Abstract
Background:
Factors underlying physiological reactions from perceived discrimination and its relation to adverse health outcomes are not completely understood. The main purpose of this study was to test the hypothesis that experiences of discrimination (recent and lifetime) correlate with biomarkers of stress, oxidative stress, and obesity among adult females.
Methods:
Data on 62 females who self-identify as African American (AA; n=31) or European American (EA; n=31) aged 21-45 years were included. Discrimination experiences (recent and lifetime) were evaluated based on a validated instrument. Stress was assessed based on hair (HC) and salivary cortisol (SC), hsC-reactive protein (hsCRP), cardiovascular markers, and LDL-cholesterol oxidation. Obesity was measured based on BMI, waist circumference, and body fat percent. Multiple linear regression analyses were performed to evaluate the influence of experiences of discrimination.
Results:
Significant differences in experiences of discrimination were observed by race (p<0.05) and were higher in AA females. Results for the multiple regression models assessing the contribution of discrimination indicate that hsCRP and pulse were significantly associated with recent experiences of discrimination, and SC, HC, hsCRP, diastolic blood pressure (DBP), and pulse were significantly associated with lifetime experiences of discrimination when adjusted for BMI and race (p<0.05). Finally, oxidation of LDL-cholesterol was significantly associated with salivary cortisol (p=0.0420) when adjusted by lifetime experiences of discrimination (p=0.0366) but not for BMI (p=0.6252).
Conclusion:
In this cross-sectional study, AA females experienced more discrimination compared to EA females. Levels of recent and lifetime experiences of discrimination were associated with some stress biomarkers. Salivary cortisol was associated with oxidation of LDL-cholesterol with shorter lag times and increased risk for cardiovascular disease.
Keywords: Discrimination, stress, health disparities, cortisol, inflammation, oxidative stress
Introduction
Racial health disparities have been ubiquitously documented in the United States with a higher prevalence of cardiovascular disease (CVD) risk in African Americans. Reports have shown greater prevalence of cardiovascular disease including obesity, stroke, diabetes, and hypertension in this population and they are more likely to suffer from four or more chronic conditions compared to whites [1, 2]. These differences are more pronounced by gender, with black women having significantly higher prevalence of obesity (54.8%) than white women (38%) [2]. There is limited understanding on the etiology of why the burden of CVD risk factors remains highest for black women compared to other gender and racial groups[3]. However, many have postulated that racial disparity in chronic prevalence in African Americans may partially result from greater chronic psychosocial ‘life’ stress, as this population is subject to repeated daily micro stressors including higher rates of poverty, social disadvantages [4], and discrimination [5]. In a world where resources are not distributed equally, the contribution of psychosocial ‘life’ stress to the health of individuals is an area deserving scientific investigation.
The way in which stressors of equal harmfulness can elicit different health-related responses among people is an area of intense scientific interest. Research has demonstrated how upstream instigating and exacerbating social and environmental factors as well as downstream health behaviors and chronic disease development factors can interact with psychosocial “life” stress to potentially account for worse health outcomes and increased disease risk in different groups [6, 7]. A psychosocial ‘life’ stress of interest among health disparities research today is discrimination, which has been reported highest among people of color, socially disadvantaged populations, and in the Midwest and southern region of the United States [8]. Since the passing of Civil Rights Act of 1964 in the U.S., little progress has been made to eliminate mistreatment of individuals based on race, color, religion, sex, or national origin [9]. Subtle ways of individual discrimination against someone based on their age, race, or gender persist as daily stressful experiences among groups. Validated psychosocial instruments facilitate quantitative evaluation of both acute and chronic discrimination and their influence on one’s psychological and physiological health [10, 11].
It has been established that the actual and/or imagined mental, physical or emotional demands of stress can result in diverse inadequate or harmful physiological responses [12]. Chronic exposure to a stressor, such as discrimination, erodes adaptive, behavioral and physiological responses over time, worsening the health of the population[13]. Physiological systems that are involved after an exposure to stress include the hypothalamic- pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS), where increased exposure to discrimination may alter cortisol levels and increase circulating inflammatory markers, leading to chronic low-grade inflammation and adverse health consequences[14, 15].
Exposure to discrimination has gained more attention as a contributor to health disparities as it appears to be associated with cumulative stress[16], higher serum cortisol, and higher levels of oxidation[17, 18]. Among a multiethnic sample of women, everyday discrimination was also found to increase risk of early coronary calcification (atherosclerosis) and metabolic syndrome risk factors such as elevated blood pressure and triglycerides [19, 20]. Other toxic forms of stress, such as childhood adversity, work-related stress, low socioeconomic status, and depression have been associated with low-grade inflammation and diseases such as hypertension[21], depression [15], metabolic syndrome[22], and obesity [23]. A gap exists in our understanding of the direct effects of discrimination on our stress response systems, cardiovascular risk factors, and chronic disease disparities.
Discrimination and its relationship with stress, obesity, and oxidative stress is not clear. Therefore, the main purpose of this study was to test the hypothesis that experiences of discrimination (recent and lifetime) correlate with biomarkers of stress, obesity, and oxidative stress among African American (AA) and European American (EA) females. Specifically, the aim of the present study was to examine first, the differences in recent and lifetime experiences of discrimination and physiological biomarkers of stress by race (AA vs. EA). The second aim was to evaluate recent and lifetime experiences of discrimination with stress biomarkers, race, and obesity. Finally, the third aim was to examine if physiological biomarkers of stress were associated with oxidative stress, which underlies cardiovascular risk. We hypothesized that AA females would exhibit higher discrimination in comparison to EA females and that the exposure to higher levels of discrimination would be positively associated with biomarkers of stress and higher oxidative stress. Identifying the physiological and cardiovascular effects of psychosocial stressors, such as stress responses caused by chronic experiences of discrimination, are essential to addressing the current health disparities among African American women.
Methodology
Participants
Participants were 62 females from Alabama aged 21-45 years, who self-identify as African American (AA; n=31) or European American (EA; n=31) from Alabama. Participant inclusion criteria included body mass index (BMI) between 19-46 kg/m2, and participants had to be sedentary or moderately active (less than two hours per week of exercise). The exercise criteria were based on previous evidence in which exercise appears to influence the stress hormones such as cortisol and would confound the study[24, 25]. Participants were recruited through multilevel marketing of University of Alabama at Birmingham (UAB) website, flyers, word of mouth, and social media (Facebook). The recruitment started in August 2014, and finished in April 2016 and the second visit was according to the availability of the participants within a window of no more to 30 days. Participants had no medical diagnosis or medication contraindicated for study participation (i.e. no one was taking diuretics, beta-blockers, calcium channel blockers, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, and others hypertension drugs) or medication known to affect body composition and metabolism. To ensure no overrepresentation and bias, recruitment was stopped once 31 females of each race (AA and EA) were enrolled.
Protocol
Data were collected during two visits. At the first visit, an interview was performed to collect information about the experiences of discrimination, and anthropometric measures, hair, and saliva samples were collected. At the second visit, blood samples were collected. All sample collections and analyses were conducted in the Core Laboratory of UAB’s, Center for Clinical and Translational Science Clinical (CCTS) Research Unit (CRU), Diabetes Research Center’s BioAnalytical Redox Biology (BARB) Core, and Nutrition and Obesity Research Center at the Department of Nutrition Sciences. The Institutional Review Board (IRB) at UAB approved this study, and the participants provided written informed consent prior to their inclusion in the study.
Blood samples
An intravenous catheter was placed to obtain blood samples and plasma was immediately collected after centrifugation at 3000 rpm for 15 minutes. Serum tubes sat at room temperature for 15 minutes to allow clotting. Serum was collected by centrifugation at 3000 rpm for 15 minutes. For low-density lipoproteins cholesterol (LDL-cholesterol) measurements, 4mL of plasma was collected. All remaining whole blood, plasma and serum were aliquotted and immediately snap frozen in liquid nitrogen for future analyses. Insulin, glucose, hsC-reactive protein (hsCRP), cholesterol, and LDL-cholesterol oxidation were measured.
Dependent variables
Stress biomarkers
Stress was assessed based on hair (HC) and salivary cortisol (SC), hsCRP, cardiovascular markers (blood pressure, pulse, insulin, and total cholesterol), and LDL-cholesterol oxidation expressed in lag time.
Hair cortisol
Long-term cortisol levels were evaluated in hair as a measure of chronic stress. Hair sample of approximately 6mm (standard pencil diameter) were cut from the vertex posterior as close to the scalp as possible. The sample was cut to three centimeters (cm) in length, reflecting roughly the prior three months, since hair is reported to grow ~1 cm/month[26]. Hair was cut and stored independently until processed, using a slightly modified procedure as described by Meyer et al[26]. Around 25-50mg of hair was washed three times with isopropanol, air dried for three days, weighed, and stored at −80°C until analysis. The dried hair samples were added to a pre weighed tube containing three 5mm steel grinding balls (Retsch) and ground using a TissueLyser II (Qiagen Venlo, Netherlands) at 25 Hz for 7 minutes. Cortisol was organically extracted using 1.5ml of methanol added to the powdered hair on using a 360 degree rotator for 18 hours at room temperature. Samples were then centrifuged at 10,000g for 5 minutes. The supernatant, 1 ml, was transferred to a clean tube and evaporated under nitrogen gas in a certified fume hood. The evaporated sample was resuspended in 400ul of phosphate buffered saline. Cortisol was assayed using 25ul of sample according to manufacturer’s protocol (Salimetrics, Salivary Cortisol Enzyme Immunoassay Kit, 1-3002, State College, PA). Data was analyzed using StatLIA Enterprise 2.2 software, and it is represented as nmol/g for hair cortisol[27].
Sample size for the HC was 31 participants for each race (AA and EA). To measure the influence of cortisol on the oxidation of cholesterol LDL in lag time (min), hair cortisol was dichotomized into low and high groups. While hair cortisol is considered a novel measurement of long term retrospective cortisol secretion, there are not well-established cutoff points[28]. Furthermore, it appears that hair cortisol may be influenced by age, hair color, body composition, pregnancy, environmental exposures, and others[27, 29]. Therefore, in this study, the sample-based percentiles were used to determine the cutoff points of low and high levels. The use of percentiles has been previously used in the literature such as in the measure of allostatic load (measure of chronic stress)[30]. For hair cortisol, high levels were set at ≥0.0040 nmol/g and low levels were set at <0.0040 nmol/g based on the sample-based percentiles (25th for low levels).
Salivary cortisol
Salivary cortisol was used as a measurement of acute stress. The saliva was collected by having the participant expectorate 5 mL into a sterile collection tube over a time period of 10-30 minutes in the morning. Collection began at a minimum of 60 minutes from their last meal. All participants thoroughly rinsed their mouth with water right before collection began. Saliva was aliquoted to 0.5mL tubes and frozen at −80°C until analysis. Preparation and assay of saliva samples was done in accordance with manufacture’s instruction (High Sensitivity Salivary Cortisol Enzyme Immunoassay kit, Salimetrics, State College, PA, USA). Data was analyzed using StatLIA Enterprise 2.2 software, and it is represented as μg/dL for salivary cortisol. To measure the influence of cortisol on the oxidation of cholesterol LDL expressed in lag time (min), salivary cortisol was dichotomized into low and high groups. Although salivary cortisol is more prevelant in epidemiological research, many confounding variables have been noted to affect this measure, such as health behaviors, race, education, and others[31]. Also, some of the cutoff points described in the literature have been established for the diagnosis of some syndromes[32]. Therefore, for this study, sample-base percentiles were used to establish the cutoff points for lower levels to keep consistency with the methodology. For salivary cortisol, high levels were set at ≥0.13 μg/dL and low levels were set at <0.13 μg/dL based on the sample-based percentile (25th for low levels). Sample size for the SC was 31 participants for each race (AA and EA).
Serum cortisol
Serum cortisol (SeC) samples require no preparation and were assayed using 25ul according to manufacturer’s instruction (Salimetrics, Salivary Cortisol Enzyme Immunoassay Kit, 1-3002, State College, PA). Data was analyzed using StatLIA Enterprise 2.2 software, and it is represented as μg/dL for serum cortisol. Sample size for the SeC was 21 participants for each race (AA and EA).
hsC- reactive protein
hsCRP was assayed with the high-sensitivity ELISA kit (030–9710s, ALPCO, Windham, NH)[33]. hsCRP was assayed with the Stanbio Sirrus using a turbidimetric procedure. Mean sensitivity is 0.50 mg/L and interassay CV is 8.9%[34]. Sample size for the hsCRP was 20 participants for each race (AA and EA).
Cardiovascular markers
Cardiovascular measures included blood pressure, pulse rate, glucose, insulin, and LDL-cholesterol. Blood pressure (both systolic blood pressure, SBP and diastolic blood pressure, DBP) measurements were performed using non-invasive, local pulse contour analysis (Dinamap Pro 200 automated cuff, GE Medical Systems). Participants were seated and remained quiet during the testing procedure. An adult-sized blood pressure cuff was placed around the non-dominant arm. The sensor was adjusted as needed to achieve the highest relative signal strength. Pulse rate was measured using non-invasive pulse wave analysis (HDI/Pulse Wave TM CR-2000, Hypertension Diagnostics, Eagan, MN). Blood samples were obtained from participants using a standard, 75 g, 2-h, 8-sample oral glucose tolerance test (OGTT). Glucose was measured using a Stanbio SIRRUS analyzer. Insulin was measured using a TOSOH immunoassay analyzer. Insulin sensitivity was calculated using the insulin and glucose serum concentrations across the test in conjunction with the arithmetic calculation validated by Matsuda and DeFronzo[35]. Lipoproteins for LDL were calculated as described by Chung et al[36]. (Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor) with modifications, a discontinuous NaCl/KBr density gradient was used in zero time point plasma for isolation of lipoproteins for LDL. A density gradient was formed by adjusting the density of plasma to 1.2g/mL with KBr and under-laying plasma below Tris buffered saline. Samples were immediately placed in vertical rotors and centrifuged in a Sorvall L160 Ultracentrifuge at 10°C, 50,000 rpm for 2.5 hours. Low-density lipoproteins, which appears as an orange band in the center of the tube were removed using a 21-gauge syringe needle and 1 ml sterile syringe and added to an Amicon Ultra centrifugal filter (Merek Millipore) and centrifuged at 9,000 rpm for 15 minutes to concentrate the LDL. The LDL was then collected, added back to the filter, filled with phosphate buffered saline (PBS) and 10 μM Diethylenetriamine-pentaacetic acid (DTPA; ACROS) to rinse and remove excess KBr and centrifuged at 9,000 rpm for 15 minutes. Endpoint cholesterol levels were determined from zero time point plasma using cholesterol standard and reagent in accordance with manufactures instruction. (Pointe Scientific, Canton, MI). Sample size for SBP, DBP, pulse rate, insulin and glucose and LDL-cholesterol were 21 participants for each race (AA and EA).
LDL-cholesterol oxidation
The LDL oxidation kinetics expressed in lag time (minutes), which is an indicator for the susceptibility of the LDL to oxidation, atherogenic potential and cardiovascular risk, were measured by following the conjugated diene formation using a spectrophotometer. A total of 50 μg/ml of LDL in PBS was added to quartz cuvettes, CuSO4 was added to give a final copper concentration of 5 μM and 0.5 μM DTPA. Measurement of conjugated diene formation at 234 nm at 5-minute intervals for 11.6 hours was started immediately after addition of copper. Shorter lag times indicate a greater susceptibility to oxidation of LDL and potential formation and propagation of oxidized LDL, increased atherogenic potential and cardiovascular risk. Sample size for LDL-cholesterol oxidation were 21 participants for each race (AA and EA).
Independent variables
Recent and Lifetime Experiences of Discrimination
A scale developed and validated by Shariff-Marco et al[10] was used to measurediscrimination. During the interview, the participants were in a comfortable and private place in which one person of the same race and gender administered the questions, and they were not asked to explain or give details regarding their responses. The scale captures four dimensions of discrimination 1) frequency of encounters with discrimination across several domains (e.g. medical care, school, work, stress and other public places); 2) timing of exposure (e.g., recent, lifetime); 3) appraisal of discrimination as stressful; and 4) responses to discrimination[10]. This survey includes two approaches; the first consists of early attribution (ask specifically about discrimination based on race/ethnicity) and the second included late attribution (ask about unfair treatment, then ask about the reasons for this unfair treatment).
The recent experiences of discrimination included experiences in the past 12 months and were divided into three sections. The first section included eight questions of “… how often have any of the following things happened to you… treated with less respect at restaurants, criticized accent or speech, people think you are not smart, people are afraid of you, people think you are dishonest, people think they are better than you, you feel threatened/harassed.” The answers to the first section included: never=0, rarely=1, sometimes=2, or often=3 (with a total maximum of 24). The second section included six questions about why you may have been treated unfairly because “… ancestry or national origin, gender or sex, or skin color, age, way you speak English, and other reason”. The answers of the second section included: yes=1 or no =0 (with a total maximum of 6). The third section included one question of “…how stressful have these experiences of unfair treatment usually been for you…” The answers of the last section included: not at all stressful=0, a little stressful=1, somewhat stressful=2, or extremely stressful=3 (with a total maximum of 3).
The lifetime experiences of discrimination included experiences during the entire lifetime and are also divided in three parts, the first section included five questions of “…how often have you been treated unfairly at… school, work, medical care, or by the police and the courts, or in other situations…” The answers of the first section included: never=0, rarely=1, sometimes=2, or often=3 (with a total maximum of 15). The second section included six questions about the reasons for discrimination “…because of your ancestry, gender or sex, race or skin color, age, the way speak English, or some other reason…” The answers of the second section included: yes=1 or no =0 (with a total maximum of 6). The last part included one question of “…how stressful have these experiences of unfair treatment usually been for you…” The answers of the last section included: not at all stressful=0, a little stressful=1, somewhat stressful=2, or extremely stressful=3 (with a total maximum of 3). Total lifetime discrimination scores (0-24) reflect the answers to the 12 questions; higher scores indicated higher lifetime discrimination. The analysis of this scale was by total score and by section (unfair treatment by place, reasons of being treated unfairly, and stress from unfair treatment.)
Obesity measurements
Obesity was measured based on body mass index (BMI), waist circumference (WC), and body fat percent (BF%). The body mass index (BMI) values were calculated for participants using height in meters (m), and weight in kilograms (kg) as follow: weight (kg)/height2 (m). Participants were weighed (Scale-tronix 6702W; Scale-tronix, Carol Stream, IL) to the nearest 0.1 kg (in minimal clothing without shoes). The measure of height was recorded without shoes using a digital stadiometer (Heightronic 235; Measurement Concepts, Snoqualmie, WA). BMI was categorized as underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5-24.99 kg/m2), overweight (BMI 25-29.99 kg/m2), and obese (BMI >30 kg/m2). Waist circumference in cm was measured at the narrowest part of the torso or the area between the ribs and iliac crest. Waist circumference measures were obtained using a flexible tape measure (Gulick II; Country Technology, Inc., Gays Mills, WI) and were recorded to the nearest 0.1 cm. The estimation of body fat percent was based on measurements of four site skinfolds (biceps, subscapular, suprailiac, and triceps) procedure described by Durnin and Womersley[37].
Covariates
Consistent with previous research, age, income, marital status, and education were included in the statistical models as factors that could influence health outcomes. Age was considered as a continuous variable. Income, marital status, and education were included as a categorical variables. The income variable was collapsed in two categories (<$20,000 and ≥20,000). Marital status was classified in three categories (divorced, never married, and married), and education was classified in 5 categories (partial high school, high school graduate, partial college, standard college graduate, and graduate professional training.) The testing of our hypotheses were based on the identification of the most parsimonious statistical models balancing statistical, physiological, and racial differences.
Statistical analysis
Descriptive statistics (mean, standard deviation (SD), and frequencies) were calculated to summarize age, marital status, income, education, body composition, and lifetime experiences of discrimination by race. Differences for absolute values in demographic characteristics, recent and lifetime experiences of discrimination, SC, and HC between AA and EA were analyzed using t and chi-squared tests. Differences by race for SBP, DBP, pulse, IR, hsCRP, SeC, and LDL-cholesterol between AA and EA were analyzed using Kruskal-Wallis test. Simple correlations were performed for body composition measurements (BMI, body fat percent, and waist circumference) and the dependent variables. The three body composition measurements BMI, WC, and BF%, showed similar correlation coefficients with the dependent variables, and BMI measure was chosen for the regression models. Multiple linear regression analyses were performed to evaluate the influence of recent and lifetime experiences of discrimination on biomarkers of stress, race, and BMI, and to evaluate the influence of stress biomarkers influence on oxidation of LDL-cholesterol in lag time. Missing values were treated by complete case analysis, in which missing values were excluded from the analysis.
The dependent variables included SBP, DBP, pulse rate, IR, LDL-Cholesterol, hsCRP, salivary cortisol (SC), Hair Cortisol (HC), Serum Cortisol (SeC), and oxidation of LDL-cholesterol in lag time. Independent variables included age, race, BMI, education, recent, and lifetime experiences of discrimination. All residuals were tested for normality after visual evaluation of residuals from the regression models. Power analysis were performed in all regression models using the R-square of each model and the predictors. The significance level was considered α=0.05 for all statistical analyses. All analyses were performed with SAS statistical software (version 9.4, 2002-2012 by SAS Institute Inc., Cary, NC).
Results
Demographic characteristics are summarized in Table 1 of the sample by race. From the total sample (n=62), females ages 21 to 45 years were included. The majority of females reported never being married, an annual income ≥$20,000, and a level of education higher than college. Significant differences were observed for BMI, waist circumference, body fat percent, and lifetime experiences of discrimination (p<0.0001). A total of 58% (n=18) of AA and 29% (n=9) of EA females were classified with obesity according to their BMI (>30 kg/m2).
Table 1.
Descriptive characteristics in a multiethnic sample of women by race
| Variables | African Americans |
European Americans |
|---|---|---|
| Percentage (n) | ||
| Marital status | ||
| Divorce | 16.13% (n= 5) | 6.45% (n= 2) |
| Never married | 61.29% (n=19) | 67.74% (n=21) |
| Married | 22.58% (n= 7) | 25.81% (n= 8) |
| Reported annual income | ||
| <$20,000 | 27.59% (n= 8) | 16.67 (n= 5) |
| ≥$20,000 | 72.41% (n=21) | 83.33 (n=25) |
| Education | ||
| Partial High School | 3.23% (n= 1) | NA |
| High School Graduate | 12.90% (n= 4) | 6.45% (n= 2) |
| Partial College | 38.71% (n=12) | 32.26% (n=10) |
| Standard College Graduate | 32.26% (n=10) | 25.81% (n= 8) |
| Graduate Professional Training | 12.90% (n= 4) | 35.48% (n=11) |
| Mean ± SD | ||
| Age (years) | 29.54±7.84 | 28.83±7.28 |
| Body composition | ||
| Body Mass Index (BMI) (Kg/m2) | 32.74± 7.52* | 27.29± 6.92 |
| Waist Circumference (WC) (cm) | 96.75±16.32* | 86.41±17.71 |
| Body fat percentage (BF%) (%) | 38.50± 5.85* | 33.90± 6.16 |
| Recent Experiences of Discrimination (total score) | 11.10±5.68 | 8.35±6.05 |
| Recent Experiences of Discrimination (by sections) | ||
| In the past 12 months… | ||
| Section 1. Treated with less respect (at restaurants, criticized accent or speech, people think not smart, people afraid of you, people think dishonest, people think better than you, threatened/harassed.) | 6.93±4.25 | 5.93±4.59 |
| Section 2. Treated unfairly because… (ancestry or national origin, gender or sex, or skin color, age, way you speak English, and other reason) | 2.24±1.82* | 0.93±1.15 |
| Section 3. Stress from unfair treatment (not at all stressful, a little stressful, somewhat stressful, or extremely stressful.) | 1.32±0.86 | 1.30±0.92 |
| Lifetime Experiences of Discrimination (total score) | 9.61±3.96* | 6.65±3.48 |
| Lifetime Experiences of Discrimination (by sections) | ||
| Section 1. Unfair treatment by place (school, work, medical care, police and the courts, and other situations.) | 4.03±2.81 | 3.06±2.46 |
| Section 2. Reasons of being treated unfairly (ancestry, gender or sex, race or skin color, age, the way speak English, or some other reason.) | 3.18±1.86* | 1.53±1.30 |
| Section 3. Stress from unfair treatment (not at all stressful, a little stressful, somewhat stressful, or extremely stressful.) | 1.73±0.72 | 1.43±0.94 |
| Total discrimination scores (recent and lifetime) | 21.00±8.63* | 14.73±9.06 |
NA, not available. Significant results are based on absolute values. T and chi-squared tests were performed to assess mean differences among African Americans (n=31) and European Americans (n=31) females. All differences were significant at
p <0.05.
Recent experiences of discrimination included a total of 15 questions divided into three sections. The first section included eight questions with score 0-3 (with a maximum 24), the second section included six questions scores 0-1 (with a maximum of 6), and the third section included one question with scores 0-3 (with a maximum of 3).
Lifetime experiences of discrimination included a total of 12 questions divided into three sections. The first section included five questions with score 0-3 (with a maximum of 15), the second section included six questions scores 0-1 (with a maximum of 6), and the third section contains one question with score 0-3 (with a maximum of 3).
Recent and lifetime experiences of discrimination and biomarkers of stress by race
Significant differences of recent and lifetime discrimination, by race (AA vs. EA) (Table 1) were revealed. Recent experiences of discrimination were not statistically significantly different by race. The analysis by section showed no significant differences for those being treated with less respect (first section) at restaurants, criticized by their accent or speech, people thinking they were not smart, people being afraid of you, people thinking you are dishonest, people thinking they are better than you, or feeling threatened/harassed by race. However, the unfair treatment did reveal racial differences (second section), where AA had significantly higher scores compared to EA females [t(1,46)=3.29, p=0.0019]. The reasons for unfair treatment included ancestry or national origin, gender or sex, or skin color, age, way you speak English, and other reasons. The main reason for unfair treatment among AA females included ancestry or national origin (18.33%, n=11), skin color (21.67%, n=13), age (16.67%, n=10), and among EA females included their sex/gender (21.67%, n=13). There were no significant differences from unfair treatment by race, but the majority of females who reported unfair treatment due to discrimination, said it was little stressful (22.92%, n=11 among AA, and 16.67%, n=8 among EA).
African American females reported higher lifetime experiences of discrimination and were statistically significantly different compared to EA females [t(1,47)=2.76, p=0.0081]. No significant racial differences for being unfairly treated in a particular location such as at school, at work, when getting medical care, with the police at the courts, and in other situations were found. Significant differences were observed by race [t(1,46)=3.84, p=0.0004] for the reasons behind the unfair treatment. The reasons for discrimination included national origin, sex/gender, skin color, the way they speak English, and other reasons. The main reason for discrimination among AA females included the combination of race and skin color (21.31%, n=13), and among the EA females included their sex/gender (14.75%, n=9). The stress felt from unfair treatment, section 3, showed no significant differences by race. However, the majority of females mentioned that their perception and evaluation of stress due to unfair treatment associated with discrimination was somewhat stressful (34.69%, n=17 among AA, and 16.33%, n=8 among EA).
Evaluation of the physiological biomarkers of stress by race revealed that AA females exhibited significantly higher levels of SBP, pulse, and HC (p<0.05) (Table 2). Although lag time (an indicator of the susceptibility of LDL to increased oxidation and atherogenic potential) was not significantly different by race; after outliers were removed (based on ± 3 SD) the lag time was significantly decreased in AA females, indicating an increased atherogenic potential and CVD risk (AA: 63.38±20.73 vs. EA 86.05±34.89, p=0.0403).
Table 2.
Cardiovascular, inflammatory, and oxidative cholesterol-LDL lag time markers in a multiethnic sample of females by race
| Variables | African American | European American |
|---|---|---|
| Mean ± SD | ||
| Cardiovascular markers | ||
| Systolic Blood Pressure (SBP) (mm Hg) | 121.35±11.72* | 113.86±13.59 |
| Diastolic Blood Pressure (DBP) (mm Hg) | 69.15±10.75 | 68.31± 9.10 |
| Pulse (beats per minute) | 78.10±12.44* | 70.72±11.02 |
| Insulin Resistance (IR) (Matsuda Index) | 4.16± 2.31 | 5.33± 3.05 |
| LDL-Cholesterol (mg/dL) | 155.88±37.61 | 155.27±31.69 |
| Inflammatory markers | ||
| High sensitivity C-reactive protein (hsCRP) | 7.11±7.64 | 3.53±4.27 |
| Stress hormones | ||
| Salivary cortisol (SC) (μg/dL) | 0.21±0.14 | 0.17±0.10 |
| Hair Cortisol (HC) (nmol/g) | 0.021±0.04* | 0.7±10−2 |
| Serum Cortisol (SeC) (μg/dL) | 1.64 ± 1.03 | 2.74±2.83 |
| Oxidative stress | ||
| LDL-Cholesterol lag time (5 μM Cu) | 71.85±32.60 | 96.00±55.96 |
Sample size for SBP, DBP, pulse, IR, hsCRP, SeC, LDL-cholesterol, and LDL-cholesterol lag time was n=21 and for SC and HC were n=31 for each race. Values are means ± SD. All differences were significant at
p <0.05. Significant results are based on absolute values. T-test were performed to assess mean differences in SC and HC and Kruskal-Wallis test were performed to assess differences in SPB, DBP, pulse, IR, hsCRP, SeC, LDL-cholesterol, and LDL-cholesterol lag time between AA and EA women.
In our study, even though SBP, IR, and LDL-cholesterol markers were not associated with recent and lifetime experiences of discrimination, the AA females showed statistically significantly higher levels of SBP compared to EA females. Levels of SBP (121.35±11.72) among AA females were clinically elevated on average (normal category for SBP: <120 mm Hg). Insulin resistance was clinically elevated among EA (5.33±3.05; normal level <4.3) and LDL-cholesterol levels were clinically higher in both AA (155.88±37.61) and EA (155.27±31.69) females (<100mg/dL).
Recent and lifetime experiences of discrimination, biomarkers of stress, race, and BMI
The influence of recent and lifetime experiences of discrimination on physiological biomarkers of stress was evaluated while considering the interactions with race and BMI. Recent experiences of discrimination showed statistically significant relationships to hsCRP and pulse, and lifetime experiences of discrimination to DBP, HC, hsCRP, and SC in our multiple regression models (Table 3). In addition to lifetime experiences of discrimination, race showed an impact on levels of SC and HC; and BMI was also associated with higher levels of hsCRP, DBP, and pulse (p<0.05). Results from the power analysis from the multiple regression models of recent experiences of discrimination showed a range of power from 0.73-0.77 and in the case of the models of lifetime experiences of discrimination the results showed a range from 0.44-0.82.
Table 3.
Regression analysis of physiological biomarkers of stress.
| Recent Experiences of Discrimination | ||||
|---|---|---|---|---|
| hsCRP [F(3,28)=19.91, p<0.001; R2=0.70] |
Pulse [F(3,32)=10.91, p<0.0001; R2=0.53] |
|||
| B Coefficient | p-value | B Coefficient | p-value | |
| Race, AA | 3.31 | 0.0030 | −2.11 | 0.5652 |
| Body Mass Index (BMI) | 0.49 | <0.0001 | 0.89 | 0.0006 |
| Recent experiences of discrimination | 0.19 | 0.0190 | 0.73 | 0.0099 |
| Lifetime Experiences of Discrimination | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Salivary cortisol [F(3,44)=2.94, p<0.0441; R2=0.1773] |
Hair cortisol [F(3,48)=5.36, p<0.0029; R2=0.2508] |
hsCRP [F(3,30)=10.91, p<0.0001; R2=0.5480] |
Diastolic blood pressure [F(3,30)=8.22, p<0.0005; R2=0.4774] |
Pulse [F(3,31)=16.42, p<0.0001; R2=0.6377] |
||||||
| B Coefficient | p-value | B Coefficient | p-value | B Coefficient | p-value | B Coefficient | p-value | B Coefficient | p-value | |
| Race, AA | −0.05 | 0.0219 | −0.006 | 0.0134 | 2.52 | 0.1502 | 4.50 | 0.0708 | 2.77 | 0.3843 |
| Body Mass Index (BMI) | −0.001 | 0.1934 | 0.0002 | 0.0902 | 0.53 | <0.0001 | 0.65 | <0.0001 | 0.18 | <0.0001 |
| Lifetime experiences of discrimination | −0.006 | 0.0223 | −0.001 | 0.0183 | 0.50 | 0.0097 | −0.58 | 0.0352 | 1.00 | 0.0069 |
All models adjusted for body mass index (BMI), and race. Models used European American females as a reference group.
Physiological stress biomarkers and oxidation of LDL-cholesterol
There were significant mean differences in lag time of LDL-cholesterol oxidation (4.52±0.51 vs. 4.19±0.42) when comparing low to high salivary cortisol levels (p=0.0354) among females (Graph 1). However, no significant differences were seen for HC (p=0.8950) and lag times. To explore cortisol’s influence on the susceptibility to oxidation of LDL-cholesterol (lag time), the lifetime experiences of discrimination were included in the models with the premise that the exposure to discrimination leads to a cascade of physiological reactions that may increase the oxidation of LDL-cholesterol. A relationship between BMI, cortisol, and the oxidation of LDL-cholesterol has previously been reported[38, 39]. Results for the multiple regression model assessing the contribution of higher levels of SC on lag time were statistically significant [F(3,36)=3.37 p<0.0298; R2=0.2347]. Lag time was significantly associated with SC (p=0.0420) when adjusted for lifetime experiences of discrimination (p=0.0366) and BMI (p=0.6252). Education was evaluated as an additional factor that may influence oxidation of LDL-cholesterol lag time. Results for the multiple regression model assessing the influence of education on lag time were statistically significant [F(2,33)=3.65, p=0.0371; R2=0.1810]. Lag time, atherogenic susceptibility, was significantly associated with lifetime experiences of discrimination (p=0.0475) but was not significantly associated with education, though showed a trend (p=0.0754).
Graph 1.
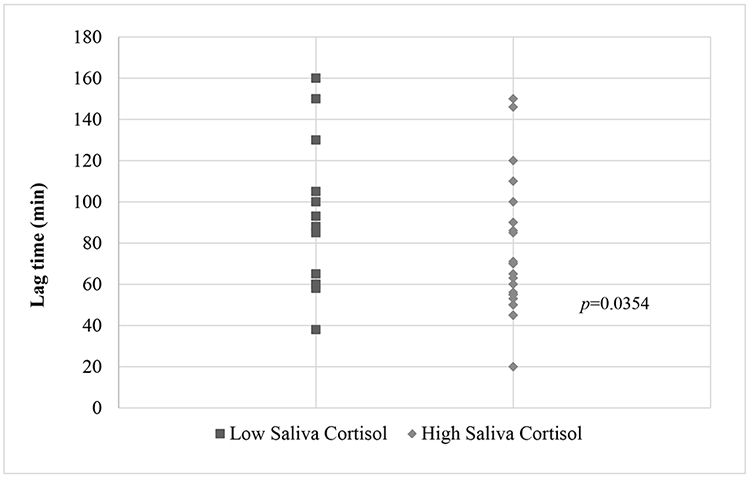
Oxidation of LDL expressed by time in minutes (Lag time) by low and high salivary cortisol
For hair cortisol, high levels were set at ≥0.0040 nmol/g and low levels were set at <0.0040 nmol/g. For saliva cortisol, high levels were set at ≥0.13 μg/dL and low levels were set at <0.13 μg/dL. The sample size for the SC was 31 participants for each race (African American and European American).
Discussion
This study investigated the correlation of experiences of discrimination (recent and lifetime) with biomarkers of physiological stress, oxidative stress, and obesity among adult females (AA vs. EA). Significantly higher discrimination levels were seen in AA compared to EA females. African American females showed significantly higher physiological markers of stress including SBP, pulse, and hair cortisol (HC) levels compare to EA females. Recent experiences of discrimination were associated with higher levels of inflammation (hsCRP) and higher pulse. Lifetime experiences of discrimination were associated with higher levels of SC, HC, hsCRP, DBP, and pulse, all of which are considered cardiovascular risk factors. Elevated levels of SC were significantly related to increased atherogenic potential of LDL cholesterol as measured by shorter lag times.
Our cohort’s socioeconomic (income and education) status is similar to what is seen nationally. African American females had lower income compared to EA females[40] and EA females had higher levels of education [41]. However, AA females in our cohort had a higher education level compared to national representation[41]. African American females had higher BMI, waist circumference, and BF% compared to EA females.
The AA females in our cohort reported higher lifetime experiences of discrimination and had more reasons to be discriminated against compared to EA females, including their race and skin color. Similarly, Lee, et al[42] showed that non-white populations reported more experiences of discrimination than their white population. Additional findings showed that there were no differences in the location where the unfair treatment occurred nor in the perception of stress from this unfair treatment for both recent and lifetime experiences of discrimination between AA and EA women. This suggests that recent and lifetime experiences of discrimination may not be specific to any particular physical location but may be more pervasive.
In our study, the exposure to recent and lifetime experiences of discrimination appears to affect the hypothalamic-pituitary axis, the sympathetic nervous system, and the cardiovascular system. Similar effects on these physiological systems have been seen when there is a chronic exposure to stress derived from racial, gender, physical environment, income, and education differences[43]. Cortisol, for example, has been proposed as a marker of stress, and in our study, exposure to higher amounts of lifetime discrimination were associated with higher levels of SC and HC. Similar results were seen in the O’Brien et al[44] study, in which discrimination influenced the HC levels of the participants. The additional influence of stress on increasing inflammatory markers has been previously studied. In our study, recent and lifetime experiences of discrimination showed a positive association with hsCRP levels. The proposed mechanism in which stress increases markers of inflammation appears to be related to catecholamines and glucocorticoid stress responses after the stress exposure[14].
Different studies have shown that biomarkers of stress such as cortisol are on average higher in individuals with obesity and several mechanisms have been described as potential contributors. Contributors include an overactivity of the HPA axis, effects on the HPA axis from inflammatory marker exposures, and an individual variation in enzymes that are involved in cortisol metabolism[45]. In our study, only DBP and hsCRP showed associations with lifetime experiences of discrimination, racial differences, and BMI. However, HC was only associated with lifetime experiences of discrimination and racial differences, but not with BMI. The stress effects on weight or excess adiposity may be explained in different ways. The non-significant relationship of BMI with cortisol levels in our sample may be related to the individual variability in glucocorticoid sensitivity, which is partly genetically determined, and may lead to higher or lower vulnerability to stressors[45] and/or other interacting behavioral factors such as activity or diet. Although LDL-cholesterol levels were high and not significantly different in our two groups (AA vs. EA), the lag time of oxidized LDL-cholesterol were shorter among AA females, which indicate greater susceptibility to oxidation and potential formation and propagation of oxidized LDL-cholesterol. The importance of the oxidized LDL-cholesterol is that it has been associated with cardiovascular diseases[46].
The increased risk for LDL-cholesterol oxidation appears to be induced by obesity[38, 39]. Van Gaal et. al.[38] for example, found obese women to have shorter lag times and less resistance to LDL oxidation compared to non-obese women. However, in our study, the increased risk for LDL-cholesterol oxidation was seen with increased levels of cortisol (measured in saliva and hair) and lifetime experience of discrimination, but not with BMI. Although both racial groups had clinically higher levels of LDL, the AA females had a significantly higher atherogenic potentional and CVD risk. Our results may be related to previous evidence, in which higher cortisol levels were associated with an increased cardiovascular risk [47] since our study revealed that increased psychosocial stress is positively associated with increased cortisol and increased susceptibility of LDL-cholesterol to oxidation. The increase of inflammatory markers contributes to an imbalance of oxidant production, where there is either excess production of ROS, inadequate antioxidant sytems, or both, which can then induce oxidative stress and ultimately lead to metabolic disease. Therefore, the salivary cortisol levels may be one indicator of someone’s cardiovascular risk based on our data. We explored the association of lifetime experience of discrimination with additional factors including education. However, we found that their level of education was not significantly associated with lag time, suggesting that a person’s level of education does not influence the effects of discrimination and associated health outcomes in our study.
During a stressful event, cortisol is released, and a person’s fight or flight response ensues by flooding the body with glucose and free fatty acids for immediate energy while suppressing insulin secretion to inhibit glucose uptake. Exposures to psychosocial stressors influence food preference for palatable, processed, "comfort” foods to deal with the physiological effects of stress[48]. Chronically, this stress response system can lead to weight gain through elevated and unused glucose and fatty acids that are potentially stored as fat if there were no increased activity “fight or flight”. Vulnerable populations experiencing chronic life stress may make poor dietary choices more frequently, preferring highly processed carbohydrates and high fat “comfort foods” that are generally low in antioxidants. A population experiencing chronic psychosocial life stress, elevated cortisol, elevated inflammatory markers, and oxidized LDL in combination with a diet poor in antioxidant properties, creates a positive feedback loop between life stress, inflammation, and oxidative stress that may overwhelm the natural antioxidant and defenses of the system.
There are some limitations in the present study. First, we did not have HDL-cholesterol measurements, which have been associated with decreased risk for cardiovascular diseases. Second, the cross-sectional design of this study did not allow for examination of trajectories of lifetime discrimination and health outcomes. Third, the sample size may have been small, and further larger studies are needed to confirm these results. Fourth, the salivary cortisol samples were taken in the morning although time from waking was not recorded. The strengths of this study include comprehensive validated instruments used to measure discrimination, use of salivary and hair cortisol as measurements of acute and chronic stress, the inclusion of two races to compare the impact of discrimination, and the inclusion of LDL-cholesterol oxidation as a cardiovascular disease risk biomarker.
This study demostrates the continued experience and perception of unfair treatment or discrimination among individuals, specifically among women in the greater Birmingham, Alabama area. However, institutional discrimination, discrimination by a society and its institutions as a whole, continues to be a very prominent matter in our American society today, negatively affecting an individual’s day to day life and health status[49]. In 2017, researchers conducted one of the largest discrimination polls to date. Among the 3,453 people polled, groups of different race, gender, and sexual identity reported discrimination when trying to rent a house, interacting with police, interviewing for a job, being considered for a promotion, going to the doctor or health clinic, and when experiencing slurs[8]. Our findings, along with previous discrimination and health disparities research, highlight the importance to take active steps in addressing and eliminating this persistent issue of discrimination within the American society, its institutions, and abroad. Recently, David R. Williams has published scientific evidence pointing to these steps needed to reduce inequalities in health, which include 1) creating communities of opportunities, 2) improving healthcare to benefit all persons and 3) raising awareness of inequalities by building political support [50], and this reiterates our drive for greater discussion among scientific and political institutions. Interventions aimed at managing the harmful stress response that ensues from recent or lifetime experiences of discrimination may be helpful in improving cardiovascular disease risks. Some of these interventions include: anti-inflammatory, anti-oxidant, and nutrient dense dietary choices; increased daily physical activity; meditation; and many others.
In conclusion, our results showed that AA females showed higher levels of discrimination and more reasons why they were treated unfairly compared to EA females. Scores of recent and lifetime experiences of discrimination were associated with some stress biomarkers. Our study shows that experiencing discrimination as a stressful life event correlates with elevated salivary and hair cortisol levels, increased inflammation, elevated blood pressure, and increased atherogenic potential of LDL-cholesterol. All of these events combined, create a perfect storm for development and exacerbation of cardiovascular disease and are a possible explanation for these health disparities. These results suggest that increased levels of discrimination, whether because of race, gender, age, or other reasons could contribute to cardiovascular disease risk and health disparities. Future research is needed to explore the relationship between chronic psychosocial stress, cortisol, inflammatory markers, dietary choices, physical activity, obesity, and CVD. Other external factors not discussed here, such as diet and exercise may mediate some of the effects of elevated stress hormones and inflammatory markers on cardiovascular disease development and warrant further investigation.
Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville (MD)2017. [PubMed] [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. NCHS Data Brief. 2017(288):1–8. Epub 2017/11/21. [PubMed] [Google Scholar]
- 3.Kanchi R, Perlman SE, Chernov C, et al. Gender and Race Disparities in Cardiovascular Disease Risk Factors among New York City Adults: New York City Health and Nutrition Examination Survey (NYC HANES) 2013-2014. J Urban Health. 2018;95(6):801–12. Epub 2018/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and socioeconomic status in adults: United States, 2005-2008. NCHS Data Brief. 2010(50):1–8. Epub 2011/01/08. [PubMed] [Google Scholar]
- 5.Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. Am J Public Health. 2003;93(2):200–8. Epub 2003/01/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler NE, Stewart J. Preface to the biology of disadvantage: socioeconomic status and health. Ann N Y Acad Sci. 2010;1186:1–4. Epub 2010/03/06. [DOI] [PubMed] [Google Scholar]
- 7.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–98. Epub 2010/11/26. [DOI] [PubMed] [Google Scholar]
- 8.Discmination in America. Experiences and Views on Affects of Discrimination Across Major Population Groups in the United States.: Robert Wood Johnson Foundation; [cited 2019 September, 11]. Available from: African American Report https://www.rwjf.org/en/library/research/2017/10/discrimination-in-america--experiences-and-views.html. [Google Scholar]
- 9.Cobbinah SS, Lewis J. Racism & Health: A public health perspective on racial discrimination. J Eval Clin Pract. 2018;24(5):995–8. Epub 2018/03/07. [DOI] [PubMed] [Google Scholar]
- 10.Shariff-Marco S, Gee GC, Breen N, et al. A mixed-methods approach to developing a self-reported racial/ethnic discrimination measure for use in multiethnic health surveys. Ethn Dis. 2009;19(4):447–53. Epub 2010/01/16. [PMC free article] [PubMed] [Google Scholar]
- 11.Shariff-Marco S, Breen N, Landrine H, et al. MEASURING EVERYDAY RACIAL/ETHNIC DISCRIMINATION IN HEALTH SURVEYS: How Best to Ask the Questions, in One or Two Stages, Across Multiple Racial/Ethnic Groups? Du Bois Rev. 2011;8(1):159–77. Epub 2011/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seplaki CL, Goldman N, Glei D, Weinstein M. A comparative analysis of measurement approaches for physiological dysregulation in an older population. Exp Gerontol. 2005;40(5):438–49. Epub 2005/05/28. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–97. Epub 2002/08/27. [DOI] [PubMed] [Google Scholar]
- 14.Rohleder N Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med. 2014;76(3):181–9. Epub 2014/03/13. [DOI] [PubMed] [Google Scholar]
- 15.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140(3):774–815. Epub 2014/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert MA, Durazo EM, Slopen N, et al. Cumulative psychological stress and cardiovascular disease risk in middle aged and older women: Rationale, design, and baseline characteristics. Am Heart J. 2017;192:1–12. Epub 2017/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richman LS, Jonassaint C. The effects of race-related stress on cortisol reactivity in the laboratory: implications of the Duke lacrosse scandal. Ann Behav Med. 2008;35(1):105–10. Epub 2008/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szanton SL, Rifkind JM, Mohanty JG, et al. Racial discrimination is associated with a measure of red blood cell oxidative stress: a potential pathway for racial health disparities. Int J Behav Med. 2012;19(4):489–95. Epub 2011/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beatty Moody DL, Chang Y, Brown C, Bromberger JT, Matthews KA. Everyday Discrimination and Metabolic Syndrome Incidence in a Racially/Ethnically Diverse Sample: Study of Women's Health Across the Nation. Psychosom Med. 2018;80(1):114–21. Epub 2017/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis TT, Everson-Rose SA, Powell LH, et al. Chronic exposure to everyday discrimination and coronary artery calcification in African-American women: the SWAN Heart Study. Psychosom Med. 2006;68(3):362–8. Epub 2006/06/02. [DOI] [PubMed] [Google Scholar]
- 21.Spruill TM. Chronic psychosocial stress and hypertension. Curr Hypertens Rep. 2010;12(1):10–6. Epub 2010/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puustinen PJ, Koponen H, Kautiainen H, Mantyselka P, Vanhala M. Psychological distress predicts the development of the metabolic syndrome: a prospective population-based study. Psychosom Med. 2011;73(2):158–65. Epub 2010/12/15. [DOI] [PubMed] [Google Scholar]
- 23.Scott KA, Melhorn SJ, Sakai RR. Effects of Chronic Social Stress on Obesity. Curr Obes Rep. 2012;1(1):16–25. Epub 2012/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill EE, Zack E, Battaglini C, et al. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest. 2008;31(7):587–91. Epub 2008/09/13. [DOI] [PubMed] [Google Scholar]
- 25.Duclos M, Tabarin A. Exercise and the Hypothalamo-Pituitary-Adrenal Axis. Front Horm Res. 2016;47:12–26. Epub 2016/06/28. [DOI] [PubMed] [Google Scholar]
- 26.Meyer J, Novak M, Hamel A, Rosenberg K. Extraction and analysis of cortisol from human and monkey hair. J Vis Exp. 2014(83):e50882. Epub 2014/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gow R, Thomson S, Rieder M, Van Uum S, Koren G. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int. 2010;196(1-3):32–7. Epub 2010/01/26. [DOI] [PubMed] [Google Scholar]
- 28.Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37(5):589–601. Epub 2011/10/07. [DOI] [PubMed] [Google Scholar]
- 29.Raul JS, Cirimele V, Ludes B, Kintz P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem. 2004;37(12):1105–11. Epub 2004/12/14. [DOI] [PubMed] [Google Scholar]
- 30.Beckie TM. A systematic review of allostatic load, health, and health disparities. Biol Res Nurs. 2012;14(4):311–46. Epub 2012/09/26. [DOI] [PubMed] [Google Scholar]
- 31.Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34(10):1423–36. Epub 2009/08/04. [DOI] [PubMed] [Google Scholar]
- 32.Kellerman RD, Rakel D. Conn's Current Therapy 2019: Elsevier; 2019. [Google Scholar]
- 33.Fisher G, Hyatt TC, Hunter GR, et al. Markers of inflammation and fat distribution following weight loss in African-American and white women. Obesity (Silver Spring). 2012;20(4):715–20. Epub 2011/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashraf AP, Fisher G, Alvarez J, et al. Associations of C-Reactive Protein to Indices of Vascular Health and the Influence of Serum 25(OH)D Status in Healthy Adults. J Nutr Metab. 2012;2012:475975. Epub 2012/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. Epub 1999/09/10. [DOI] [PubMed] [Google Scholar]
- 36.Chung BH, Segrest JP, Cone JT, et al. High resolution plasma lipoprotein cholesterol profiles by a rapid, high volume semi-automated method. J Lipid Res. 1981;22(6):1003–14. Epub 1981/08/01. [PubMed] [Google Scholar]
- 37.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32(1):77–97. Epub 1974/07/01. [DOI] [PubMed] [Google Scholar]
- 38.Van Gaal LF, Vertommen J, De Leeuw IH. The in vitro oxidizability of lipoprotein particles in obese and non-obese subjects. Atherosclerosis. 1998;137 Suppl:S39–44. Epub 1998/08/07. [DOI] [PubMed] [Google Scholar]
- 39.Vincent HK, Taylor AG. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int J Obes (Lond). 2006;30(3):400–18. Epub 2005/11/23. [DOI] [PubMed] [Google Scholar]
- 40.Semega JL, Fontenot KR. U.S. Census Boreau, Current Population Reports. Income and Poverty in the United States: 2016. U.S. Government Printing Office; Washington, DC: 2017. [Google Scholar]
- 41.Ryan CL, Bauman K. Educational Attainment in the United States: 2015. U.S. Department of Commerce. Economics and Statistics Administration; 2016. [Google Scholar]
- 42.Lee RT, Perez AD, Boykin CM, Mendoza-Denton R. On the prevalence of racial discrimination in the United States. PLoS One. 2019;14(1):e0210698. Epub 2019/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Djuric Z, Bird CE, Furumoto-Dawson A, et al. Biomarkers of Psychological Stress in Health Disparities Research. Open Biomark J. 2008;1:7–19. Epub 2008/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Brien KM, Meyer J, Tronick E, Moore CL. Hair cortisol and lifetime discrimination: Moderation by subjective social status. Health Psychol Open. 2017;4(1):2055102917695176. Epub 2017/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Valk ES, Savas M, van Rossum EFC. Stress and Obesity: Are There More Susceptible Individuals? Curr Obes Rep. 2018;7(2):193–203. Epub 2018/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao S, Liu J. Association between circulating oxidized low-density lipoprotein and atherosclerotic cardiovascular disease. Chronic Dis Transl Med. 2017;3(2):89–94. Epub 2017/10/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manenschijn L, Schaap L, van Schoor NM, et al. High long-term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. J Clin Endocrinol Metab. 2013;98(5):2078–83. Epub 2013/04/19. [DOI] [PubMed] [Google Scholar]
- 48.Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol. 2011;300(6):R1266–77. Epub 2011/03/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams DR, Mohammed SA. Racism and Health I: Pathways and Scientific Evidence. Am Behav Sci. 2013;57(8). Epub 2013/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams DR, Cooper LA. Reducing Racial Inequities in Health: Using What We Already Know to Take Action. Int J Environ Res Public Health. 2019;16(4). Epub 2019/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]


