Abstract
Genome-wide association studies (GWASs) have identified hundreds of single nucleotide polymorphisms (SNPs) associated with type 2 diabetes (T2D) and coronary artery disease (CAD), respectively. Nevertheless, these studies were generally performed for single-trait/disease and failed to assess the pleiotropic role of the identified variants. To identify novel functional loci and the pleiotropic relationship between CAD and T2D, the targeted cFDR analysis on CpG-SNPs was performed by integrating two independent large and multi-centered GWASs with summary statistics of T2D (26,676 cases and 132,532 controls) and CAD (60,801 cases and 123,504 controls). Applying the cFDR significance threshold of 0.05, we observed a pleiotropic enrichment between T2D and CAD by incorporating pleiotropic effects into a conditional analysis framework. We identified 79 novel CpG-SNPs for T2D, 61 novel CpG-SNPs for CAD, and 18 novel pleiotropic loci for both traits. Among these novel CpG-SNPs, 33 of them were annotated as methylation quantitative trait locus (meQTL) in whole blood, and ten of them showed expression QTL (eQTL), meQTL, and metabolic QTL (metaQTL) effects simultaneously. To the best of our knowledge, we performed the first targeted cFDR analysis on CpG-SNPs, and our findings provided novel insights into the shared biological mechanisms and overlapped genetic heritability between T2D and CAD.
Keywords: Type 2 diabetes, Coronary artery disease, Genome-wide association study (GWAS), DNA methylation, CpG-SNP, cFDR
Introduction
Diabetes mellitus is a chronic disease that occurs when the pancreas can not produce enough insulin (type 1 diabetes, T1D), or when the body can not effectively use the insulin (type 2 diabetes, T2D) (Association 2014). In 2016, about 38 million deaths were directly or indirectly caused by diabetes. The most common cause of death among patients with diabetes (especially T2D) is cardiovascular disease, responsible for 70% of deaths (Szuszkiewicz-Garcia and Davidson 2014). T2D is one of the major risk factors for coronary artery diseases (CAD) (Naito and Miyauchi 2017), which is associated with two to fourfold increased mortality risk from CAD. Abundant evidence has pointed out that T2D and CAD share many common primary risk factors including hypertension, smoking, hyperlipemia, hyperglycemia, and dysbetalipoproteinemia, as well as several potential risk factors such as obesity, low physical activity, cardiovascular family history, gender, and age (Kannel 1985; Norhammar and Schenck-Gustafsson 2013).
Although plenty of genetic loci have been identified for association with T2D or CAD by genome-wide association studies (GWASs) (Nelson et al. 2017; Scott et al. 2017), most of these identified single nucleotide polymorphisms (SNPs) were located in the intron region, and only a subset of them may affect transcription process. However, these specific functional variants were generally unknown. To search for additional novel functional genetic loci for T2D and CAD, one feasible strategy is to focus on specific variants that may potentially affect regulatory factors such as DNA methylation.
DNA methylation, also known as cytosine methylation, is a major epigenetic modification of vertebrate genomes and has profound impacts on human genetic disorders (Bogdanovic and Lister 2017). CpG site is the primary substrate for methyl transfer reactions and, therefore, SNPs that can introduce or disrupt a CpG site (CpG-SNPs) may dramatically alter the methylation status at the affected loci (Shoemaker et al. 2010; Zhi et al. 2013). Targeted association analysis of CpG-SNPs has been demonstrated as an efficient strategy to identify novel functional variants associated with complex diseases and traits, such as T2D (Dayeh et al. 2013), breast cancer (Harlid et al. 2011), hypertriglyceridemia (de Toro-Martin et al. 2016), schizophrenia (Bani-Fatemi et al. 2013), and osteoporosis (Qiu et al. 2018).
Another limitation of GWAS is that the identified SNPs can only explain a small proportion of the heritability (10% for T2D and 13.3% for CAD) (Nikpay et al. 2015). This phenomenon of failing to explain a substantial proportion of the heritability of complex phenotypes is often described as the “missing heritability” problem (Pei et al. 2014). To excavate the “missing heritability”, one effective way is expanding the sample sizes. However, this approach can be highly expensive and time-consuming (Stahl et al. 2012). Recently, Andreassen et al. (Andreassen et al. 2013) developed a genetic-pleiotropy-informed conditional false discovery rate (cFDR) method by leveraging two independent GWASs from associated traits in a conditional analysis. This method has been widely applied to different genetically inherited diseases and phenotypes (Peng et al. 2017a; Wang et al. 2017b; Zeng et al. 2017; Zhou et al. 2017; Hu et al. 2018).
In this study, we performed a targeted cFDR analysis on CpG-SNPs for T2D and CAD to identify novel functional gene loci and the pleiotropic relationship between these traits by integrating two independent GWASs (Nikpay et al. 2015; Scott et al. 2017). Our findings provided novel insights into the shared biological mechanisms and overlapped genetic heritability between T2D and CAD.
Methods
GWAS datasets
Two independent GWASs (Nikpay et al. 2015; Scott et al. 2017) with summary statistics for T2D and CAD were downloaded from publicly available datasets. The dataset for T2D contained meta-analysis summary statistics of 26,676 T2D cases and 132,532 control subjects from 18 studies of European ancestry performed by DIAGRAM Consortium (Scott et al. 2017). The dataset for CAD contained association summary statistics of 60,801 cases and 123,504 controls from 48 studies for a GWAS meta-analysis of CAD conducted by CARDIoGRAM Consortium (Nikpay et al. 2015). Both of the datasets consisted of the summary statistics for each SNP with the p values that have undergone genomic control at the individual study level. Further details of these two studies are described in the previous publications (Nikpay et al. 2015; Scott et al. 2017).
Identification of potentially functional CpG‑SNPs
The identification of potentially functional CpG-SNPs was described in our previous study (Qiu et al. 2018). In brief, an SNP was defined as a CpG-SNP if it introduces or disrupts a CpG site, and we identified common CpG-SNPs in the human genome by interrogating common variants [minor allele frequency (MAF) > 0.05] from human reference genome (hg19) (Genomes Project et al. 2010), and our in-house whole-genome high-coverage deep re-sequencing study (Shen et al. 2013). A total of 3,811,642 common CpG-SNPs were identified throughout the human genome.
Data processing
By overlapping the 7,362,687 common SNPs in the T2D and CAD meta-analysis with the identified CpG-SNPs, a total of 2,236,577 common CpG-SNPs with association summary statistics for both traits were retrieved. Next, we performed the linkage disequilibrium (LD) based pruning (r2 ≥ 0.2) through PLINK 1.9 (Lin et al. 2018) and identified 93,408 independent CpG-SNPs, which were used for the downstream cFDR analysis.
Statistical analysis
To estimate the pleiotropic enrichment of association compared to that expected under the null hypothesis, we first constructed a conditional Q–Q plot by successively conditioning the principal trait on the SNPs with varying strengths of association in the conditional trait, then followed by a constructed fold-enrichment plots to further estimate the pleiotropic enrichment between CAD and T2D. The cFDR approach is well-established and has been widely applied to integrate independent GWASs with summary statistics and assess whether a random SNP is associated with the principal phenotype given that the observed p values for the principal and conditional traits are both smaller than two pre-defined disease-specific significance thresholds. Next, we computed the conjunction cFDR (ccFDR) to determine the pleiotropic loci. Finally, we applied conditional and conjunctional Manhattan plots to visualize the localization of the independent loci associated with T2D conditional on CAD and vice versa, as well as independent loci with a pleiotropic effect on both traits. An SNP or gene is defined as a novel one if it has not been reported in previous GWASs (Nikpay et al. 2015; Scott et al. 2017) or our previous cFDR study (Zhang et al. 2018b). Detailed information is present in the supplementary materials.
Function annotation of the pleiotropic CpG-SNPs
To explore the functional role of the identified pleiotropic CpG-SNPs in T2D and CAD, we annotated each pleiotropic CpG-SNP to various DNA features or regulatory elements using HaploReg (https://www.broadinstitute.org/mammals/haploreg/haploreg.php) and SNPnexus (https://www.snp-nexus.org/). Both tools retrieved the ENCODE (Rosenbloom et al. 2013) and RoadMap (Dayem Ullah et al. 2018) annotations for the queried CpG-SNPs as well as their LD proxy variants (r2 ≥ 0.8).
Next, we tested whether the identified CpG-SNPs or their LD proxy variants (r2 ≥ 0.8) have expression quantitative trait loci (eQTL), methylation QTL (meQTL) or metabolic QTL (metaQTL) effects. The eQTL hits were obtained from HaploReg based on GTEx and other eQTL results; independent cis- and trans- meQTLs in whole blood were obtained from Bonder’s study (Bonder et al. 2016) (https://genenetwork.nl/biosqtlbrowser/); and metaQTLs were obtained from SNiPA (https://snipa.helmholtz-muenchen.de/snipa3/) which summarized recently published metaQTL studies.
Gene ontology (GO) enrichment and protein–protein interaction
The GOEAST program (https://omicslab.genetics.ac.cn/GOEAST/) was applied to identify significantly enriched gene ontology terms among the list of genes associated with pleiotropic CpG-SNPs. The p values were calculated by hypergeometric tests and adjusted for multiple comparisons by stringent Yekutieli method (FDR under dependency) (Yekutieli and Benjamini 1999). Next, to partially explore and characterize the functional relationship of the identified genes, the corresponding protein association networks were constructed using the STRING 10.5 database (https://string-db.org/).
Mendelian randomization analysis
To further explore the causal effect of T2D on CAD, we applied two-sample Mendelian randomization (MR) analysis using T2D as exposure and CAD as outcome through “Two-SampleMR” package (https://github.com/MRCIEU/TwoSampleMR) (Hemani et al. 2018). Similar to Chen’s study (Chen et al. 2018), instead of testing at the whole genome level, we selected out a subset of genes to operate “gene-specific MR”. In detail, we first mapped T2D-related CpG-SNPs to their corresponding genes. Then, we extracted all the SNPs mapped to these genes in the original T2D GWAS dataset as a candidate for exposure instruments while using CAD as the outcome. Similar to our previous study (Zhang et al. 2018a), the candidate instrumental SNPs should meet the selection criteria where: (1) SNPs have significant association with the exposure (T2D, p ≤ 5 × 10−8); (2) among the SNPs that in high LD (r2 ≥ 0.01), only the SNP with the highest effect size (β value) will be retained. Finally, 17 SNPs were included as instrumental variables (Table S5), and MR was performed using MR Egger, inverse-variance weighted (IVW), weighted median, and weighted mode.
Results
Assessment of pleiotropic enrichment
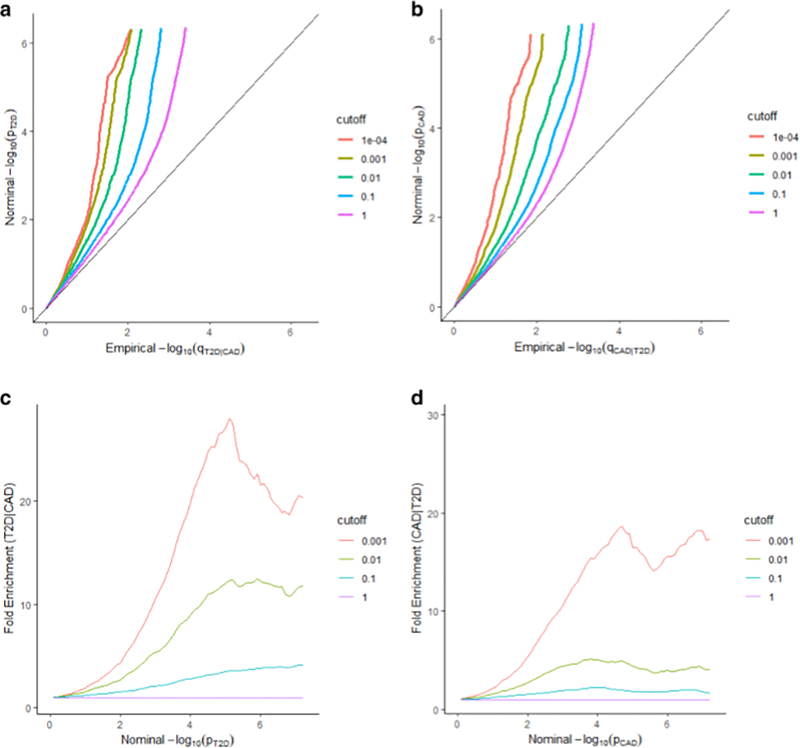
We observed a clear separation between the different Q-Q curves, and the proportion of actual effects in T2D varied considerably across different levels of association for CAD (Fig. 1a, c), which indicated a strong enrichment of T2D-associated SNPs. A similar enrichment pattern was also identified in CAD conditioned on T2D (Fig. 1b, d). We observed an evident upward shift from the expected baseline. It clearly demonstrated the pleiotropy between T2D and CAD (T2D|CAD, CAD|T2D), where T2D conditioned on CAD (T2D|CAD) achieved the most significant pleiotropic enrichment, as an enrichment fold over 20 was observed in Y-axis while comparing the most stringent subset to all SNPs.
Fig. 1.
Stratified Q–Q plots and fold-enrichment plots. Stratified Q–Q plots of nominal vs. empirical − log 10(p) values in principal trait below the standard GWAS threshold of p ≤ 5 × 10−8 as a function of the significance of the association with conditional trait at the level of p ≤ 1, p ≤ 0.1, p ≤ 0.01, p ≤ 0.001, and p ≤ 0.0001, respectively. a T2D as a function of the significance of the association with CAD, and b CAD as a function of the significance of the association with T2D. Fold-enrichment plots of enrichment vs. nominal − log 10(p) values (corrected for inflation) corresponding to levels of p ≤ 1, p ≤ 0.1, p ≤ 0.01, p ≤ 0.001, respectively in c T2D below the standard GWAS threshold of p ≤ 5 × 10−8 as a function of significance of the association with CAD; and in d CAD below the standard GWAS threshold of p ≤ 5 × 10−8 as a function of significance with T2D. Dashed lines indicate the null-hypothesis
T2D loci identified by cFDR
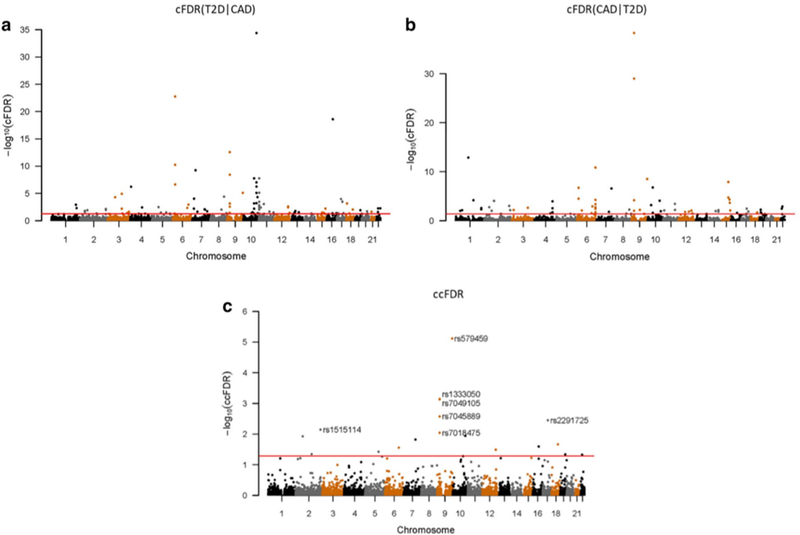
We identified 98 significant (cFDR ≤ 0.05) CpG-SNPs associated with T2D conditioned on CAD (Fig. 2a), including 79 novel SNPs that have not been reported in association with T2D in previous studies (Scott et al. 2017; Zhang et al. 2018b) (Table S1). Using a more conservative threshold of cFDR ≤ 0.01, 55 CpG-SNPs (37 novel CpG-SNPs) remained as significant variants.
Fig. 2.
Conditional Manhattan plot. SNPs with − log 10(cFDR) ≥ 1.3 (cFDR ≤ 0.05) for a T2D given CAD (T2D|CAD) and b CAD given T2D (CAD|T2D), or c − log 10(ccFDR) ≥ 1.3 (ccFDR ≤ 0.05) are shown above the red line
We performed a series of bioinformatics analyses to explore the potential regulatory function for these CpG-SNPs. Assuming these CpG-SNPs were partially effective through DNA methylation, we analyzed the meQTL effects of identified CpG-SNPs, where 13 of them showed significant meQTL effects in whole blood (Table S3). In addition, we investigated whether these CpG-SNPs were also associated with T2D through metabolomics regulation. Interestingly, 11 CpG-SNPs showed metaQTL effects, including 4 SNPs (i.e., rs7723, rs1596972, rs17202418, rs2237892) whose associated metabolites (e.g., 1-oleoylglycerophosphocholine, 2-methylbutyroylcarnitine, gamma-glutamylvaline, etc.) have direct connections with the susceptibility of T2D (Table S4) (Lontchi-Yimagou et al. 2013; Leitner et al. 2017; Zhao and Li 2019). Notably, 7 novel CpG-SNPs (i.e., rs10786044, rs2066612, rs6770420, rs7004862, rs7094128, rs7723, and rs7732628) showed meQTL, eQTL, and metaQTL effects simultaneously and several of them were enriched in enhancer/promoter elements in various tissues (Table 2).
Table 2.
Functional annotation for ten CpG-SNPs showing significant effects in meQTL, eQTL, and metaQTL
| rsID | GENCODE genes | Traits | meQTL (p) | eQTL hits | metaQTL (metabolites) | Promoter histone marks | Enhancer histone marks | DNAse | Proteins bound | Motifs changed |
|---|---|---|---|---|---|---|---|---|---|---|
| rs579459 | ABO | Pleiotropic | 1.45E–09 | Five hits | ADp | BLD | Gl | Four tissues | NFYA, POL2 | Hmx, Nkx2 |
| rsl 1172113 | LRP1 | CAD | 1.43E–07 | Four hits | SM C18:1 | Eight tissues | 15 tissues | 17 tissues | FOX Al | AP-2, Hiel, PU.l |
| rs3105748 | SLC22A3 | CAD | 7.67E–13 | Four hits | Succinylcarnitine | 6 tissues | Four altered motifs | |||
| rsl 0786044 | AL161652.1 | T2D | 9.93E–05 | Whole blood | 2-Hydroxypalmi- tate |
Five tissues | RAPI, ZNF263 | HNF6, ZEB1 | ||
| rs2066612 | DLEU1 | T2D | 4.06E–07 | Whole blood | X-11847 | BHLHE40, Foxa, HEY1 | ||||
| rs6770420 | EIF5A2 | T2D | 1.04E–08 | Whole blood | Ursodeoxycholate | Hsf, SIX5 | ||||
| rs7004862 | 1NTS8 | T2D | ≤ 1.00E–50 | 23 hits | SM C24:0 | BLD | HDAC2,Mef2 | |||
| rs7094128 | LRRC20 | T2D | 5.05E–08 | Fiboblast, bood | 1-Methylxanthine | BLD | IPSC, ADRL | AP-4, HEN1, Lmo2-complex | ||
| rs7723 | TMED4 | T2D | 2.14E–47 | 9 hits | 1-Oleoylglycero | Four tissues | Four tissues | Four altered motifs | ||
| rs7732628 | CTC-564N23.3 | T2D | 6.41E–05 | Heart artery | X-12206 | FAT, PANC | 15 tissues | CFOS | AP-1, ERalpha-a |
meQTL methylation quantitative trait locus (including associated SNPs with an LD r2≥0.8, Table S3), eQTL expression quantitative trait locus, metaQTL metabòlic quantitative trait locus, DNAse deoxyribonuclease, Adp ADpSGEGDFXAEGGGVR, 1 -oleoylglycero 1-oleoylglycerophosphocholine
CAD loci identified by cFDR
Likewise, we identified 80 significant (cFDR ≤ 0.05) CpG-SNPs associated with CAD conditioned on T2D (Fig. 2b, Table S2), including 61 novel variants for CAD. Using a more conservative threshold of cFDR ≤ 0.01, 49 significant loci remained (32 novel SNPs).
By exploring the potential regulatory functions of these significant CpG-SNPs, we found 15 CpG-SNPs showing significant meQTL effects in whole blood (Table S3), and 9 CpG-SNPs associated with various metabolites (Table S4), including some candidates (e.g., butyrylcarnitine, ethylmalonate, cholesterol, etc.) that have been implicated in the pathophysiology of CAD. In particular, two novel CpG-SNPs, rs11172113 and rs3105748, showed meQTL, eQTL, and metaQTL effects simultaneously (Table 2).
Pleiotropic loci in T2D and CAD identified with ccFDR
To further investigate whether any of the SNPs were associated with both T2D and CAD, we computed ccFDR and constructed a ccFDR Manhattan plot (Fig. 2c). Finally, 18 novel pleiotropic CpG-SNPs reached the significance level (ccFDR ≤ 0.05, 8 CpG-SNPs with ccFDR ≤ 0.01) and were annotated to 17 different genes (Table 1). None of these CpG-SNPs have been identified as pleiotropic loci for T2D and CAD in our previous study (Zhang et al. 2018b), yet 6 SNPs have been associated with CAD or T2D separately (Nikpay et al. 2015; Zhang et al. 2018b). Then we analyzed the eQTL, meQTL and metaQTL effects of identified pleiotropic CpG-SNPs and found 8 SNPs have at least one effect. Importantly, one pleiotropic CpG-SNP, rs579459, showed meQTL, eQTL, and metaQTL effects simultaneously, as well as other predicted functions such as alteration of transcription factor binding motif (Table 2).
Table 1.
Conjunction cFDR for 18 pleiotropic CpG-SNPs in T2D and CAD (ccFDR ≤ 0.05)
| rsID | Chr | Pos | Allele | Gene | Location | eQTL/meQTL/metaQTL | SNP type | Gene type | cFDR_CAD | cFDR_T2D | ccFDR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs7604944 | 2 | 65319288 | C/T | SPRED2 | Intronic | Novel | Novel | 4.70E–02 | 2.57E–02 | 4.70E–02 | |
| rs2252641 | 2 | 145043894 | T/C | TEX41 | Non-coding intronic | CAD | Novel | 3.24E–02 | 2.46E–03 | 3.24E–02 | |
| rsl515114 | 2 | 226233671 | A/G | AC062015.1 | 48.3 kb upstream | eQTL (adipose) | Novel | Novel | 4.64E–39 | 7.20E–04 | 7.20E–04 |
| rs1428387 | 5 | 123369550 | C/T | CEP120 | Intronic | Novel | Novel | 3.55E–03 | 3.20E–04 | 3.55E–03 | |
| rs1262557 | 6 | 126733443 | C/T | RPS4XP9 | 49.6 kb upstream | Novel | Novel | 9.10E–03 | 2.60E–13 | 9.10E–03 | |
| rs17405606 | 7 | 107630918 | C/T | WBP1LP2 | 1.4 kb upstream | Novel | Novel | 9.40E–30 | 7.40E–04 | 7.40E–04 | |
| rs7049105 | 9 | 22028802 | A/G | AL359922.1, CDKN2B-AS1 | Intronic, non-coding intronic | eQTL (blood), meQTL (LD tors 10120688) | CAD | Novel, confirmed | 4.58E–02 | 1.20E–02 | 4.58E–02 |
| rs1333050 | 9 | 22125914 | C/T | CDKN2B-AS1 | 4.8 kb upstream | CAD | Confirmed | 2.96E–09 | 7.65E–06 | 7.65E–06 | |
| rs7045889 | 9 | 22133252 | G/A | CDKN2B-AS1 | 12.2 kb upstream | Novel | Confirmed | 9.39E–03 | 1.19E–02 | 1.19E–02 | |
| rs7018475 | 9 | 22137686 | T/G | CDKN2B-AS1 | 16.6 kb upstream | T2D | Confirmed | 2.16E–02 | 8.90E–03 | 2.16E–02 | |
| rs579459 | 9 | 133278724 | T/C | ABO | 3.5 kb upstream | eQTL (5 hits), meQTL, metaQTL | CAD | Novel | 1.51E–02 | 8.23E–03 | 1.51E–02 |
| rs7904519 | 10 | 113014168 | A/G | TCF7L2 | Intronic | eQTL (artery), meQTL (LD to rs7077247) | T2D | Novel | 3.78E–02 | 2.97E–02 | 3.78E–02 |
| rs1169302 | 12 | 120994499 | T/G | HNF1A | Intronic | eQTL (3 hits), meQTL (LD to rs2259816) | Novel | Novel | 2.55E–02 | 2.53E–19 | 2.55E–02 |
| rs9940128 | 16 | 53766842 | G/A | FTO | Intronic | Novel | Novel | 3.58E–02 | 4.50E–02 | 4.50E–02 | |
| rs2291725 | 17 | 48961770 | T/C | GIP | Coding nonsyn | eQTL (29 hits), meQTL (LD to rs3895874) | Novel | Novel | 6.73E–05 | 2.66E–03 | 2.66E–03 |
| rs521663 | 18 | 60155007 | T/C | AC090771.2 | Non-coding intronic | Novel | Novel | 7.05E–03 | 7.20E–03 | 7.20E–03 | |
| rs10408179 | 19 | 45653746 | T/C | RN7SL836P | 1.9 kb upstream | eQTL (blood), meQTL (LD tors 10406431) | Novel | Novel | 1.14E–02 | 4.05E–35 | 1.14E–02 |
| rs4823044 | 22 | 29528836 | T/C | THOC5,AC005529.1 | Intronic, non-coding intronic | eQTL (33 hits), meQTL (LD to rs9614006) | Novel | Novel | 2.76E–02 | 4.44E–03 | 2.76E–02 |
The allele was exhibited as reference allele/alter allele; SNP type and gene type means whether identified CpG-SNPs and genes have been reponed in previous GWAS or in our previous related cFDR studies
chr chromosome, Pos chromosomal position (GRCh38/hg38), eQTL expression quantitative trait locus, meQTL methylation quantitative trait locus (including associated SNPs with an LD r2≥0.8, Table S3), metaQTL metabolic quantitative trait locus, T2D type 2 diabetes, CAD coronary artery disease, cFDR conditional false discovery rate, ccFDR conjunctional conditional false discovery rate
GO enrichment analysis and protein–protein interaction analysis
By performing GO enrichment analysis for genes that were nearest to the identified significant CpG-SNPs, we revealed that genes associated with T2D were significantly enriched in biological processes of “pancreas development” (p = 9.73 × 10−6) and “regulation of cellular ketone metabolic process” (p = 3.4 × 10−5), and genes associated with CAD were enriched in GO terms like “apolipoprotein binding” (p = 0.014) (Table 3).
Table 3.
Gene ontology (GO) lerms enriched for SNP-annotated genes with FDR ≤ 0.05
| Traits | GO terms | Term description | Gene counts | FDR |
|---|---|---|---|---|
| T2D | GO.0031016 | Pancreas development | 9 | 9.73E–06 |
| GO.0010565 | Regulation of cellular ketone metabolic process | 11 | 3.40E–05 | |
| GO.0031018 | Endocrine pancreas development | 7 | 3.40E–05 | |
| GO.0048522 | Positive regulation of cellular process | 49 | 3.40E–05 | |
| GO.0048518 | Positive regulation of biological process | 53 | 3.62E–05 | |
| CAD | GO.0034185 | Apolipoprotein binding | 3 | 1.40E–02 |
| GO.0005515 | Protein binding | 24 | 1.50E–02 | |
| Pleiotropic | GO:0043102 | Amino acid salvage | 2 | 3.51E–02 |
| GO:0071265 | l-Methionine biosynthetic process | 2 | 3.57E–02 | |
| GO:0046883 | Regulation of hormone secrction | 4 | 4.14E–02 | |
| GO:0071267 | l-Methionine salvage | 2 | 4.38E–02 |
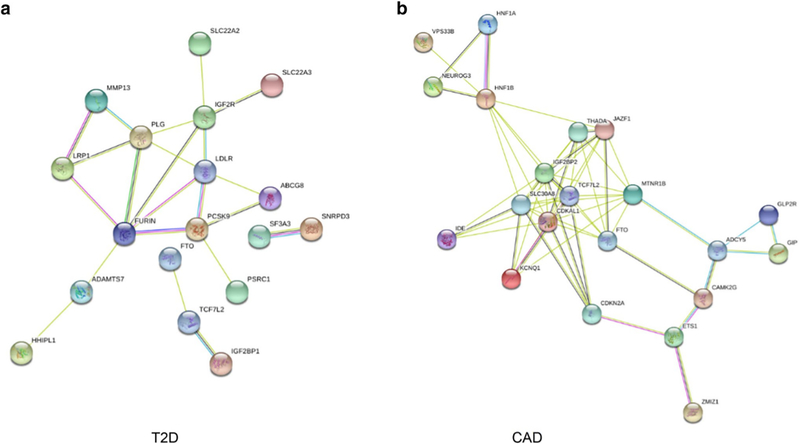
To further explore and visualize the functional partnership among identified T2D- and CAD-targeted genes, we conducted protein–protein interaction analysis with STRING 10.5 database (Fig. 3). In the protein network plot of T2D (Fig. 3a), proteins including PCSK9, LDLR, PLG, MMP13, and IGF2R, have very close contact and have been proven to have a strong relationship with diabetes (Graham et al. 2013; Chanprasertyothin et al. 2015; Eroglu et al. 2016; Ference et al. 2016; Ribeiro et al. 2016). In the protein network of CAD (Fig. 3b), proteins including MTNR1B, GLP2R, GIP, ETS1, and CAMK2G, have close contact and have been associated with CAD (Jiang et al. 2015; Winsvold et al. 2015; Amare et al. 2017; Iacobellis et al. 2017; Gong et al. 2018).
Fig. 3.
Functional protein association network analysis. Connections are based on co-expression and experimental evidence with a STRING 10.5 summary score above 0.4. Each filled node denotes a gene; edges between nodes indicate protein–protein interactions between protein products of the corresponding genes in a T2D and b CAD. Different edge colors represent the types of evidence for the association
The causal effect of T2D on CAD
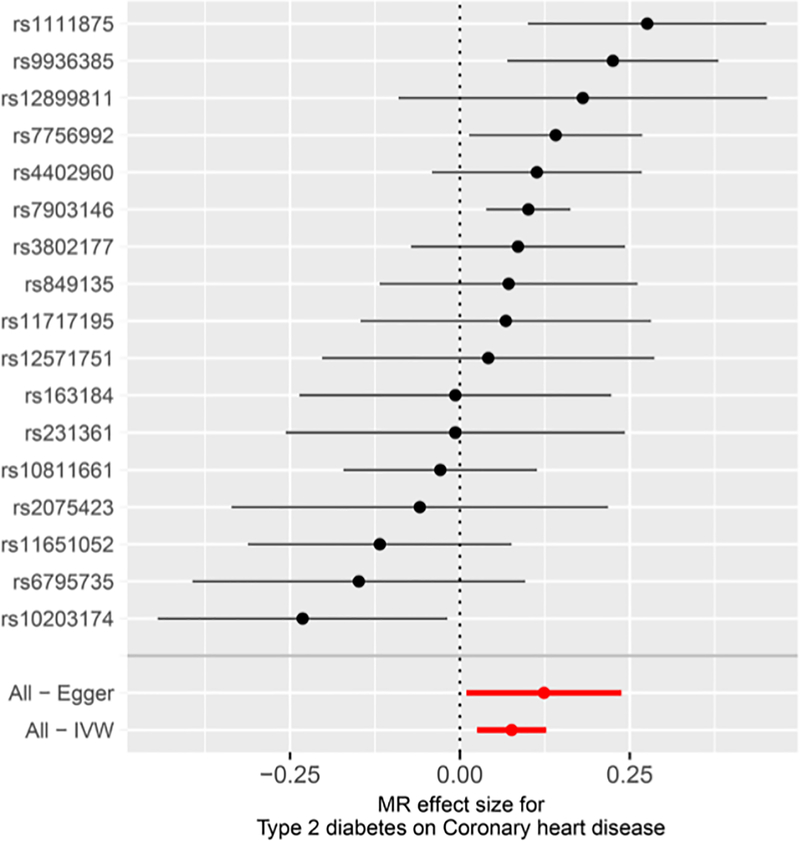
Though the causal relationship between T2D and CAD has been studied on the whole genome level (Dale et al. 2017), the key genes that contribute to such a causality remain unknown. To investigate the effect of specific genes on the causality between T2D and CAD, we applied a gene-specific two-sample MR analysis (see “Methods”) to characterize the causal effect of T2D on CAD. After gene selection, LD assessment, and data harmonization, a total of 17 T2D-related SNPs annotated to 16 different genes (including THADA, CDKN2B-AS1, MTNR1B, etc.) were selected as instrumental variables (the detailed information was summarized in Table S5). The selected instrumental SNPs showed no direct evidence of significant association (Table S5) with CAD, suggesting that these instrument variables are less likely to violate the “no horizontal pleiotropy” assumption (i.e., instrumental variables affect the outcome through a pathway other than the exposure) (Hemani et al. 2017). This was further supported by the results from the MR-Egger regression test where the estimated value for the intercept term was null for T2D and CAD (β = − 0.006, p = 0.374). These results indicated that horizontal pleiotropy does not heavily influence our results. We detected a significant causal relationship of these selected genes between T2D and CAD using MR Egger and IVW methods as main models while using simple mode, weighted median, and weighted mode as additional validations (Figs. 4, S1–S3, Table 4). The result suggests that these genes may be the key factors of the causality between T2D and CAD.
Fig. 4.
Forest plot of gene-specific MR estimates T2D on CAD. The causal effect of T2D on CAD estimated by MR analysis on 16 T2D-associated genes was expressed by IVW [β = 0.076, 95% CI (0.05, 0.09), p = 0.003] and MR Egger [β = 0.124, 95% CI (0.066, 0.154), p = 0.050]
Table4.
Genetic association between T2D and CAD by Mendelian randomization analysis on 16 T2D-associated genes
| Method | nSNP | β (95% CI) | p |
|---|---|---|---|
| MR Egger | 17 | 0.124 (0.066, 0.154) | 0.050 |
| Weighted median | 17 | 0.096 (0.067, 0.111) | 0.001 |
| Inverse variance weighted | 17 | 0.076 (0.05, 0.09) | 0.003 |
| Simple mode | 17 | 0.074 (0.019, 0.103) | 0.200 |
| Weighted mode | 17 | 0.099 (0.071,0.114) | 0.003 |
Detailed SNPs information is exhibited in Table S5
nSNP number of SNPs applied in the test, β effect size, 95% CI 95% confidence interval, p p value
Discussion
Our study represents the first targeted cFDR analysis for CpG-SNPs that are associated with both CAD and T2D. In this study, by leveraging the power of two independent GWAS datasets from T2D and CAD, and by identifying functional CpG-SNPs, we discovered 98 CpG-SNPs for T2D and 80 CpG-SNPs for CAD. Most of these genes have not been reported to be significantly associated with T2D or CAD before. By applying the ccFDR methods, we further identified 18 novel CpG-SNPs associated with both T2D and CAD.
CpG-SNPs have been suggested as an important mechanism where genetic variants can affect gene expression and function via epigenetic mechanisms (Tsuboi et al. 2017; Qiu et al. 2018). Therefore, it is necessary to analyze the genetic effects of CpG-SNPs on different levels. Under this consideration, as well as to investigate other epigenomic functions of CpG-SNPs, we analyzed eQTL, meQTL, and metaQTL effects of identified CpG-SNPs. Interestingly, we identified 10 T2D-/CAD- associated CpG-SNPs that showed eQTL, meQTL, and metaQTL effects (Table 2), suggesting that these genetic loci may play essential roles in T2D and/or CAD pathogenesis. These CpG-SNPs were annotated to ten different genes, including two novel genes DLEU1 and EIF5A2, which have not been significantly associated with either T2D or CAD in previous studies. The DLEU1 gene participates in the biological processes of circulating OCSFA (odd-numbered chain saturated fatty acids) metabolism (de Oliveira Otto et al. 2018) and can alter CDK1 expression (Wang et al. 2017a), which is related to diabetes (Zhao and Li 2019). EIF5A2 is a direct and functional target of miR-203 (Deng et al. 2016), and c-Jun/miR-203/SOCS3 signaling pathway is associated with insulin resistance (Zhou et al. 2015). In addition, we found one pleiotropic CpG-SNP, rs579459, showing meQTL, eQTL, and metaQTL effects simultaneously. rs579459 was annotated to the ABO gene, which has recently been suggested to have a critical role in metabolic disease (Suhre et al. 2011) and CAD (Chen et al. 2016). Furthermore, the metabolite (ADpSGEGDFXAEGGGVR) associated with rs579459 belongs to phosphorylated fibrinogen peptides A (FPA), which has been implicated in the pathogenesis of T2D (Yang et al. 2018). These facts suggested that rs579459 (representing ABO) may be a crucial locus in connecting pathogenesis of both T2D and CAD. We also identified several genes (SLC22A2, KCNQ1, CDKN2B-AS1, and CDKAL1) contained multiple significant CpG-SNPs, implying that multiple neigh-boring CpG-SNPs may synergistically mediate the DNA methylation and gene expression of the target genes. Interestingly, SLC22A2 only showed a suggestive association (p = 7.159 × 10−5) with CAD in previous GWAS (Higgins et al. 1996), but it reached genome-wide significance (cFDR = 1.40 × 10−11) in our study. SLC22A2 plays a significant role in lipid metabolism (Ober et al. 2009), which is an essential factor for CAD (Ma et al. 2019).
By functional annotation using GO enrichment, we identified several terms involved in the pathogenesis of T2D such as “pancreas development” and “regulation of cellular ketone metabolic process”, suggesting that the CpG-SNPs might be associated with T2D via regulation of beta-cell development in pancreas and may also regulate ketone metabolism in T2D patients. We also identified terms associated with CAD, such as “apolipoprotein binding”, indicating the regulation of apolipoprotein activity and lipid metabolism might be one of the mechanisms relating CpG-SNPs to the CAD (Pechlaner et al. 2017). Interestingly, the enriched biological functions in the pleiotropic genes were mostly related to l-methionine metabolism, which was previously found in relation to both diseases (Yin et al. 2018; Zaghloul et al. 2019). By protein–protein interaction network, we constructed functional networks for T2D and CAD. Specially, we found several key proteins that contribute to the network of both diseases, including FTO, IGF, GLP, and SLC. By the gene-specific MR analysis, we also partially conclude the causality between T2D-related genes, including FTO, IGF2BP2, and SLC30A8, implying that these genes could be the key factors in the co-regulation of T2D and CAD. Those findings shed light on the biological understanding of how CpG-SNPs co-regulate the pathogenesis of T2D and CAD.
Our study presents several advantages. First, compared with our previous cFDR studies (Lv et al. 2017; Peng et al. 2017b; Zhang et al. 2018b), by focusing on potential functional CpG-SNPs, false-negative rates of missing functional SNPs may be reduced. Second, by applying the cFDR method, the statistical power was increased because the integration of two large GWAS datasets can provide an increased effective sample size. Although the meta-analysis of GWAS data can achieve a similar purpose, it only allows for more powerful detection of loci with the same direction of allelic effects in the phenotypes. The cFDR method, on the other hand, allows for detecting loci regardless of their effect directions and has the ability to identify pleiotropic loci for both traits. Third, by integrating evidence from eQTL, meQTL, and metaQTL studies, we further prioritized a list of strong candidates of functional variants contributing to the pathogenesis of T2D and/or CAD. Inevitably, our study also has some limitations. First, we could not provide information about the effect estimates of pleiotropic loci on the phenotypes since we only had access to summary statistics but not genotypes of individual GWASs. If we could obtain the raw genotype data in the future, we will perform a linear regression for the novel SNPs and ones identified earlier, so that we could estimate how much our findings would contribute to the total heritability. Second, though the meQTL effects of CpG-SNPs were investigated, we only used the summary data from one study (Bonder et al. 2016), which will underestimate the portion of meQTL loci. And third, although we conducted the gene-specific MR method to investigate the roles of specific genes in the causal relationship between T2D and CAD, the effect of individual gene remains unknown. Therefore, we cannot determine the rank of the importance among these genes. Also, the causal effects of methylation and metabolomics on these traits are still unknown. In our future study, we will consider applying multivariable MR method into our study, which is capable of measuring multiple risk factors (Burgess and Thompson 2015).
In conclusion, we performed a targeted cFDR analysis for potential functional CpG-SNPs and detected several novel pleiotropic CpG-SNPs of potential functions for T2D and CAD. Our findings provided novel insights into mutual genetic mechanisms underlying the pathogenesis of T2D and CAD, which may lay a foundation for further biological experiments and clinical studies.
Supplementary Material
Acknowledgments
Funding This research was partially supported or benefited by grants from the National Institutes of Health (R01AR059781, P20GM109036, R01MH107354, R01MH104680, R01GM109068, R01AR069055, U19AG055373, R01AG061917), the Franklin D. Dickson/Missouri Endowment, and the Edward G. Schlieder Endowment and the Drs. W. C. Tsai and P. T. Kung Professorship in Biostatistics from Tulane University, the National Natural Science Foundation of China (Nos. 81370974, 81500056), the Natural Science Foundation of Hunan Province, China (No. 2016JJ3182), and Central South Univesity (2018zzts886).
Footnotes
Conflict of interest ZW and CQ wrote the main manuscript text; XL, YL, and XW conducted data analysis; WL, QW prepared all figures; LJZ and KL prepared supplementary information; the study was designed and supervised by HS, HWD, and SYT. All authors reviewed the manuscript. All authors have no conflicts of interest to declare.
Compliance with ethical standards
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00438-020-01651-3) contains supplementary material, which is available to authorized users.
Data availability
The sequencing data generated from the “whole-genome high-coverage deep re-sequencing study (Shen et al. 2013)” and the list of identified CpG-SNP are available from the corresponding author on request. The T2D GWAS dataset used in this study was obtained from DIAGRAM Consortium (https://diagram-consortium.org/downloads.html). The CAD GWAS dataset used in this study was obtained from CARDIoGRAM Consortium (https://www.cardiogramplusc4d.org/data-downloads/).
References
- Amare AT, Schubert KO, Klingler-Hoffmann M, Cohen-Woods S, Baune BT (2017) The genetic overlap between mood disorders and cardiometabolic diseases: a systematic review of genome wide and candidate gene studies. Transl Psychiatry 7:e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen OA, Thompson WK, Schork AJ, Ripke S, Mattingsdal M, Kelsoe JR, Kendler KS, O’Donovan MC, Rujescu D, Werge T, Sklar P, Psychiatric Genomics C, Bipolar D, Schizophrenia Working G, Roddey JC, Chen CH, McEvoy L, Desikan RS, Djurovic S, Dale AM (2013) Improved detection of common variants associated with schizophrenia and bipolar disorder using pleiotropy-informed conditional false discovery rate. PLoS Genet 9:e1003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AD (2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37:S81–S90 [DOI] [PubMed] [Google Scholar]
- Bani-Fatemi A, Goncalves VF, Zai C, de Souza R, Le Foll B, Kennedy JL, Wong AH, De Luca V (2013) Analysis of CpG SNPs in 34 genes: association test with suicide attempt in schizophrenia. Schizophr Res 147:262–268 [DOI] [PubMed] [Google Scholar]
- Bogdanovic O, Lister R (2017) DNA methylation and the preservation of cell identity. Curr Opin Genet Dev 46:9–14 [DOI] [PubMed] [Google Scholar]
- Bonder MJ, Luijk R, Zhernakova DV, Moed M, Deelen P, Vermaat M, van Iterson M, van Dijk F, van Galen M, Bot J, Slieker RC, Jhamai PM, Verbiest M, Suchiman HED, Verkerk M, van der Breggen R, van Rooij J, Lakenberg N, Arindrarto W, Kielbasa SM, Jonkers I, van’t Hof P, Nooren I, Beekman M, Deelen J, van Heemst D, Zhernakova A, Tigchelaar EF, Swertz MA, Hofman A, Uitterlinden AG, Pool R, van Dongen J, Hottenga JJ, Stehouwer CDA, van der Kallen CJH, Schalkwijk CG, van den Berg LH, van Zwet EW, Mei H, Li Y, Lemire M, Hudson TJ, Slagboom PE, Wijmenga C, Veldink JH, van Greevenbroek MMJ, van Duijn CM, Boomsma DI, Isaacs A, Jansen R, van Meurs JBJ, Hoen PAC, Franke L, Heijmans BT (2016) Disease variants alter transcription factor levels and methylation of their binding sites. Nat Genet 49:131. [DOI] [PubMed] [Google Scholar]
- Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44:512–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Thompson SG (2015) Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol 181:251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanprasertyothin S, Jongjaroenprasert W, Ongphiphadhanakul B (2015) The association of soluble IGF2R and IGF2R gene polymorphism with type 2 diabetes. J Diabetes Res 2015:216383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Yang SH, Xu H, Li JJ (2016) ABO blood group system and the coronary artery disease: an updated systematic review and meta-analysis. Sci Rep 6:23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XF, Zhu DL, Yang M, Hu WX, Duan YY, Lu BJ, Rong Y, Dong SS, Hao RH, Chen JB, Chen YX, Yao S, Thynn HN, Guo Y, Yang TL (2018) An osteoporosis risk SNP at 1p36.12 acts as an allele-specific enhancer to modulate LINC00339 expression via long-range loop formation. Am J Hum Genet 102:776–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale CE, Fatemifar G, Palmer TM, White J, Prieto-Merino D, Zabaneh D, Engmann JEL, Shah T, Wong A, Warren HR, McLachlan S, Trompet S, Moldovan M, Morris RW, Sofat R, Kumari M, Hypponen E, Jefferis BJ, Gaunt TR, Ben-Shlomo Y, Zhou A, Gentry-Maharaj A, Ryan A, Mutsert R, Noordam R, Caulfield MJ, Jukema JW, Worrall BB, Munroe PB, Menon U, Power C, Kuh D, Lawlor DA, Humphries SE, Mook-Kanamori DO, Sattar N, Kivimaki M, Price JF, Davey Smith G, Dudbridge F, Hingorani AD, Holmes MV, Casas JP (2017) Causal associations of adiposity and body fat distribution with coronary heart disease, stroke subtypes, and type 2 diabetes mellitus: a mendelian randomization analysis. Circulation 135:2373–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayeh TA, Olsson AH, Volkov P, Almgren P, Ronn T, Ling C (2013) Identification of CpG-SNPs associated with type 2 diabetes and differential DNA methylation in human pancreatic islets. Diabetologia 56:1036–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayem Ullah AZ, Oscanoa J, Wang J, Nagano A, Lemoine NR, Chelala C (2018) SNPnexus: assessing the functional relevance of genetic variation to facilitate the promise of precision medicine. Nucleic Acids Res 46:W109–w113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Otto MC, Lemaitre RN, Sun Q, King IB, Wu JHY, Manichaikul A, Rich SS, Tsai MY, Chen YD, Fornage M, Weihua G, Aslibekyan S, Irvin MR, Kabagambe EK, Arnett DK, Jensen MK, McKnight B, Psaty BM, Steffen LM, Smith CE, Riserus U, Lind L, Hu FB, Rimm EB, Siscovick DS, Mozaffarian D (2018) Genome-wide association meta-analysis of circulating odd-numbered chain saturated fatty acids: results from the CHARGE Consortium. PLoS ONE 13:e0196951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Toro-Martin J, Guenard F, Tchernof A, Deshaies Y, Perusse L, Biron S, Lescelleur O, Biertho L, Marceau S, Vohl MC (2016) A CpG-SNP located within the ARPC3 gene promoter is associated with hypertriglyceridemia in severely obese patients. Ann Nutr Metab 68:203–212 [DOI] [PubMed] [Google Scholar]
- Deng B, Wang B, Fang J, Zhu X, Cao Z, Lin Q, Zhou L, Sun X (2016) MiRNA-203 suppresses cell proliferation, migration and invasion in colorectal cancer via targeting of EIF5A2. Sci Rep 6:28301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu Z, Harman E, Vardarli E, Kayikcioglu M, Vardarli AT (2016) LDLR C1725T gene polymorphism frequency in type 2 diabetes mellitus patients with dyslipidemia. J Clin Med Res 8:793–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, Voros S, Giugliano RP, Davey Smith G, Fazio S, Sabatine MS (2016) Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 375:2144–2153 [DOI] [PubMed] [Google Scholar]
- Genomes Project C, Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, Hurles ME, McVean GA (2010) A map of human genome variation from population-scale sequencing. Nature 467:1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Qiu C, Huang D, Zhang Y, Yu S, Zeng C (2018) Integrative functional analysis of super enhancer SNPs for coronary artery disease. J Hum Genet 63:627–638 [DOI] [PubMed] [Google Scholar]
- Graham JG, Zhang X, Goodman A, Pothoven K, Houlihan J, Wang S, Gower RM, Luo X, Shea LD (2013) PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes. Tissue Eng Part A 19:1465–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlid S, Ivarsson MI, Butt S, Hussain S, Grzybowska E, Eyfjord JE, Lenner P, Forsti A, Hemminki K, Manjer J, Dillner J, Carlson J (2011) A candidate CpG SNP approach identifies a breast cancer associated ESR1-SNP. Int J Cancer 129:1689–1698 [DOI] [PubMed] [Google Scholar]
- Hemani G, Tilling K, Davey Smith G (2017) Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet 13:e1007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC (2018) The MR-Base platform supports systematic causal inference across the human phenome. Elife 7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M, Province M, Heiss G, Eckfeldt J, Ellison RC, Folsom AR, Rao DC, Sprafka JM, Williams R (1996) NHLBI Family Heart Study: objectives and design. Am J Epidemiol 143:1219–1228 [DOI] [PubMed] [Google Scholar]
- Hu Y, Tan LJ, Chen XD, Liu Z, Min SS, Zeng Q, Shen H, Deng HW (2018) Identification of novel potentially pleiotropic variants associated with osteoporosis and obesity using the cFDR method. J Clin Endocrinol Metab 103:125–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobellis G, Camarena V, Sant DW, Wang G (2017) Human epicardial fat expresses glucagon-like peptide 1 and 2 receptors genes. Horm Metab Res 49:625–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Huang S, Li X, Li X, Zhang Y, Chen ZY (2015) Tyrosine kinase receptor B protects against coronary artery disease and promotes adult vasculature integrity by regulating Ets1-mediated VE-cadherin expression. Arterioscler Thromb Vasc Biol 35:580–588 [DOI] [PubMed] [Google Scholar]
- Kannel WB (1985) Lipids, diabetes, and coronary heart disease: insights from the Framingham Study. Am Heart J 110:1100–1107 [DOI] [PubMed] [Google Scholar]
- Leitner DR, Fruhbeck G, Yumuk V, Schindler K, Micic D, Woodward E, Toplak H (2017) Obesity and type 2 diabetes: two diseases with a need for combined treatment strategies—EASO can lead the way. Obes Facts 10:483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Peng C, Greenbaum J, Li ZF, Wu KH, Ao ZX, Zhang T, Shen J, Deng HW (2018) Identifying potentially common genes between dyslipidemia and osteoporosis using novel analytical approaches. Mol Genet Genomics 293:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP (2013) Diabetes mellitus and inflammation. Curr Diab Rep 13:435–444 [DOI] [PubMed] [Google Scholar]
- Lv WQ, Zhang X, Zhang Q, He JY, Liu HM, Xia X, Fan K, Zhao Q, Shi XZ, Zhang WD, Sun CQ, Deng HW (2017) Novel common variants associated with body mass index and coronary artery disease detected using a pleiotropic cFDR method. J Mol Cell Cardiol 112:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Waldmann E, Ooi EMM, Chan DC, Barrett HPR, Watts GF, Parhofer KG (2019) Lipoprotein (a) and Low-density lipoprotein apolipoprotein B metabolism following apheresis in patients with elevated lipoprotein(a) and coronary artery disease. Eur J Clin Invest 49:e13053. [DOI] [PubMed] [Google Scholar]
- Naito R, Miyauchi K (2017) Coronary artery disease and type 2 diabetes mellitus current treatment strategies and future perspective. Int Heart J 58:475–480 [DOI] [PubMed] [Google Scholar]
- Nelson CP, Goel A, Butterworth AS, Kanoni S, Webb TR, Marouli E, Zeng L, Ntalla I, Lai FY, Hopewell JC, Giannakopoulou O, Jiang T, Hamby SE, Di Angelantonio E, Assimes TL, Bottinger EP, Chambers JC, Clarke R, Palmer CNA, Cubbon RM, Ellinor P, Ermel R, Evangelou E, Franks PW, Grace C, Gu D, Hingorani AD, Howson JMM, Ingelsson E, Kastrati A, Kessler T, Kyriakou T, Lehtimaki T, Lu X, Lu Y, Marz W, McPherson R, Metspalu A, Pujades-Rodriguez M, Ruusalepp A, Schadt EE, Schmidt AF, Sweeting MJ, Zalloua PA, Al Ghalayini K, Keavney BD, Kooner JS, Loos RJF, Patel RS, Rutter MK, Tomaszewski M, Tzoulaki I, Zeggini E, Erdmann J, Dedoussis G, Bjorkegren JLM, Consortium E-C, CardioGramplusC4D, Group UKBCCCw, Schunkert H, Farrall M, Danesh J, Samani NJ, Watkins H, Deloukas P (2017) Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat Genet 49:1385–1391 [DOI] [PubMed] [Google Scholar]
- Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, Webb TR, Zeng L, Dehghan A, Alver M, Armasu SM, Auro K, Bjonnes A, Chasman DI, Chen S, Ford I, Franceschini N, Gieger C, Grace C, Gustafsson S, Huang J, Hwang SJ, Kim YK, Kleber ME, Lau KW, Lu X, Lu Y, Lyytikainen LP, Mihailov E, Morrison AC, Pervjakova N, Qu L, Rose LM, Salfati E, Saxena R, Scholz M, Smith AV, Tikkanen E, Uitterlinden A, Yang X, Zhang W, Zhao W, de Andrade M, de Vries PS, van Zuydam NR, Anand SS, Bertram L, Beutner F, Dedoussis G, Frossard P, Gauguier D, Goodall AH, Gottesman O, Haber M, Han BG, Huang J, Jalilzadeh S, Kessler T, Konig IR, Lannfelt L, Lieb W, Lind L, Lindgren CM, Lokki ML, Magnusson PK, Mallick NH, Mehra N, Meitinger T, Memon FU, Morris AP, Nieminen MS, Pedersen NL, Peters A, Rallidis LS, Rasheed A, Samuel M, Shah SH, Sinisalo J, Stirrups KE, Trompet S, Wang L, Zaman KS, Ardissino D, Boerwinkle E, Borecki IB, Bottinger EP, Buring JE, Chambers JC, Collins R, Cupples LA, Danesh J, Demuth I, Elosua R, Epstein SE, Esko T, Feitosa MF, Franco OH, Franzosi MG, Granger CB, Gu D, Gudnason V, Hall AS, Hamsten A, Harris TB, Hazen SL, Hengstenberg C, Hofman A, Ingelsson E, Iribarren C, Jukema JW, Karhunen PJ, Kim BJ, Kooner JS, Kullo IJ, Lehtimaki T, Loos RJF, Melander O, Metspalu A, Marz W, Palmer CN, Perola M, Quertermous T, Rader DJ, Ridker PM, Ripatti S, Roberts R, Salomaa V, Sanghera DK, Schwartz SM, Seedorf U, Stewart AF, Stott DJ, Thiery J, Zalloua PA, O’Donnell CJ, Reilly MP, Assimes TL, Thompson JR, Erdmann J, Clarke R, Watkins H, Kathiresan S, McPherson R, Deloukas P, Schunkert H, Samani NJ, Farrall M (2015) A comprehensive 1000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet 47:1121–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norhammar A, Schenck-Gustafsson K (2013) Type 2 diabetes and cardiovascular disease in women. Diabetologia 56:1–9 [DOI] [PubMed] [Google Scholar]
- Ober C, Nord AS, Thompson EE, Pan L, Tan Z, Cusanovich D, Sun Y, Nicolae R, Edelstein C, Schneider DH, Billstrand C, Pfaffinger D, Phillips N, Anderson RL, Philips B, Rajagopalan R, Hatsukami TS, Rieder MJ, Heagerty PJ, Nickerson DA, Abney M, Marcovina S, Jarvik GP, Scanu AM, Nicolae DL (2009) Genome-wide association study of plasma lipoprotein(a) levels identifies multiple genes on chromosome 6q. J Lipid Res 50:798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechlaner R, Tsimikas S, Yin X, Willeit P, Baig F, Santer P, Oberhollenzer F, Egger G, Witztum JL, Alexander VJ, Willeit J, Kiechl S, Mayr M (2017) Very-low-density lipoprotein-associated apolipoproteins predict cardiovascular events and are lowered by inhibition of APOC-III. J Am Coll Cardiol 69:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei YF, Zhang L, Papasian CJ, Wang YP, Deng HW (2014) On individual genome-wide association studies and their meta-analysis. Hum Genet 133:265–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Lou HL, Liu F, Shen J, Lin X, Zeng CP, Long JR, Su KJ, Zhang L, Greenbaum J, Deng WF, Li YM, Deng HW (2017a) Enhanced identification of potential pleiotropic genetic variants for bone mineral density and breast cancer. Calcif Tissue Int 101:489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Shen J, Lin X, Su KJ, Greenbaum J, Zhu W, Lou HL, Liu F, Zeng CP, Deng WF, Deng HW (2017b) Genetic sharing with coronary artery disease identifies potential novel loci for bone mineral density. Bone 103:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Shen H, Fu X, Xu C, Deng H (2018) Meta-analysis of genome-wide association studies identifies novel functional CpG-SNPs associated with bone mineral density at lumbar spine. Int J Genom 2018:6407257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro M, Lopez de Figueroa P, Nogueira-Recalde U, Centeno A, Mendes AF, Blanco FJ, Carames B (2016) Diabetes-accelerated experimental osteoarthritis is prevented by autophagy activation. Osteoarthr Cartil 24:2116–2125 [DOI] [PubMed] [Google Scholar]
- Rosenbloom KR, Sloan CA, Malladi VS, Dreszer TR, Learned K, Kirkup VM, Wong MC, Maddren M, Fang R, Heitner SG, Lee BT, Barber GP, Harte RA, Diekhans M, Long JC, Wilder SP, Zweig AS, Karolchik D, Kuhn RM, Haussler D, Kent WJ (2013) ENCODE data in the UCSC Genome Browser: year 5 update. Nucleic Acids Res 41:D56–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RA, Scott LJ, Magi R, Marullo L, Gaulton KJ, Kaakinen M, Pervjakova N, Pers TH, Johnson AD, Eicher JD, Jackson AU, Ferreira T, Lee Y, Ma C, Steinthorsdottir V, Thorleifsson G, Qi L, Van Zuydam NR, Mahajan A, Chen H, Almgren P, Voight BF, Grallert H, Muller-Nurasyid M, Ried JS, Rayner NW, Robertson N, Karssen LC, van Leeuwen EM, Willems SM, Fuchsberger C, Kwan P, Teslovich TM, Chanda P, Li M, Lu Y, Dina C, Thuillier D, Yengo L, Jiang L, Sparso T, Kestler HA, Chheda H, Eisele L, Gustafsson S, Franberg M, Strawbridge RJ, Benediktsson R, Hreidarsson AB, Kong A, Sigurethsson G, Kerrison ND, Luan J, Liang L, Meitinger T, Roden M, Thorand B, Esko T, Mihailov E, Fox C, Liu CT, Rybin D, Isomaa B, Lyssenko V, Tuomi T, Couper DJ, Pankow JS, Grarup N, Have CT, Jorgensen ME, Jorgensen T, Linneberg A, Cornelis MC, van Dam RM, Hunter DJ, Kraft P, Sun Q, Edkins S, Owen KR, Perry JRB, Wood AR, Zeggini E, Tajes-Fernandes J, Abecasis GR, Bonnycastle LL, Chines PS, Stringham HM, Koistinen HA, Kinnunen L, Sennblad B, Muhleisen TW, Nothen MM, Pechlivanis S, Baldassarre D, Gertow K, Humphries SE, Tremoli E, Klopp N, Meyer J, Steinbach G, Wennauer R, Eriksson JG, Mnnisto S, Peltonen L, Tikkanen E, Charpentier G, Eury E, Lobbens S, Gigante B, Leander K, McLeod O, Bottinger EP, Gottesman O, Ruderfer D, Bluher M, Kovacs P, Tonjes A, Maruthur NM, Scapoli C, Erbel R, Jockel KH, Moebus S, de Faire U, Hamsten A, Stumvoll M, Deloukas P, Donnelly PJ, Frayling TM, Hattersley AT, Ripatti S, Salomaa V, Pedersen NL, Boehm BO, Bergman RN, Collins FS, Mohlke KL, Tuomilehto J, Hansen T, Pedersen O, Barroso I, Lannfelt L, Ingelsson E, Lind L, Lindgren CM, Cauchi S, Froguel P, Loos RJF, Balkau B, Boeing H, Franks PW, Barricarte Gurrea A, Palli D, van der Schouw YT, Altshuler D, Groop LC, Langenberg C, Wareham NJ, Sijbrands E, van Duijn CM, Florez JC, Meigs JB, Boerwinkle E, Gieger C, Strauch K, Metspalu A, Morris AD, Palmer CNA, Hu FB, Thorsteinsdottir U, Stefansson K, Dupuis J, Morris AP, Boehnke M, McCarthy MI, Prokopenko I, Replication DIG, Meta-analysis C (2017) An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes 66:2888–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Li J, Zhang J, Xu C, Jiang Y, Wu Z, Zhao F, Liao L, Chen J, Lin Y, Tian Q, Papasian CJ, Deng HW (2013) Comprehensive characterization of human genome variation by high coverage whole-genome sequencing of forty four Caucasians. PLoS ONE 8:e59494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker R, Deng J, Wang W, Zhang K (2010) Allele-specific methylation is prevalent and is contributed by CpG-SNPs in the human genome. Genome Res 20:883–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl EA, Wegmann D, Trynka G, Gutierrez-Achury J, Do R, Voight BF, Kraft P, Chen R, Kallberg HJ, Kurreeman FA, Kathiresan S, Wijmenga C, Gregersen PK, Alfredsson L, Siminovitch KA, Worthington J, de Bakker PI, Raychaudhuri S, Plenge RM (2012) Bayesian inference analyses of the polygenic architecture of rheumatoid arthritis. Nat Genet 44:483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wagele B, Altmaier E, Deloukas P, Erdmann J, Grundberg E, Hammond CJ, de Angelis MH, Kastenmuller G, Kottgen A, Kronenberg F, Mangino M, Meisinger C, Meitinger T, Mewes HW, Milburn MV, Prehn C, Raffler J, Ried JS, Romisch-Margl W, Samani NJ, Small KS, Wichmann HE, Zhai G, Illig T, Spector TD, Adamski J, Soranzo N, Gieger C (2011) Human metabolic individuality in biomedical and pharmaceutical research. Nature 477:54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuszkiewicz-Garcia MM, Davidson JA (2014) Cardiovascular disease in diabetes mellitus: risk factors and medical therapy. Endocrinol Metab Clin N Am 43:25–40 [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Nagatomo T, Gohno T, Higuchi T, Sasaki S, Fujiki N, Kurosumi M, Takei H, Yamaguchi Y, Niwa T, Hayashi SI (2017) Single CpG site methylation controls estrogen receptor gene transcription and correlates with hormone therapy resistance. J Steroid Biochem Mol Biol 171:209–217 [DOI] [PubMed] [Google Scholar]
- Wang LL, Sun KX, Wu DD, Xiu YL, Chen X, Chen S, Zong ZH, Sang XB, Liu Y, Zhao Y (2017a) DLEU1 contributes to ovarian carcinoma tumourigenesis and development by interacting with miR-490–3p and altering CDK1 expression. J Cell Mol Med 21:3055–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Lin X, Li DY, Zhou R, Greenbaum J, Chen YC, Zeng CP, Peng LP, Wu KH, Ao ZX, Lu JM, Guo YF, Shen J, Deng HW (2017b) Linking Alzheimer’s disease and type 2 diabetes: novel shared susceptibility genes detected by cFDR approach. J Neurol Sci 380:262–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsvold BS, Nelson CP, Malik R, Gormley P, Anttila V, Vander Heiden J, Elliott KS, Jacobsen LM, Palta P, Amin N, de Vries B, Hamalainen E, Freilinger T, Ikram MA, Kessler T, Koiranen M, Ligthart L, McMahon G, Pedersen LM, Willenborg C, Won HH, Olesen J, Artto V, Assimes TL, Blankenberg S, Boomsma DI, Cherkas L, Davey Smith G, Epstein SE, Erdmann J, Ferrari MD, Gobel H, Hall AS, Jarvelin MR, Kallela M, Kaprio J, Kathiresan S, Lehtimaki T, McPherson R, Marz W, Nyholt DR, O’Donnell CJ, Quaye L, Rader DJ, Raitakari O, Roberts R, Schunkert H, Schurks M, Stewart AF, Terwindt GM, Thorsteinsdottir U, van den Maagdenberg AM, van Duijn C, Wessman M, Kurth T, Kubisch C, Dichgans M, Chasman DI, Cotsapas C, Zwart JA, Samani NJ, Palotie A (2015) Genetic analysis for a shared biological basis between migraine and coronary artery disease. Neurol Genet 1:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SS, Chang YJ, Zhang H, Yu X, Shang W, Chen GQ, Chen DDY, Gu ZY (2018) Enrichment of phosphorylated peptides with metalorganic framework nanosheets for serum profiling of diabetes and phosphoproteomics analysis. Anal Chem 90:13796–13805 [DOI] [PubMed] [Google Scholar]
- Yekutieli D, Benjamini Y (1999) Resampling-based false discovery rate controlling multiple test procedures for correlated test statistics. J Stat Plan Inference 82:171–196 [Google Scholar]
- Yin J, Ren W, Chen S, Li Y, Han H, Gao J, Liu G, Wu X, Li T, Woo Kim S, Yin Y (2018) Metabolic regulation of methionine restriction in diabetes. Mol Nutr Food Res 62:e1700951. [DOI] [PubMed] [Google Scholar]
- Zaghloul A, Iorgoveanu C, Desai A, Balakumaran K, Chen K (2019) Methylenetetrahydrofolate reductase polymorphism and premature coronary artery disease. Cureus 11:e5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng CP, Chen YC, Lin X, Greenbaum J, Chen YP, Peng C, Wang XF, Zhou R, Deng WM, Shen J, Deng HW (2017) Increased identification of novel variants in type 2 diabetes, birth weight and their pleiotropic loci. J Diabetes 9:898–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Greenbaum J, Zhang W-D, Sun C-Q, Deng H-W (2018a) Age at menarche and osteoporosis: a Mendelian randomization study. Bone 117:91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Liu HM, Lv WQ, He JY, Xia X, Zhang WD, Deng HW, Sun CQ (2018b) Additional common variants associated with type 2 diabetes and coronary artery disease detected using a pleiotropic cFDR method. J Diabetes Complic 32:1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Li W (2019) Gene coexpression network analysis identified potential biomarkers in gestational diabetes mellitus progression. Mol Genet Genomic Med 7:e00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi D, Aslibekyan S, Irvin MR, Claas SA, Borecki IB, Ordovas JM, Absher DM, Arnett DK (2013) SNPs located at CpG sites modulate genome-epigenome interaction. Epigenetics 8:802–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Liu W, Gu M, Zhou H, Zhang G (2015) Helicobacter pylori infection causes hepatic insulin resistance by the c-Jun/miR-203/SOCS3 signaling pathway. J Gastroenterol 50:1027–1040 [DOI] [PubMed] [Google Scholar]
- Zhou R, Lin X, Li DY, Wang XF, Greenbaum J, Chen YC, Zeng CP, Lu JM, Ao ZX, Peng LP, Bai XC, Shen J, Deng HW (2017) Identification of novel genetic loci for osteoporosis and/or rheumatoid arthritis using cFDR approach. PLoS ONE 12:e0183842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data generated from the “whole-genome high-coverage deep re-sequencing study (Shen et al. 2013)” and the list of identified CpG-SNP are available from the corresponding author on request. The T2D GWAS dataset used in this study was obtained from DIAGRAM Consortium (https://diagram-consortium.org/downloads.html). The CAD GWAS dataset used in this study was obtained from CARDIoGRAM Consortium (https://www.cardiogramplusc4d.org/data-downloads/).