Abstract
RvE1 (1) is an endogenous lipid mediator with very potent anti-inflammatory activity, which is due to the inhibition of neutrophil chemotaxis and inflammatory cytokine production and the promotion of macrophage phagocytosis. On the basis of the conformational analysis of RvE1, we designed its four cyclopropane congeners (2a–d), in which the conformationally flexible terminal C1–C4 moiety of RvE1 was rigidified by introducing stereoisomeric cyclopropanes. The four congeners and also RvE1 were efficiently synthesized via a common synthetic route. The evaluation of the anti-inflammatory effects of the compounds in mice resulted in the identification of trans-β-CP-RvE1 (2d), which was significantly more active than RvE1, as a potential lead for anti-inflammatory drugs of a novel mechanism of action.
Keywords: Resolvin E1, anti-inflammatory, cyclopropane congener, proresolving lipid mediator
Resolvin E1 (RvE1, 1), one of the four resolvin E-series, is an ω-3 fatty acid eicosapentaenoic acid (EPA) metabolite with very potent anti-inflammatory activity identified by Serhan et al. (Figure 1).1,2 Its remarkable anti-inflammatory effects are due to the inhibition of neutrophil chemotaxis and inflammatory cytokine production and the promotion of macrophage phagocytosis. Resolvins are widely studied,3,4 yet only a few analogues of RvE1 are reported, and its structure–activity relationship is almost unknown despite its remarkable biological activity.5−7 In our continuous medicinal chemistry study on resolvins,8−15 here we report the synthesis and anti-inflammatory effects of RvE1 and its cyclopropane congeners.
Figure 1.
Structures of resolvin E-series.
In the RvE1 structure, five unsaturated bonds significantly restrict the conformation of the molecule, and the three hydroxy groups at the 5-, 12-, and 18-allylic positions also restrict the conformation due to allylic strain (Figure 2). Therefore, the C5–C12 and C14–C20 moieties seem to be rather rigid structures. In contrast, the C1–C4 moiety is conformationally flexible due to the continuous unsubstituted methylene chain. The unsubstituted C13 methylene also contributes to the conformational flexibility around this position. Thus the structure of RvE1 comprises three parts: the rather conformationally rigid C5–C12 and C14–C20 parts connected by the C13-methylene and the conformationally flexible C1–C4 part. Conformations analyzed by computational calculations with MacroModel supported the above speculation on the conformationally rigid and flexible parts of RvE1. The terminal carboxy group in RvE1 is likely essential for its biological activity, similar to other lipid mediators. Thus the relative spatial arrangement of the carboxy group to the rigid unsaturated parts might be key to the bioactive conformation. The calculated stable structure of RvE1 suggests the four stable conformations A–D for the flexible C1–C4 moiety, and one or some of these might be the bioactive forms (Figure 2).
Figure 2.

Stable conformation of RvE1.
We took advantage of the characteristic structural properties of cyclopropane, the smallest rigid ring, to restrict the 3D structure of various biologically active compounds, allowing us to improve the biological activity and to identify the bioactive conformations.16−19 Previously, we successfully applied the cyclopropane strategy to develop stable equivalents of RvE2.9 We thought that by introducing a cyclopropane ring into the flexible C1–C4 terminal moiety of RvE1, the relative positioning of the key terminal carboxy group in the molecule could be differentially restricted depending on the stereochemistry of the cyclopropane moiety (Figure 3). Thus we designed a series of cyclopropane congeners of RvE1, that is, 2a (cis-α-CP-RvE1), 2b (cis-β-CP-RvE1), 2c (trans-α-CP-RvE1), and 2d (trans-β-CP-RvE1), in which the C2–C4 moiety of RvE1 was replaced with cyclopropanes having different stereochemistries. For each CP-RvE1, MacroModel calculations were performed to obtain the stable conformation.20 Each CP-RvE1 with a different stereochemistry assumed a 3D structure different in the C1–C4 moiety (Figure 3). Importantly, in the most stable conformations of the four congeners, the relative positioning of the carboxy group in the molecule effectively mimicked those in the four stable conformations of RvE1 obtained by calculations (Figure 2): A, B, C, and D in RvE1 were nearly superimposable to A′ in 2a, B′ in 2b, C′ in 2c, and D′ in 2d, respectively, in their calculated stable conformations. Thus we planned to synthesize the congeners 2a–d and evaluate their anti-inflammatory effects, which might provide a lead for new anti-inflammatory drugs and also some insight into the bioactive form of RvE1.
Figure 3.

Design of the four CP-RvE1s (2a–d) mimicking the stable conformations of the C1–C4 moiety of RvE1 (1). The four stable conformations A–D of RvE1 (lower left) and the most stable conformations A′−D′ of the four CP-RvE1s (lower right).
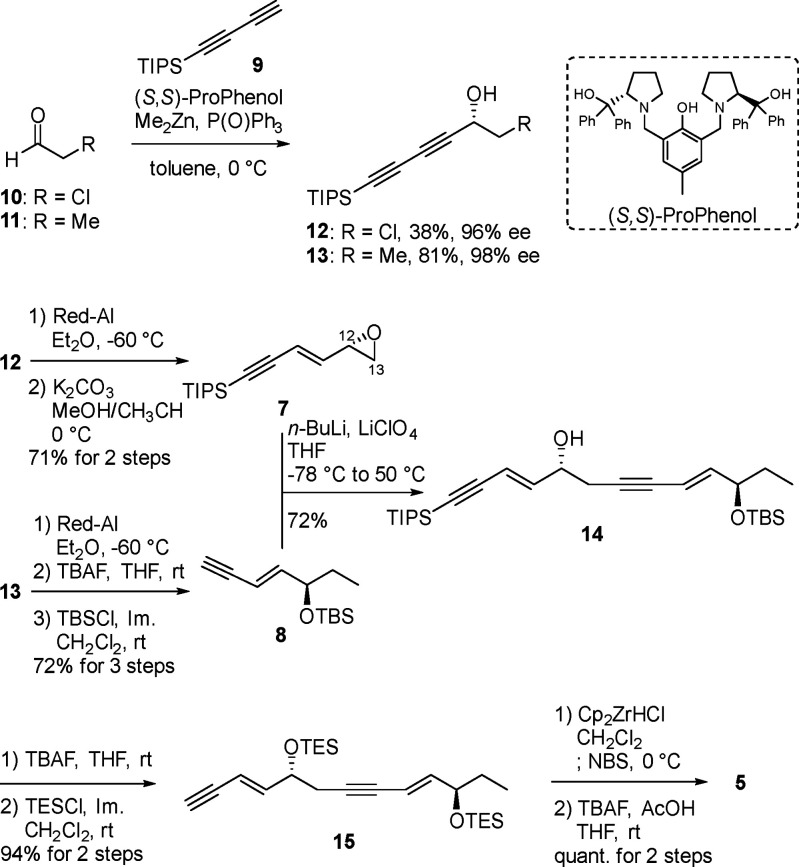
Although some total syntheses of RvE1 have been reported,21−24 we required an alternative synthetic route in which the C2–C4 moiety would be readily replaceable to effectively provide not only RvE1 itself but also its cyclopropane congeners 2a–d. Scheme 1 shows our retrosynthetic analysis of RvE1 (1) and CP-RvE1s (2a–d). All of the target compounds would be synthesized via Sonogashira coupling with trans-dienyl bromide 5 as a common key intermediate by changing the partner alkynes, that is, compound 3 or cyclopropane units 4a–d. All four stereoisomeric units 4a–d would be prepared from the corresponding stereoisomers 6a–d, which we previously developed as versatile cyclopropane units with stereochemical diversity.25,26 The dienyl bromide 5 was expected to be obtained by an epoxy ring opening of epoxide 7 with enyne 8. Both 7 and 8 would be obtained from diyne 9 and chloroacetaldehyde (10) or propanal (11) by adopting Trost’s ProPhenol-catalyzed diynylation.27,28 Thus the two chiral centers in 5 would be efficiently constructed by the same catalytic reaction with diyne 9.
Scheme 1. Retrosynthetic Analysis of RvE1 (1) and Its Cyclopropane Congeners 2a–d.
On the basis of the retrosynthesis, we first focused on the synthesis of the key intermediate 5, as shown in Scheme 2. When chloroacetaldehyde (10) or propanal (11) was subjected to the ProPhenol-catalyzed diynylation with 9 according to the previous report,27,28 the desired adduct 12 or 13 was obtained with high optical purity, respectively. The internal alkyne of 12 was regio- and stereoselectively reduced with Red-Al, and the resulting chlorohydrin was converted to epoxide 7. The internal alkyne of 13 was similarly regio- and stereoselectively reduced, the terminal silyl group of the resulting allylic alcohol was removed, and the hydroxy group was protected with a tert-butyldimethylsilyl (TBS) group to give 8. We next investigated the regioselective epoxy ring-opening reaction of epoxide 7 with lithiated enyne prepared from 8 in tetrahydrofuran (THF). The reaction with BF3·Et2O gave the desired allylic alcohol 14 in only low yield, where the undesired regioisomeric ring-opening at C12 predominantly occurred. When LiClO4 was used as the Lewis acid,29 however, the desired 14 was obtained as a single product in 72% yield. After manipulation of the silyl protecting groups of 14, the resulting 15 was treated with Schwartz’s reagent and N-bromosuccinimide to give the corresponding trans-dienyl bromide regio- and stereoselectively,30,31 of which the two triethylsilyl groups were simultaneously removed to afford the key common intermediate 5.
Scheme 2. Synthesis of the Common Intermediate 5.
Preparation of the cis-cyclopropane units 4a and 4b started from the chiral cyclopropanes 6a and 6b, respectively (Scheme 3).25,26 Nucleophilic addition to the aldehyde 6a with trimethylsilyl acetylene and BuLi was not stereoselective; however, the desired alcohol 16a was obtained in a pure form after silica gel column chromatography. After protecting-group manipulation to form alkyne 17a, its successive treatment with Dess–Martin periodinane32,33 and under Pinnik oxidation conditions afforded 18a, of which the MOM protecting group of the hydroxy group was changed to a pivaloyl group to give the desired methyl ester 4a. The stereoisomeric ester 4b was similarly obtained from 6b. Starting from the chiral trans-cyclopropane 6c or 6d, the corresponding trans-cyclopropane unit 4c or 4d, respectively, was also prepared (Supporting Information).
Scheme 3. Preparation of the cis-Cyclopropane Units 4a and 4b.
The synthetic route of RvE1 is shown in Scheme 4. Sonogashira coupling of the bromide 5 with alkyne 3(34) was performed to afford the coupling product 19 in excellent yield. Partial reduction of 19 was achieved under conditions using Zn(Cu/Ag)35,36 but was not reproducible. The reduction conditions reported by Hansen using Zn (Cu/Ag) in the presence of trimethylsilyl chloride (TMSCl) as a zinc activator and Et3SiH as a hydrogen source37 successfully provided the pentaene 20 in excellent yield with good reproducibility. Finally, the methyl ester of 20 was hydrolyzed to furnish RvE1 (1).
Scheme 4. Synthesis of RvE1 (1) and CP-RvE1 (2a–d).
We synthesized CP-RvE1s (2a–d) by the same procedure used for the synthesis of RvE1, as shown in Scheme 4. Sonogashira coupling of units 4a–d with dienyl bromide 5, partial reduction using the zinc reagent, and finally hydrolysis of the esters afforded the four target CP-RvE1s (2a–d).
Finally, we evaluated the anti-inflammatory activity of cis-α-CP-RvE1 (2a), cis-β-CP-RvE1 (2b), trans-α-CP-RvE1 (2c), and trans-β-CP-RvE1 (2d) as well as the parent proresolving mediator RvE1 using an in vivo mouse model of bacteria-induced peritonitis (Figure 4).38−40 We have observed that the increase in the total number of cells in the peritoneal cavity during the acute inflammation after the intraperitoneal (ip) administration of heat-killed Propionibacterium acnes (P. acnes), a Gram-positive bacterium, is almost entirely due to the neutrophil increase.40 The P. acnes-induced influx of neutrophils was significantly (∼30%) suppressed by 300 pg of RvE1 injected intraperitoneally into mice. The anti-inflammatory effects of cis-β-CP-RvE1 (2b) and trans-α-CP-RvE1 (2c) were significantly reduced compared with that of RvE1; however, cis-α-CP-RvE1 (2a) and trans-β-CP-RvE1 (2d) showed remarkable anti-inflammatory effects in this system. In particular, trans-β-CP-RvE1 was significantly more active (∼50% suppression) than parent RvE1. Thus the introduction of a cyclopropane into the terminal C1–C4 moiety of RvE2 changed the anti-inflammatory effects depending on the cyclopropane stereochemistry. These results suggest that the bioactivity of RvE1 can be related to the relative positioning of the carboxy terminal C1–C4 moiety in the molecule, and also the bioactive forms of the C1–C4 moiety in RvE1 might be analogous to conformations A and D, which were mimicked by cis-α-CP-RvE1 (2a) and trans-β-CP-RvE1 (2d). However, it should be noted that the biological activity evaluated in this study is an in vivo effect, which can be affected not only by the pharmacodynamic effect on the target biomolecule but also by the pharmacokinetic effect of compounds. Therefore, additional studies including an in vitro activity evaluation and a pharmacokinetic profile will be conducted in due course.
Figure 4.

Anti-inflammatory activity of RvE1 (1) and CP-RvE1s (2a–d) in mice. % suppression of neutrophil number in ip administration of 300 pg of RvE1 or CP-RvE1s to a mouse (n = 3–5, pooled from three independent experiments). Student’s two-tailed t tests were performed for statistical analyses of the data.
In conclusion, we designed the four cyclopropane congeners 2a–d of RvE1, in which the conformationally flexible but biologically important C1–C4 moiety of RvE1 was rigidified by introducing stereoisomeric cyclopropanes. The computational calculations suggested that the relative positioning of the C1-carboxy group in the four congeners effectively mimicked that of the four stable conformations of RvE1. RvE1 and its four congeners were systematically synthesized using the common key intermediate 5. The biological evaluation of the congeners showed that the anti-inflammatory effects can be changed depending on the cyclopropane stereochemistry. In particular, trans-β-CP-RvE1 (2d) was significantly more active than parent RvE1; therefore, it may be a lead for anti-inflammatory drugs of a novel mechanism of action.
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists (B) (25860087, H.F.), a Scientific Research Grant (C) (17K08360, H.F.), Scientific Research Grants (A) (15H02495 and 19H01014, S.S.) from the Japan Society for the Promotion of Science, and a research grant from Takeda Science Foundation (H.F.). Partial support was provided by the Platform Project for Supporting Drug Discovery and Life Science Research (BINDS) from AMED under grant number JP18am0101093.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00639.
The authors declare no competing financial interest.
Supplementary Material
References
- Serhan C. N.; Petasis N. A. Resolvins and Protectins in Inflammation Resolution. Chem. Rev. 2011, 111, 5922–5943. 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K.; Sommer N.; Hache K.; Hecker A.; Reiche S.; Schneck E.; Weissmann N.; Seeger W.; Hecker M. Resolvin E1 Improves Mitochondrial Function in Human Alveolar Epithelial Cells during Severe Inflammation. Lipids 2019, 54, 53–65. 10.1002/lipd.12119. [DOI] [PubMed] [Google Scholar]
- Quiros M.; Feier D.; Birkl D.; Agarwal R.; Zhou D. W.; Garcia A. J.; Parkos C. A.; Nusrat A. Resolvin E1 is a pro-repair molecule that promotes intestinal epithelial wound healing. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 9477–9482. 10.1073/pnas.1921335117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M.; Oh S. F.; Chonan T.; Hong S.; Elangovan S.; Sun Y.-P.; Uddin J.; Petasis N. A.; Serhan C. N. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J. Biol. Chem. 2006, 281, 22847–22854. 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- Hong S.; Porter T. F.; Lu Y.; Oh S. F.; Pillai P. S.; Serhan C. N. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J. Immunol. 2008, 180, 3512–3519. 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- Oh S. F.; Pillai P. S.; Recchiuti A.; Yang R.; Serhan C. N. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J. Clin. Invest. 2011, 121, 569–581. 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A.; Fukuda H.; Shiida N.; Tanaka N.; Furugen A.; Ogura J.; Shuto S.; Mano N.; Yamaguchi H. Determination of ω-6 and ω-3 PUFA metabolites in human urine samples using UPLC/MS/MS. Anal. Bioanal. Chem. 2015, 407, 1625–1639. 10.1007/s00216-014-8412-5. [DOI] [PubMed] [Google Scholar]
- Fukuda H.; Muromoto R.; Takakura Y.; Ishimura K.; Kanada R.; Fushihara D.; Tanabe M.; Matsubara K.; Hirao T.; Hirashima K.; Abe H.; Arisawa M.; Matsuda T.; Shuto S. Design and Synthesis of Cyclopropane Congeners of Resolvin E2, an Endogenous Proresolving Lipid Mediator, as Its Stable Equivalents. Org. Lett. 2016, 18, 6224–6227. 10.1021/acs.orglett.6b02612. [DOI] [PubMed] [Google Scholar]
- Deyama S.; Shimoda K.; Suzuki H.; Ishikawa Y.; Ishimura K.; Fukuda H.; Hitora-Imamura N.; Ide S.; Satoh M.; Kaneda K.; Shuto S.; Minami M. Resolvin E1/E2 ameliorate lipopolysaccharide-induced depression-like behaviors via ChemR23. Psychopharmacology 2018, 235, 329–336. 10.1007/s00213-017-4774-7. [DOI] [PubMed] [Google Scholar]
- Unno Y.; Sato Y.; Fukuda H.; Ishimura K.; Ikeda H.; Watanabe M.; Tansho-Nagakawa S.; Ubagai T.; Shuto S.; Ono Y. Resolvin E1, but not resolvins E2 and E3, promotes fMLF-induced ROS generation in human neutrophils. FEBS Lett. 2018, 592, 2706–2715. 10.1002/1873-3468.13215. [DOI] [PubMed] [Google Scholar]
- Deyama S.; Shimoda K.; Ikeda H.; Fukuda H.; Shuto S.; Minami M. Resolvin E3 attenuates lipopolysaccharide-induced depression-like behavior in mice. J. Pharmacol. Sci. 2018, 138, 86–88. 10.1016/j.jphs.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Sato M.; Aoki-Saito H.; Fukuda H.; Ikeda H.; Koga Y.; Yatomi M.; Tsurumaki H.; Maeno T.; Saito T.; Nakakura T.; Mori T.; Yanagawa M.; Abe M.; Sako Y.; Dobashi K.; Ishizuka T.; Yamada M.; Shuto S.; Hisada T. Resolvin E3 attenuates allergic airway inflammation via the interleukin-23–interleukin-17A pathway. FASEB J. 2019, 33, 12750–12759. 10.1096/fj.201900283R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y.; Fukuda H.; Muromoto R.; Hirashima K.; Ishimura K.; Fujiwara K.; Ishihara J.; Matsuda T.; Watanabe M.; Shuto S. Design and Synthesis of Benzene Congeners of Resolvin E2, a Proresolving Lipid Mediator, as Its Stable Equivalents. ACS Med. Chem. Lett. 2020, 11, 479–484. 10.1021/acsmedchemlett.9b00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H.; Ikeda H.; Muromoto R.; Hirashima K.; Ishimura K.; Fujiwara K.; Aoki-Saito H.; Hisada T.; Watanabe M.; Ishihara J.; Matsuda T.; Shuto S. Synthesis of Resolvin E3, a Proresolving Lipid Mediator, and Its Deoxy Derivatives: Identification of 18-Deoxy-resolvin E3 as a Potent Anti-Inflammatory Agent. J. Org. Chem. 2020, 85, 14190–14200. 10.1021/acs.joc.0c01701. [DOI] [PubMed] [Google Scholar]
- Kawamura S.; Unno Y.; List A.; Mizuno A.; Tanaka M.; Sasaki T.; Arisawa M.; Asai A.; Groll M.; Shuto S. Potent proteasome inhibitors derived from the unnatural cis-cyclopropane isomer of Belactosin A: synthesis, biological activity, and mode of action. J. Med. Chem. 2013, 56, 3689–3700. 10.1021/jm4002296. [DOI] [PubMed] [Google Scholar]
- Kawamura S.; Unno Y.; Tanaka M.; Sasaki T.; Yamano A.; Hirokawa T.; Kameda T.; Asai A.; Arisawa M.; Shuto S. Investigation of the noncovalent binding mode of covalent proteasome inhibitors around the transition state by combined use of cyclopropylic strain-based conformational restriction and computational modeling. J. Med. Chem. 2013, 56, 5829. 10.1021/jm400542h. [DOI] [PubMed] [Google Scholar]
- Mizuno A.; Kameda T.; Kuwahara T.; Endoh H.; Ito Y.; Yamada S.; Hasegawa K.; Yamano A.; Watanabe M.; Arisawa M.; Shuto S. Cyclopropane-Based Peptidomimetics Mimicking Wide-Ranging Secondary Structures of Peptides: Conformational Analysis and Their Use in Rational Ligand Optimization. Chem. - Eur. J. 2017, 23, 3159. 10.1002/chem.201605312. [DOI] [PubMed] [Google Scholar]
- For a review, see:Mizuno A.; Matsui K.; Shuto S. From Peptides to Peptidomimetics: A Strategy Based on the Structural Features of Cyclopropane. Chem. - Eur. J. 2017, 23, 14394–14409. 10.1002/chem.201702119. [DOI] [PubMed] [Google Scholar]
- The stable conformations of RvE1 were calculated using Maestro 9.9 MacroModel. The conditions of the calculation are as follows: solvent: water, force field: OPLS2005. There are four conformations within 1.5 kcal/mol from the energy of the most stable conformation of RvE1.
- Ogawa N.; Kobayashi Y. Total synthesis of resolvin E1. Tetrahedron Lett. 2009, 50, 6079–6082. 10.1016/j.tetlet.2009.08.061. [DOI] [Google Scholar]
- Allard M.; Barnes K.; Chen X.; Cheung Y.-Y.; Duffy B.; Heap C.; Inthavongsay J.; Johnson M.; Krishnamoorthy R.; Manley C.; Steffke S.; Varughese D.; Wang R.; Wang Y.; Schwartz C. E. Total synthesis of Resolvin E1. Tetrahedron Lett. 2011, 52, 2623–2626. 10.1016/j.tetlet.2011.03.035. [DOI] [Google Scholar]
- Urbitsch F.; Elbert B. L.; Llaveria J.; Streatfeild P. E.; Anderson E. A. A Modular, Enantioselective Synthesis of Resolvins D3, E1, and Hybrids. Org. Lett. 2020, 22, 1510–1515. 10.1021/acs.orglett.0c00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesman J. I.; Tungen J. E.; Vik A.; Hansen T. V. Stereoselective synthesis of the specialized pro-resolving and anti-inflammatory mediator resolvin E1. Tetrahedron 2020, 76, 130821. 10.1016/j.tet.2019.130821. [DOI] [Google Scholar]
- Kazuta K.; Matsuda A.; Shuto S. Development of versatile cis- and trans-dicarbon-substituted chiral cyclopropane units: synthesis of (1S,2R)- and (1R,2R)-2-aminomethyl-1-(1H-imidazol-4-yl)cyclopropanes and their enantiomers as conformationally restricted analogues of histamine. J. Org. Chem. 2002, 67, 1669–1677. 10.1021/jo010852x. [DOI] [PubMed] [Google Scholar]
- Watanabe M.; Kazuta Y.; Hayashi H.; Yamada S.; Matsuda A.; Shuto S. Stereochemical diversity-oriented conformational restriction strategy. Development of potent histamine H3 and/or H4 receptor antagonists with an imidazolylcyclopropane structure. J. Med. Chem. 2006, 49, 5587–5596. 10.1021/jm0603318. [DOI] [PubMed] [Google Scholar]
- Trost B. M.; Chan V. S.; Yamamoto D. Enantioselective prophenol-catalyzed addition of 1,3-diynes to aldehydes to generate synthetically versatile building blocks and diyne natural products. J. Am. Chem. Soc. 2010, 132, 5186–5192. 10.1021/ja910656b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost B. M.; Quintard A. Asymmetric catalytic alkynylation of acetaldehyde: Application to the synthesis of (+)-tetrahydropyrenophorol. Angew. Chem., Int. Ed. 2012, 51, 6704–6708. 10.1002/anie.201203035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini M.; Crotti P.; Favero L.; Macchia F. Stereo- and regioselective metal salt-catalyzed alkynylation of 1,2-epoxides. Tetrahedron Lett. 1991, 32, 6617–6620. 10.1016/0040-4039(91)80237-Z. [DOI] [Google Scholar]
- Hart D. W.; Schwartz J. Hydrozirconation. Organic synthesis via organozirconium intermediates. Synthesis and rearrangement of alkylzirconium(IV) complexes and their reaction with electrophiles. J. Am. Chem. Soc. 1974, 96, 8115–8116. 10.1021/ja00833a048. [DOI] [Google Scholar]
- Schwartz J.; Labinger J. A. Hydrozirconation: A New Transition Metal Reagent for Organic Synthesis. Angew. Chem., Int. Ed. Engl. 1976, 15, 333–340. 10.1002/anie.197603331. [DOI] [Google Scholar]
- Dess D. B.; Martin J. C. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 1983, 48, 4155–4156. 10.1021/jo00170a070. [DOI] [Google Scholar]
- Dess D. B.; Martin J. C. A useful 12-I-5 triacetoxyperiodinane (the Dess-Martin periodinane) for the selective oxidation of primary or secondary alcoh ols and a variety of related 12-I-5 species. J. Am. Chem. Soc. 1991, 113, 7277–7287. 10.1021/ja00019a027. [DOI] [Google Scholar]
- Götz K.; Liermann J. C.; Thines E.; Anke H.; Opatz T. Structure elucidation of hypocreolide A by enantioselective total synthesis. Org. Biomol. Chem. 2010, 8, 2123–2130. 10.1039/c001794a. [DOI] [PubMed] [Google Scholar]
- Boland W.; Schroer N.; Sieler C.; Feigel M. Sterospecific Syntheses and Spectroscopic Properties of Isomeric 2,4,6,8-Undecatetraenes. New Hydrocarbons from the Marine Brown Alga Giffordia mitchellae. Part IV. Helv. Chim. Acta 1987, 70, 1025–1040. 10.1002/hlca.19870700415. [DOI] [Google Scholar]
- Gueugnot S.; Alami M.; Linstrumelle G.; Mambu L.; Petit Y.; Larchevêque M. An efficient total synthesis of 5-(S)-HETE. Tetrahedron 1996, 52, 6635–6646. 10.1016/0040-4020(96)00316-X. [DOI] [Google Scholar]
- Mohamed Y. M. A.; Hansen T. V. Z-Stereoselective semi-reduction of alkynes: modification of the Boland protocol. Tetrahedron 2013, 69, 3872–3877. 10.1016/j.tet.2013.03.038. [DOI] [Google Scholar]
- Białecka A.; Mak M.; Biedroń R.; Bobek M.; Kasprowicz A.; Marcinkiewicz J. S. Different Pro-Inflammatory and Immunogenic Potentials of Propionibacterium Acnes and Staphylococcus Epidermidis: Implications for Chronic Inflammatory Acne. Arch. Immunol. Ther. Exp. (Warsz) 2005, 53, 79–85. [PubMed] [Google Scholar]
- Ananias R. Z.; Rodrigues E. G.; Braga E. G.; Squaiella C. C.; Mussalem J. S.; Longhini A. L.; Travassos L. R.; Longo-Maugéri I. M. Modulatory Effect of Killed Propionibacterium Acnes and Its Purified Soluble Polysaccharide on Peritoneal Exudate Cells From C57Bl/6 Mice: Major NKT Cell Recruitment and Increased Cytotoxicity. Scand. J. Immunol. 2007, 65, 538–548. 10.1111/j.1365-3083.2007.01939.x. [DOI] [PubMed] [Google Scholar]
- Hirashima K.; Muromoto R.; Minoguchi H.; Matsumoto T.; Kitai Y.; Kashiwakura J.; Shimoda K.; Oritani K.; Matsuda T. The mechanism of Tyk2 deficiency-induced immunosuppression in mice involves robust IL-10 production in macrophages. Cytokine+ 2020, 130, 155077. 10.1016/j.cyto.2020.155077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.