) is a multifunctional 25 kDa protein, ligand-operated and acting as chaperone at the endoplasmic reticulum (ER) membranes. S1R agonists have a diversity of S1R-mediated signaling functions that are broadly neuroprotective, prosurvival, and antiapoptotic; however, not much is known about the exact mechanisms of action at the molecular level. Nonetheless, S1R expression and function is associated with various neurological disorders, including Alzheimer’s (AD), amyotrophic lateral sclerosis/frontotemporal dementia, and Huntington’s diseases (HD). Furthermore, the interactions between the S1R and ion channels effect regulation of functional properties and the expression of some sodium, potassium, calcium, and TRP ion channels. S1R binds to ligands with diverse structures, including psychostimulant drugs such as cocaine, methamphetamine, dextromethorphan, haloperidol, fluoxetine, and donepezil. The behavioral phenotype of knockout S1R in mice revealed gender-related anxiety, depressive-like and memory alterations, which implied that the central nervous system appears to be the primary site of S1R activity and modulation. Therefore, S1R could have application to a potentially wide range of CNS diseases.
The sigma-2 receptor (S2R) is an endoplasmic reticulum (ER) transmembrane protein with a binding site determined to have the molecular identity as TMEM97, a biomarker for rapidly dividing cells, which is overexpressed in multiple tumor types. Distinction between agonists and antagonists at the S2R remains uncertain; however, the agonists appear to be potent anticancer agents. S2R antagonists in mouse models have been suggested as potential Alzheimer’s disease therapeutics based on their ability to prevent oligomer binding to neurons and their effects in preventing neurodegeneration, synapse loss and cognition. In addition, selective S2R ligands based on a norbenzomorphans scaffold have shown to be effective in mouse models of Alzheimer’s disease.
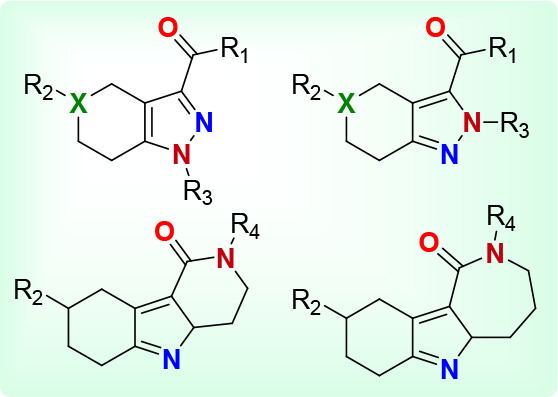
In spite of these advances, establishing specific roles for S1R and S2R has been difficult, which has increased the likelihood for off-target effects. As a consequence, the need exists for a more useful set of ligands that are closely related structurally but are potent and selective for either of these receptors. This Patent Highlight showcases a new class of small molecules having a tetrahydroindazole structure that functions as a modulator of S1R and S2R, which are useful as therapeutics for the treatment of Alzheimer’s disease, cancer, and other neurological conditions associated with the S1R and S2R activity.
Definitions
X is either C or N,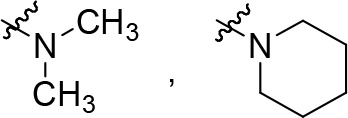
R1 is selected from some of the following: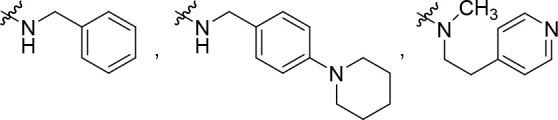
R2 is selected from some of the following: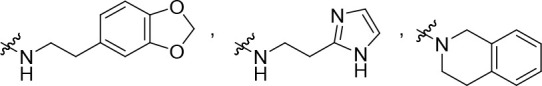
R3 is selected from the following: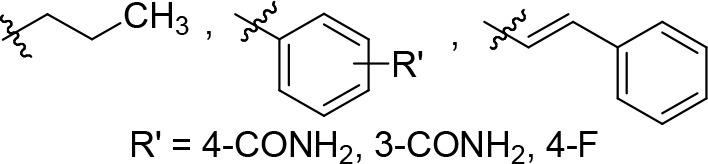
R4 = H or Me
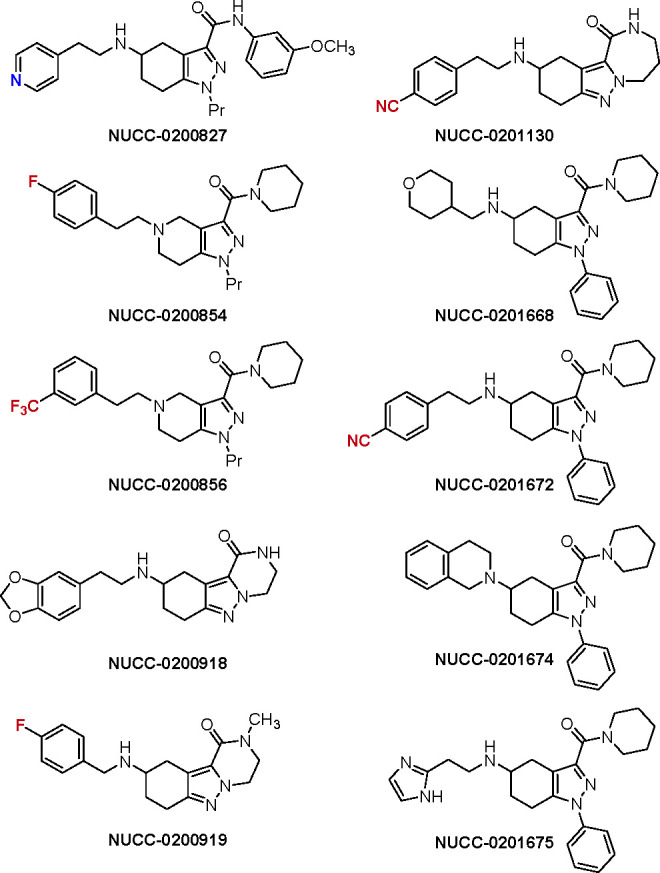
Key Structures
Biological Data
The table belows show S1R and S2R binding
activity, where Ki of +++ = < 1 μM;
++ = 1–10 μM and selectivity can be tuned by structural
modification.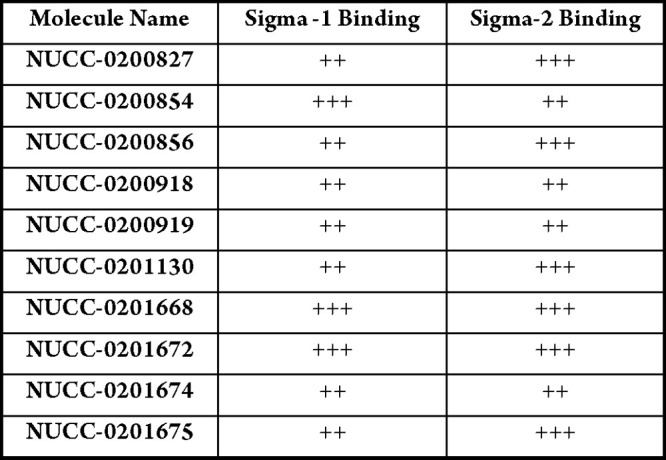
Recent Review Articles
-
1.
Agha H.; McCurdy C. R.. RSC Med. Chem. 2020, DOI: 10.1039/d0md00186d.
-
2.
Herrando-Grabulosa M.; Gaja-Capdevila N.; Vela J. M.; Navarro X.. Br. J. Pharmacol. 2020, DOI: 10.1111/bph.15224.
-
3.
Ma W.; Chen A.; Xie X.; Huang Y.. Neuropharmacology 2020, 108342.
-
4.
Zeng C.; Riad A.; Mach R. H.. Cancers 2020, 12, 1877.
-
5.
Vavers E.; Zvejniece L.; Maurice T.; Dambrova M.. Front. Pharmacol. 2019, 10, 223.
-
6.
Ryskamp D. A.; Korban S.; Zhemkov V.; Kraskovskaya N.; Bezprozvanny I.. Front. Neurosci. 2019, 13862.
The author declares no competing financial interest.


