Abstract
Clinical translation of drug-drug interaction (DDI) studies is limited, and knowledge gaps across different types of DDI evidences make it difficult to consolidate and link them to clinical consequences. Consequently, we developed information retrieval (IR) models to retrieve DDI and drug-gene interaction (DGI) evidences from 25 million PubMed abstracts, and distinguish DDI evidences into in-vitro pharmacokinetics (PK), and clinical PK and pharmacodynamics (PD) studies for FDA-approved and withdrawn drugs. Additionally, information extraction models were developed to extract DDI- and DGI-pairs from the IR-retrieved abstracts. An overlapping analysis identified 986 DDI-pairs between all three types of evidences. Another 2,157 and 13,012 DDI-pairs, and 3,173 DGI-pairs were identified from known clinical PK-PD DDI, clinical PD DDI, and DGI evidences, respectively. By integrating DDI and DGI evidences, we discovered 119 and 18 new pharmacogenetic hypotheses associated with CYP3A and CYP2D6, respectively. Some of these DGI evidences can also aid us in understanding DDI mechanisms.
Keywords: Bioinformatics, drug-drug interactions, adverse drug reactions, discovery
INTRODUCTION
Drug-drug Interactions (DDIs) are one of the major causes of adverse drug events (ADEs) and have been demonstrated as a public health burden.1, 2 With increasing rates of poly-pharmacy, the incidence of DDIs is most likely to increase, and thus drug interaction research remains essential.3 Current DDI studies investigate different but complimentary scopes of drug interactions: in-vitro pharmacokinetics (in-vitro PK), clinical pharmacokinetics (clinical PK), and clinical pharmacodynamics (clinical PD).4–6 In-vitro PK studies investigate DDI related molecular mechanisms such as metabolic enzymes or drug transporter proteins using recombinant systems or cell/tissue models. Clinical PK studies, on the other hand, evaluate whether one objective drug’s exposure is changed due to the co-administrated precipitant drug. The molecular mechanisms of clinical PK DDIs are not necessarily known, unless the two drugs are either known substrates/inhibitors/inducers of an enzyme. Clinical PD studies investigate whether the objective drug’s efficacy or adverse drug events (ADEs) are changed because of the co-administrated precipitant.7 In-vitro PK experiments can be easily connected to pharmacogenetics (PG) studies because of their shared proteins and genes but this is not necessarily true in case of clinical PK or PD DDI studies.8, 9
The goal of translational research in relation to DDI and PG studies is to achieve a comprehensive understanding of the PD, PK, and molecular mechanisms underlying drug effects in order to achieve clinical utility. However, it usually takes a long time to accomplish this overarching goal because of existing barriers between different scientific domains.4 A salient example is tamoxifen, whose CYP2D6 metabolic pathway was initially discovered in-vitro in 2003.10 The genetic effects of CYP2D6 on the exposure of tamoxifen and its active metabolites was later published in 2007.11 The PK interactions between tamoxifen and anti-fungals such as fluconazole were subsequently revealed in 2009.12 And finally, the combined effect of the CYP2D6 genotypes and drug inhibitors on tamoxifen efficacy and ADEs (hot flashes) was determined in 2010.13 This example clearly demonstrates the association between DDI and drug-gene interaction (DGI) studies, however, it also shows the extended duration of DDI and PG research needed to achieve translational goals.
The translational landscape of drug interactions research has created an enormous opportunity for the field of informatics. The diverse and independent scientific disciplines involved in DDI and DGI research make it difficult to provide comprehensive evidences for all drugs. Despite the existence of several databases, none of them have been successful in linking all the available information. DrugBank is probably the only database that comes close to identifying and including both DDI and DGI evidences.14 However, it’s PK and PD DDI evidences lack details on magnitude of drug exposure change and clinical phenotypes, respectively. DGI evidences in DrugBank include a drug’s relationship with metabolic enzymes or transporter proteins but not the effect of pharmacogenetics on PK and PD effects. On the other hand, PharmGKB is designed to provide PG evidences on PK and PD outcomes, but no DDI evidences.15 DiDB includes a collection of in-vitro PK and clinical PK DDI evidences, but very limited PD DDI and PG evidences.16 Therefore, it is of greatest translational research interest to consolidate these evidences in order to promote discovery of knowledge gaps between discordant DDI and DGI studies.5, 6
Text mining, as an efficient knowledge discovery tool has been extensively applied to mine drug interaction signals from the biomedical literature.17–21 For example, Percha and Altman have developed a novel classification model to map all drug-gene interactions (DGIs) in MEDLINE abstracts, and discover new drug-gene relationships.18 Previously, our group generated new DDI pairs by mining the PubMed literature using known cytochrome P450 (CYP450) probe substrates and inhibitors, and identifying all existing CYP450 substrates and inhibitors from in-vitro experiments.17 Recently, we also developed a DDI and DGI corpus with the goal of developing a new text mining algorithm and evaluating the performance of the text mining analyses separately for in-vitro and clinical PK DDI evidences.21 However, we did not investigate the overlapping or non-overlapping evidences between the two. None of the existing informatics analyses have fully investigated the translational landscape of DDI and PG studies and the knowledge gaps that exist between them, nor have they differentiated between in-vitro and clinical PK, and clinical PD evidences in the published DDI and PG studies.
In this paper, a text mining approach was utilized to differentially screen in-vitro PK DDI, clinical PK DDI, and clinical PD DDI, and DGI evidences, followed by an overlapping analysis. Our aim was to investigate and identify knowledge gaps among in-vitro PK, clinical PK, and clinical PD studies, and translate the literature-based discovery evidences between DDI and DGI studies.
METHODS
A detailed description of the methods involved in the development of the text mining approach is presented in the supplemental file. A brief description is included below.
Lexica construction
Lexica comprising of drug names, enzymes, action terms, and ADE terms were prepared. Based on the drug groups in DrugBank, FDA approved and withdrawn drugs (2403 generic names) were extracted for text mining. For drug enzyme terms, 94 symbol names and synonyms (350 terms in total) were collected from Gene ontology,22 HUGO Gene Nomenclature Committee (HGNC),23 and The Human Cytochrome P450 (CYP) Allele Nomenclature Database.24 The action terms that describe the drug and enzyme relationships (i.e. inhibition or induction) were collected from our PK ontology21 and the recent work by Percha and Altman.18 The 19,550 preferred terms (PTs) of adverse drug reactions were normalized from 70,177 lowest level terms (LLTs) in The Medical Dictionary for Regulatory Activity (MedDRA) database.25
Corpus construction
Two types of corpora, including information retrieval (IR) and information extraction (IE) corpora were constructed for retrieving DDI and DGI abstracts and extracting DDI/DGI pairs, respectively. The IR corpus has 300 manually curated DDI abstracts in each one of the in-vitro PK, clinical PK, and clinical PD studies. For PG studies, 3,429 DGI relevant abstracts were collected from PharmGKB. The IE corpus consists of 210 in-vitro PK DDI, 218 clinical PK DDI, 140 clinical PD DDI, and 395 DGI abstracts. In the IE corpus, terms such as drugs, enzymes and relationships between drug-drug/drug-gene pairs were annotated. The details of the data collection (Table S1), text annotation, and annotation evaluation process are provided in the supplemental file.
Text mining schemes
As shown in Figure 1, text mining for each type of DDI or DGI evidence was accomplished in two stages: Information Retrieval (IR) and Information Extraction (IE). In the IR stage, an optimal model that maximizes recall rate of identifying relevant abstracts for each type of study was built using the IR corpus. The document-level classifier was trained upon N1 positive DDI abstracts and N2 randomly selected negative abstracts. And, the performance of the document-level classifier was evaluated using the testing dataset (N3 DDI abstracts and N4 negative abstracts). The data collection statistics for the IR models is shown in Table 1. After the optimal IR models were built, 25 million abstracts were screened and relevant DDI and DGI abstracts were identified.
Figure 1.
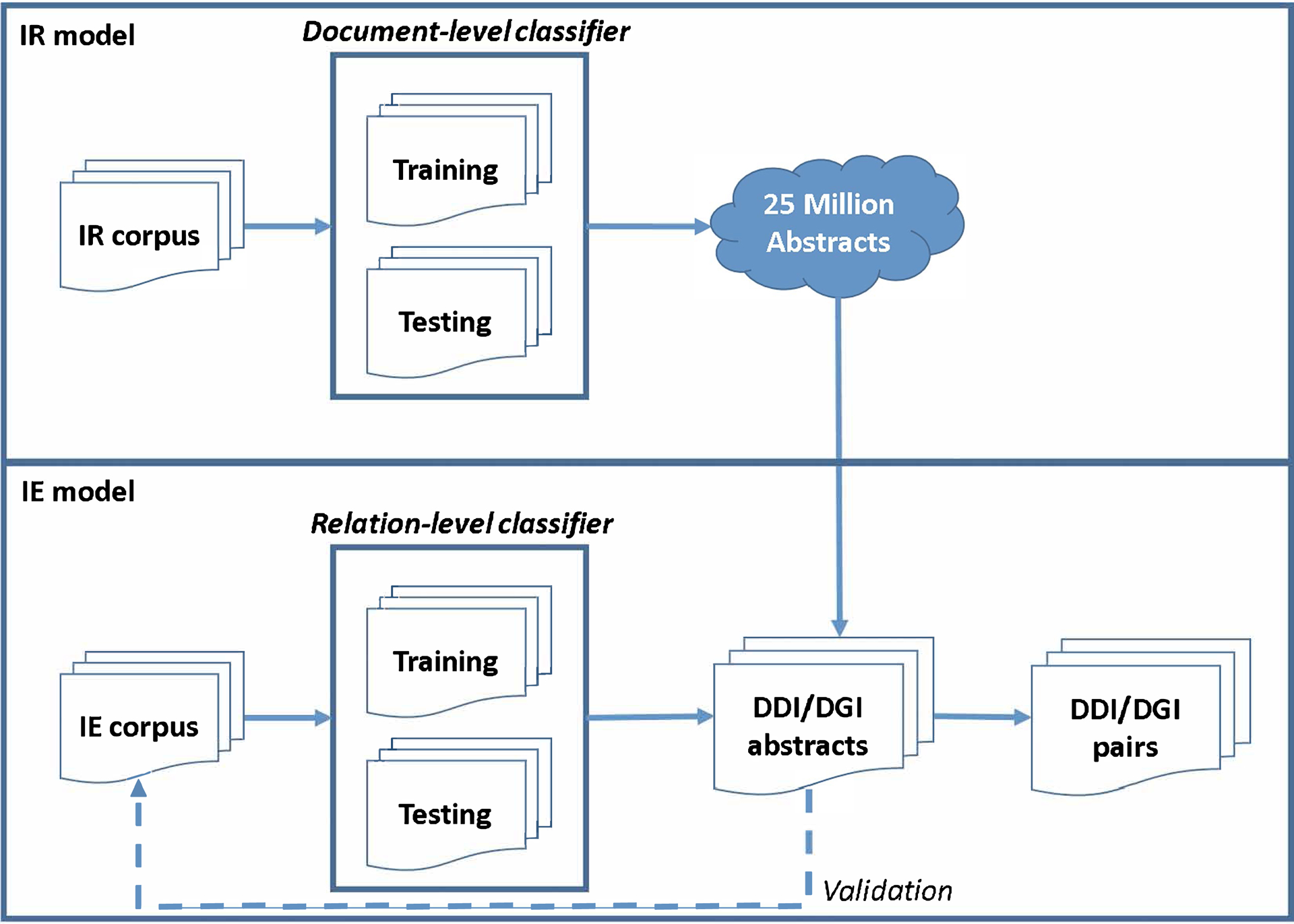
Text mining pipeline for the information retrieval and information extraction tasks
Table 1.
Data collection statistics for IR models
| Training data | Testing data | |||
|---|---|---|---|---|
| Study types | Positive (N1) | Negative (N2) | Positive (N3) | Negative (N4) |
| DDI | ||||
| In-vitro PK | 150* | 10,000 | 150* | 800† |
| Clinical PK | ||||
| Clinical PD | ||||
| DGI | 1,700 | 1,700 | 1,729 | 8,300 |
IR: information retrieval, PK: pharmacokinetics, PD: pharmacodynamics, DGI: drug-gene interactions
The 150 abstracts were different for training and testing as well as each of the in-vitro PK, clinical PK, and clinical PD DDI studies
Among 800 negative abstracts, 500 were single-drug or nutrition-related abstracts and 300 were randomly selected abstracts
In the IE stage, an optimal model that maximizes F-measure of extracting relation pairs was built using the IE corpus. The DDI or DGI relationship classifiers were built upon 60% of the true entity relation pairs in the IE corpus (i.e. training data) and the remaining 40% were used for performance evaluation (i.e. testing data). Finally, using the optimal IE models, DDI and DGI pairs were extracted from their respective abstracts retrieved in IR stage.
IR Model Development
IR was implemented in Weka.26 String attributes in each abstract were converted into a set of attributes representing word occurrence information from the text using “StringToWordVector” module. Within the module, a set of word features converted from the normal text were extracted using IteratedLovinsStemmer, stopwordsHandler, NGramTokenizer (1–3), lowerCaseTokens and wordsToKeep (1000). The statistics for these word features, including term frequency-inverse document frequency and output word counts were prepared using TFTransform, IDFTransfrom, and outputWordCounts. Subject to the optimization of recall rate, sequential minimal optimization (SMO) was utilized for the text classification.
IE Model Development
IE of the DDI and DGI pairs was achieved in two steps: entity recognition and normalization, and relation pair extraction.
Entity Recognition and Normalization
The relevant entities, including drugs, enzymes, ADEs and interaction terms were tagged using name-entity recognition (NER) by string-matching against the lexica. Extracted drugs, enzymes, and ADEs were normalized to generic drug names, gene symbol names, and preferred terms in MeDRA, respectively. Interaction terms were normalized to their stemmed forms.
Relation pair extraction
The existing text mining methods recognize a piece of text that contains a semantic property of interest and extracts syntactic relations between entities in a single sentence using natural language processing.27–32 Different from these works, we developed a feature-based approach to extract DDI/DGI pairs from context in an entire abstract. If N unique drug names are mentioned in an abstract, there are N*(N-1)/2 possible drug combinations that may or may not have interactions. Our IE model was built to predict the interaction relationship between each drug combination and optimize the F-measure.
In our DDI IE models, 16 features were created. These features capture syntactic, statistic, and scientific patterns from drug interactions present in the text. They were mainly derived from three types of information (entity location, entity statistics, and entity background knowledge). The location features provided location information for drug entities and interacting terms, or their co-occurrence, i.e., drug pairs co-occurring in the title sentence or the same sentence or the relative distance between drug pairs and interacting terms. The statistical features offer the frequency of drugs, drug pairs, and drug co-occurrence in a sentence or cross sentences. And, the knowledge features supply the background knowledge of drug pair relations, such as enzyme-substrate/inhibitor relationships and anatomical therapeutic chemical (ATC) classification information.
To perform the DDI IE task, we customized five groups of feature sets from the 16 features using different strategies (Table S2 and S3). Three manual (G1, G2, and G5) and two statistically (G3 and G4) determined group sets were adopted for the three types of studies. For G3 and G4, a stepwise regression model was used to determine statistically significant features, with G4 also involving 2-way interaction terms. To maximize the F-measure for prediction, the optimal combination of 5 feature groups and 7 popular classifiers [J48, Naïve Bayes, SMO, Logistic Regression, Random Forest, Logistic Model Trees (LMT), and Iterative Classifier Optimizer (ICO)] were explored for each study type. The details of the feature creation and selection are described in the section under “Experimental settings” in the supplemental file.
To perform DGI IE, all descriptive structures for the drug-gene relationships were identified from PubMed abstracts.18 Based on their findings, two important types of terms, including interacting verbs (e.g. inhibit) and mechanism terms (e.g. methylation), were included to characterize all dependency paths for DGI presentations. Four types of features (50 features) were created: 1) 44 features were scored based on the relative location, distance, and negation of each combination of 22 verb or 22 mechanism terms, (2) co-occurrence frequency of each combination or each combination with verb terms, (3) relative position of the bracket containing drugs or enzymes, and (4) the order of the drug, gene, and verb/mechanism terms present in the sentences (Table S4). For this task, logistic regression was utilized for both feature selection and DGI prediction.
Hypotheses generation
By integrating the DDI and DGI evidences discovered through screening of the biomedical literature, and implementing a translational research method to discover knowledge gaps in drug interaction studies, we generated research hypotheses to: 1) understand the hazards of specific drugs given certain genetic polymorphisms, and 2) explore molecular mechanisms of drug interactions (Figure 2).
Figure 2. Hypotheses generation.
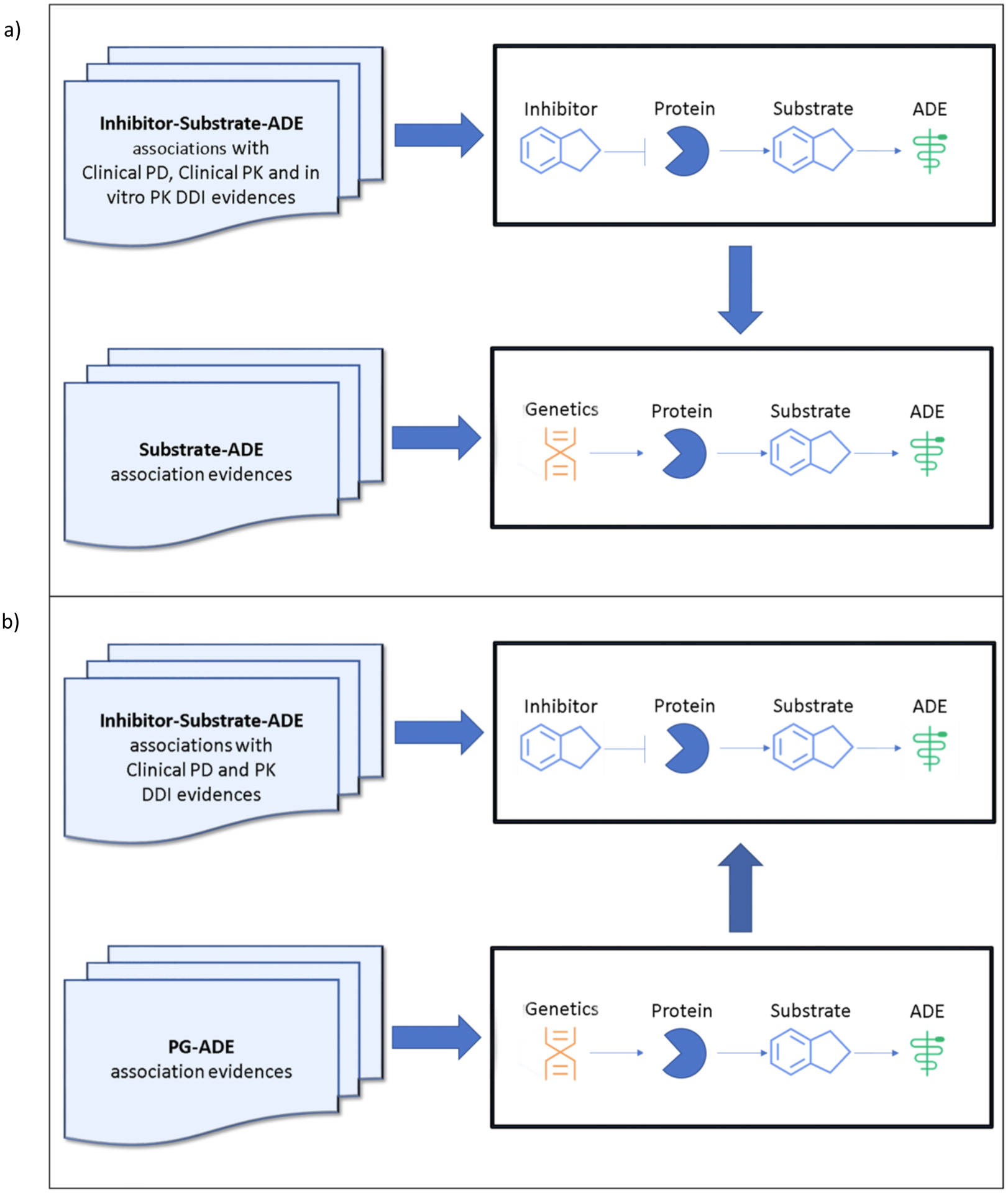
a) Translate drug-drug interaction (DDI) signals to predict genetic effects related to Adverse Drug Events (ADEs) and
b) Translate drug-gene interaction (DGI) signals to predict molecular mechanisms of DDI
Translate DDI signals into pharmacogenetic hypotheses
A knowledge discovery method was used to translate DDI signals into pharmacogenetic hypotheses. The process included the examination of evidences to determine whether drug D1 changed drug D2’s efficacy or ADEs (i.e. PD DDI), D2’s exposure (i.e. PK DDI), or inhibited D2’s metabolic enzyme E (in-vitro PK). If these DDI effects were noted, we then hypothesized that the functional genetic polymorphisms of E may be associated with D2’s efficacy or ADEs.
Translate DGI signals into DDI mechanistic hypotheses
To explore unknown mechanisms involving drug interactions with only clinical PK and PD evidences, a discovery method was proposed to translate DGI signals into DDI mechanisms. The process included the evaluation of both drugs (D1 and D2) to discern their shared target genes and ADEs. If D1 and D2 were reported to interact and had common interacting genes, we hypothesized that their interaction may be synergistic or antagonistic for a given ADE.
RESULTS
The number of abstracts from each type of study as well as the recall, F-measure and validity related statistics are presented below. Figure 3 presents the number of DDI and PG abstracts retrieved and the DDI and DGI pairs extracted from each type of study. The Venn diagram in Figure 3 shows the overlap of drug pairs from the three types of DDI studies to help identify potential knowledge gaps between DDI and DGI evidences. The data related to the Venn Diagram and the DGI associated ADEs are presented in the supplemental excel files “Venn diagram data and statistics.xlsx” and “DGI-ADE information.xlsx”.
Figure 3.
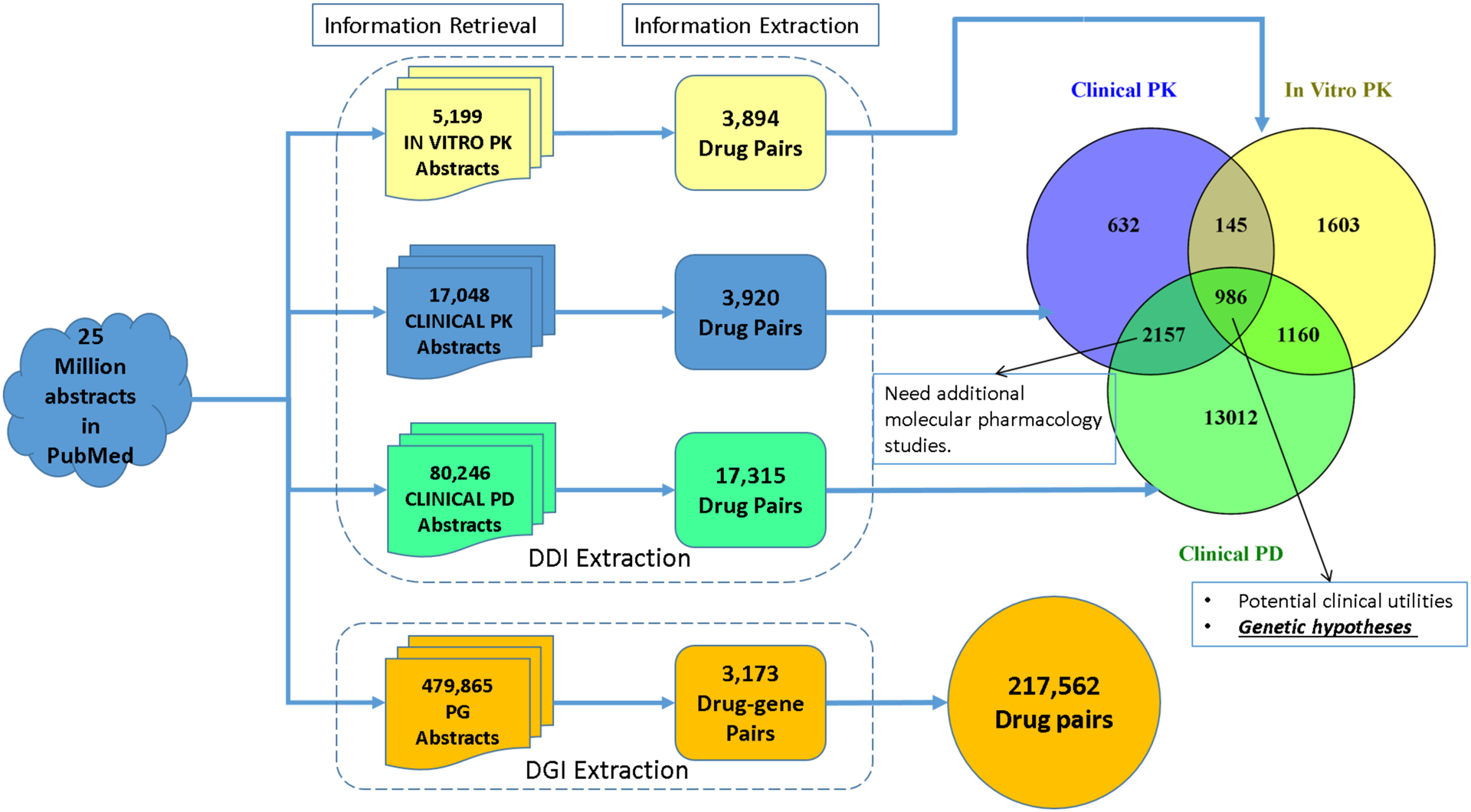
Results from the information retrieval and information extraction stages accompanied by a Venn diagram illustrating the overlap between the different DDI studies
Information Retrieval (IR): identifying DDI and DGI relevant abstracts from MEDLINE
Using our recently developed corpus, the optimal IR models were built for each study type. The F-measures for the performance of the IR models were 0.94, 0.84, 0.70, and 0.78, respectively; and the recall rates were 0.98, 0.99, 0.86, and 0.97 for in-vitro PK DDI, clinical PK DDI, clinical PD DDI, and PG, respectively (Table S5). Using these optimally trained models, a large-scale IR analysis of 25 million MEDLINE abstracts (1975–2015) was conducted. Studies involving animal models were removed using MeSH terms under the tree “B01.050” (Animal). We retrieved 5,199 in-vitro PK, 17,048 clinical PK, 80,246 clinical PD DDI, and 479,865 PG abstracts (Figure 3). To further demonstrate the performance of these IR models, studies in the IE corpora (210, 218, 140, and 395 abstracts for in-vitro PK, clinical PK, clinical PD, and PG studies) were used since there is no overlap between the IR and IE corpora. Recall rates for these IE studies were determined to be 1.00, 0.96, 0.99, and 0.94, respectively.
Information Extraction (IE): identifying DDI and DGI pairs from the MEDLINE Abstracts
To extract DDI and DGI pairs from the MEDLINE abstracts identified in the IR step, IE models were customized and optimized. The DDI extraction performances for each of the in-vitro PK, clinical PK, and clinical PD studies were compared across five feature sets (G1-G5) and seven classifiers and are presented in Tables S6, S7, and S8, respectively. The optimal F-measure for in-vitro PK studies was 0.83 using feature group 5 (G5) and the Naïve Bayes classifier; the optimal F-measure for clinical PK studies was 0.85 using feature group 1 (G1) and the Iterative Classifier Optimizer (ICO); and the optimal F-measure for clinical PD studies was 0.73 using feature group 1 (G1) and the Naïve Bayes classifier. For DGI IE model, 50 features were trained over logistic linear regression classifier to reach the optimal F-measure of 0.82 (Table S9). All four optimized IE models were then applied to the relevant DDI/DGI abstracts retrieved from the previous IR stage. The IE analysis focused on FDA approved and withdrawn drugs, and identified 3,894, 3,920, and 17,315 unique in-vitro PK, clinical PK, and clinical PD DDI pairs, respectively, and 3,173 unique DGI pairs (Figure 3). Using 3,173 retrieved DGI pairs, 217,562 drug pairs were further generated when both drugs shared enzyme relationships.
The overlap and knowledge gap among DDI Evidences
With the drug pairs extracted in the IE stage, the Venn diagram shown in Figure 3 was constructed to present the overlapping DDIs. A total of 986 unique drug pairs were found to overlap between all three study types. Another 2,157 DDIs represented the overlap between clinical PK and clinical PD studies. Lastly, 13,012 DDI pairs were found to only have clinical PD evidence.
Another overlapping analysis was performed to compare “extracted DDIs” from DDI IE to “predicted DDIs” from DGI IE. In Table 2, 94.8% of the 986 DDI pairs shared by all three DDI study types were predicted by DGI results. Other types of DDI evidences that overlap well with DGI predicted DDIs include: “clinical PK – in-vitro PK” (95.9%), “clinical PD – in-vitro PK” (86.6%), and “in-vitro PK” (85.2%). For the remaining DDIs without in-vitro PK evidences, DGI does not predict DDIs well, and the overlapping percentages are below 70%. Only 42.7% of the clinical PD DDIs were predicted from DGI results.
Table 2.
Overlapping analysis for DDI studies
| Venn Diagram Area | No. of DDIs in LS result | No. of DDIs predicted by DGI (%) | No. of DDIs found in DrugBank (%) | Top 20 DDIs (Found in DrugBank/ Validated DDIs) |
|---|---|---|---|---|
| Full in-vitro PK area | 3,894 | 3,443 (88.4) | 2,594 (66.6) | 9/17 |
| Full clinical PK area | 3,920 | 2,980 (76.0) | 2,734 (69.8) | 16/20 |
| Full clinical PD area | 17,315 | 8,991 (51.9) | 8,296 (47.9) | 13/19 |
| Overlap of clinical PD – clinical PK – in-vitro PK | 986 | 935 (94.8) | 867 (87.9) | 19/20 |
| Overlap of clinical PK – in-vitro PK | 145 | 139 (95.9) | 112 (77.2) | 19/20 |
| Overlap of clinical PD – in-vitro PK | 1,160 | 1,004 (86.6) | 785 (67.7) | 19/20 |
| Overlap of clinical PD – clinical PK | 2,157 | 1,494 (69.3) | 1,406 (65.2) | 13/19 |
| Only clinical PK | 632 | 412 (65.2) | 349 (55.2) | 13/14 |
| Only in-vitro PK | 1,603 | 1,365 (85.2) | 830 (51.8) | 11/11 |
| Only clinical PD | 13,012 | 5,558 (42.7) | 5,238 (40.3) | 8/18 |
| Venn Diagram Area | No. of DDIs in Venn Diagram Area | No. of DDIs found in DrugBank (%) | Type of Evidence | |
| Clinical PD – clinical PK – in-vitro PK | 6,683 | 3,271 (48.9) | PK Evidence | |
| Clinical PD – in-vitro PK | ||||
| Clinical PK | ||||
| Clinical PD – clinical PK – in-vitro PK | 17,315 | 4,016 (23.2) | PD Evidence | |
| Clinical PD – in-vitro PK | ||||
| Clinical PD | ||||
No.: Number, DDI: drug-drug interactions, LS: literature search, DGI: drug-gene interactions, PK: pharmacokinetics, PD: pharmacodynamics
Comparing DDI text mining evidences to DDI data in the DrugBank database
DDI text mining performance was also evaluated by comparing the results with DDI data from the DrugBank database. For our comparison analysis, we only focused on FDA approved and withdrawn drugs. Between 222,409 DrugBank DDIs and 19,695 text-mined DDIs, 9,587 DDIs overlapped. We compared the overlapping DDIs under the sub-groups defined by the three DDI evidence types. In Table 2, DDI pairs with all three types of evidences from our text mining analysis overlapped the most with DrugBank DDIs (~88%), while DDI pairs with only clinical PD evidence had the lowest overlapping rate (~40%).
To demonstrate the validity of our text mined DDI evidences, top 20 DDI pairs in each study type were evaluated manually (Table 2). DDI pairs were ranked by their reporting frequencies in different PubMed abstracts. Among top 20 DDIs from in-vitro PK, clinical PK, and clinical PD studies, 17, 20, and 19 pairs were manually validated as true DDIs, respectively. However, only 9, 16, and 13 of these DDIs were found to be reported in the DrugBank. Additionally, for the top 20 DDI pairs in the overlapping areas among two or three evidence types, almost all them were validated in our manual review but only few of these DDIs were reported in DrugBank. DDI pairs that did not overlap with DrugBank data were also manually reviewed for validity. Among the top 20 DDIs from our 119 three-way overlapped DDIs, 17 were found to have confirmed DDI evidences in the literature. Similarly, all of the top 20 DDIs with overlapping clinical PD and clinical PK evidences were confirmed to have DDI evidences in the literature.
Translate DDI signals into genetics hypotheses
The 986 DDI pairs shared among three types of DDI studies were translated into genetic hypotheses with respect to their ADEs. Among these 986 DDIs, 865 (87.8%), 481 (48.8%), 193 (19.6%), 419 (42.5%), and 365 (37%) were associated with CYP3A, CYP2D6, CYP2C8, CYP2C9, and CYP2C19, respectively, with some DDIs involving more than one CYP450 enzymes. In our following genetic hypothesis generation analysis, we focused on CYP3A and CYP2D6 as they were responsible for 88% and 49% of the 986 DDIs, respectively.
CYP3A related DDIs had 68 distinctive substrates, and CYP2D6 related DDIs had 25 different substrates. Based on these CYP3A and CYP2D6 substrates, 552 and 192 ADE terms were found to co-occur in their clinical PD DDI abstracts, respectively. Similarly, 199 and 57 ADE terms related with the 68 CYP3A and 25 CYP2D6 substrates from DGI abstracts were retrieved. The common ADE terms from both DDI and DGI abstracts were considered as potential CYP3A or CYP2D6 genes related ADEs. These common DDI and DGI ADEs were further evaluated through manual review. Overall, 150 and 31 genetic hypotheses were generated from the 68 CYP3A substrates and 25 CYP2D6 substrates, respectively. Out of these, 31 CYP3A-related and 13 CYP2D6-related PG evidences were reported in published pharmacogenetics studies (Table 3). As a result, 119 and 18 new pharmacogenetic hypotheses were generated for CYP3A and CYP2D6, respectively.
Table 3.
Novel or tested pharmacogenetics hypotheses related to CYP3A and CYP2D6 genes
| Gene | Substrate (S) | Inhibitors (I) | DDI dosing recommendations in drug labels (S/I) | Adverse drug events | Novel genetic hypotheses | PMIDs for tested hypotheses |
|---|---|---|---|---|---|---|
| CYP3A | Alprazolam | Nefazodone/Fluoxetine/Ritonavir | n/a | Sedation | Yes | - |
| Amprenavir | Ritonavir | 1200 mg/200 mg o.d, 600 mg/100 mg b.i.d | Diarrhea | Yes | - | |
| Hyperlipidemia | Yes | - | ||||
| Nausea | Yes | - | ||||
| Oral paresthesias | Yes | - | ||||
| Rash | Yes | - | ||||
| Vomiting | Yes | - | ||||
| Atazanavir | Ritonavir | 300 mg/100 mg o.d, 400 mg/100 mg o.d if given with other HIV drugs | Cholelithiasis | Yes | - | |
| Diarrhea | Yes | - | ||||
| Hyperlipidemia | Yes | - | ||||
| Increase in total and subcutaneous fat | Yes | - | ||||
| Metabolic syndrome | Yes | - | ||||
| Upper respiratory tract infection | Yes | - | ||||
| Vomiting | Yes | - | ||||
| Hyperbilirubinemia | No | 28599374 | ||||
| Nephrolithiasis | No | 25151207 | ||||
| Atorvastatin | Itraconazole/Ticagrelor/Ritonavir | >20 mg atorvastatin to be given with caution; no adjustment with Ticagrelor; minimum required dose with ritonavir | Rhabdomyolysis | Yes | ||
| Atorvastatin | Itraconazole/Ritonavir | >20 mg atorvastatin to be given with caution; minimum required dose with ritonavir | Myopathy | No | 28812116, 17289397, 15900215 | |
| Carbamazepine | Ritonavir | n/a | Ataxia | Yes | - | |
| Cerivastatin | Cyclosporine | n/a | Myopathy | Yes | - | |
| Rhabdomyolysis | Yes | - | ||||
| Cisapride | Ketoconazole/Clarithromycin/Diltiazem | Contraindicated; n/a for diltiazem | QTc prolongation | Yes | - | |
| Cisapride | Clarithromycin/Diltiazem | Contraindicated; n/a for diltiazem | Syncope | Yes | - | |
| Cisapride | Ketoconazole/Clarithromycin | Contraindicated | Ventricular arrythmias | Yes | - | |
| Colchicine | Clarithromycin | 0.3 or 0.6 mg colchicine; dose and frequency depend on the condition treated | Death | Yes | - | |
| Gi toxicity | Yes | - | ||||
| Multiorgan failure | Yes | - | ||||
| Myelosuppression | Yes | - | ||||
| Pancytopenia | Yes | - | ||||
| Cyclosporine | Telaprevir | n/a | Cytomegalovirus reactivation | Yes | - | |
| Acute rejection | No | 27653228, 18444945, 29043387 | ||||
| Cyclosporine | Ketoconazole/Nefazodone | n/a | Nephrotoxicity | No | 22388796 | |
| Darunavir | Ritonavir | 800 mg/100 mg o.d, 600 mg/100 mg b.i.d | Diarrhea | Yes | - | |
| Headache | Yes | - | ||||
| Hyperlipidemia | Yes | - | ||||
| Increase in alt | Yes | - | ||||
| Increase in total and subcutaneous fat | Yes | - | ||||
| Lipoma | Yes | - | ||||
| Metabolic syndrome | Yes | - | ||||
| Nasopharyngitis | Yes | - | ||||
| Nausea | Yes | - | ||||
| Upper respiratory tract infection | Yes | - | ||||
| Diazepam | Cimetidine | n/a | Sedation | Yes | - | |
| Docetaxel | Ketoconazole | n/a | Less febrile neutropenia | Yes | - | |
| Less neutrophil suppression | Yes | - | ||||
| Anemia | No | 25495407, 17410042 | ||||
| Diarrhea | No | 25495407, 17410042 | ||||
| Edema | No | 19332043 | ||||
| Fatigue | No | 21468756, 17410042 | ||||
| Increase in liver function tests | No | 25495407 | ||||
| Myelosuppression | No | 25495407, 17545536, 19332043, 16765145, 17410042 | ||||
| Nausea | No | 25495407, 17410042 | ||||
| Neuropathy | No | 27574448, 17410042 | ||||
| Everolimus | Voriconazole | Avoid voriconazole | Pneumonia | Yes | ||
| Fentanyl | Fluconazole/Voriconazole | n/a | Respiratory depression | No | 21223952 | |
| Imatinib | Docetaxel | n/a | Diarrhea | Yes | - | |
| Nausea | Yes | - | ||||
| Vomiting | Yes | - | ||||
| Indinavir | Ritonavir | 800 mg/100 mg b.i.d, 800 mg/200 mg b.i.d | Alopecia | Yes | - | |
| Flank pain | Yes | - | ||||
| Hematuria | Yes | - | ||||
| Hyperlipidemia | Yes | - | ||||
| Nausea | Yes | - | ||||
| Nephrolithiasis | Yes | - | ||||
| Vomiting | Yes | - | ||||
| Levomethadyl acetate | Ketoconazole | n/a | Prolongation of miosis | Yes | - | |
| Lopinavir | Ritonavir | 800 mg/200 mg o.d, 400 mg/100 mg b.i.d, 600 mg/150 mg If given with other HIV drugs | Asthenia | Yes | - | |
| Diarrhea | Yes | - | ||||
| Gastritis | Yes | - | ||||
| Headache | Yes | - | ||||
| Hyperglycemia | Yes | - | ||||
| Hyperinsulinemia | Yes | - | ||||
| Hyperlipidemia | Yes | - | ||||
| Lipoma | Yes | - | ||||
| Rash | Yes | - | ||||
| Lovastatin | Ritonavir/Telaprevir/Boceprevir | Contraindicated | Myalgia | Yes | - | |
| Lovastatin | Itraconazole/Ritonavir | Contraindicated | Myopathy | Yes | - | |
| Lovastatin | Itraconazole/Cyclosporine/Ritonavir | Contraindicated; avoid cyclosporine | Rhabdomyolysis | Yes | - | |
| Methadone | Fluvoxamine | n/a | Opioid withdrawal | No | 21902501 | |
| Midazolam | Ketoconazole/Saquinavir | Avoid ketoconazole; n/a for saquinavir | Cognitive impairment | Yes | - | |
| Midazolam | Posaconazole | Avoid posaconazole | Diarrhea | Yes | - | |
| Midazolam | Ketoconazole/Ritonavir/Diltiazem/Verapamil/Ranitidine/Erythromycin | Avoid ketoconazole/diltiazem/verapamil/erythromycin; n/a for ranitidine; ritonavir contraindicated | Sedation | No | 17786417 | |
| Nelfinavir | Ritonavir | n/a | Diarrhea | Yes | - | |
| Nausea | Yes | - | ||||
| Rash | Yes | - | ||||
| Paclitaxel | Cyclosporine | n/a | Nail changes | Yes | - | |
| Neurotoxicity | No | 23640974, 20212519 | ||||
| Vomiting | No | 15901749 | ||||
| Paclitaxel | Cyclosporine/Pazopanib | n/a | Diarrhea | Yes | - | |
| Nausea | No | 15901749 | ||||
| Paclitaxel | Cyclosporine/Pazopanib/Verapamil | n/a | Neutropenia | No | 12454106, 26179145, 15901749 | |
| Paclitaxel | Pazopanib | n/a | Abscess | Yes | - | |
| Dysgeusia | Yes | - | ||||
| Fatigue | Yes | - | ||||
| Gastrointestinal perforations | Yes | - | ||||
| Hair color changes | Yes | - | ||||
| Hepatotoxicity | Yes | - | ||||
| Hyperbilirubinemia | Yes | - | ||||
| Hypertension | Yes | - | ||||
| Myalgia | Yes | - | ||||
| Alopecia | No | 24025145 | ||||
| Myelosuppression | No | 26179145, 21702053, 15901749 | ||||
| Neuropathy | No | 25398452 | ||||
| Paclitaxel | Pazopanib/Verapamil | n/a | Thrombocytopenia | No | 15901749 | |
| Pimozide | Clarithromycin | Contraindicated | QTc prolongation | Yes | - | |
| Quetiapine | Ritonavir/Atazanavir | 1/6th the usual dose | Delirium | Yes | - | |
| Sedation | Yes | - | ||||
| Weight gain | Yes | - | ||||
| Quinidine | Cimetidine | n/a | QT, QTc, QRS AND RR prolongation | Yes | - | |
| Saquinavir | Ritonavir/Nelfinavir | 1000 mg/100 mg b.i.d; n/a for nelfinavir | Abdominal discomfort | Yes | - | |
| Diarrhea | Yes | - | ||||
| Dysphagia | Yes | - | ||||
| Headache | Yes | - | ||||
| Nausea | Yes | - | ||||
| Saquinavir | Ritonavir | 1000 mg/100 mg b.i.d, | Asthenia | Yes | - | |
| Hyperlipidemia | Yes | - | ||||
| Increase in liver function tests | Yes | - | ||||
| Paresthesias | Yes | - | ||||
| QTc prolongation | Yes | - | ||||
| Simvastatin | Amiodarone | <20 mg/day | Acute renal failure | Yes | - | |
| Azotemia | Yes | - | ||||
| Simvastatin | Diltiazem | n/a | Hepatitis | Yes | - | |
| Simvastatin | Amiodarone/Diltiazem | <20 mg/day; n/a for diltiazem | Hepatotoxicity | Yes | - | |
| Simvastatin | Clarithromycin/Amiodarone/Ritonavir/Itraconazole/Cyclosporine/Verapamil | Avoid clarithromycin/itraconazole; <20 mg/day with amiodarone and verapamil; <10 mg/day with cyclosporine; ritonavir contraindicated | Rhabdomyolysis | Yes | - | |
| Simvastatin | Ritonavir/Telaprevir/Boceprevir | Contraindicated | Myalgia | No | 16321621 | |
| Simvastatin | Diltiazem/Clarithromycin/ Ritonavir/ Amiodarone/Itraconazole/Cyclosporine/Verapamil | Avoid clarithromycin/itraconazole; <20 mg/day with amiodarone and verapamil; <10 mg/day with cyclosporine; n/a for diltiazem; ritonavir contraindicated | Myopathy | No | 17289397, 22122820 | |
| Sirolimus | Cyclosporine | 2 mg or 5 mg per day maintenance based on immunologic risk | Hypertension | Yes | - | |
| Hypertriglyceridemia | Yes | - | ||||
| Myelosuppression | Yes | - | ||||
| Nephrotoxicity | Yes | - | ||||
| Diabetes mellitus | No | 28245187 | ||||
| Hypercholesterolemia | No | 21441846 | ||||
| Tacrolimus | Fluconazole/Voriconazole | 1/3rd the usual dose followed by adjustments based on blood concentration levels | Hepatotoxicity | No | 24438215 | |
| Hyperglycemia | No | 25966085 | ||||
| Nephrotoxicity | No | 17495880, 20526235, 23574377, 19644155, 19067682, 21677300, 25201288, 26856709, 27977332, 27217047, 29539600 | ||||
| Terfenadine | Nefazodone/Ketoconazole/Itraconazole | n/a | QTc prolongation | Yes | - | |
| Torsades de pointes | Yes | - | ||||
| Tipranavir | Ritonavir | 500 mg/200 mg b.i.d | Diarrhea | Yes | - | |
| Headache | Yes | - | ||||
| Hepatotoxicity | Yes | - | ||||
| Hyperlipidemia | Yes | - | ||||
| Nausea | Yes | - | ||||
| Trazodone | Ritonavir | n/a | Dizziness | Yes | - | |
| Fatigue | Yes | - | ||||
| Hypotension | Yes | - | ||||
| Nausea | Yes | - | ||||
| Sedation | Yes | - | ||||
| Syncope | Yes | - | ||||
| Triazolam | Nefazodone/Ritonavir | Contraindicated | Sedation | Yes | - | |
| Verapamil | Cimetidine | n/a | Increase in negative dromotropic effect | Yes | - | |
| CYP2D6 | Codeine | Quinidine | n/a | Psychomotor effects | Yes | - |
| Pupillary effects | Yes | - | ||||
| Respiratory depression | No | 22492761, 18713907 |
||||
| Dextromethorphan | Quinidine | 20 mg/10 mg o.d for 7 days and then b.i.d | Diarrhea | Yes | - | |
| Falls | Yes | - | ||||
| QTc prolongation | Yes | - | ||||
| Urinary tract infection | Yes | - | ||||
| Imipramine | Fluoxetine | n/a | QTc prolongation | Yes | - | |
| Lidocaine | Propafenone | n/a | Negative inotropic effect | Yes | - | |
| Central nervous system side effects | Yes | - | ||||
| Metoprolol | Cimetidine | n/a | Fatigue | No | 2049246, 24637943 | |
| Anxiety | Yes | - | ||||
| Asthenia | Yes | - | ||||
| Sweating | Yes | - | ||||
| Metoprolol | Diphenhydramine | n/a | Systolic blood pressure | No | 24637943 | |
| Metoprolol | Diphenhydramine/Verapamil | n/a | Heart rate reduction | No | 19037197, 18784654, 24637943, 24193112, 29095089 | |
| Metoprolol | Propafenone | n/a | Nightmares | Yes | - | |
| Left ventricular failure | Yes | - | ||||
| Metoprolol | Verapamil | n/a | Atrioventricular block | No | 3437726 | |
| Systemic arterial pressure | No | 19037197, 24637943 | ||||
| Mexiletine | Quinidine | n/a | Prolongation of refractory period and conduction | Yes | - | |
| Propafenone | Quinidine | Avoid quinidine | Heart rate reduction | No | 7595187 | |
| Propranolol | Cimetidine | n/a | Heart rate reduction | No | 9399616, 12728976 | |
| Risperidone | Ritonavir | n/a | Dystonia | No | 20599499 | |
| Parkinsonism | No | 20599499 | ||||
| Coma | Yes | - | ||||
| Akathisia | Yes | - | ||||
| Timolol | Paroxetine | n/a | Heart rate reduction | No | 7474246, 20925579 | |
| Venlafaxine | Fluoxetine | n/a | Serotonin syndrome | No | 19822698, 23799451, 16958828, 10780263 | |
| Zuclopenthixol | Fluoxetine | n/a | Extrapyramidal side effects | No | 12107620 | |
| Dystonia | Yes | - |
DDI: drug-drug interactions, PMID: PubMed ids for journal articles, n/a: not available, o.d: once a day, b.i.d: twice a day
Translate DGI signals into DDI molecular mechanistic hypotheses
Among the 2,157 DDIs shared between clinical PD and PK DDI evidences, 1,497 DDI pairs shared the same metabolic enzymes (i.e. CYPs and UGTs) in their drug-gene-interactions. Therefore, these 1,497 DDI pairs potentially have a pharmacokinetics drug interactions mechanism. Among the remaining 660 DDIs, 68 DDI pairs were found to share the same molecular pathways, 38 DDI pairs shared common genes, and 12 DDI pairs shared common genetic variants. The 38 DDI pairs with shared genes were reviewed further to determine if they shared the same ADEs, whether the risk of the shared ADEs were increased by these DDIs, and whether any in-vitro cell culture studies had investigated their DDI mechanisms. After manual review, seven of 38 DDI pairs were validated to have increased ADEs, and three had additional DDI evidence from in-vitro experiments (Table 4).
Table 4.
Novel or tested molecular mechanistic hypotheses
| Drug 1 | Drug 2 | DDI dosing recommendations in drug labels (D1/D2) | Drug-drug interaction effects | Shared genes | Novel molecular mechanistic hypotheses | PMIDs for tested hypotheses |
|---|---|---|---|---|---|---|
| Ramipril | Hydrochlorothiazide | n/a | Improved blood pressure reduction | ACE rs4359 or rs4344 | Yes | - |
| Azathioprine | Mercaptopurine | n/a | An increased risk of pancreatitis |
HLA-DQA1 (*02:01 allele), HLA-DRB1 (*07:01 allele) |
Yes | - |
| Etanercept | Methotrexate | 50 mg etanercept once weekly | Increased efficacy in patients with rheumatoid arthritis |
ATP5F1E rs1059150, HLA-E rs1264457, KLRC1 rs7301582 |
Yes | - |
| Amlodipine | Benazepril | 2.5 mg/10 mg o.d, 10 mg/40 mg o.d maintenance | Improved blood pressure reduction | ACE rs1799752 | Yes | - |
| Raltitrexed | Irinotecan | n/a | Increased incidence of asthenia |
TYMS
rs45445694 |
No | 9607593 |
| Cisplatin | Pemetrexed | 500 mg/m2 i.v and 75 mg/m2 i.v | Improved response rate in mesothelioma |
ABCC2
rs2273697, MTHFR rs1801133, SLC19A1 rs1051298 |
No | 16898269, 22562354 |
| Carbamazepine | Levetiracetam | n/a | Leads to beneficial anticonvulsant pharmacodynamic interactions |
SCN1A
rs2298771 |
No | 24211788 |
DDI: drug-drug interactions, PMID: PubMed ids for journal articles, n/a: not available, o.d: once a day, i.v: intra-venous administration
DISCUSSION
Adverse drug events caused by drug interactions are a critical issue for prescriptions. In clinical practice, prescription decision support typically stems from in-vivo and clinical evidence. However, there is high variability in drug responses, which are affected by both genetic and environmental factors. Therefore, studying genetic or molecular mechanisms underlying DDIs is essential to help: 1) understand the hazards of specific drugs given certain genetic polymorphisms, and 2) explore molecular mechanisms of such interactions. Today, more than 2,000 genetic tests are currently available, but not every drug is covered and the tests can be expensive.33 To address these challenges, we introduced a translational research method to discover knowledge gaps in drug interaction studies. Utilizing the results from our large-scale screening, two sets of hypotheses were generated by 1) translating DDI signals into genetic information for adverse drug events and 2) translating DGI signals into molecular mechanistic hypotheses.
To demonstrate the process of pharmacogenetic hypotheses generation, evaluation, and validation, we use the example of tacrolimus, a CYP3A substrate. Among 87 clinical PD DDI abstracts showing the interaction evidences between tacrolimus and CYP3A inhibitors such as ketoconazole, clarithromycin, cyclosporine or ritonavir, 141 ADE terms were identified and extracted. From these results, we assume that 141 genetic hypotheses can be generated for tacrolimus. Another 153 ADEs were extracted from DGI abstracts related to tacrolimus and CYP3A. A total of 25 ADE terms were common between the DDI and DGI abstracts. From these, three ADEs (nephrotoxicity, hepatotoxicity, and hyperglycemia) were validated and found to be associated with the CYP3A5 polymorphism, rs776746 (PharmGKB level 2A or level 3 evidence).34–37 Thus, we were able to validate our tacrolimus ADEs-related genetic hypotheses, underscoring the accuracy of our text mining algorithm. More importantly, the generation of 119 new pharmacogenetic hypotheses highlights the significance of our translational research method in enabling the discovery of potentially new genetic mechanisms that may otherwise not have been explored through conventional DDI research methods. An example of this is simvastatin-induced rhabdomyolysis. Even though there are reported evidences for the association between CYP3A4 and CYP3A5 genetic polymorphisms and simvastatin induced mild myopathy symptoms,38 there is no reported study on the association between CYP3A and the more severe form of myopathy, i.e. rhabdomyolysis.
Similar to the PG hypotheses, molecular mechanistic hypotheses were validated or determined to be new through our manual review process. For example, the combination of cisplatin and pemetrexed showed improved response rate in mesothelioma patients in a randomized phase III trial.39 Both drugs share the ABCC2, MTHFR, and SLC19A1 target genes among them (PharmGKB level 4 evidence). Patients with ABCC2 rs2273697 (AA or AG genotype) were reported to have improved overall and progression-free survival when treated with either cisplatin or pemetrexed compared to patients with the ABCC2 GG genotype.40 The synergistic PD DDI between cisplatin and pemetrexed has been demonstrated in in-vitro studies over multiple cancer cell lines (MCF7, A549, and PA1 cells).41 In particular, when MCF7 cells were incubated with pemetrexed for 24 h followed by cisplatin for 24 h, synergistic inhibition of cell proliferation was noted. Similar synergistic effects were also observed in the A549 and PA1 cell lines. Another example is the interaction between etanercept and methotrexate which results in improved response in rheumatoid arthritis patients especially with the ATP5E rs1059150 (GG), HLA-E rs1264457 (AA), or KLRC1 rs7301582 (CT or TT) variants (PharmGKB level 3 evidence). However, no in-vitro experiments have been reported that illustrate their DDI mechanisms. This etanercept/methotrexate example demonstrates that our translational discovery method can also generate novel pharmacodynamic DDI mechanisms.
Our study has both strengths and limitations. Our text mining algorithm enabled us to screen ~25 million MEDLINE abstracts in order to retrieve DDI and DGI evidences. We were also able to extract a substantial number of DDI and DGI pairs from the literature, and distinguish them based on the type of study involved. However, we only focused on drug pairs and the CYP450-related enzymes and genes. Therefore, we could not evaluate DDIs or ADEs associated with high dimensional drug combinations or their interactions with other enzymes and drug transporters. Our algorithm was also designed to identify co-occurrence of drugs and ADE terms, as such, a manual review process was required to verify and confirm these associations. Additionally, our algorithm does no collect information on drug dosage or sample size from individual studies at the moment but we are planning to add this information along with the information on drug transporters and other enzymes in the future. Despite these limitations, our study provides a tremendous amount of information on DDIs, DGIs and ADEs and allowed us to generate several novel genetic hypotheses.
In conclusion, a text mining pipeline was developed to extract DDI evidences from the biomedical literature in the current study. Initially, golden standard corpora for DDIs and DGIs were created to facilitate the text mining development. Subsequently, a large-scale analysis was conducted to identify knowledge gaps in DDI and DGI research, which were then used to generate hypotheses in order to identify novel genetic mechanisms involving drug interactions and predict potential molecular mechanistic DDI mechanisms.
Supplementary Material
Table S1. Inclusion-exclusion criteria for the abstract validation process
Table S2. FDA’s probe features
Table S3. Feature selections for the three DDI study types
Table S4. Categorization of drug-gene-verb presentation order
Table S5. Performance evaluation of the IR task
Table S6. Performance evaluation of IE for in-vitro PK DDI studies
Table S7. Performance evaluation of IE for clinical PK DDI studies
Table S8. Performance evaluation of IE for clinical PD DDI studies
Table S9. Performance evaluation of IE for DGI studies
Figure S1 Examples of the calculation for the average angle of a drug pair to interaction verbs (∠_i,j,k,s)
STUDY HIGHLIGHTS.
• What is the current knowledge on the topic?
Several studies have explored different informatics approaches to mine drug interactions data from the biomedical literature. However, none of them have distinguished the DDI evidences into in-vitro PK, clinical PK, and clinical PD studies, which can impede the translational scope of drug interactions research.
• What question did this study address?
The goal of this study was to retrieve and extract DDI and DGI evidences from the biomedical literature and distinguish the DDI study types into in-vitro PK, clinical PK and clinical PD studies. Additionally, the integrated DDI and DGI evidences were used to determine knowledge gaps that could enable the generation of novel DDI and ADE related hypotheses.
• What does this study add to our knowledge?
This study adds to the existing knowledge by providing 1) a novel algorithm that extracts drug interaction evidences from diverse DDI and DGI studies, 2) a method to distinguish the different types of DDI studies, and 3) an integrated drug interactions data that enables knowledge discovery through generation of novel genetic hypotheses or molecular DDI mechanisms.
• How might this change clinical pharmacology of translational science?
The integrated knowledge generated by our study is valuable for translational research in drug interaction studies as it can facilitate future studies that help in improving our understanding of DDI-related ADEs through the detection of novel genetic or molecular mechanisms. The validated hypotheses can then be evaluated for potential clinical applications in the future.
Funding sources
This work is supported by grants from the National Library of Medicine (R01LM011945), and the National Institute of General Medical Sciences (R01GM104483).
Footnotes
Conflict of Interest
All authors declare no competing interests for this work.
REFERENCES
- 1.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A & Schwartzman A National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report, 1–20, 4 (2010). [PubMed] [Google Scholar]
- 2.Niska R, Bhuiya F & Xu J National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Report, 1–31 (2010). [PubMed] [Google Scholar]
- 3.Hajjar ER, Cafiero AC & Hanlon JT Polypharmacy in elderly patients. Am J Geriatr Pharmacother 5, 345–51 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Hennessy S & Flockhart DA The need for translational research on drug-drug interactions. Clin Pharmacol Ther 91, 771–3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce R, Collins C, Horn J & Kalet I Computing with evidence Part II: An evidential approach to predicting metabolic drug-drug interactions. J Biomed Inform 42, 990–1003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyce R, Collins C, Horn J & Kalet I Computing with evidence Part I: A drug-mechanism evidence taxonomy oriented toward confidence assignment. J Biomed Inform 42, 979–89 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prueksaritanont T et al. Drug-drug interaction studies: regulatory guidance and an industry perspective. AAPS J 15, 629–45 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crews KR et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin Pharmacol Ther 91, 321–6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilke RA et al. The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin Pharmacol Ther 92, 112–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desta Z, Ward BA, Soukhova NV & Flockhart DA Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 310, 1062–75 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Goetz MP et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat 101, 113–21 (2007). [DOI] [PubMed] [Google Scholar]
- 12.In brief: Tamoxifen and SSRI interaction. Med Lett Drugs Ther 51, 45 (2009). [PubMed] [Google Scholar]
- 13.Hertz DL, McLeod HL & Irvin WJ Jr. Tamoxifen and CYP2D6: a contradiction of data. Oncologist 17, 620–30 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wishart DS et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res 34, D668–72 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whirl-Carrillo M et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther 92, 414–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drug Interaction Database. <https://www.druginteractioninfo.org/solutions/drug-interaction-database/>.
- 17.Duke JD et al. Literature based drug interaction prediction with clinical assessment using electronic medical records: novel myopathy associated drug interactions. PLoS Comput Biol 8, e1002614 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Percha B & Altman RB Learning the Structure of Biomedical Relationships from Unstructured Text. PLoS Comput Biol 11, e1004216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Percha B, Garten Y & Altman RB Discovery and explanation of drug-drug interactions via text mining. Pac Symp Biocomput, 410–21 (2012). [PMC free article] [PubMed] [Google Scholar]
- 20.Tari L, Anwar S, Liang S, Cai J & Baral C Discovering drug-drug interactions: a text-mining and reasoning approach based on properties of drug metabolism. Bioinformatics 26, i547–53 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu HY et al. An integrated pharmacokinetics ontology and corpus for text mining. BMC Bioinformatics 14, 35 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gene Ontology Consortium: going forward. Nucleic Acids Res 43, D1049–56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.HUGO Gene Nomenclature Committee at the European Bioinformatics Institute. <http://www.genenames.org>.
- 24.Sim SC & Ingelman-Sundberg M The Human Cytochrome P450 (CYP) Allele Nomenclature website: a peer-reviewed database of CYP variants and their associated effects. Hum Genomics 4, 278–81 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown EG, Wood L & Wood S The medical dictionary for regulatory activities (MedDRA). Drug Saf 20, 109–17 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Witten IH, Frank E, Hall MA & Pal CJ Data Mining: Practical machine learning tools and techniques (Morgan Kaufmann: 2016). [Google Scholar]
- 27.Percha B, Garten Y, and Altman RB Discovery and explanation of drug-drug interactions via text mining. Pacific Symp Biocomput, 410–21 (2012). [PMC free article] [PubMed] [Google Scholar]
- 28.Segura-Bedmar I, Martinez P & de Pablo-Sanchez C Using a shallow linguistic kernel for drug-drug interaction extraction. Journal of biomedical informatics 44, 789–804 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Segura-Bedmar I, Martinez P & de Pablo-Sanchez C A linguistic rule-based approach to extract drug-drug interactions from pharmacological documents. BMC Bioinformatics 12 Suppl 2, S1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segura-Bedmar I, Martinez P & Herrero-Zazo M Lessons learnt from the DDIExtraction-2013 Shared Task. J Biomed Inform 51, 152–64 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Segura-Bedmar I, Martınez P & Sánchez-Cisneros D The 1st DDIExtraction-2011 challenge task: Extraction of Drug-Drug Interactions from biomedical texts. Challenge Task on Drug-Drug Interaction Extraction 2011, 1–9 (2011). [Google Scholar]
- 32.Tari L, Anwar S, Liang S, Cai J, Baral C Discovering drug-drug interactions: a text-mining and reasoning approach based on properties of drug metabolism. Bioinformatics 26, i547–53 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Genetic Testing: MedlinePlus. <https://medlineplus.gov/genetictesting.html>.
- 34.Quaglia M, Terrazzino S, Boldorini R, Stratta P & Genazzani AA Severe acute nephrotoxicity in a kidney transplant patient despite low tacrolimus levels: a possible interaction between donor and recipient genetic polymorphisms. J Clin Pharm Ther 38, 333–6 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Li N, Wang MX & Lu SC Benefits of minimizing immunosuppressive dosage according to cytochrome P450 3A5 genotype in liver transplant patients: findings from a single-center study. Genet Mol Res 14, 3191–9 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Xue F et al. CYP3A5 genotypes affect tacrolimus pharmacokinetics and infectious complications in Chinese pediatric liver transplant patients. Pediatr Transplant 18, 166–76 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Kuypers DR, Naesens M, de Jonge H, Lerut E, Verbeke K & Vanrenterghem Y Tacrolimus dose requirements and CYP3A5 genotype and the development of calcineurin inhibitor-associated nephrotoxicity in renal allograft recipients. Ther Drug Monit 32, 394–404 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Yang WH et al. Simvastatin-induced myopathy with concomitant use of cyclosporine: case report. Int J Clin Pharmacol Ther 49, 772–7 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Janne PA et al. Pemetrexed alone or in combination with cisplatin in previously treated malignant pleural mesothelioma: outcomes from a phase IIIB expanded access program. J Thorac Oncol 1, 506–12 (2006). [PubMed] [Google Scholar]
- 40.Goricar K, Kovac V & Dolzan V Clinical-pharmacogenetic models for personalized cancer treatment: application to malignant mesothelioma. Sci Rep 7, 46537 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kano Y et al. Schedule-dependent interactions between pemetrexed and cisplatin in human carcinoma cell lines in vitro. Oncol Res 16, 85–95 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Inclusion-exclusion criteria for the abstract validation process
Table S2. FDA’s probe features
Table S3. Feature selections for the three DDI study types
Table S4. Categorization of drug-gene-verb presentation order
Table S5. Performance evaluation of the IR task
Table S6. Performance evaluation of IE for in-vitro PK DDI studies
Table S7. Performance evaluation of IE for clinical PK DDI studies
Table S8. Performance evaluation of IE for clinical PD DDI studies
Table S9. Performance evaluation of IE for DGI studies
Figure S1 Examples of the calculation for the average angle of a drug pair to interaction verbs (∠_i,j,k,s)


