INTRODUCTION
Early studies in viral hepatitis noted 2 distinct patterns of infection, suggesting the involvement of multiple hepatitis viruses.1 For several years, these 2 patterns of infection were dubbed “infectious hepatitis” for clinically apparent infection and “serum hepatitis” for clinically inapparent infection. After discovery of the Australia antigen, serum hepatitis was renamed hepatitis B, and infectious hepatitis was renamed hepatitis A.2 The hepatitis A virus (HAV) was characterized in 1973 by Feinstone and colleagues.3 It is transmitted by a fecal-oral route, causing a self-limited infectious hepatitis, but can also cause large epidemics through person-to-person contact.4 HAV is a positive-sense RNA virus in the Herpavirus genus of the Picornaviridae family, with 4 genotypes characterized in humans. It is a nonenveloped small (27 nm) particle in an icosahedral shape.
Most HAV infections that occur in developing countries are not clinically apparent and cause no symptoms, likely because of partial immunity in endemic areas. In contrast, infections in developed countries often are characterized by jaundice and an acute hepatitis, especially in adolescents and adults. HAV cannot cause chronic infection, unlike hepatitis B and C.4 Diagnostic testing for HAV is readily available as is a commercial HAV vaccine5 (Table 1).
Table 1.
Hepatitis A in a nutshell
| Epidemiology | Estimated 1.4 million cases per year globally Infections can be sporadic or epidemic |
| Transmission | Fecal-oral via Person-to-person contact Consumption of contaminated food or water |
| Diagnosis | Presence of IgM antibodies to HAV |
| Treatment | Supportive |
| Classical presentation | Children Asymptomatic Adults Jaundice, hyperbilirubinemia, RUQ pain, anorexia |
| Unusual presentations | Relapsing hepatitis Prolonged cholestasis Acute liver failure |
| Prevention | HAV vaccine |
| Postexposure prophylaxis | HAV vaccine HAV immune globulin |
Abbreviation: RUQ, right upper quadrant.
EPIDEMIOLOGY
HAV infections are seen around the globe, with greater prevalence in developing countries and low-income regions.6 HAV is hyperendemic in sub-Saharan Africa and South Asia, with nearly no at-risk adults because of the frequency of early childhood exposure. Intermediate endemicity is seen in Latin America, the Middle East, North Africa, Eastern Europe, and middle-income regions in Asia.7 Countries with stronger economies, such as the United States and countries in Western Europe, have lower rates of HAV infection, but the susceptibility of their nonimmune adult population becoming ill from HAV is much higher than the lower-income countries.8,9 There is a paradoxic effect with regards to HAV in countries that are demonstrating upward mobility intheir economy. The first HAV exposure in their citizens occurs later in life when compared with lower-income countries, ultimately posing a difficult public health problem in their HAV epidemiologic transition.10
HAV is transmitted feco-orally between people in close contact with each other.11 Transmission commonly occurs from children to their parents, one of the reasons that daycare centers are often implicated in HAV spread.12 HAV from food and water contamination often involves a food service worker who did not appropriately wash hands and sanitize after defecation.13 Fresh produce can be a culprit in spreading HAV infection, because the virus is difficult to wash off surfaces of fruits and vegetables.14 Contaminated water, whether by inadequate chlorination or by poor irrigation infrastructure, leads to both contained and epidemic infections.15 Transient viremia after initial acquisition is responsible for rarely seen parenteral transmission.16 Risk factors in developed countries include men having sex with men (MSM), travel to an endemic country, and intravenous drug use.17–19 Developed countries remain with locales where HAV is endemic, as seen in Native American tribes in the western United States.20
HAV outbreaks occur and commonly are associated with poor sanitary conditions. Outbreaks are often related to water contamination and inadequate sewage disposal in both developing and developed countries.21 Outbreaks in higher-income nations are often linked to a source of contaminated food or water.22 Shellfish are associated with HAV transmission because of their water filtration effect, which effectively concentrates the virus, and have been the cause of prior large epidemics.23
A recent HAV outbreak that reached international attention was the San Diego outbreak, with 590 confirmed cases between November 22, 2016 and June 21, 2018 of genotype 1b HAV in San Diego, California. Most cases were either boys or men, and major risk factors included homelessness, injection drug use, and MSM. Approximately 17% of cases were hepatitis C virus coinfected, and 5% of cases were hepatitis B virus (HBV) coinfected. The San Diego outbreak, and more recently, an outbreak in Michigan highlight that the epidemiology of HAV outbreaks may be shifting from contaminated food and water to poor sanitation revolving around homelessness, overcrowding, and injection drug use.9 The same pattern is being seen in the now ongoing Kentucky outbreak, with more than four thousand infections and 43 deaths.24 The outbreak is largely spread by patients using drugs and without stable housing, as was the case in San Diego. Challenges in hepatitis A vaccination in rural Kentucky and limited funding and resources to acquire vaccine were thought to be the major reasons this outbreak is now the largest and deadliest in the United States.25
NATURAL HISTORY
After a nonimmune subject acquires HAV, the virus is taken up through the enterohepatic circulation and enters the liver, where it replicates.26,27 HAV has been shown to be able to infect enteric cells in culture,28 but there seems to be no evidence of significant replication in the gut. Virions can be detected in stool and blood before onset of symptoms. Several days later, serum transaminases rise. Prodromal symptoms occur about a month following exposure and can consist of fever, malaise, nausea, vomiting, and anorexia. Prodromal symptoms are common in adult infections but not as much in children.4 Adult infections will typically also be characterized by jaundice, diarrhea, and hyperbilirubinemia, peaking 7 to 10 days after the onset of jaundice. Jaundice will typically resolve much faster than the malaise and anorexia, which can last for months. Pediatric infections will often be asymptomatic or have very few symptoms.29
UNUSUAL CLINICAL MANIFESTATIONS
Acute liverfailure from HAV is rare, occurring in about 1 in every 300 cases, and very infrequently results in death or the need for liver transplantation.30 A relapsing hepatitis is infrequently seen in adult infections with recurrent symptoms typically occurring within 6 months of prior infection. Relapsing hepatitis is characterized by shedding of HAV in the stool and elevated transaminases. Occasionally, the elevation in transaminases will be asymptomatic.31 Hepatitis A–associated prolonged cholestasis has also been reported after infection, for periods up to 1 year.32,33 Extrahepatic manifestations previously reported include rash, kidney injury, myocarditis, and Guillain-Barre syndrome.34
DIAGNOSIS
Diagnosis of HAV infection is typically confirmed by serologic evidence of a recent infection, that is, detection of immunoglobulin M (IgM) antibodies against HAV. Concordance between assays is high, but there is about a 10% reported rate of discrepancy.35 IgM antibodies typically peak about a month after exposure and can persist for up to a year. False negative results can be seen in early infection, while the patient is viremic and interval antibody retesting should be considered.26,36 False positive results have been reported in a variety of scenarios, including patients with rheumatoid factor or other autoimmune disease.37 IgM antibodies to HAV can be detected in the setting of recent HAV immunization or recent HAV vaccine boosting.38,39 IgG response typically follows IgM response after 1 week, typically persists for life, and confers neutralizing activity to future HAV exposures. Durability of IgG response may be limited in immunosuppression, as previously demonstrated in human immunodeficiency virus (HIV) -infected persons with an absence of detectable HAV antibodies several years after vaccination40 (Fig. 1).
Fig. 1.
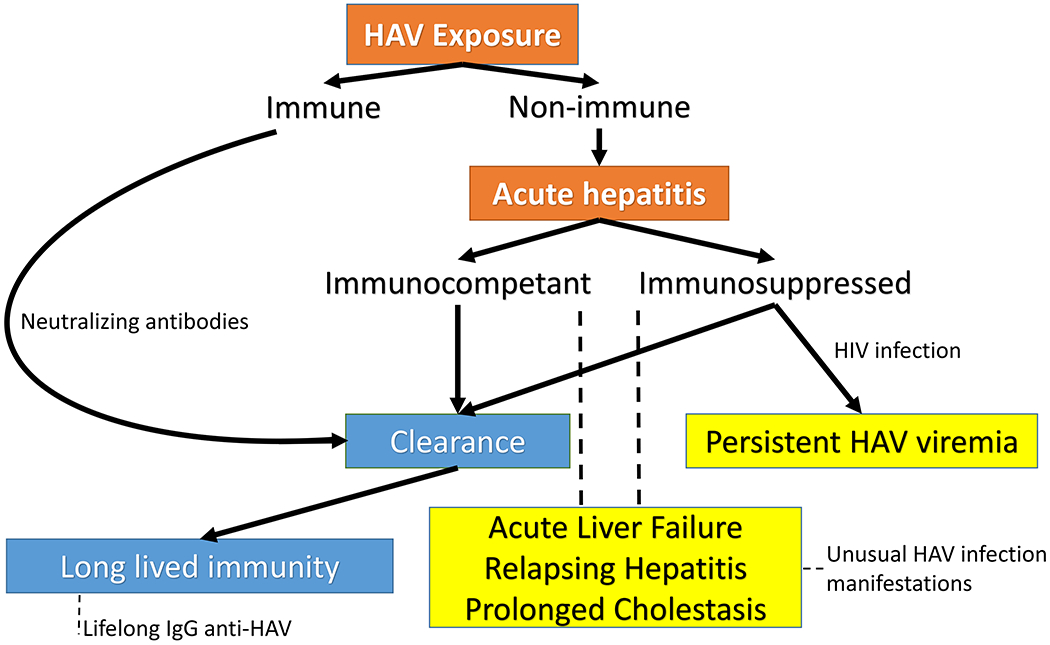
Effects of HAV exposure in immunocompetent and immunosuppressed patients. Patients immunized against HAV before exposure can clear the virus via preformed neutralizing antibodies. Nonimmune patients who are immunocompetent typically clear the virus after the infectious hepatitis, whereas immunosuppressed patients may sustain a chronic viremia. In rare cases, both immunocompetent and immunosuppressed hosts may manifest an unusual presentation of HAV, including but not limited to liver failure, relapsing hepatitis, and prolonged cholestasis.
Serum detection of HAV RNA can be technically done but rarely used in diagnosis of acute hepatitis A infection. Hepatitis A viremia is detectable in serum of immunocompetent hosts within a few days of infection and persists for 3 to 4 weeks.41 Immunosuppressed patients may have persistent hepatitis A viremia beyond 4 weeks.42 HAV RNA has also been detected in stool and saliva of infected hosts, but at much lower concentrations than serum.43 RNA can be detected by real-time polymerase chain reaction (PCR) or nested PCR methods. Molecular characteristics of HAV, although not important in diagnosis, have been used in epidemiologic studies and can assist in phylogenetic analysis of early outbreaks and epidemics.35
TREATMENT
The treatment of acute hepatitis A is supportive.44 Liver failure from hepatitis A is rare, but is estimated to occur in less than 5% of cases.45 Immediate referral to a transplant center is critical for cases of HAV-associated fulminant liver failure.
Therapeutics has been previously investigated for cases of HAV-associated liver failure. ALF-5755, a C-type lectin, was administered to 10 subjects with HAV-associated liver failure. There was no evidence of improvement in transplant-free survival rate.46 N-acetylcysteine, although shown to be very effective for acetaminophen-induced liver failure, does not seem to confer any benefit for HAV-associated acute liver failure.47
Interferon (IFN) as a treatment of acute hepatitis A infection has been previously evaluated and shown to be effective in cell cultures.48 Case reports of IFN treatment of acute hepatitis A are limited, and its utility is unclear.49 Direct-acting antivirals have been evaluated in cell culture systems and shown to have potential effectiveness in inhibiting HAV replication and in antiviral activity.50–52 Drug development and clinical trials are limited by difficulty in enrolling subjects before they resolve their infection to measure potential outcomes of intervention.
PREVENTION
Sanitation measures play an important role in HAV infection prevention, including but not limited to careful attention to hygiene, particularly in the food service industry.53 Food service workers who are ill with jaundice of unclear cause should be restricted from work. Hospitalized patients with HAV should be on enteric precautions for 1 week after the onset of jaundice, when viral shedding in the stool is at its highest.54
Prevention of HAV by vaccine is the standard approach across much of the world, and many countries have adopted universal vaccination against HAV in their children55 (Table 2). HAV vaccine is also a mainstay of postexposure prophylaxis.56 The United States offers 2 commercially available hepatitis A vaccines and 1 combination HAV-HBV vaccine.57 The HAV vaccine is typically administered in 2 doses, 6 months apart, whereas the HAV-HBV vaccine usually requires 3 doses.58 Live-attenuated HAV vaccines are used in China with good success.59
Table 2.
Vaccine preparations in the United States and populations that are recommended to receive HAV vaccine
| Vaccine Name | Protects Against | Dosing Schedule | Relevant Populations |
|---|---|---|---|
| Havrix | HAV | Two doses 6 mo apart | Children 1–2 y old |
| Vaqta | HAV | Two doses 6 mo apart | Travel to endemic area |
| Twinrix | HAV, HBV | Three doses at 0, 1, and 6 mo | MSM PWID Chronic liver disease Health care workers |
Abbreviation: PWID, people who inject drugs.
The efficacy of both live-attenuated and inactivated vaccines has been well established in large trials across the globe collectively encompassing nearly 750,000 patients.60 Both vaccines confer a protective effect against hepatitis A when given before exposure. Immunogenicity of the 2 HAV vaccines in the United States, when compared head to head, was equal.61 An antibody titer greater than or equal to 20 mIU/mL is thought to be protective.62 Lower protection rates following vaccination are observed in immunosuppressed persons, including HIV infection, inflammatory bowel disease, and organ transplant recipients.63
Nonvaccinated persons traveling to HAV endemic regions should have a single dose of vaccine before their departure.64 Persons with chronic liver disease, the elderly, and the immunocompromised should receive both vaccine and immunoglobulin at 0.02 mL/kg at a separate injection site.56,65 Postexposure prophylaxis for HAV is best achieved with either HAV vaccine or immunoglobulin, and both seem to be equally effective.66,67
SUMMARY
HAV continues to be a global health issue, with the highest rates in lower-income countries. Infections are typically linked with contaminated food or water and almost always tied to poor sanitary conditions. Treatment is supportive, and there are no drug therapies available for acute hepatitis A infection. Vaccination is the most effective form of prevention and is also used in postexposure prophylaxis. Universal vaccination for HAV in children should be adopted whenever possible and has been shown to reduce HAV burden around the globe.
KEY POINTS.
Hepatitis A occurs around the world and causes an acute hepatitis, which is typically subclinical.
Clinical illness is not common in endemic areas because of early childhood exposures, but poses a large risk to travelers from nonendemic areas.
Diagnosis of hepatitis A typically requires serologic testing.
Jaundice, anorexia, right upper quadrant pain, and elevated alanine aminotransferase levels are classically described in acute hepatitis A.
Hepatitis A is preventable through vaccination.
Footnotes
DISCLOSURE
The authors have nothing to disclose.
REFERENCES
- 1.Krugman S, Ward R, Giles JP, et al. Infectious hepatitis: detection of virus during the incubation period and in clinically inapparent infection. N Engl J Med 1959; 261:729–34. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg BS, Gerstley BJ, Hungerford DA, et al. A serum antigen (Australia antigen) in Down’s syndrome, leukemia, and hepatitis. Ann Intern Med 1967;66(5): 924–31. [DOI] [PubMed] [Google Scholar]
- 3.Feinstone SM, Kapikian AZ, Purceli RH. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science 1973;182(4116):1026–8. [DOI] [PubMed] [Google Scholar]
- 4.Martin A, Lemon SM. Hepatitis A virus: from discovery to vaccines. Hepatology 2006;43(2 Suppl 1):S164–72. [DOI] [PubMed] [Google Scholar]
- 5.Innis BL, Snitbhan R, Kunasol P, et al. Protection against hepatitis A by an inactivated vaccine. JAMA 1994;271(17):1328–34. [PubMed] [Google Scholar]
- 6.Jacobsen KH. Globalization and the changing epidemiology of hepatitis A virus. Cold Spring Harb Perspect Med 2018;8(10). 10.1101/cshperspect.a031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine 2010;28(41):6653–7. [DOI] [PubMed] [Google Scholar]
- 8.Havelaar AH, Kirk MD, Torgerson PR, et al. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med 2015;12(12):e1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wooten DA. Forgotten but not gone: learning from the hepatitis A outbreak and public health response in San Diego. Top Antivir Med 2019;26(4):117–21. [PMC free article] [PubMed] [Google Scholar]
- 10.Zakaria S, Fouad R, Shaker O, et al. Changing patterns of acute viral hepatitis at a major urban referral center in Egypt. Clin Infect Dis 2007;44(4):e30–6. [DOI] [PubMed] [Google Scholar]
- 11.Lemon SM. Type A viral hepatitis. New developments in an old disease. N Engl J Med 1985;313(17):1059–67. [DOI] [PubMed] [Google Scholar]
- 12.Klevens RM, Miller JT, Iqbal K, et al. The evolving epidemiology of hepatitis A in the United States: incidence and molecular epidemiology from population-based surveillance, 2005-2007. Arch Intern Med 2010;170(20):1811–8. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz NG, Revillion M, Roque-Afonso AM, et al. A food-borne outbreak of hepatitis A virus (HAV) infection in a secondary school in Upper Normandy, France, in November 2006. Euro Surveill 2008;13(22). pii=18885. [PubMed] [Google Scholar]
- 14.Croci L, De Medici D, Scalfaro C, et al. The survival of hepatitis A virus in fresh produce. Int J Food Microbiol 2002;73(1):29–34. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad T, Adnan F, Nadeem M, et al. Assessment of the risk for human health of enterovirus and hepatitis A virus in clinical and water sources from three metropolitan cities of Pakistan. Ann Agric Environ Med 2018;25(4):708–13. [DOI] [PubMed] [Google Scholar]
- 16.Hettmann A, Juhasz G, Dencs A, et al. Phylogenetic analysis of a transfusion-transmitted hepatitis A outbreak. Virus Genes 2017;53(1):15–20. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka S, Kishi T, Ishihara A, et al. Outbreak of hepatitis A linked to European outbreaks among men who have sex with men in Osaka, Japan, from March to July 2018 Hepatol Res 2019;49(6):705–10. [DOI] [PubMed] [Google Scholar]
- 18.Aasheim ET, Seymour M, Balogun K, et al. Acute hepatitis A in an elderly patient after care worker travel to high endemicity country. Hum Vaccin Immunother 2013;9(11):2480–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster M, Ramachandran S, Myatt K, et al. Hepatitis A virus outbreaks associated with drug use and homelessness-California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep 2018;67(43):1208–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheek JE, Hennessy TW, Redd JT, et al. Epidemic assistance from the Centers for Disease Control and Prevention involving American Indians and Alaska Natives, 1946-2005. Am J Epidemiol 2011;174(11 Suppl):S89–96. [DOI] [PubMed] [Google Scholar]
- 21.Bizri AR, Fares J, Musharrafieh U. Infectious diseases in the era of refugees: hepatitis A outbreak in Lebanon. Avicenna J Med 2018;8(4):147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purpari G, Macaluso G, Di Bella S, et al. Molecular characterization of human enteric viruses in food, water samples, and surface swabs in Sicily. Int J Infect Dis 2019;80:66–72. [DOI] [PubMed] [Google Scholar]
- 23.Xu ZY, Li ZH, Wang JX, et al. Ecology and prevention of a shellfish-associated hepatitis A epidemic in Shanghai, China. Vaccine 1992;10(Suppl 1):S67–8. [DOI] [PubMed] [Google Scholar]
- 24.Kentucky’s Hepatitis A outbreak surpasses 3,000 cases. 2018. Available at: https://www.wlky.com/article/kentuckys-hepatitis-a-outbreak-surpasses-3000-cases/25592667. Accessed March 8, 2019.
- 25.Kentucky’s ‘too low and too slow’ response to nation’s worst hepatitis A outbreak. 2019. Available at: https://www.courier-journal.com/story/news/investigations/2019/02/21/kentucky-hepatitis-response-too-slow-deadly-outbreak-worst-in-united-states/2453874002/. Accessed March 8, 2019.
- 26.Lemon SM, Walker CM. Hepatitis A virus and hepatitis E virus: emerging and reemerging enterically transmitted hepatitis viruses. Cold Spring Harb Perspect Med 2018;9(6):a031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker CM. Adaptive immune responses in hepatitis A virus and hepatitis E virus infections. Cold Spring Harb Perspect Med 2018;9(9):a033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathiesen LR, Moller AM, Purcell RH, et al. Hepatitis A virus in the liver and intestine of marmosets after oral inoculation. Infect Immun 1980;28(1):45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin EC, Jeong SH. Natural history, clinical manifestations, and pathogenesis of hepatitis A. Cold Spring Harb Perspect Med 2018;8(9). 10.1101/cshperspect.a031708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JD, Cho EJ, Ahn C, et al. A model to predict 1-month risk of transplant or death in hepatitis a-related acute liver failure. Hepatology 2019;70(2):621–9. [DOI] [PubMed] [Google Scholar]
- 31.Cuthbert JA. Hepatitis A: old and new. Clin Microbiol Rev 2001;14(1):38–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiff ER. Atypical clinical manifestations of hepatitis A. Vaccine 1992;10(Suppl 1):S18–20. [DOI] [PubMed] [Google Scholar]
- 33.Munoz-Martinez SG, Diaz-Hernandez HA, Suarez-Flores D, et al. Atypical manifestations of hepatitis A virus infection. Rev Gastroenterol Mex 2018;83(2): 134–43. [DOI] [PubMed] [Google Scholar]
- 34.Allen O, Edhi A, Hafeez A, et al. A very rare complication of hepatitis A infection: acute myocarditis–a case report with literature review. Case Rep Med 2018;2018: 3625139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park SH, Kim EJ, Lee JH, et al. Molecular characterization of hepatitis A virus isolated from acute gastroenteritis patients in the Seoul region of Korea. Eur J Clin Microbiol Infect Dis 2009;28(10):1177–82. [DOI] [PubMed] [Google Scholar]
- 36.Lee HK, Kim KA, Lee JS, et al. Window period of anti-hepatitis A virus immunoglobulin M antibodies in diagnosing acute hepatitis A. Eur J Gastroenterol Hepatol 2013;25(6):665–8. [DOI] [PubMed] [Google Scholar]
- 37.Tennant E, Post JJ. Production of false-positive immunoglobulin M antibodies to hepatitis A virus in autoimmune events. J Infect Dis 2016;213(2):324–5. [DOI] [PubMed] [Google Scholar]
- 38.Fayol V, Ville G. Evaluation of automated enzyme immunoassays for several markers for hepatitis A and B using the Abbott IMx analyser. Eur J Clin Chem Clin Biochem 1991;29(1):67–70. [PubMed] [Google Scholar]
- 39.Castrodale L, Fiore A, Schmidt T. Detection of immunoglobulin M antibody to hepatitis A virus in Alaska residents without other evidence of hepatitis. Clin Infect Dis 2005;41(9):e86–8. [DOI] [PubMed] [Google Scholar]
- 40.Crum-Cianflone NF, Wilkins K, Lee AW, et al. Long-term durability of immune responses after hepatitis A vaccination among HIV-infected adults. J Infect Dis 2011;203(12):1815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes JA, Fontaine MJ, Gonzalez CL, et al. Case report of a transfusion-associated hepatitis A infection. Transfusion 2014;54(9):2202–6. [DOI] [PubMed] [Google Scholar]
- 42.Lin KY, Chen GJ, Lee YL, et al. Hepatitis A virus infection and hepatitis A vaccination in human immunodeficiency virus-positive patients: a review. World J Gastroenterol 2017;23(20):3589–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshi MS, Bhalla S, Kalrao VR, et al. Exploring the concurrent presence of hepatitis A virus genome in serum, stool, saliva, and urine samples of hepatitis A patients. Diagn Microbiol Infect Dis 2014;78(4):379–82. [DOI] [PubMed] [Google Scholar]
- 44.Chalmers TC, Eckhardt RD, Reynolds WE, et al. The treatment of acute infectious hepatitis. Controlled studies of the effects of diet, rest, and physical reconditioning on the acute course of the disease and on the incidence of relapses and residual abnormalities. J Clin Invest 1955;34(7, Part II):1163–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee WM, Squires RH Jr, Nyberg SL, et al. Acute liver failure: summary of a workshop. Hepatology 2008;47(4):1401–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nalpas B, Ichai P, Jamot L, et al. A proof of concept, phase II randomized European trial, on the efficacy of ALF-5755, a novel extracellular matrix-targeted antioxidant in patients with acute liver diseases. PLoS One 2016; 11(3):e0150733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gunduz H, Karabay O, Tamer A, et al. N-acetyl cysteine therapy in acute viral hepatitis. World J Gastroenterol 2003;9(12):2698–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crance JM, Leveque F, Chousterman S, et al. Antiviral activity of recombinant interferon-alpha on hepatitis A virus replication in human liver cells. Antiviral Res 1995;28(1):69–80. [DOI] [PubMed] [Google Scholar]
- 49.Yoshiba M, Inoue K, Sekiyama K. Interferon for hepatitis A. Lancet 1994; 343(8892):288–9. [DOI] [PubMed] [Google Scholar]
- 50.Morris TS, Frormann S, Shechosky S, et al. In vitro and ex vivo inhibition of hepatitis A virus 3C proteinase by a peptidyl monofluoromethyl ketone. Bioorg Med Chem 1997;5(5):797–807. [DOI] [PubMed] [Google Scholar]
- 51.Blaum BS, Wunsche W, Benie AJ, et al. Functional binding of hexanucleotides to 3C protease of hepatitis A virus. Nucleic Acids Res 2012;40(7):3042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang X, Kanda T, Nakamoto S, et al. The JAK2 inhibitor AZD1480 inhibits hepatitis A virus replication in Huh7 cells. Biochem Biophys Res Commun 2015;458(4): 908–12. [DOI] [PubMed] [Google Scholar]
- 53.Trujillo-Ochoa JL, Viera-Segura O, Fierro NA. Challenges in management of hepatitis A virus epidemiological transition in Mexico. Ann Hepatol 2018;18(1):14–22. [DOI] [PubMed] [Google Scholar]
- 54.Koenig KL, Shastry S, Burns MJ. Hepatitis A virus: essential knowledge and a novel identify-isolate-inform tool for frontline healthcare providers. West J Emerg Med 2017;18(6):1000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Souto FJD, de Brito WI, Fontes CJF. Impact of the single-dose universal mass vaccination strategy against hepatitis A in Brazil. Vaccine 2019;37(6):771–5. [DOI] [PubMed] [Google Scholar]
- 56.Nelson NP, Link-Gelles R, Hofmeister MG, et al. Update: recommendations of the advisory committee on immunization practices for use of hepatitis A vaccine for postexposure prophylaxis and for preexposure prophylaxis for international travel. MMWR Morb Mortal Wkly Rep 2018;67(43):1216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Leary ST, Kimberlin DW. Update from the advisory committee on immunization practices. J Pediatric Infect Dis Soc 2018;7(3):181–7. [DOI] [PubMed] [Google Scholar]
- 58.Beran J, Kervyn D, Wertzova V, et al. Comparison of long-term (10 years) immunogenicity of two- and three-dose regimens of a combined hepatitis A and B vaccine in adolescents. Vaccine 2010;28(37):5993–7. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization. Global advisory committee on vaccine safety, 16-17 June 2010. Geneva, Switzerland: World Health Organization; 2010. Report No.: 85. [Google Scholar]
- 60.Irving GJ, Holden J, Yang R, et al. Hepatitis A immunisation in persons not previously exposed to hepatitis A. Cochrane Database Syst Rev 2012;(7):CD009051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Braconier JH, Wennerholm S, Norrby SR. Comparative immunogenicity and tolerance of Vaqta and Havrix. Vaccine 1999;17(17):2181–4. [DOI] [PubMed] [Google Scholar]
- 62.Lemon SM, Binn LN. Serum neutralizing antibody response to hepatitis A virus. J Infect Dis 1983;148(6):1033–9. [DOI] [PubMed] [Google Scholar]
- 63.Garcia Garrido HM, Wieten RW, Grobusch MP, et al. Response to hepatitis A vaccination in immunocompromised travelers. J Infect Dis 2015;212(3):378–85. [DOI] [PubMed] [Google Scholar]
- 64.Hepatitis A questions and answers for health professionals. 2018. Available at: http://www.cdc.gov/hepatitis/hav/havfaq.htm#c2. Accessed March 8, 2019.
- 65.Vento S, Garofano T, Renzini C, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med 1998; 338(5):286–90. [DOI] [PubMed] [Google Scholar]
- 66.Victor JC, Monto AS, Surdina TY, et al. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med 2007;357(17):1685–94. [DOI] [PubMed] [Google Scholar]
- 67.Betz TG. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N Engl J Med 2008;358(5):531 [author reply: 532]. [DOI] [PubMed] [Google Scholar]


