Abstract
Although hematopoietic prostaglandin D synthase (H-PGDS) is an attractive target for treatment of a variety of diseases, including allergic diseases and Duchenne muscular dystrophy, no H-PGDS inhibitors have yet been approved for treatment of these diseases. Therefore, the development of novel agents having other modes of action to modulate the activity of H-PGDS is required. In this study, a chimeric small molecule that degrades H-PGDS via the ubiquitin-proteasome system, PROTAC(H-PGDS)-1, was developed. PROTAC(H-PGDS)-1 is composed of two ligands, TFC-007 (that binds to H-PGDS) and pomalidomide (that binds to cereblon). PROTAC(H-PGDS)-1 showed potent activity in the degradation of H-PGDS protein via the ubiquitin-proteasome system and in the suppression of prostaglandin D2 (PGD2) production. Notably, PROTAC(H-PGDS)-1 showed sustained suppression of PGD2 production after the drug removal, whereas PGD2 production recovered following removal of TFC-007. Thus, the H-PGDS degrader—PROTAC(H-PGDS)-1—is expected to be useful in biological research and clinical therapies.
Keywords: Prostaglandin D2, ubiquitin-proteasome system, protein knockdown, PROTACs
Overproduction of PGD2 is related to a variety of diseases, including allergic diseases,1,2 physiological sleep,3 and Duchenne muscular dystrophy.4 H-PGDS is one of the enzymes involved in PGD2 synthesis; therefore, H-PGDS is a potential therapeutic target for such diseases, for example, in the nasal mucosa of patients with allergic rhinitis.5 In vivo studies have demonstrated that H-PGDS inhibition is effective in the treatment of allergic inflammation.6−8 To date, several types of H-PGDS inhibitors have been developed as therapies for allergic and inflammatory responses.9 However, the advancement of these inhibitors into clinical studies has not been satisfactory, probably due to the differences of pharmacokinetics and pharmacodynamics between preclinical animals and humans. Thus, the development of novel agents for clinical investigation with modes of action other than H-PGDS inhibition is required.
In recent years, innovative chimeric drugs, PROTACs (proteolysis targeting chimeras), and SNIPERs (specific and non-genetic inhibitor of apoptosis protein [IAP]-dependent protein erasers), which enable the degradation of target proteins via the ubiquitin-proteasome system (UPS), have been developed. These drugs are chimeric molecules, composed of target protein ligands and E3 ligase ligands, which recruit a target protein in proximity to an E3 ligase to induce protein degradation.10−13 SNIPERs recruit IAP ubiquitin ligase, while PROTACs recruit other E3 ligases, such as cereblon (CRBN) and von Hippel–Lindau ubiquitin ligase. Notably, PROTACs and SNIPERs against the same target protein show different activities of degradation,14 suggesting that the appropriate combination of target protein and E3 ligase is important for development of potent degraders. Some degraders have been reported to show more durable suppression of the cellular responses caused by the target protein than small molecule inhibitors.14−16 A variety of PROTACs and SNIPERs have been developed for the treatment of cancer that targeted related proteins, such as transcriptional regulators, nuclear receptors, and protein kinases.12,17,18 As a new mechanism of action for regulating the activity of H-PGDS, the development of degraders targeting the H-PGDS protein is an attractive approach for the treatment of chronic allergic diseases. In the present study, we developed the chimeric small molecule PROTAC(H-PGDS)-1, which had potent activity for the degradation of H-PGDS protein via the UPS and in the suppression of PGD2 production.
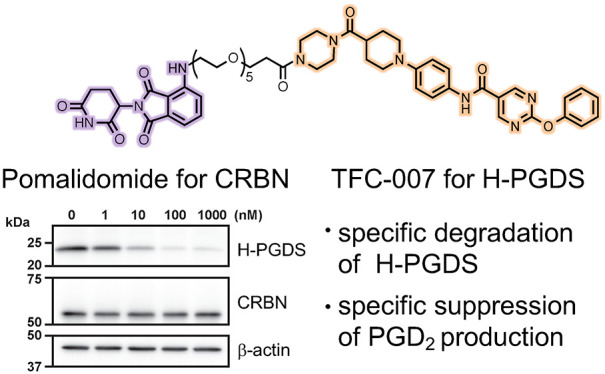
Among the representative H-PGDS selective inhibitors, HQL-79,19 F092,20 BSPT,21 TAS-204,7 and TFC-007,8 TAS-204, and TFC-007 show high inhibitory activity against H-PGDS enzyme (with IC50 values of 23 nM (in vitro) and 83 nM (in vitro), respectively, Figure 1a).7,8 The binding mode between H-PGDS and F092 (and HQL-79) has been revealed by X-ray diffraction.19,20 From the above information, we focused on the chemical structures of F092 and TFC-007, which contain the same N-phenyl-5-pyrimidinecarboxamide moiety, to design a chimeric molecule. In the X-ray crystal structure of the ligand binding domain of H-PGDS and F092 (PDB: 5YWX), the N-phenyl-5-pyrimidinecarboxamide moiety faces to the inside and the 2-pyrrolidone moiety is orientated to the outside of the protein (Figure 1b). We hypothesized that the elongated structure of TFC-007, similar to F092, would be suitable as a target protein ligand and the morpholine moiety of TFC-007 would face the surface of the protein. Thus, we designed a TFC-007-based PROTAC by the replacement of the morpholine moiety with a piperazine moiety for linking with the E3 ligase ligand. As the E3 ligase ligand, pomalidomide was selected, which binds to CRBN. CRBN is a broadly expressed protein and forms part of the cullin-4-containing E3 ubiquitin ligase complex. The structures of PROTAC(H-PGDS)-1 and the negative control PROTAC(H-PGDS)-2 with N-methylated pomalidomide, which is considered to have considerably reduced binding affinity to CRBN,15 are shown in Figure 1c. The synthetic routes for PROTAC(H-PGDS)-1 and PROTAC(H-PGDS)-2 are given in the Supporting Information (Schemes S1 and S2, respectively).
Figure 1.
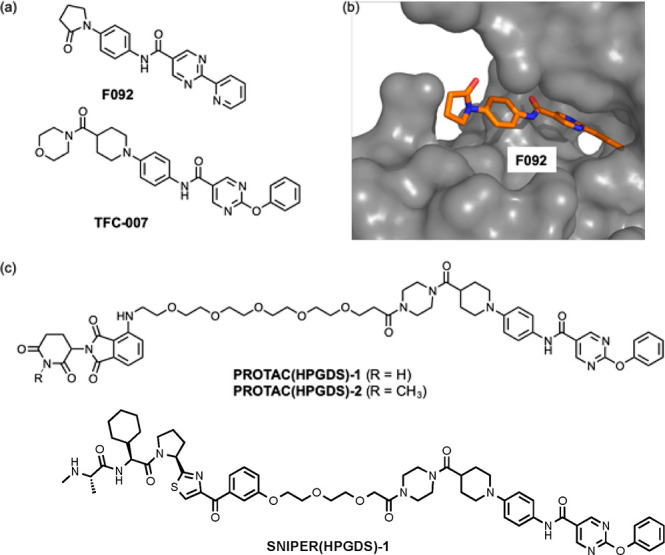
(a) Chemical structures of the H-PGDS inhibitors F092 and TFC-007. (b) X-ray crystal structure of H-PGDS with F092 (PDB: 5YWX). (c) Chemical structures of PROTAC(H-PGDS)-1, PROTAC(H-PGDS)-2, and SNIPER(H-PGDS)-1.
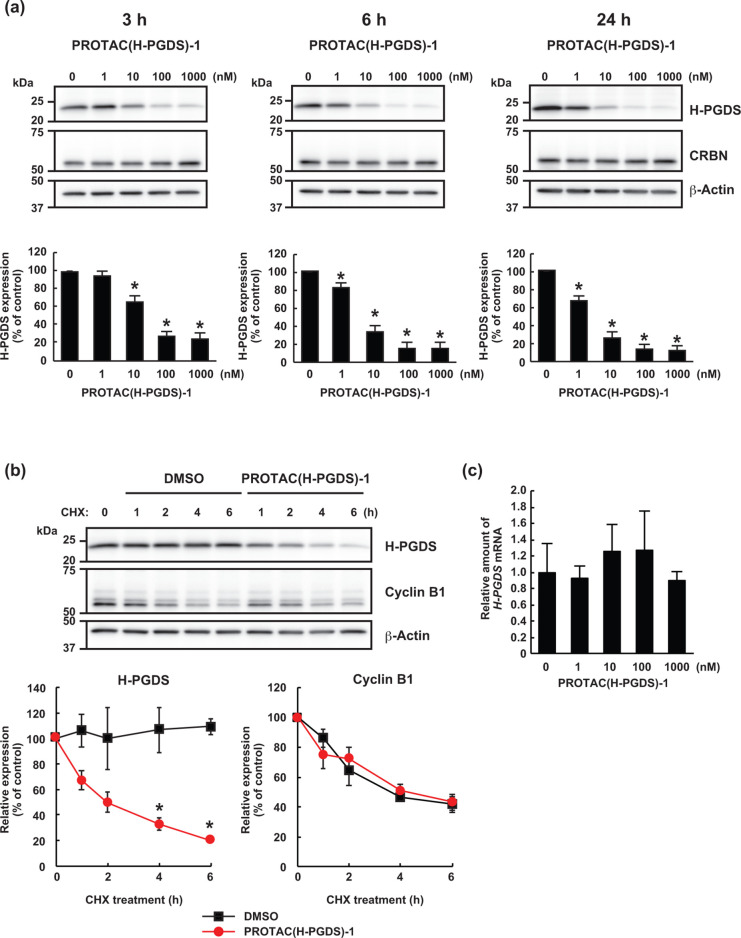
To examine the effect of PROTAC(H-PGDS)-1 on H-PGDS protein levels, human KU812 cells expressing H-PGDS protein were treated with graded concentrations of PROTAC(H-PGDS)-1 for 3 h (Figure 2a). Effective reduction of H-PGDS protein by PROTAC(H-PGDS)-1 was observed at concentrations ≥10 nM, and the maximum activity was observed at 100–1000 nM. Additionally, PROTAC(H-PGDS)-1, at concentrations ≥10 nM, showed more potent activity in the reduction of the H-PGDS protein when the cells were incubated with PROTAC(H-PGDS)-1 for 6 or 24 h (Figure 2a). Similar protein reduction activity for PROTAC(H-PGDS)-1 was also observed in MEG-01s cells expressing H-PGDS protein (Figure S9). This reduction activity was not observed for SNIPER(H-PGDS)-1, in which TFC-007 is conjugated to an IAP ligand LCL161 derivative to recruit IAPs as E3 ligase13 (Figure 1c, Figure S10). While PROTAC(H-PGDS)-1 showed potent activity in H-PGDS protein reduction, PROTAC(H-PGDS)-1 had little effect on the protein levels of mPGES-1 (a major enzyme for PGE2 synthesis)22 and AKR-1B1 (a major enzyme for PGF2α synthesis)23,24 (Figure S11), suggesting that PROTAC(H-GDS)-1 may specifically reduce H-PGDS and not other prostaglandin synthases.
Figure 2.
PROTAC(H-PGDS)-1 is a degrader for H-PGDS protein. (a) KU812 cells were incubated with the indicated concentration of PROTAC(H-PGDS)-1 for the indicated time. The H-PGDS/β-actin ratio was normalized by the vehicle control as 100. The data in the bar graph are means ± SD (n = 3). (b) Turnover of H-PGDS protein in KU812 cells after treatment with 10 μg/mL cycloheximide (CHX) in the presence or absence of 100 nM PROTAC(H-PGDS)-1 for the indicated periods. The H-PGDS/β-actin and cyclin B1/β-actin ratios were normalized by the time 0 control as 100. The data in the graphs are means ± SD (n = 3). (c) Expression of H-PGDS mRNA in KU812 cells. Cells were incubated with the indicated concentration of PROTAC(H-PGDS)-1 for 6 h. Expression levels are relative to vehicle treatment, which was arbitrarily set to 1. The data in the bar graph are means ± SD (n = 3). *P < 0.01 compared with vehicle-treated control in a two-tailed Student’s t test.
To understand the mechanism for the reduction of H-PGDS protein by PROTAC(H-PGDS)-1, we examined the turnover of H-PGDS protein after PROTAC(H-PGDS)-1 treatment. When KU812 cells were treated with the protein synthesis inhibitor cycloheximide in KU812 cells, the levels of H-PGDS protein were dramatically decreased within 6 h in the PROTAC(H-PGDS)-1-treated cells but were retained in the control cells (Figure 2b). In contrast, the turnover rate of cyclin B1 was not affected by PROTAC(H-PGDS)-1 treatment. These results indicated that PROTAC(H-PGDS)-1 induces a reduction in H-PGDS protein levels. Furthermore, the levels of H-PGDS mRNA in KU812 cells were not affected by PROTAC(H-PGDS)-1 (Figure 2c). These results indicated that PROTAC(H-PGDS)-1 induces the degradation of H-PGDS protein.
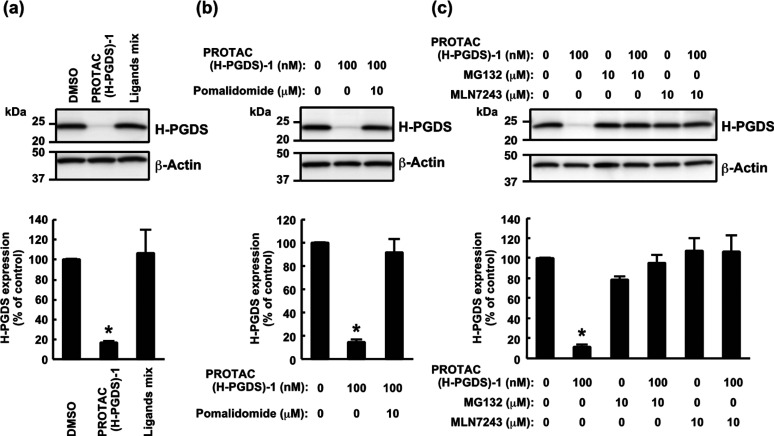
To explore the mechanism of PROTAC(H-PGDS)-1-induced degradation of the H-PGDS protein, we first examined the effect of TFC-007 and pomalidomide on the H-PGDS protein. While addition of PROTAC(H-PGDS)-1 resulted in dramatic degradation of H-PGDS protein, the combination of TFC-007 and pomalidomide (1 μM each) did not effectively decrease the amount of H-PGDS protein (Figure 3a), indicating that linking the two ligands into a single molecule is critically important for the degradation of the H-PGDS protein. PROTAC(H-PGDS)-1 was designed to recruit CRBN for the degradation of H-PGDS protein. A competition assay using an excess amount of pomalidomide diminished the protein degradation activity of PROTAC(H-PGDS)-1 (Figure 3b), indicating that CRBN binding was required for the protein degradation.
Figure 3.
Involvement of the ubiqutin-proteasome system in the PROTAC(H-PGDS)-1-induced degradation of H-PGDS protein. (a) KU812 cells were incubated with 1 μM PROTAC(H-PGDS)-1 or the ligand mixture (TFC-007 and pomalidomide, 1 μM each) for 6 h. (b) Competition assay using an excess amount of pomalidomide with PROTAC(H-PGDS)-1 in KU812 cells. Cells were incubated with 100 nM PROTAC(H-PGDS)-1 and/or 10 μM pomalidomide for 6 h. (c) Effect of MG132 and MLN7243 on the protein knockdown activity of PROTAC(H-PGDS)-1 in KU812 cells. Cells were incubated with 100 nM PROTAC(H-PGDS)-1 in the presence or absence of 10 μM MG132 or 10 μM MLN7243 for 6 h. The H-PGDS/β-actin ratio was normalized by the vehicle control as 100. The data in the bar graphs are means ± SD (n = 3). *P < 0.01 compared to vehicle-treated control in a two-tailed Student’s t test.
To investigate the involvement of the UPS in the degradation of H-PGDS protein by PROTAC(H-PGDS)-1, we used the proteasome inhibitor MG132 and the ubiquitin-activating enzyme inhibitor MLN7243. The PROTAC(H-PGDS)-1-induced degradation of H-PGDS protein was suppressed by the inhibitors (Figure 3c), suggesting that the degradation of H-PGDS protein requires the UPS.
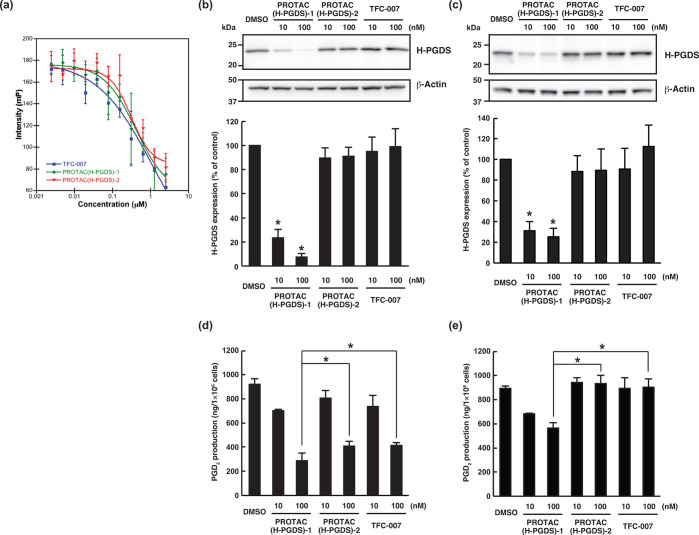
As discussed above, PROTAC(H-PGDS)-1 is a potent H-PGDS protein degrader that is dependent on the UPS. In addition to the degradation, PROTAC(H-PGDS)-1 may also inhibit the enzymatic activity of H-PGDS; this is because it contains the TFC-007 moiety, which inhibits H-PGDS enzymatic activity. To investigate the importance of H-PGDS degradation for the effect of PGD2 production, we developed an inactive form of PROTAC(H-PGDS)-1 as a control compound. PROTAC(H-PGDS)-2 is composed of TFC-007 and N-methylated pomalidomide, which is unable to recruit CRBN.15 Using competitive binding assay with a fluorescence probe, we investigated the binding affinity to H-PGDS. TFC-007, PROTAC(H-PGDS)-1, and PROTAC(H-PGDS)-2 showed a similar affinity toward H-PGDS with IC50 values of 0.32, 0.32, and 0.30 μM, respectively (Figure 4a). We also investigated the inhibitory activity of the compounds against the H-PGDS enzyme in vitro. PROTAC(H-PGDS)-1 and PROTAC(H-PGDS)-2 had slightly higher IC50 values, 266 nM for PROTAC(H-PGDS)-1 and 320 nM for PROTAC(H-PGDS)-2, than TFC-007 (71 nM, Figure S12). Then, we examined the degradation activity of the compounds against H-PGDS protein. KU812 cells were treated with PROTAC(H-PGDS)-1, PROTAC(H-PGDS)-2, or TFC-007 for 24 h and then washed and incubated with compound-free medium. PROTAC(H-PGDS)-1 resulted in a significant H-PGDS degradation (Figure 4b) and maintained the H-PGDS degradation for up to 6 h (Figure 4c), suggesting that PROTAC(H-PGDS)-1 was a potent degrader and inhibitor of H-PGDS and that the degradation activity of PROTAC(H-PGDS)-1 was prolonged after its removal. In contrast, neither PROTAC(H-PGDS)-2 nor TFC-007 affected H-PGDS protein levels (Figure 4b,c), suggesting that PROTAC(H-PGDS)-2 and TFC-007 inhibited enzymatic activity but did not degrade H-PGDS. Finally, we investigated the effect of PROTAC(H-PGDS)-1, PROTAC(H-PGDS)-2, and TFC-007 on the production of PGD2 in KU812 cells. In line with the inhibition of the H-PGDS enzyme activity (Figure 4a, Figure S12), treatment with PROTAC(H-PGDS)-1 for 24 h suppressed production of PGD2 the same as TFC-007 and more effectively than PROTAC(H-PGDS)-2 (Figure 4d). PROTAC(H-PGDS)-2 was considered to possess similar physical properties to PROTAC(H-PGDS)-1 such as solubility and cell permeability because of their similar structures. PROTAC(H-PGDS)-1 shows slightly stronger activity than PROTAC(H-PGDS)-2 to suppress production of PGD2, suggesting that the dual mechanism of enzyme inhibition and protein degradation gives a more potent compound than an enzyme inhibition alone. At 6 h after compound removal, PGD2 production recovered in the cells treated with PROTAC(H-PGDS)-2 or TFC-007, whereas it remained significantly suppressed in the cells treated with PROTAC(H-PGDS)-1 (Figure 4e).
Figure 4.
Comparison of the degradation and inhibition of H-PGDS. (a) Fluorescence polarization assays of the binding affinity between H-PGDS and TFC-007, PROTAC(H-PGDS)-1, and PROTAC(H-PGDS)-2. (b, c) KU812 cells were incubated with the indicated concentration of the compounds for 24 h (b) and then washed four times to remove the compounds and incubated in compound-free medium for 6 h (c). The H-PGDS/β-actin ratio was normalized by the vehicle control as 100. The data in the bar graph are means ± SD (n = 3). *P < 0.01 compared to vehicle-treated control in a two-tailed Student’s t test. (d, e) KU812 cells were incubated with the indicated concentration of the compounds for 24 h. Then, the cells were incubated with 5 μM A23187 in the presence of each compound for 30 min (d), or the cells were washed four times to remove the compounds, incubated in compound-free medium for 6 h, and incubated with 5 μM A23187 for 30 min (e). PGD2 levels in the medium were measured. The data in the bar graph are means ± SD (n = 3). *P < 0.05 in a two-tailed Student’s t test.
In conclusion, a potent degrader of the H-PGDS protein, PROTAC(H-PGDS)-1, was successfully developed by conjugating TFC-007 (H-PGDS ligand) to pomalidomide (E3 ligase, CRBN ligand). PROTAC(H-PGDS)-1 effectively induced the selective degradation of H-PGDS protein via the UPS and showed sustained suppression of PGD2 production. The degrader PROTAC(H-PGDS)-1 with a new mechanism of action is expected to be as effective, or more effective, than conventional inhibitors and may allow a reduction in the number of doses for the treatment of chronic inflammation. To clinically develop an H-PGDS degrader, it is necessary to evaluate the safety, pharmacokinetics, and pharmacodynamics of the H-PGDS degrader in vivo. Further structure-based improvement of the H-PGDS degraders by replacement with other linkers and ligands for E3 ligase and/or H-PGDS is in progress in our laboratory.
Acknowledgments
Victoria Muir, PhD, from the Edanz Group (https://en-author-services.edanzgroup.com/ac) edited a draft of this manuscript.
Glossary
Abbreviations
- H-PGDS
hematopoietic prostaglandin D synthase
- PGD2
prostaglandin D2
- SNIPER
specific and non-genetic IAP-dependent protein eraser
- IAP
inhibitor of apoptosis protein
- PROTACs
proteolysis targeting chimeras
- UPS
ubiquitin-proteasome system
- CRBN
cereblon
- CHX
cycloheximide
- mPGES-1
microsomal prostaglandin E synthase 1
- AKR-1B1
aldo-keto reductase family 1 member B
- PGE2
prostaglandin E2
- PGF2α
prostaglandin F2α
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00605.
Synthetic procedures for all compounds listed in this manuscript and the protocols for in vitro assays (binding assay, protein degradation assay) (PDF)
Author Contributions
H.Y. and N.S. contributed equally to this work. H.Y., N.S., K.A., M.N., and Y.D. designed the research and wrote the paper. H.Y., N.S., M.N., Y.M., K.F., and T.I. performed the experiments and analyzed results. All authors discussed the results and commented on the manuscript.
This study was supported in part by grants from Japan Agency for Medical Research and Development (20mk0101120j0003 to Y.D., 20ak0101073j0604 to M.N., and 20ak0101073j0904 to Y.D.); Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology (JSPS/MEXT KAKENHI Grants Number JP18K07311 to N.S., JP17K08385 to Y.D., and JP18H05502 to M.N. and Y.D.); TERUMO Foundation for Life Sciences and Arts (to Y.D.); Takeda Science Foundation (to N.S. and Y.D.); the Naito Foundation (to Y.D.); the Sumitomo Foundation (to Y.D.); and the Novartis Foundation (Japan) for the Promotion of Science (to Y.D.).
The authors declare no competing financial interest.
Supplementary Material
References
- Lewis R. A.; Soter N. A.; Diamond P. T.; Austen K. F.; Oates J. A.; Roberts L. J. Prostaglandin D2 Generation after Activation of Rat and Human Mast Cells with Anti-IgE. J. Immunol. 1982, 129, 1627–1631. [PubMed] [Google Scholar]
- Matsuoka T.; Hirata M.; Tanaka H.; Takahashi Y.; Murata T.; Kabashima K.; Sugimoto Y.; Kobayashi T.; Ushikubi F.; Aze Y.; Eguchi N.; Urade Y.; Yoshida N.; Kimura K.; Mizoguchi A.; Honda Y.; Nagai H.; Narumiya S. Prostaglandin D2 as a Mediator of Allergic Asthma. Science 2000, 287, 2013–2017. 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- Urade Y.; Hayaishi O. Biochemical, Structural, Genetic, Pysiological, and Pathophysiological Features of Lipocalin-type Prostaglandin D Synthase. Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol. 2000, 1482, 259–271. 10.1016/S0167-4838(00)00161-8. [DOI] [PubMed] [Google Scholar]
- Takeshita E.; Komaki H.; Shimizu-Motohashi Y.; Ishiyama A.; Sasaki M.; Takeda S. A Phase I Study of TAS-205 in Patients with Duchenne Muscular Dystrophy. Ann. Clin. Transl. Neurol. 2018, 5, 1338–1349. 10.1002/acn3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittchen S.; Heinemann A. Therapeutic Potential of Hematopoietic Prostaglandin D2 Synthase in Allergic Inflammation. Cells 2019, 8, 619. 10.3390/cells8060619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aritake K.; Kado Y.; Inoue T.; Miyano M.; Urade Y. Structural and Functional Characterization of HQL-79, an Orally Selective Inhibitor of Human Hematopoietic Prostaglandin D Synthase. J. Biol. Chem. 2006, 281, 15277–15286. 10.1074/jbc.M506431200. [DOI] [PubMed] [Google Scholar]
- Kajiwara D.; Aoyagi H.; Shigeno K.; Togawa M.; Tanaka K.; Inagaki N.; Miyoshi K. Role of Hematopoietic Prostaglandin D Synthase in Biphasic Nasal Obstruction in Guinea Pig Model of Experimental Allergic Rhinitis. Eur. J. Pharmacol. 2011, 667, 389–395. 10.1016/j.ejphar.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Nabe T.; Kuriyama Y.; Mizutani N.; Shibayama S.; Hiromoto A.; Fujii M.; Tanaka K.; Kohno S. Inhibition of Hematopoietic Prostaglandin D Synthase Improves Allergic Nasal Blockage in Guinea Pigs. Prostaglandins Other Lipid Mediators 2011, 95, 27–34. 10.1016/j.prostaglandins.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Thurairatnam S. Hematopoietic prostaglandin D synthase inhibitors. Prog. Med. Chem. 2012, 51, 97–133. 10.1016/B978-0-12-396493-9.00004-2. [DOI] [PubMed] [Google Scholar]
- Hughes S. J.; Ciulli A. Molecular Recognition of Ternary Complexes: a New Dimension in the Structure-guided Design of Chemical Degraders. Essays Biochem. 2017, 61, 505–516. 10.1042/EBC20170041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A. C.; Crews C. M. Induced Protein Degradation: an Emerging Drug Discovery Paradigm. Nat. Rev. Drug Discovery 2017, 16, 101–14. 10.1038/nrd.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohoka N.; Shibata N.; Hattori T.; Naito M. Protein Knockdown Technology: Application of Ubiquitin Ligase to Cancer Therapy. Curr. Cancer Drug Targets 2016, 16, 136–146. 10.2174/1568009616666151112122502. [DOI] [PubMed] [Google Scholar]
- Naito M.; Ohoka N.; Shibata N. SNIPERs-Hijacking IAP activity to induce protein degradation. Drug Discovery Today: Technol. 2019, 31, 35–42. 10.1016/j.ddtec.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Shibata N.; Shimokawa K.; Nagai K.; Ohoka N.; Hattori T.; Miyamoto N.; Ujikawa O.; Sameshima T.; Nara H.; Cho N.; Naito M. Pharmacological Difference between Degrader and Inhibitor against Oncogenic BCR-ABL Kinase. Sci. Rep. 2018, 8, 13549. 10.1038/s41598-018-31913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J.; Qian Y.; Altieri M.; Dong H.; Wang J.; Raina K.; Hines J.; Winkler J. D.; Crew A. P.; Coleman K.; Crews C. M. Hijacking the E3 Ubiquitin Ligase Cereblon to Efficiently Target BRD4. Chem. Biol. 2015, 22, 755–763. 10.1016/j.chembiol.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You I.; Erickson E. C.; Donovan K. A.; Eleuteri N. A.; Fischer E. S.; Gray N. S.; Toker A. Discovery of an AKT Degrader with Prolonged Inhibition of Downstream Signaling. Cell Chem. Biol. 2020, 27, 66–73. 10.1016/j.chembiol.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burslem G. M.; Crews C. M. Proteolysis-Targeting Chimeras as Therapeutics and Tools for Biological Discovery. Cell 2020, 181, 102–114. 10.1016/j.cell.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei H.; Peng Y.; Zhao Q.; Chen Y. Small Molecule PROTACs: an Emerging Technology for Targeted Therapy in Drug Discovery. RSC Adv. 2019, 9, 16967–16976. 10.1039/C9RA03423D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobutoshi M.; Kosuke A.; Ayumi T.; Masanori H.; Kumi H.; Kazuhiko M.; Masatoshi H.; Ichiro H.; Yoshiyuki K.; Tadato T.; Hiromichi N.; Matsushita N.; Aritake K.; Takada A.; Hizue M.; Hayashi K.; Mitsui K.; Hayashi M.; Hirotsu I.; Kimura Y.; Tani T.; Nakajima H. Pharmacological Studies on the Novel Antiallergic Drug HQL-79: II. Elucidation of Mechanisms for Antiallergic and Antiasthmatic Effects. Jpn. J. Pharmacol. 1998, 78, 11–22. 10.1254/jjp.78.11. [DOI] [PubMed] [Google Scholar]
- Takaya D.; Inaka K.; Omura A.; Takenuki K.; Kawanishi M.; Yabuki Y.; Nakagawa Y.; Tsuganezawa K.; Ogawa N.; Watanabe C.; Honma T.; Aritake K.; Urade Y.; Shirouzu M.; Tanaka A. Characterization of Crystal Water Molecules in a High-affinity Inhibitor and Hematopoietic prostaglandin D Synthase Complex by Interaction Energy Studies. Bioorg. Med. Chem. 2018, 26, 4726–4734. 10.1016/j.bmc.2018.08.014. [DOI] [PubMed] [Google Scholar]
- Inoue T.; Okano Y.; Kado Y.; Aritake K.; Irikura D.; Uodome N.; Okazaki N.; Kinugasa S.; Shishitani H.; Matsumura H.; Kai Y.; Urade Y. First Determination of the Inhibitor Complex Structure of Human Hematopoietic Prostaglandin D Synthase. J. Biochem. 2004, 135, 279–283. 10.1093/jb/mvh033. [DOI] [PubMed] [Google Scholar]
- Hara S.; Kamei D.; Sasaki Y.; Tanemoto A.; Nakatani Y.; Murakami M. Prostaglandin E Synthases: Understanding their Pathophysiological Roles through Mouse Genetic Models. Biochimie 2010, 92, 651–659. 10.1016/j.biochi.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Kabututu Z.; Manin M.; Pointud J. C.; Maruyama T.; Nagata N.; Lambert S.; Lefrançois-Martinez A. M.; Martinez A.; Urade Y. Prostaglandin F2alpha Synthase Activities of Aldo-keto Reductase 1B1, 1B3 and 1B7. J. Biochem. 2009, 145, 161–168. 10.1093/jb/mvn152. [DOI] [PubMed] [Google Scholar]
- Watanabe K. Recent Reports about Enzymes Related to the Synthesis of Prostaglandin (PG) F(2) (PGF(2α) and 9α, 11β-PGF(2)). J. Biochem. 2011, 150, 593–596. 10.1093/jb/mvr116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.