Abstract
Introduction
Limitations in inflammatory bowel disease (IBD) care necessitate greater patient activation and self-efficacy, measures associated with positive health outcomes.
Methods
We assessed change in patient activation and general self-efficacy from baseline to 12 months through our TELEmedicine for IBD trial, a multicenter, randomized controlled trial consisting of a web-based monitoring system that interacts with participants via text messaging. A total of 222 adults with IBD who had experienced an IBD flare within 2 years prior to the trial were randomized into either a control arm that received standard care (SC) or an intervention arm that completed self-testing through the TELE-IBD system every other week (EOW) or weekly (W).
Results
Changes in self-efficacy scores were not significantly different between control and experimental groups. Patient activation scores were significantly different between standard care and the TELE-IBD EOW group only (p = 0.03).
Conclusions
Use of remote monitoring did not improve self-efficacy or patient activation compared to routine care.
Keywords: Telemedicine, Inflammatory bowel disease, Patient activation, Self-efficacy
Introduction
Inflammatory bowel disease (IBD), comprised of ulcerative colitis (UC) and Crohn’s disease (CD), poses a significant burden of illness to approximately 1.6 million US adults [1] and has a growing international incidence, including among populations that are not traditionally affected by IBD [2]. Patients with IBD suffer from chronic inflammation of the gastrointestinal tract resulting in debilitating symptoms such as bloody stools, abdominal pain, and fatigue. IBD symptoms often manifest as “flares” followed by periods of remission, severely reducing IBD patients’ quality of life and at times resulting in surgery [2].
While treatment exists for IBD, a number of factors reduce the efficacy of IBD care. These include but are not limited to heterogeneity of phenotypes resulting in wide variation in care [3], low patient adherence to medications [4, 5], costs of and access to treatment [6, 7], low patient knowledge of IBD [8, 9], and inadequate monitoring of side effects [10]. In addition, much of medical care for IBD involves office visits that are unlikely to coincide with flares. These limitations indicate a need for a greater role of IBD patients in their own disease management.
Patient activation and self-efficacy are key mediators of self-care and have been shown to correlate with patient behaviors and outcomes which address some of the shortcomings of traditional IBD care. Patient activation—the process of empowering patients with knowledge, skills, and motivation to effectively manage their own health care [11]—is a mutable quality [12] that predicts positive self-management behaviors for chronic diseases [12] avoidance of unhealthy behaviors [13], and fewer hospitalizations and emergency room (ER) visits [13]. Similarly, self-efficacy—confidence in one’s ability to perform specific behaviors and shape one’s own outcomes [14]—has been shown to correlate with patient adherence to treatment [15] and positive health behaviors such as healthy eating and physical activity [16]. Thus, activated patients with a high sense of self-efficacy are better equipped to self-manage their IBD symptoms.
Telemedicine enables providers to monitor, treat, educate, and support IBD patients managing their symptoms, possibly increasing patients’ involvement in their own care and confidence in their ability to perform self-care. We have previously shown that a telemedicine system for managing IBD is both feasible and well accepted among patients [17] and other research demonstrates that patients that accept care via telemedicine have greater patient activation [18]. Research on the effects of telemedicine on self-efficacy and patient activation shows mixed results, suggesting a need for more studies on these relationships [19–21]. The precise relationship between a telemedicine system for IBD patients and self-efficacy and patient activation remains unclear.
The present study aims to assess change in self-efficacy and patient activation from baseline to 12 months between participants receiving remote monitoring compared to standard of care during the TELEmedicine for Patients with IBD (TELE-IBD) trial [22]. We hypothesized that participants receiving the TELE-IBD intervention would have greater self-efficacy and patient activation from baseline to 12 months compared to participants receiving standard care.
Methods
Study Design
The TELE-IBD trial is a 1-year, multicenter, randomized controlled trial that has previously been described in detail [23]. The aim of the present study is to analyze changes in general self-efficacy and patient activation, secondary outcomes of the trial. Participants were enrolled in the trial from 2013 to 2015 and randomized into either a control arm that received standard care (SC) or one of two interventional arms. Participants in the interventional arms completed selfassessment of symptoms, weight, and side effects either weekly (TELE-IBD W) or every other week (TELE-IBD EOW) based on group assignment. General self-efficacy and patient activation for all participants were assessed during study visits at baseline, 6 months, and one year.
Study Setting
Patients were recruited from three tertiary IBD referral centers, University of Maryland (UM), University of Pittsburgh Medical Center (UPMC), and Vanderbilt University (VU).
Study Participants: Inclusion and Exclusion Criteria
Inclusion criteria included: (1) documented IBD based on usual diagnostic criteria [24], (2) at least one documented IBD flare up (defined as an increase in IBD symptoms sufficient to warrant a change in medication dose or addition of a new medication) in the 2 years prior to the baseline study visit, and (3) at least 18 years of age.
Exclusion criteria included: inability to speak and/or read English; inability to comply with the study protocol as determined by site investigators; presence of an ileostomy, colostomy, ileoanal pouch anastomosis, or ileorectal anastomosis; imminent surgery; history of short bowel syndrome; uncontrolled medical or psychiatric disease; and pregnancy.
TELE-IBD Intervention
The TELE-IBD system has previously been described in detail [23]. The system includes a mobile phone through which participants interface with research staff and providers and respond to prompts related to their IBD. Patient data are presented to providers and research staff on a web-based platform that is used to send individualized action plans to participants and email alerts to research staff if certain criteria are met. Participants also receive educational messages twice weekly in the TELE-IBD W group and weekly in the TELE-IBD EOW group. The system was developed by the UM IBD Program and CircleLink Health, LLC.
Data Collection
Participants completed study visits at baseline, 6 months, and 12 months while continuing routine clinical visits. Demographic information and clinical characteristics were collected at the baseline visit. Disease activity, disease-specific and general quality of life, clinical knowledge (CC), locus of control (LOC), and mental health were assessed at each visit. The Harvey Bradshaw Index (HBI) was used to assess disease activity in patients with Crohn’s disease [25]. The Simple Clinical Colitis Activity Index (SCCAI) was used to assess disease activity for patients with UC. Active disease was indicated by an HBI of 5 or greater or an SCCAI of 3 or greater [26]. General quality of life was assessed using the 36-item Short Form Health Survey, and disease-specific quality of life was assessed using the Inflammatory Bowel Disease Questionnaire. Rotter’s LOC scale was used to assess LOC, which refers to one’s perception of the extent to which oneself or external forces determine the events that occur in one’s life [27]. To assess participants’ knowledge of their disease, participants completed the Crohn’s and Colitis Knowledge (CCKNOW) survey and received a score from 0 to 24 [28]. The 5-item Mental Health Inventory (MHI 5) questionnaire, a subscale of the 36-item Short Form Health Survey, was used to assess participants’ mental health [29].
Assessment of Outcomes
The General Self-Efficacy Scale (GSE) was used to measure self-efficacy, a confidence in one’s ability to overcome barriers and accomplish a goal [30]. Participant responses to each of the 10 items on the GSE were scored based on a 4-point scale (1 = not at all true, 2 = hardly true, 3 = moderately true, 4 = exactly true) and then summed. GSE scores ranged from 10 to 40, with higher GSE scores indicating greater participant self-efficacy.
Patient activation was measured using the 13-item Patient Activation Measure (PAM), a validated measure of a patient’s knowledge, skills, and motivation to manage their own health care [11, 31]. The PAM is comprised of 13 items that are scored on a 4-point scale (1 = disagree strongly, 2 = disagree, 3 = agree, 4 = agree strongly) and an additional “N/A” option. The sum score is converted to a 0–100 scale based on a calibration table, and participants are stratified into 4 activation levels based on their adjusted PAM score with higher levels indicating greater activation (level 1 = the patient may not believe their role is important, level 2 = the patient lacks confidence and knowledge to act, level 3 = the patient begins to take action, level 4 = the patient can act through stresses) [31].
Statistical Methods
Demographics and baseline disease-related clinical information were collected for all participants and compared across groups. GSE and PAM scores were the primary outcome and were analyzed as continuous variables. A standard t test was used to assess change in PAM and GSE scores between groups. Potential covariates identified included age, sex, race, insurance status, diagnosis, disease duration and activity, study site, baseline MHI 5 score, baseline knowledge score, baseline GSE score, and baseline PAM score. Oneway analysis of variance (ANOVA) was used to compare covariate distributions between exposure groups. Pearson’s Chi-square was used to compare covariate distributions between outcome groups. Stratum-specific estimates from one-way ANOVA were compared for qualitative differences between strata to assess for effect measure modification.
In order to test confounding, we individually adjusted for each covariate in a linear regression model, with group assignment as the independent variable and change in PAM and GSE scores as the dependent variable. Percent changes in beta coefficient estimates were calculated. A value of 10% was used as the threshold for significance.
Ethical Considerations
The UMB Human Research Protection Office, UPMC Office of Research, and VU Human Research Protection Program approved the study. A Data Safety and Monitoring Board (DSMB) was established to review study procedures and monitor enrollment, quality of data collection, adverse events, and patient safety.
Results
Three hundred forty-eight participants met eligibility criteria and were enrolled in the trial.
Ten participants withdrew from the standard care arm, 16 from the TELE-IBD EOW, and 22 from TELE-IBD W. Only participants who completed PAM and GSE surveys at baseline and 12 months were included in the analysis, yielding a total of 187 participants (Fig. 1).
Fig. 1.
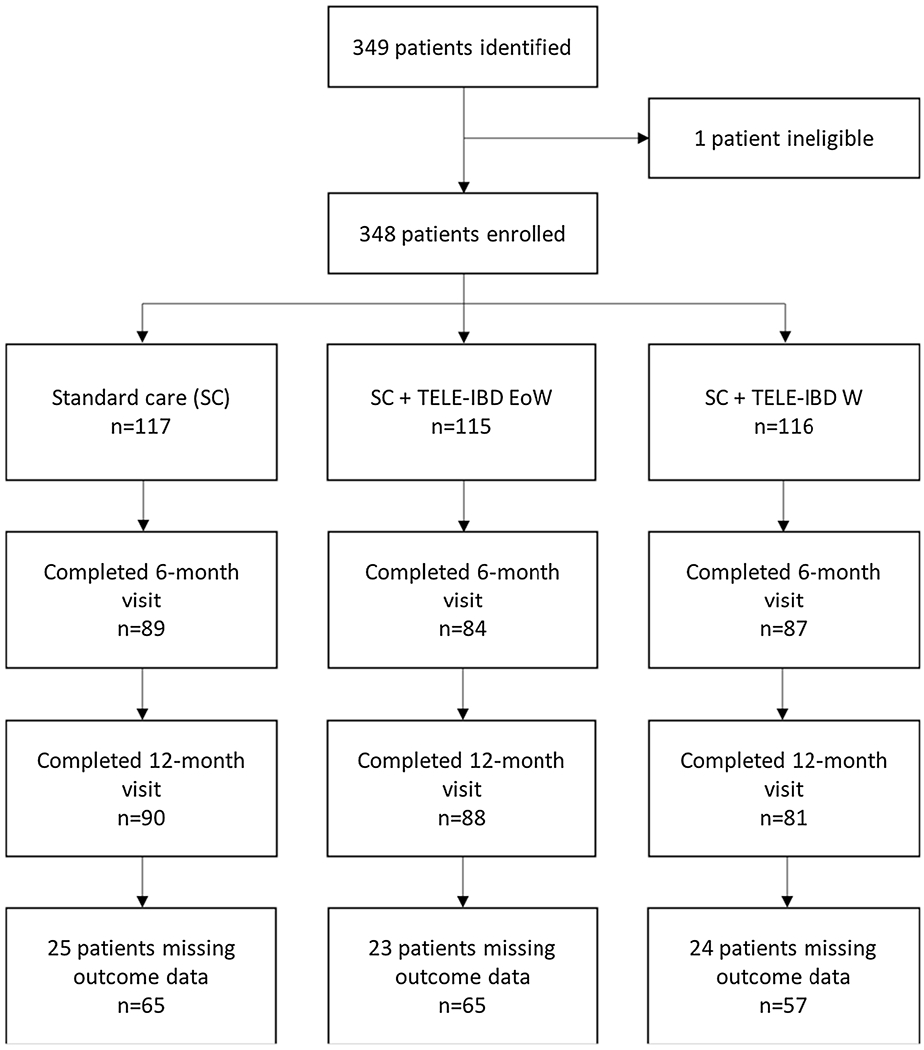
Number of participants in the TELE-IBD Trial by arm and study visit
Demographic and Clinical Characteristics of Participants in the TELE-IBD Trial
Demographic and disease-related characteristics for the overall sample and exposure groups are shown in Table 1. Participants were 93.6% white and 59.4% female. Mean age was 39.3 ± 11.9 years. Enrollment by site varied as follows: 103 (55.1%) at UM, 56 (29.9%) at UPMC, and 28 (15.0%) at VU. Eighty-three (44.4%) participants had active disease at the baseline visit. Significant differences were found among exposure groups at baseline for LOC (p < 0.05) and GSE (p < 0.0001). For LOC, 61.5% (n = 40) of the standard care arm, 63.1% (n=41) of the TELE-IBD EOW arm, and 52.6% (n = 21) of the TELE-IBD W arm had baseline LOC scores between 0 and 10. For GSE, 32.3% (n = 21) of the standard care arm, 40.0% (n = 26) of the TELE-IBD EOW arm, and 40.4% (n = 23) of the TELE-IBD W arm had baseline GSE scores between 10 and 30.
Table 1.
Demographics and clinical characteristics of participants of the TELEmedicine for patients with inflammatory bowel disease (TELE-IBD) Trial with available baseline and follow-up general self-efficacy and patient activation measure scores
| Variable | Overall (n = 222) | Standard care (n = 75) | Biweekly testing (n = 75) | Weekly testing (n = 72) |
|---|---|---|---|---|
| Age (mean ± SD) | 39.3 ± 11.9 | 40.2 ± 11.0 | 39.7 ± 13.0 | 37.7 ± 11.6 |
| Sex (n, %) | ||||
| Male | 76 (40.6) | 24 (36.9) | 25 (38.5) | 27 (47.4) |
| Female | 111 (59.4) | 41 (63.1) | 40 (61.5) | 30 (52.6) |
| Race (n, %) | ||||
| White | 175 (93.6) | 59 (90.8) | 62 (95.4) | 54 (94.7) |
| Non-white | 12 (6.4) | 6 (9.2) | 3 (4.6) | 3 (5.3) |
| Diagnosis (n, %) | ||||
| Crohn | 129 (69.0) | 46 (70.8) | 47 (72.3) | 36 (63.2) |
| UC/IC | 58 (31.0) | 19 (29.2) | 18 (27.7) | 21 (36.8) |
| Disease duration (mean ± SD) | 13.1 ± 9.2 | 13.7 ± 10.0 | 12.4 ± 9.3 | 13.1 ± 8.3 |
| Disease activity (n, %)a | ||||
| Remission | 104 (55.6) | 32 (49.2) | 37 (56.9) | 35 (61.4) |
| Active | 83 (44.4) | 33 (50.8) | 28 (43.1) | 22(38.6) |
| Site (n, %) | ||||
| UM | 103 (55.1) | 34 (52.3) | 38 (58.5) | 31 (54.4) |
| UPMC | 56 (29.9) | 19 (29.2) | 18 (27.7) | 19 (33.3) |
| VU | 28 (15.0) | 12 (18.5) | 9 (13.8) | 7 (12.3) |
| Mental healtha (mean ± SD) | 75.4 ± 17.0 | 76.4 ± 15.9 | 74.4 ± 17.9 | 75.3 ± 17.3 |
| Locus of controla (mean ± SD) | 9.9 ± 3.8 | 9.7 ± 3.8 | 9.9 ± 3.9 | 10.1 ± 3.7 |
| Baseline knowledgea (mean ± SD) | 12.8 ± 4.3 | 12.7 ± 4.4 | 13.0 ± 4.8 | 12.6 ± 3.6 |
| GSE baselinea (mean ± SD) | 33.3 ± 11.8 | 32.6 ± 3.8 | 32.5 ± 3.9 | 32.4 ± 4.0 |
| PAM baseline*a (mean ± SD) | 69.8 ± 16.5 | 69.9 ± 13.9 | 70.8 ± 16.0 | 70.2 ± 14.4 |
The Harvey Bradshaw Index (HBI) was used to assess disease activity in Crohn patients. The Simple Clinical Colitis Activity Index (SCCAI) was used to assess disease activity for UC patients. Rotter’s LOC scale was used to assess LOC. The Crohn’s and Colitis Knowledge (CCKNOW) survey was used to assess participants’ knowledge of their disease. The 5-item Mental Health Inventory (MHI 5) questionnaire, a subscale of the 36-item Short Form Health Survey, was used to assess participants’ mental health and quality of life
P value between groups is less than 0.05
Association of Frequency of Self-Assessment with Change in PAM and GSE Scores
GSE scores improved in all arms during the trial (+ 0.99 ± 2.9 in standard care, + 0.52 ± 3.3 in TELE-IBD EOW, + 0.49 ± 4.1 in TELE-IBD W). Participants in the standard care arm showed higher increases than those in the interventional arm; however, these differences were not significant (p = 0.40 for SC vs TELE-IBD EOW, p = 0.44 for SC vs TELE-IBD W). PAM scores improved in both standard care (+ 4.7 ± 13.9) and TELE-IBD W (+ 5.3 ± 18.0), but decreased in the TELE-IBD EOW arm (− 1.1 ± 16.6). Changes in PAM scores were significantly different between standard care and the TELE-IBD EOW group only (p = 0.03 for standard care vs TELE-IBD EOW, p = 0.82 for standard care vs TELE-IBD W).
Significant differences in change in GSE and PAM scores were observed among strata of covariates (Table 2). Participants with a diagnosis of UC had higher increases in GSE compared to those participants with CD (1.0 ± 3.5 vs 0.5 ± 3.4, p = 0.03). Participants with a baseline GSE score of 10–30 showed significantly higher changes in GSE scores than those with a baseline GSE score of 31–40 (1.8 ± 3.1 vs – 0.02 ± 3.5, p = 0.02). Similarly, participants with PAM scores corresponding to level 1 patient activation showed the greatest increases in PAM scores compared to other groups with higher PAM scores (27.5 ± 15.7 for level 1, 15.0 ± 17.4 for level 2, 3.5 ± 15.4 for level 3, – 1.8 ± 13.8 for level 4, p < 0.0001).
Table 2.
Covariates associated with outcome
| Variable | n | GSE score mean difference (mean ± SD) | p value | PAM score mean difference (mean ± SD) | p value |
|---|---|---|---|---|---|
| Diagnosis | |||||
| CD | 129 | 0.5 ± 3.4 | 0.03 | 2.8 ± 17.0 | 0.84 |
| UC/IC | 58 | 1.0 ± 3.5 | 2.9 ± 14.8 | ||
| Baseline GSE | |||||
| 10–30 | 70 | 1.8 ± 3.1 | 0.02 | 2.3 ± 17.1 | 0.41 |
| 31–40 | 117 | −0.02 ± 3.5 | 3.2 ± 15.9 | ||
| Baseline PAM | |||||
| Level 1 | 7 | 1.3 ± 4.2 (n = 7) | 0.24 | 27.5 ± 15.7 (n = 7) | <0.0001 |
| Level 2 | 20 | 1.9 ± 2.5 (n = 20) | 15.0 ± 17.4 (n = 20) | ||
| Level 3 | 62 | 0.7 ± 3.4 (n = 62) | 3.5 ± 15.4 (n = 68) | ||
| Level 4 | 98 | 0.4 ± 3.2 (n = 85) | −1.8 ± 13.8 (n = 89) |
Assessment for Effect Measure Modification and Confounding
For PAM, no potential effect modifiers were identified for either standard care compared to TELE-IBD W or standard care compared to TELE-IBD EOW. Effect modifier assessment for GSE showed that LOC was a potential effect modifier in the TELE-IBD W compared to standard care arm. No effect modifiers were identified for GSE for the comparison between TELE-IBD EOW and standard of care arm.
Based on their effect on the association between exposure and outcome, LOC, baseline GSE, and baseline PAM were included in the final model as potential cofounders (Table 3). After adjusting for confounding variables, participants with baseline GSE scores between 10 and 30 increased GSE scores by 1.8 points (p = 0.001).
Table 3.
Adjusted change in general self-efficacy and patient activation measure score in participants of the TELEmedicine for inflammatory bowel disease (TELE-IBD) trial
| Variable | Adjusted estimate change in GSE (ß coeff, SE) | p value | Adjusted estimate change in PAM (ß coeff, SE) | p value |
|---|---|---|---|---|
| Arm | ||||
| Standard | 0.56, 0.61 | 0.36 | Ref | |
| EOW | −0.01, 0.61 | 0.98 | −3.96, 2.59 | 0.13 |
| Weekly | Ref | 1.94, 2.68 | 0.47 | |
| LOC | ||||
| 0–10 | Ref | Ref | ||
| 11–21 | 0.64, 0.51 | 0.22 | 1.36, 2.25 | 0.55 |
| Baseline GSE | ||||
| 10–30 | 1.83, 0.55 | 0.001 | Ref | |
| 31–40 | Ref | 5.25, 2.43 | 0.03 | |
| Baseline PAM | ||||
| Level 1 | 1.51, 1.49 | 0.31 | 31.3, 5.97 | <0.001 |
| Level 2 | 0.19, 1.35 | 0.89 | 18.6, 3.63 | <0.001 |
| Level 3 | 0.53, 1.36 | 0.70 | 7.1, 2.51 | 0.005 |
| Level 4 | Ref | Ref | ||
Changes in PAM scores in the TELE-IBD EOW and TELE-IBD W arms were – 3.96 and 1.94, respectively. These changes were not statistically significant compared to standard care (p = 0.13, p = 0.47, respectively). Participants with baseline GSE scores between 31 and 40 increased PAM scores by 5.25 points (p = 0.03). Similarly, participants with baseline PAM scores corresponding to level 1 activation showed a 31.3 point increase in PAM score (p < 0.001), those with baseline level 2 activation showed a 18.6 point increase (p < 0.001), and those with baseline level three activation showed a 7.1 point increase (p = 0.005).
Discussion
Change in PAM and GSE scores was assessed from baseline to 12 months for participants in the TELE-IBD trial. All arms experienced modest increases in GSE scores; however, neither interventional arms had a significantly larger improvement than the control group. Standard care and TELE-IBD W arms experienced increases in PAM scores while the TELE-IBD EOW arm showed a significant reduction in PAM. After adjusting for LOC scores, baseline PAM, and baseline GSE as potential confounders, differences among groups were no longer significant. Participants with lower baseline GSE scores demonstrated significantly higher improvements in both GSE and PAM. Similarly, participants with lower baseline PAM scores showed significantly higher increases in PAM scores.
To our knowledge, no other trial has assessed the effect of telemedicine on patient activation and general self-efficacy for IBD patients. However, similar studies have been done in other chronic illnesses. Trief et al. [32] found that a telemedicine intervention improved self-efficacy and health outcomes among diabetics. Similarly, Clarke et al. [33] reported that patients with low baseline mental health function experienced the largest gains in overall distress, depression, and anxiety after receipt of a telemedicine intervention, mirroring our findings of large improvements among patients with lower baseline GSE and PAM scores. Several studies have reported increases in patient activation after a telemedicine intervention [18, 20, 21, 34]. The TELE-IBD EOW arm in our trial surprisingly showed a reduction in PAM from baseline to 12 months; however, Ledford et al. [35] also reported a negative effect of telemedicine on pregnant mothers receiving maternity care. The mixed results of the effect of telemedicine on PAM and GSE are at least partially explained by the heterogeneity of telemedicine interventions and the contexts in which they are applied. After adjustment for baseline confounding, there were no differences in GSE or PAM between the standard care and intervention groups.
The strengths of our study include the large sample size and the randomized control trial design. In addition, we have previously shown that our TELE-IBD intervention is both feasible and agreeable for patients [17]. Our study had a number of limitations. Most participants had baseline PAM scores corresponding to the highest PAM level (n = 98, 52.4%), and the mean baseline GSE score of our sample was higher than the national average of the USA [36]. Thus, our failure to observed significant changes in PAM and GSE in our study may be due to our study population having less room for improvement in patient activation and self-efficacy. Similarly, our population was selected from patients receiving care at tertiary IBD referral centers. Patients in referral centers are likely not representative of patients in the community with less severe disease. Further, standard of care in all three centers include integrated, multidisciplinary, expert IBD care which may decrease the effect of the intervention in the experimental arms of the study.
Last, a disease-specific self-efficacy measure may have more accurately assessed how our intervention impacted self-efficacy with regard to self-management of IBD.
Overall, use of remote monitoring through text messaging as an adjunct to care did not improve GSE or PAM compared to routine care in referral centers for IBD. Patients with lower baseline PAM and GSE scores demonstrated the greatest improvements in patient activation and self-efficacy. Further research is needed to elucidate factors that increase self-efficacy and patient activation among IBD patients, including whether targeted intervention with telemedicine in high-risk groups is more effective than standard care.
Acknowledgments
Funding This research was supported by the Agency for Healthcare Research and Quality (1R01HS018975-01A1) and the University of Maryland General Clinical Research Centers Program. Zaid Bilgrami was supported by the Program for Research Initiated by Students and Mentors (PRISM) at the University of Maryland School of Medicine.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Dahlhamer JM. Prevalence of inflammatory bowel disease among adults aged ≥ 18 years—United States, 2015: MMWR. Morbidity and mortality weekly report; 2016;65. [DOI] [PubMed] [Google Scholar]
- 2.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. [DOI] [PubMed] [Google Scholar]
- 3.Altschuler A, Collins B, Lewis JD, et al. Gastroenterologists’ attitudes and self-reported practices regarding inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:992–999. [DOI] [PubMed] [Google Scholar]
- 4.Sewitch MJ, Abrahamowicz M, Barkun A, et al. Patient non-adherence to medication in inflammatory bowel disease. Am J Gastroenterol. 2003;98:1535. [DOI] [PubMed] [Google Scholar]
- 5.Jackson CA, Clatworthy J, Robinson A, Horne R. Factors associated with non-adherence to oral medication for inflammatory bowel disease: a systematic review. Am J Gastroenterol. 2010;105:525. [DOI] [PubMed] [Google Scholar]
- 6.Ediger JP, Walker JR, Graff L, et al. Predictors of medication adherence in inflammatory bowel disease. Am J Gastroenterol. 2007;102:1417. [DOI] [PubMed] [Google Scholar]
- 7.Rogala L, Miller N, Graff LA, et al. Population-based controlled study of social support, self-perceived stress, activity and work issues, and access to health care in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:526–535. [DOI] [PubMed] [Google Scholar]
- 8.Fishman LN, Barendse RM, Hait E, Burdick C, Arnold J. Self-management of older adolescents with inflammatory bowel disease: a pilot study of behavior and knowledge as prelude to transition. Clin Pediatr. 2010;49:1129–1133. [DOI] [PubMed] [Google Scholar]
- 9.Baars JE, Siegel CA, van’t Spijker A, Markus T, Kuipers EJ, van der Woude CJ. Inflammatory bowel disease-patients are insufficiently educated about the basic characteristics of their disease and the associated risk of colorectal cancer. Dig Liver Dis. 2010;42:777–784. [DOI] [PubMed] [Google Scholar]
- 10.Cross RK, Lapshin O, Finkelstein J. Patient subjective assessment of drug side effects in inflammatory bowel disease. J Clin Gastroenterol. 2008;42:244–251. [DOI] [PubMed] [Google Scholar]
- 11.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hibbard JH, Mahoney ER, Stock R, Tusler M. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42:1443–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene J, Hibbard JH. Why does patient activation matter? an examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med. 2012;27:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandura A Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191. [DOI] [PubMed] [Google Scholar]
- 15.Maeda U, Shen BJ, Schwarz ER, Farrell KA, Mallon S. Self-efficacy mediates the associations of social support and depression with treatment adherence in heart failure patients. Int J Behav Med. 2013;20:88–96. [DOI] [PubMed] [Google Scholar]
- 16.King DK, Glasgow RE, Toobert DJ, et al. Self-efficacy, problem solving, and social-environmental support are associated with diabetes self-management behaviors. Diabetes Care. 2010;33:751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cross RK, Finkelstein J. Feasibility and acceptance of a home telemanagement system in patients with inflammatory bowel disease: a 6-month pilot study. Dig Dis Sci. 2007;52:357–364. 10.1007/s10620-006-9523-4 [DOI] [PubMed] [Google Scholar]
- 18.Viers BR, Pruthi S, Rivera ME, et al. Are patients willing to engage in telemedicine for their care: a survey of preuse perceptions and acceptance of remote video visits in a urological patient population. Urology. 2015;85:1233–1240. [DOI] [PubMed] [Google Scholar]
- 19.Ciere Y, Cartwright M, Newman SP. A systematic review of the mediating role of knowledge, self-efficacy and self-care behaviour in telehealth patients with heart failure. J Telemed Telecare. 2012;18:384–391. [DOI] [PubMed] [Google Scholar]
- 20.Kim JY, Wineinger NE, Steinhubl SR. The influence of wireless self-monitoring program on the relationship between patient activation and health behaviors, medication adherence, and blood pressure levels in hypertensive patients: a substudy of a randomized controlled trial. J Med Internet Res. 2016;18:e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oddone EZ, Gierisch JM, Sanders LL, et al. A coaching by telephone intervention on engaging patients to address modifiable cardiovascular risk factors: a randomized controlled trial. J Gen Intern Med. 2018;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cross RK, Langenberg P, Regueiro MD, et al. 903-a randomized controlled trial of telemedicine for patients with inflammatory bowel disease (Tele-IBD). Gastroenterology. 2018;154:S–177. [DOI] [PubMed] [Google Scholar]
- 23.Cross RK, Jambaulikar G, Langenberg P, et al. TELEmedicine for patients with inflammatory bowel disease (TELE-IBD): design and implementation of randomized clinical trial. Contemp Clin Trials. 2015;42:132–144. [DOI] [PubMed] [Google Scholar]
- 24.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol. 1989;24:2–6. [DOI] [PubMed] [Google Scholar]
- 25.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;315:514. [DOI] [PubMed] [Google Scholar]
- 26.Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr Gen Appl. 1966;80:1. [PubMed] [Google Scholar]
- 28.Eaden JA, Abrams K, Mayberry JF. The Crohn’s and Colitis knowledge score: a test for measuring patient knowledge in inflammatory bowel disease. Am J Gastroenterol. 1999;94:3560. [DOI] [PubMed] [Google Scholar]
- 29.Berwick DM, Murphy JM, Goldman PA, Ware JE Jr, Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Med Care. 1991;1:169–176. [DOI] [PubMed] [Google Scholar]
- 30.Schwarzer R, Jerusalem M. Generalized self-efficacy scale In: Weinman J, Wright S, Windsor JM, eds. Measures in Health Psychology: A User’s Portfolio. Causal and Control Beliefs. Berkshire: NFER-NELSON; 1995:35. [Google Scholar]
- 31.Health Insigina. Patient activation measure (PAM) 13 TM License Materials copyright. Portland: Insigna Health LLC; 2011. [Google Scholar]
- 32.Trief PM, Teresi JA, Eimicke JP, Shea S, Weinstock RS. Improvement in diabetes self-efficacy and glycaemic control using telemedicine in a sample of older, ethnically diverse individuals who have diabetes: the IDEATel project. Age Ageing. 2009;38:219–225. [DOI] [PubMed] [Google Scholar]
- 33.Clarke J, Proudfoot J, Birch MR, et al. Effects of mental health self-efficacy on outcomes of a mobile phone and web intervention for mild-to-moderate depression, anxiety and stress: secondary analysis of a randomised controlled trial. BMC Psychiatry. 2014;14:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michaud TL, Siahpush M, Schwab RJ, et al. Remote patient monitoring and clinical outcomes for postdischarge patients with type 2 diabetes. Popul Health Manag. 2018;21:387–394. [DOI] [PubMed] [Google Scholar]
- 35.Ledford CJ, Womack JJ, Rider HA, et al. Unexpected effects of a system-distributed mobile application in maternity care: a randomized controlled trial. Health Educ Behav. 2017;45:323–330. [DOI] [PubMed] [Google Scholar]
- 36.Scholz U, Doña BG, Sud S, Schwarzer R. Is general self-efficacy a universal construct? psychometric findings from 25 countries. Eur J Psychol Assess. 2002;18:242. [Google Scholar]


