Abstract
Background
Two-dimensional speckle tracking echocardiography (2D-STE) is a novel and non-invasive technique for the diagnosis of coronary artery disease (CAD). This retrospective study from a single center aimed to identify myocardial ischemia using 2D-STE in CAD patients identified by angiography.
Material/Methods
From March 1 to November 30, 2019, 690 patients in Beijing Hospital were enrolled. After angiography, 346 patients were diagnosed with CAD. Reduction in vessel diameter of ≥50% by stenosis in at least 1 major coronary artery or its main branch was considered CAD. Analysis of 2D-STE was performed using EchoPAC version 201.
Results
The global strain was significantly impaired in CAD patients (P<0.01). Global longitudinal peak strain (GLPS) was analyzed in layers. For GLPS of the epicardium, the odds ratio (OR) was 1.297 (1.217–1.382; P=0.002), the area under the curve (AUC) was 0.727, and the cut-off value was −16.95; sensitivity and specificity were 73.7% and 63.0%, respectively. For GLPS of the middle layer, the OR was 1.260 (1.192–1.333; P<0.001), the AUC was 0.732, and the cut-off value was −20.95; sensitivity and specificity were 82.4% and 56.2%, respectively. For GLPS of the endocardium, the OR was 1.193 (1.137–1.251; P<0.001), the AUC was 0.708, and the cut-off value was −22.95; sensitivity and specificity were 82.9% and 52.9%, respectively.
Conclusions
The findings from this study support the clinical application of 2D-STE in patient populations with suspected myocardial ischemia due to CAD. Therefore, 2D-STE combined with ECG monitoring may have a future role for early screening of CAD patients.
Keywords: Coronary Vessels, Echocardiography, Myocardial Ischemia
Background
Non-invasive modalities that are convenient and efficient, especially for early screening, can play a key role in the control of coronary artery disease (CAD). Conventional echocardiography mainly relies on detecting decreased ventricular wall motion by the echocardiologist using the naked eyes, and only provides reliable results in some patients with CAD and myocardial infarction [1]. Thus, it is difficult to apply this procedure in all kinds of CAD. Two-dimensional speckle tracking echocardiography (2D-STE) is a novel technique and the results are based on the deformation of the myocardium. Recently, studies have found that 2D-STE can be used in clinical practice to diagnose coronary heart disease in a specific population with acute myocardial infarction, angina pectoris, and chronic coronary heart disease, especially in outpatient departments [2–4]. With 2D-STE, cardiologists can implement timely interventional strategies in patients with non-ST-segment elevation myocardial infarction [5]. 2D-STE has an extensive application value in clinical outpatient management, whether for screening patients or for evaluating postoperative rehabilitation. Therefore, this retrospective study from a single center aimed to identify myocardial ischemia using 2D-STE in patients with CAD identified by coronary artery angiography.
Nevertheless, in a multicenter prospective study in Israel, the longitudinal strain of 2D-STE was not sufficient to rule out acute coronary syndrome (ACS) in the emergency department [6]. The diagnostic efficacy of 2D-STE for patients with CAD varies between studies, as some studies reported high specificity and sensitivity, while others reported low values [7–9]. Routine application of 2D-STE in patients with coronary heart disease is still controversial.
2D-STE is convenient, cost effective, and readily available compared with exercise electrocardiograph (ECG), cardiovascular magnetic resonance (CMR), and angiography. The latest guideline on non–ST-segment elevation acute coronary syndromes (NSTE-ACS) suggests that using GLPS might improve the diagnostic value of conventional echocardiography [8]. This guideline does not have sufficient standardizations to recommend the routine use of 2D-STE in all patients with CAD [10]. Thus, this study aimed to investigate the diagnostic value of 2D-STE for coronary heart disease in a large population of patients who underwent angiography. Our focus was to provide more evidence of the clinical application of 2D-STE in patients suspected of having coronary artery disease.
Material and Methods
Population
This is a cross-sectional study. We enrolled all consecutive patients referred for coronary angiography for the first time from March to November 2019 in Beijing Hospital. All patients provided informed written consent to participate in this study. All procedures complied with the principles of the Declaration of Helsinki and were approved by the Beijing Hospital Ethics Committee. The study was registered in ClinicalTrials (NCT03905200).
The inclusion criteria were: 1) patients with ischemic symptoms (chest pain or discomfort (angina) suspected to be caused by myocardial ischemia) or positive examination results; 2) patients aged >18 years; and 3) patients with sinus rhythm.
The exclusion criteria were: 1) Patients with prior history of CAD; 2) patients with ST-elevation myocardial infarction, 3) patients with moderate to severe valve disease, malignant arrhythmia, hypertrophic cardiomyopathy, or dilated cardiomyopathy; 4) patients with other end-stage diseases (a serious disease of organs and systems other than the cardiovascular system, with a life expectancy of less than one year); or 5) patients with poor echocardiographic images.
Conventional Echocardiography
All echocardiograms were obtained using Vivid E9 (GE Healthcare; Horten, Norway) with a M5SC transducer. Conventional echocardiography was performed by a doctor blinded to the results of other tests (ECG, creatine kinase, and troponin). The left ventricular (LV) end-diastolic diameter, septal wall thickness, and LV posterior wall thickness were obtained from the parasternal long-axis view using 2D echocardiography. Early (E) peak velocity, atrial (A) diastolic filling velocity, and deceleration time of the E wave were measured using pulsed-wave Doppler. The E/A ratio was calculated. The left ventricular ejection fraction was determined using the biplane Simpson method [31].
STE
Patients underwent echocardiography before coronary angiography, which was performed by the same experienced sonographer, using GE Vivid E9. Images of the left ventricle (short-axis views at basal, middle and apical levels; apical two-, three-, and four-chamber views) were obtained at end-expiration at 50–70 frames/s. Offline analysis was performed by an independent analyst, using EchoPAC version 201 (GE Medical Systems, Chicago, IL).
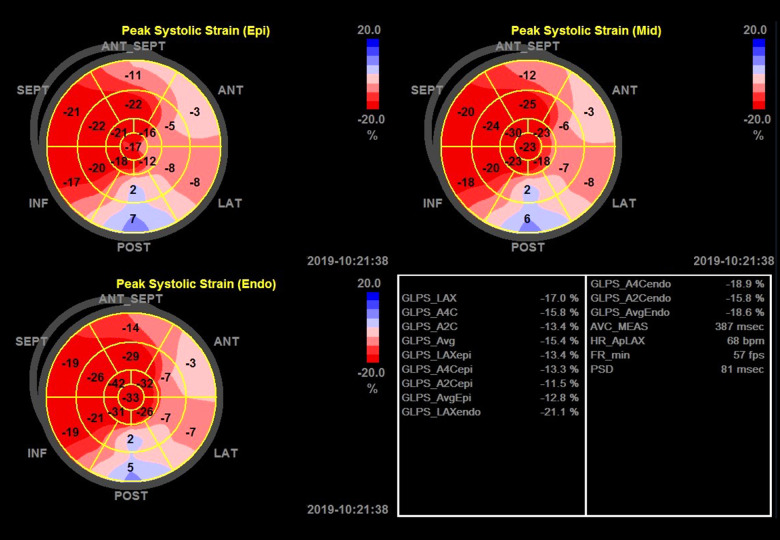
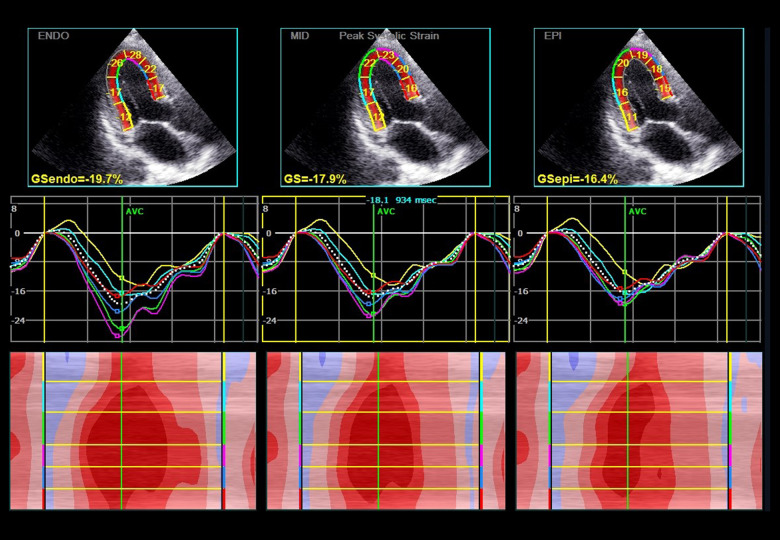
At end-systole, the endocardial border of each view was manually traced. The region of interest (ROI) was readjusted if tracking was unsatisfactory. Poor-quality images were excluded from further analysis. The global longitudinal peak strain (GLPS) was analyzed. GLPS in 3 layers – GLPS (epicardium, epi), GLPS average (GLPSavg), and GLPS (endocardium, endo) – were analyzed separately in the regression model. GLPSavg indicates the middle layer. In most studies, it also represents the whole GLPS of the left ventricle [2,11,29]. Global circumferential strain (GCS) was obtained from basal, middle, and apical short views. Peak strain deviation (PSD) and other parameters were also obtained (Figures 1, 2).
Figure 1.
Global and layer longitudinal peak strain assessed by 2D-STE in a patient with CAD in the bull’s eyes plots. The strain plots depict the peak systolic strain of epicardium layer, middle layer, and endocardium layer. The peak systolic strain in the 17 segments of the left ventricle are shown in the bulls-eye plots. The right lower panel indicates the global longitudinal peak systolic strain value of the left apical axis view, apical 4-chamber view, apical 2-chamber view, and the average values. ANT – anterior; GLPS – global lateral pulse strain; INF – inferior; LAT – lateral; SEPT – septum; LAX – apical long axis; A4C – apical 4-chamber; A2C – apical 2-chamber; epi – epicardium; endo – endocardium; AVC – atrioventricular contraction; PSD – peak strain deviation.
Figure 2.
Layer longitudinal peak strain assessed by 2D-STE in a patient with CAD. The first-row panels indicate the global and segment strain from apical long-axis view in endocardium, middle layer, and epicardium. The second-row panels indicate the corresponding segmental strain traces. The lower panels indicate the corresponding qualitative color M-mode strain referring to the 6 consecutive myocardial segments. Dark red, normal strain; light red, decreased strain; and pink color, strongly reduced strain. ENDO – endocardium; MID – middle layer; EPI – epicardium; GS – global strain.
Coronary Angiography
Patients were sent to the catheter laboratory after undergoing echocardiography. Coronary angiography was performed by an experienced interventional cardiologist. Angiograms were assessed by 2 experienced senior interventional cardiologists. Reduction ≥50% in luminal diameter due to stenosis in at least 1 major coronary artery or its main branch was considered CAD. The procedure was based on 2015 European Society of Cardiology (ESC) Guidelines [8].
Statistical Analysis
Proportions were compared between CAD and non-CAD groups using the chi-square test. Continuous variables were compared using the t test. Univariate analysis was performed on baseline characteristics: age, sex, hypertension, diabetes mellitus (DM), hypercholesterolemia, smoking, CAD family history, heart rate, diastolic blood pressure, body mass index (BMI), GLPS (epi), GLPSavg, GLPS (endo), and PSD. Logistic regression analysis was performed on specific characteristics that were significantly different between the 2 groups: sex, DM, smoking, diastolic blood pressure, GLPS (epi), GLPSavg, GLPS (endo), PSD, GCS basal, and GCS apical.
Receiver operating characteristic (ROC) curves were constructed, and the area under the curve (AUC) was calculated. GLPSavg indicates the middle layer of the myocardium and is derived using a special algorithm involving the whole myocardium. From the ROC constructed for GLPS (epi), GLPSavg, and GLPS (endo), we obtained the optimal cut-off value with the highest sensitivity and specificity. Intra-observer and inter-observer reproducibility was assessed in 20 random patients using the Bland-Altman method. A P value of <0.05 was considered statistically significant. Analyses were performed using SPSS Statistics version 22.0 software (IBM Corp., Armonk, NY, USA).
Patient and Public Involvement
Patients and the public were not involved in the design, recruitment, or implementation of the study.
Results
In our study, a total of 690 patients were enrolled. Overall, 68 patients were excluded due to poor image quality or absence of angiography. Of the remaining 622 patients, 346 had CAD and 276 did not have CAD.
Table 1 presents the baseline characteristics of the patients. GLPS (epi), GLPSavg, and GLPS (endo) were significantly more impaired in the CAD group than in the non-CAD group (P<0.01). PSD was not significantly different between the 2 groups. Absolute values of GCS apical and GCS basal decreased significantly in the CAD group compared with the non-CAD group (P<0.01), whereas the absolute value of the GCS in the middle planes showed no significant difference between the 2 groups.
Table 1.
Baseline characteristics of the population.
| Non-CAD (n=276) | CAD (n=346) | χ2/t | P | |
|---|---|---|---|---|
| Age, y | 64.22±9.22 | 64.19±9.88 | 0.048 | 0.962 |
| Male (%) | 135 (48.9) | 251 (72.5) | 36.41 | <0.001 |
| Hypertension (%) | 183 (66.3) | 230 (66.5) | 0.002 | 0.965 |
| DM (%) | 81 (29.3) | 151 (43.6) | 13.413 | <0.001 |
| Hypercholesterolemia (%) | 200 (72.5) | 262 (75.7) | 0.853 | 0.356 |
| Smoke (%) | 94 (34.1) | 193 (55.8) | 29.152 | <0.001 |
| CAD family history | 85 (30.8) | 111 (32.1) | 0.117 | 0.732 |
| Herat rate (bpm) | 75.38±13.36 | 74.5±11.21 | 0.890 | 0.374 |
| Systolic blood pressure | 134.9±16.67 | 135.08±17.15 | −0.136 | 0.892 |
| Diastolic blood pressure | 79.33±11.31 | 77.38±10.96 | 2.169 | 0.03 |
| BMI | 25.73±3.96 | 26.03±6.16 | −0.721 | 0.471 |
| GLPS (epi) | −18.57±3.22 | −15.74±3.32 | −10.725 | <0.001 |
| GLPSavg | −21.79±3.78 | −18.52±3.74 | −10.787 | <0.001 |
| GLPS (endo) | −24.57±4.31 | −21.23±4.27 | −9.654 | <0.001 |
| PSD | 51.25±22.77 | 54.08±26.31 | −1.414 | 0.158 |
| GS apical | −30.45±12.28 | −28.20±12.36 | −2.263 | 0.024 |
| GS mid | −23.23±9.08 | −22.37±8.96 | −1.18 | 0.238 |
| GS basal | −16.77±7.30 | −14.89±7.14 | −3.225 | 0.001 |
Continuous data are presented as mean±SD. Categorical data are presented as percentage n (%). DM – diabetes mellitus; CAD – coronary artery disease; BMI – body mass index; GLPS (epi) – global longitudinal peak strain of epi-myocardium; GLPSavg – average global longitudinal peak strain; GLPS (endo) – global longitudinal peak strain of endo-myocardium; PSD – peak strain dispersion; GS – global strain.
Diagnostic Performance of GLPS (epi)
In Table 2, multifactor regression analysis results showed that DM [odds ratio (OR) 1.819 (1.242–2.664; P=0.002), diastolic blood pressure, and GLPS (epi) [OR 1.297 (1.217–1.382; P=0.002) per 1% decrease] were independent risk factors for CAD. Diastolic blood pressure was an independent protective factor, while diabetes and GLPS (epi) were independent risk factors. In the regression model, smoking, GCS apical, and GCS basal were not independent predictors of CAD. In Figure 3 and Table 3, the predictive value analysis showed that the AUC was 0.727, indicating a good predictive value, and the cut-off value was −16.95, with sensitivity and specificity of 73.7% and 63%, respectively. In addition, the AUC of the regression model was 0.777, indicating that the regression model had a good effect.
Table 2.
Regression model (A) including global longitudinal peak strain epicardium (GLPSepi).
| B | S.E. | Wald | P | OR | OR 95% CI | ||
|---|---|---|---|---|---|---|---|
| Upper | Lower | ||||||
| DM | 0.598 | 0.195 | 9.449 | 0.002 | 1.819 | 1.242 | 2.664 |
| Smoke | 0.278 | 0.232 | 1.435 | 0.231 | 1.320 | 0.838 | 2.081 |
| Diastolic blood pressure | −0.023 | 0.008 | 7.390 | 0.007 | 0.977 | 0.961 | 0.994 |
| GLPS (epi) | 0.260 | 0.032 | 64.33 | <0.001 | 1.297 | 1.217 | 1.382 |
| GS apical | −0.004 | 0.008 | 0.227 | 0.634 | 0.996 | 0.981 | 1.012 |
| GS basal | 0.007 | 0.013 | 0.286 | 0.593 | 1.007 | 0.981 | 1.034 |
DM – diabetes mellitus; GLPSavg (epi) – global longitudinal peak strain of epi-myocardium; GS – global circumferential strain.
Figure 3.
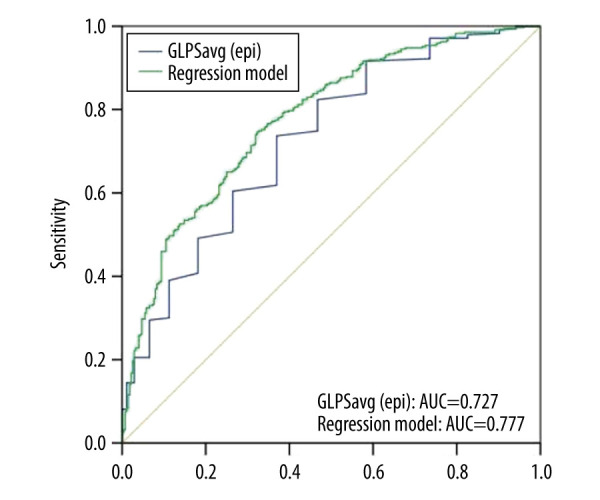
Receiver operating characteristic (ROC) curves for predicting coronary artery disease (CAD). ROC curves of global longitudinal peak strain epicardium (GLPSepi) (blue line) and regression model (A) (green line). AUC indicates the area under the curve.
Table 3.
Predictive value analysis of GLPS.
| AUC | 95% CI | P | Sensitivity (%) | Specificity (%) | Cutoff value | |
|---|---|---|---|---|---|---|
| Regression model (A) | ||||||
| GLPS (epi) | 0.727 | 0.688–0.767 | <0.001 | 73.7 | 63.0 | −17.95 |
| Regression model | 0.777 | 0.741–0.814 | <0.001 | 74.6 | 67.8 | – |
| Regression model (B) | ||||||
| GLPSavg | 0.732 | 0.693–0.772 | <0.001 | 82.4 | 56.2 | −21.95 |
| Regress model | 0.780 | 0.744–0.817 | <0.001 | 81.5 | 61.2 | – |
| Regression model (C) | ||||||
| GLPS (endo) | 0.708 | 0.668–0.749 | <0.001 | 82.9 | 52.9 | −24.95 |
| Regress model | 0.767 | 0.729–0.804 | <0.001 | 53.5 | 85.5 | – |
GLPS (epi) – global longitudinal peak strain of epi-myocardium; GLPSavg – average global longitudinal peak strain; GLPS (endo) – global longitudinal peak strain of endo-myocardium.
Diagnostic Performance of GLPSavg
Multifactor regression analysis results showed that DM [OR 1.825 (1.246–2.675; P=0.002)], diastolic blood pressure, and GLPSavg [OR 1.260 (1.192–1.333; P=0.000) per 1% decrease] were independent factors of CAD (Table 4). Diastolic blood pressure was an independent protective factor, while DM and GLPSavg were independent risk factors. In the regression model, smoking, GCS apical, and GCS basal were not independent predictors of CAD. In Figure 4 and Table 3, the predictive value analysis showed that the AUC was 0.727, indicating a high predictive value, and the cut-off value was −20.95, with sensitivity and specificity of 82.4% and 56.2%, respectively. The AUC of the regression model was 0.732, indicating that the regression model had a good effect.
Table 4.
Regression model (B) including global longitudinal peak strain average (GLPSavg).
| B | S.E. | Wald | P | OR | OR 95% CI | ||
|---|---|---|---|---|---|---|---|
| Upper | Lower | ||||||
| Gender | −0.976 | 0.245 | 15.865 | <0.001 | 0.377 | 0.233 | 0.609 |
| DM | 0.602 | 0.195 | 9.528 | 0.002 | 1.825 | 1.246 | 2.675 |
| Smoke | 0.287 | 0.233 | 1.515 | 0.218 | 1.333 | 0.844 | 2.105 |
| Diastolic BP | −0.023 | 0.008 | 7.587 | 0.006 | 0.977 | 0.961 | 0.993 |
| GLPSavg | 0.231 | 0.029 | 65.772 | <0.001 | 1.260 | 1.192 | 1.333 |
| GS apical | −0.006 | 0.008 | 0.505 | 0.477 | 0.994 | 0.979 | 1.01 |
| GS basal | 0.007 | 0.013 | 0.255 | 0.613 | 1.007 | 0.981 | 1.034 |
DM – diabetes mellitus; BP – blood pressure; GLPSavg – average global longitudinal peak strain; GS – global circumferential strain.
Figure 4.
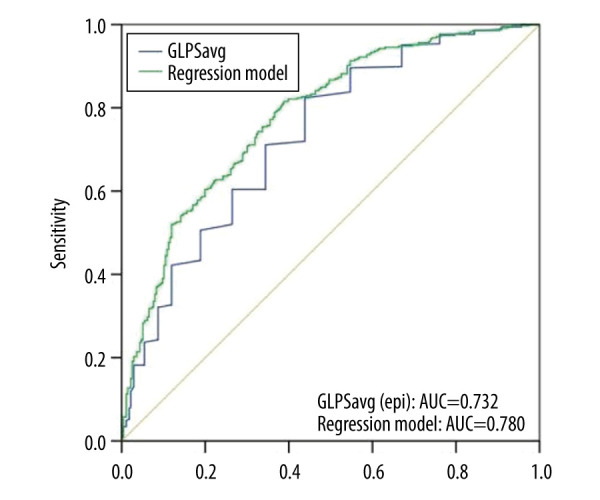
Receiver operating characteristic (ROC) curvesfor predicting CAD. ROC curves of global longitudinal peak strain average (GLPSavg) (blue line) and regression model (B) (green line). AUC indicates the area under the curve.
Diagnostic Performance of GLPS (endo)
The multifactor regression analysis results showed that DM [OR 1.846 (1.267–2.689; P<0.001)], diastolic blood pressure, and GLPS (endo) [OR 1.193 (1.137–1.251; P<0.001) per 1% decrease] were independent factors for CAD (Table 5). Diastolic blood pressure was an independent protective factor, while diabetes and GLPS (endo) were independent risk factors. Predictive value analysis showed that the AUC was 0.708, indicating a good predictive value, and the cut-off value was −22.95 with sensitivity and specificity of 82.9% and 52.9%, respectively (Figure 5, Table 3). The AUC of the regression model was 0.767, indicating that the regression model had a good effect.
Table 5.
Regression model (C) including global longitudinal peak strain endocardium (GLPSendo).
| B | S.E. | Wald | P | OR | OR 95% CI | ||
|---|---|---|---|---|---|---|---|
| Upper | Lower | ||||||
| Gender | −0.981 | 0.241 | 16.552 | <0.001 | 0.375 | 0.234 | 0.601 |
| DM | 0.613 | 0.192 | 10.199 | 0.001 | 1.846 | 1.267 | 2.689 |
| Smoke | 0.284 | 0.230 | 1.528 | 0.216 | 1.328 | 0.847 | 2.083 |
| Diastolic BP | −0.023 | 0.008 | 7.347 | 0.007 | 0.978 | 0.962 | 0.994 |
| GLPS (endo) | 0.176 | 0.024 | 52.863 | <0.001 | 1.193 | 1.137 | 1.251 |
| GS apical | −0.004 | 0.008 | 0.227 | 0.633 | 0.996 | 0.981 | 1.011 |
| GS basal | 0.011 | 0.013 | 0.733 | 0.392 | 1.011 | 0.986 | 1.038 |
DM – diabetes mellitus; BP – blood pressure; GLPS(endo) – global longitudinal peak strain of endocardium; GS – global circumferential strain.
Figure 5.
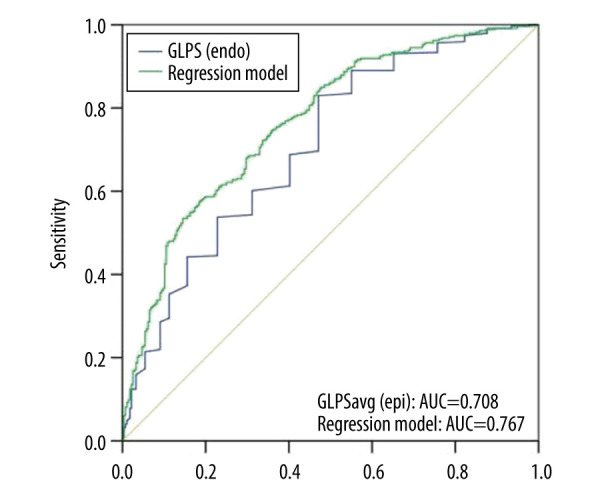
Receiver operating characteristic (ROC) curves for predicting CAD. ROC curves of global longitudinal peak strain endocardium (GLPSendo) (blue line) and regression model C (green line). AUC indicates the area under the curve.
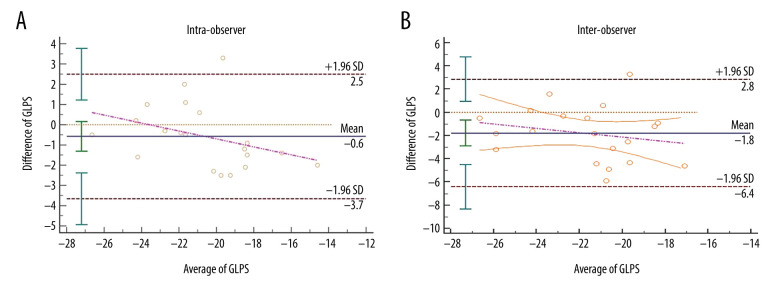
Reproducibility
The mean difference±standard deviations (SDs) for the intra-observer agreement was −0.6±3.1% and was not significantly different (Figure 6A). The mean difference±SDs for the inter-observer agreement was −1.8±4.6% which was significantly different (Figure 6B).
Figure 6.
Bland-Altman analysis for reproducibility. Intra-observer variability of global longitudinal peak strain (GLPS) (A) and inter-observer variability of GLPS (B). Mean indicates mean difference. GLPS indicates the global longitudinal peak strain.
Discussion
In our study, GLPS was significantly impaired in all layers in the CAD patients. GCS in the basal and apical planes was reduced in the CAD group, whereas GCS in the middle plane was not different between the CAD and the non-CAD groups. In the regression model, GCS had no predictive value, while GLPS in all 3 layers had a good predictive value. Furthermore, the value was greater when modeled in conjunction with other parameters. Previous studies have reported impairment of strain beginning from the endocardium in CAD patients [2,11]. However, in this study, epicardial wall deformation was more sensitive to coronary stenosis. However, based on pathophysiological considerations, the endocardial myocardium is more vulnerable to myocardial ischemia. The discrepancy may be due to geometric causes of wall deformation.
Myocardial function is damaged at the cellular level when ischemia starts. Different myocardial layers respond differently to ischemia [11]. Ever since Heimdal et al applied STE to analyze the deformation of myocardial tissue in 1998, the technique has proved to be useful in various heart diseases [12]. Longitudinal strain is one of the sensitive markers of CAD [13]. Strain measured using tissue Doppler imaging (TDI) are angle-dependent and depend greatly on the experience of the doctors using the machine [14]. Introduction of the novel 2D-STE can resolve the problems associated with TDI and conventional echocardiography; therefore, the strain value assessed by 2D-STE is more accurate and reliable.
In a recent study, the strain attained a sensitivity of 77% and a specificity of 93%, which predicted 70% of CAD cases [7]. Another study showed that GLPS had a high sensitivity (83%) and specificity (77%) in identifying patients with obstructive CAD [15]. A study on 150 NSTE-ACS patients showed a high performance of GLPS in predicting CAD (AUC=0.92) [16]. In our study, the performance of GLPS was not as satisfactory as in previous studies, yet it was acceptable. This might be due to the heterogeneity of the population. GLPS decreases differently according to the levels of myocardial ischemia in different CAD patients.
Several studies have proven that speckle tracking echocardiography has great value in the diagnosis and prognosis of coronary heart disease [11,16–22]. Biering-Sorensen et al found that the absolute value of GLPS was significantly lower in patients with CAD and was an independent predictor of CAD [7]. In a study of patients with acute myocardial infarction, GCS was able to predict acute coronary occlusion [5]. In our study, GCS in the basal and apical planes was reduced in patients with CAD, but it could not predict CAD. Furthermore, STE could even predict the final infarct size [23]. Haugaa et al found that peak strain dispersion could predict ventricular arrhythmias after infarction [18, 24]. Gjesdal et al [25] discovered that GLS was valuable in the identification of small- and medium-sized infarcts in patients with chronic ischemic heart disease. Hagemann et al [11] found that layer-specific GLS was significantly impaired in patients with significant CAD. The results of these studies are consistent with the findings of our study.
As a novel echocardiography technology, 2D-STE can detect ischemic region quantitatively, while conventional echocardiography assesses myocardial ischemia based on the operator’s naked eyes. Compared with exercise ECG and CMR, 2D-STE is much more available and requires a shorter procedure and post-processing time [26]. It also has more diagnostic value in disabled patients who cannot undergo exercise ECG, coronary computed tomographic angiography, or CMR. As a non-invasive modality, 2D-STE has the advantages of reduced economic and biological costs [27,28].
However, echocardiography interpretations are subjective. In our study, echocardiography showed good intra-observer reproducibility, but there were inconsistencies between different observers. The reason might be that different researchers have different understandings of the definition of the endocardial border. The operator must have sufficient experience to make an accurate measurement. We chose several STE parameters according to the reference and analysis. There are still relevant parameters of territory strain that have not been analyzed in the article; thus, more detailed information is needed. We did not analyze non-ischemic factors, such as diabetes and hypertension, which may reduce strain values. We defined coronary artery disease as at least 50% stenosis of coronary vessels. However, the results may have been different if the criteria were 75% or 90% stenosis, or in different types of CAD patients. Research with larger samples and more detailed analysis may produce more information on the diagnostic value of STE. In this study, we used only 1 method (EchoPAC version 201) and vendor (GE). The results might have been different using different vendors. Finally, STE is a developing technique that is still limited by image quality, patient condition, and software issues. It is a semi-automated and semi-quantitative technique. Automation is the future of 2D-STE.
Conclusions
The findings from this study support the clinical application of 2D-STE in patient populations with suspected myocardial ischemia due to CAD. Therefore, 2D-STE combined with ECG monitoring may have a future role for early screening of patients with coronary heart disease.
Footnotes
Conflict of Interests
None.
Source of support: Fang Wang was supported by grants from the Beijing Municipal Science and Technology Commission for Scientific Research (BMSTC; Z161100000516053), and the 13th Five-year National Science and Technology Major Project (NSTMP; 2017ZX09304026). BMSTC, CHDRP, and NSTMP had no input to the design, execution, analysis, or writing of the study
References
- 1.Takeuchi M, Wu VC. Application of left ventricular strain to patients with coronary artery disease. Curr Opin Cardiol. 2018;33(5):464–69. doi: 10.1097/HCO.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 2.Skaarup KG, Iversen A, Jorgensen PG, et al. Association between layer-specific global longitudinal strain and adverse outcomes following acute coronary syndrome. Eur Heart J Cardiovasc Imaging. 2018;19(12):1334–42. doi: 10.1093/ehjci/jey004. [DOI] [PubMed] [Google Scholar]
- 3.Baron T, Christersson C, Hjorthen G, et al. Changes in global longitudinal strain and left ventricular ejection fraction during the first year after myocardial infarction: Results from a large consecutive cohort. Eur Heart J Cardiovasc Imaging. 2018;19(10):1165–73. doi: 10.1093/ehjci/jex260. [DOI] [PubMed] [Google Scholar]
- 4.Badano LP, Muraru D. The good, the bad, and the ugly of using left ventricular longitudinal myocardial deformation by speckle-tracking echocardiography to assess patients after an acute myocardial infarction. Circ Cardiovasc Imaging. 2017;10(7):e006693. doi: 10.1161/CIRCIMAGING.117.006693. [DOI] [PubMed] [Google Scholar]
- 5.Grenne B, Eek C, Sjoli B, et al. Acute coronary occlusion in non-ST-elevation acute coronary syndrome: Outcome and early identification by strain echocardiography. Heart. 2010;96(19):1550–56. doi: 10.1136/hrt.2009.188391. [DOI] [PubMed] [Google Scholar]
- 6.Shiran A, Blondheim DS, Shimoni S, et al. Two-dimensional strain echocardiography for diagnosing chest pain in the Emergency Room: A multicentre prospective study by the Israeli echo research group. Eur Heart J Cardiovasc Imaging. 2017;18(9):1016–24. doi: 10.1093/ehjci/jew168. [DOI] [PubMed] [Google Scholar]
- 7.Liang HY, Cauduro S, Pellikka P, et al. Usefulness of two-dimensional speckle strain for evaluation of left ventricular diastolic deformation in patients with coronary artery disease. Am J Cardiol. 2006;98(12):1581–86. doi: 10.1016/j.amjcard.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 8.Roffi M, Patrono C, Collet JP, et al. [2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC)]. G Ital Cardiol (Rome) 2016;17(10):831–72. doi: 10.1714/2464.25804. [DOI] [PubMed] [Google Scholar]
- 9.Scharrenbroich J, Hamada S, Keszei A, et al. Use of two-dimensional speckle tracking echocardiography to predict cardiac events: Comparison of patients with acute myocardial infarction and chronic coronary artery disease. Clin Cardiol. 2018;41(1):111–18. doi: 10.1002/clc.22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smiseth OA, Torp H, Opdahl A, et al. Myocardial strain imaging: How useful is it in clinical decision making? Eur Heart J. 2016;37(15):1196–207b. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagemann CA, Hoffmann S, Hagemann RA, et al. Usefulness of layer-specific strain in diagnosis of coronary artery disease in patients with stable angina pectoris. Int J Cardiovasc Imaging. 2019;35(11):1989–99. doi: 10.1007/s10554-019-01652-3. [DOI] [PubMed] [Google Scholar]
- 12.Heimdal A, Stoylen A, Torp H, Skjaerpe T. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr. 1998;11(11):1013–19. doi: 10.1016/s0894-7317(98)70151-8. [DOI] [PubMed] [Google Scholar]
- 13.Blessberger H, Binder T. Two-dimensional speckle tracking echocardiography: Clinical applications. Heart. 2010;96(24):2032–40. doi: 10.1136/hrt.2010.199885. [DOI] [PubMed] [Google Scholar]
- 14.Urheim S, Edvardsen T, Torp H, et al. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation. 2000;102(10):1158–64. doi: 10.1161/01.cir.102.10.1158. [DOI] [PubMed] [Google Scholar]
- 15.Nucifora G, Schuijf JD, Delgado V, et al. Incremental value of subclinical left ventricular systolic dysfunction for the identification of patients with obstructive coronary artery disease. Am Heart J. 2010;159(1):148–57. doi: 10.1016/j.ahj.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 16.Caspar T, Samet H, Ohana M, et al. Longitudinal 2D strain can help diagnose coronary artery disease in patients with suspected non-ST-elevation acute coronary syndrome but apparent normal global and segmental systolic function. Int J Cardiol. 2017;236:91–94. doi: 10.1016/j.ijcard.2017.02.068. [DOI] [PubMed] [Google Scholar]
- 17.Biering-Sorensen T, Biering-Sorensen SR, et al. Global longitudinal strain by echocardiography predicts long-term risk of cardiovascular morbidity and mortality in a low-risk general population: The Copenhagen City Heart Study. Circ Cardiovasc Imaging. 2017;10(3):e005521. doi: 10.1161/CIRCIMAGING.116.005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eek C, Grenne B, Brunvand H, et al. Strain echocardiography and wall motion score index predicts final infarct size in patients with non-ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging. 2010;3(2):187–94. doi: 10.1161/CIRCIMAGING.109.910521. [DOI] [PubMed] [Google Scholar]
- 19.Ersboll M, Valeur N, Mogensen UM, et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2013;61(23):2365–73. doi: 10.1016/j.jacc.2013.02.061. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Wu WC, Ma H, Wang H. Usefulness of layer-specific strain for identifying complex CAD and predicting the severity of coronary lesions in patients with non-ST-segment elevation acute coronary syndrome: Compared with Syntax score. Int J Cardiol. 2016;223:1045–52. doi: 10.1016/j.ijcard.2016.08.277. [DOI] [PubMed] [Google Scholar]
- 21.Liang YJ, Zhang Q, Fung JW, et al. Impact of reduction in early- and late-systolic functional mitral regurgitation on reverse remodelling after cardiac resynchronization therapy. Eur Heart J. 2010;31(19):2359–68. doi: 10.1093/eurheartj/ehq134. [DOI] [PubMed] [Google Scholar]
- 22.Atici A, Barman HA, Durmaz E, et al. Predictive value of global and territorial longitudinal strain imaging in detecting significant coronary artery disease in patients with myocardial infarction without persistent ST-segment elevation. Echocardiography. 2019;36(3):512–20. doi: 10.1111/echo.14275. [DOI] [PubMed] [Google Scholar]
- 23.Diao KY, Yang ZG, Ma M, et al. The diagnostic value of global longitudinal strain (GLS) on myocardial infarction size by echocardiography: A systematic review and meta-analysis. Sci Rep. 2017;7(1):10082. doi: 10.1038/s41598-017-09096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haugaa KH, Grenne BL, Eek CH, et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc Imaging. 2013;6(8):841–50. doi: 10.1016/j.jcmg.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Gjesdal O, Hopp E, Vartdal T, et al. Global longitudinal strain measured by two-dimensional speckle tracking echocardiography is closely related to myocardial infarct size in chronic ischaemic heart disease. Clin Sci (Lond) 2007;113(6):287–96. doi: 10.1042/CS20070066. [DOI] [PubMed] [Google Scholar]
- 26.Colla J, Martin J, Eilbert W, Wishnoff M. Bedside use of speckle tracking echocardiography in the Emergency Department to identify acute myocardial infarction. J Emerg Med. 2019;56(5):530–35. doi: 10.1016/j.jemermed.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Picano E. Economic and biological costs of cardiac imaging. Cardiovasc Ultrasound. 2005;3:13. doi: 10.1186/1476-7120-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altiok E, Tiemann S, Becker M, et al. Myocardial deformation imaging by two-dimensional speckle-tracking echocardiography for prediction of global and segmental functional changes after acute myocardial infarction: A comparison with late gadolinium enhancement cardiac magnetic resonance. J Am Soc Echocardiogr. 2014;27(3):249–57. doi: 10.1016/j.echo.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]