Important Compound Classes
Title
Aza-heterobicyclic Inhibitors of MAT2A and Methods of use for Treating Cancer
Patent Publication Number
WO 2020/139991 A1
Publication Date
July 02, 2020
Priority Application
US 62/785,519
Priority Date
December 27, 2018
Inventors
Konteatis, Z. D.; Li, M.; Reznik, S. K.; Sui, Z.
Assignee Company
Agios Pharmaceutical, Inc. (US); 88 Sidney Street, Cambridge, MA 02139, United States
Disease Area
Cancer
Biological Target
Methionine adenosyltransferase isoform 2A (MAT2A)
Summary
S-Adenosyl-l-methionine (AdoMet or SAM) is the primary donor in biochemical transmethylation reactions. SAM is an essential amino acid in animals that plays a crucial role in immune disorders owing to its activities in epigenetic regulation, especially DNA methylation. The cellular enzyme, methionine adenosyltransferase (MAT), also known as S-adenosylmethionine synthetase, catalyzes the synthesis of S-adenosyl methionine from methionine and ATP. The liver plays a major role in the disposal of methionine and SAM utilization, mainly for the synthesis of phosphatidylcholine and creatine which are then utilized by the rest of the body. Furthermore, disruption of the methionine cycle causes methyl donor imbalance and results in fatty liver, hypermethionemia, hyperhomocysteinemia, and increased risk for hepatocellular carcinoma.
The MAT1A and MAT2A genes are encoded by two distinct catalytic MAT isoforms, while MAT2B encodes a regulatory MAT2A subunit. Expression level of MAT1A is specifically found in the adult liver, while MAT2A is widely distributed. Hypomethylation of the MAT2A promoter and histone acetylation is found to cause upregulation of MAT2A expression. Whereas, the downregulation of MAT1A and the up-regulation of MAT2A occur in hepatocellular carcinoma. As a result, antineoplastic therapy uses small interfering RNA to substantially suppresses growth and induces apoptosis in the hepatoma cells.
Some cancer cell lines that are deficient in methylthioadenosine phosphorylase (MTAP), an enzyme widely expressed in normal tissues that catalyzes the conversion of methylthioadenosine into adenine and 5-methylthioribose-phosphate, are particularly sensitive to the inhibition of MAT2A. Many human and murine malignant cells lack MTAP activity, and its deficiency is found in diseases such as nonsmall cell lung cancers (NSCLCs), pancreatic cancers, head and neck cancers, leukemias, gliomas, melanomas, bladder cancers, endometrial cancers, astrocytomas, osteosarcomas, myxoid chondrosarcomas, ovarian cancers, breast cancers, soft tissue sarcomas, non-Hodgkin lymphoma, and mesotheliomas.
The 9p21 chromosomal region is of interest because it is frequently deleted in a variety of cancers, including NSLC, leukemias, melanomas, pancreatic cancers, gliomas, and mesothelioma. Many tumors possess deletions that involve a 170 kb region containing MTAP, pl4ARF, and P16INK4A. The deletion process often inactivates more than one gene. For instance, deletion of the MTAP gene, but not p16INK4A is indicative of a cancer at an early stage of development, whereas deletion of the genes encoding for p16 and MTAP is indicative of a more advanced cancer.
The present Patent Highlight discloses exemplary compounds that inhibit MAT2A and are useful for treating various cancers, including therapeutics that are refractory to standard treatments such as radiation therapy, chemotherapy, surgery, and hormonal therapy.
Definitions
L = O, S, NR or a bond;
R1 = C1–C6-alkyl, C2–C6-alkenyl, C3–C6-carbocyclyl;
R2 = R3 = (C2–C6)alkynyl, C6–C10-aryl, 5- to 10-membered heteroaryl;
R4 = H, C1–C6-alkyl, −OH, −CN, halo;
R5 = H, C1–C6-alkoxy, C2–C6-alkenyl, −CN, halo.
Key Structures
Biological Assay
PiColorLock Gold kit was used to measure inhibition potency, and Cell Titer Glo was used to test the impart of test compound on cancer growth.
Biological Data
The table below shows representative
compounds that inhibit SAM with an IC50 according to the
following: A = < 100 nM, B = > 100 nM, and <1 μM, C
=
≥1 μM.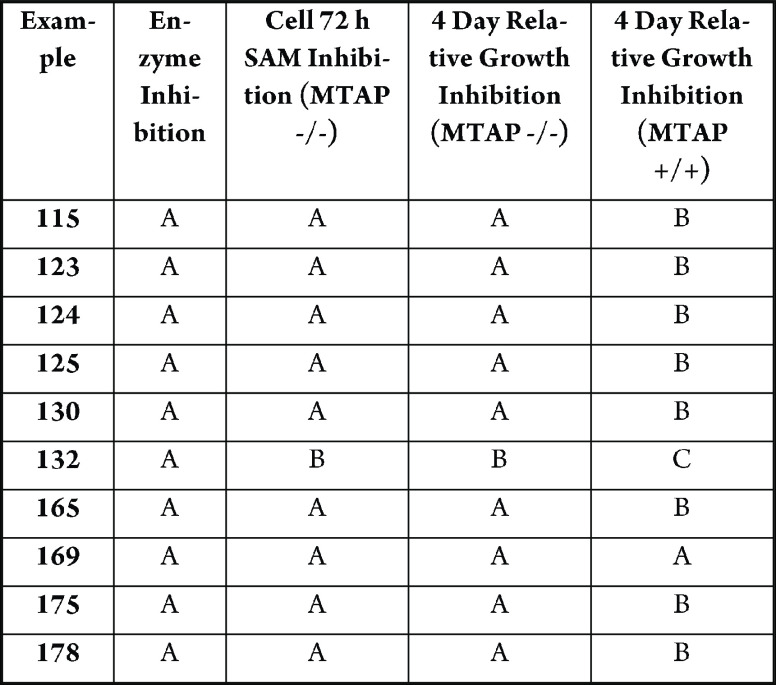
Recent Review Articles
-
1.
Rittle J.ACS Cent. Sci. 2019, 5, 1741..
-
2.
Maldonado L. Y.; Arsene D.; Mato J. M.; Lu S. C.. Exp. Biol. Med. 2018, 243, 107..
-
3.
Gao J.; Cahill C. M.; Huang X.; Roffman J. L.; Lamon-Fava S.; Fava M.; Mischoulon D.; Rogers J. T.. Neurotherapeutics 2018, 15, 156..
-
4.
Sharma A.; Sharma A.; Gerbarg P.; Bottiglieri T.; et al.. J. Clin. Psychiatry 2017, 78, 656..
The author declares no competing financial interest.




