Abstract
Background:
Transbronchial lung cryobiopsy (TBLC) is an emerging technique in the diagnostic approach to diffuse parenchymal lung diseases. However, the role of TBLC in smoking-related Interstitial Lung Diseases (ILDs) is still under discussion.
Objectives:
The aim of the present study was to describe our experience with TBLC in diagnostic work-up of patients with smoking-related ILDs.
Method:
We retrospectively reviewed data of patients evaluated in a tertiary hospital ILDs outpatient clinic, who underwent TBLC, from September 2014 to December 2019. TBLC was performed in accordance with the 2018 expert statement from the Cryobiopsy Working Group.
Results:
Forty-five patients (25 men [55.6%]) with a mean age of 53.9 years [SD, 9.1] were included. The most frequent radiological pattern was ground glass opacity (42 patients). TBLC was performed in different segments of the same lobe in 38 patients and in two lobes in 7 patients. The mean maximal diameter of the samples was 5.2 mm (range, 3–16 mm [SD 2.0]). Pneumothorax occurred in seven patients (15%) and moderate bleeding occurred in one patient. A specific pathological diagnosis was achieved in 43 of 45 patients. The most frequent histopathologic pattern found was desquamative interstitial pneumonia (33 patients), followed by smoking-related interstitial fibrosis (7 patients), respiratory bronchiolitis - ILD (1 patient) and pulmonary Langerhans cell histiocytosis (1 patient). Two patients had alternative diagnosis (Pneumoconiosis and Interstitial Pneumonia with unspecific features) and one patient had normal lung parenchyma. A definitive multidisciplinary team (MDT) diagnosis was reached in 95.5% (43 of 45 cases). Two patients were submitted to additional diagnostic techniques.
Conclusions:
The results from this series support TBLC as a safe procedure with a meaningful diagnostic value in the context of a MDT approach of smoking-related ILDs. (Sarcoidosis Vasc Diffuse Lung Dis 2020; 37 (4): e2020013)
Keywords: transbronchial lung cryobiopsy, smoking-related interstitial lung diseases, diagnostic yield, complications, diffuse lung diseases, surgical lung biopsy
Introduction
Cigarette smoking is implicated in a heterogeneous spectrum of diffuse parenchymal lung diseases (DPLD) referred to as smoking-related interstitial lung diseases (ILDs) (1-3). Lung diseases that have a causal association with tobacco exposure include respiratory bronchiolitis - interstitial lung disease (RB-ILD), desquamative interstitial pneumonia (DIP), pulmonary Langerhans cell histiocytosis (PLCH) and acute eosinophilic pneumonia (AEP) (3). Smoking-related interstitial fibrosis (SRIF) is a relatively newly appreciated entity, with distinct histopathologic features that has gained prominence. Its clinical ramifications and radiologic features are still unclear, however it should be considered in differential diagnosis of RB-ILD and DIP (4-5).
With exception of AEP that has an acute onset, on clinical presentation, patients usually complain about insidious dyspnea and cough over the course of weeks to months (1). On imaging, RB-ILD shows upper lung predominance of the findings, characterized by the evidence of low attenuation centriacinar nodules and ground-glass opacities (GGOs). The radiological findings in DIP are lower lobe predominant and characterized by GGOs and reticular opacities interposed with relatively normal lung zones. PLCH presents with a mix of nodules and bizarre shaped cysts on upper lobes. Imaging findings of AEP are predominant in the lower lungs, showing diffuse consolidations, GGOs and ill-defined centrilobular nodules (1,3,6).
The current approach of smoking-related ILDs includes a multidisciplinary team (MDT) diagnosis combining clinical, radiological, and pathologic features (7-9).
In smoking-related ILDs, namely in RB-ILD, PLCH and AEP, in active smokers with suggestive clinical, radiological and bronchoalveolar lavage (BAL) features, lung biopsy is usually not required (3). Attempts at obtaining tissue should be reserved for ambiguity in diagnosis.
Surgical lung biopsy (SLB) has been considered as the definitive mean of obtaining adequate biopsy specimens. However, side effects of SLB such as prolonged air leakage, infections, acute exacerbation and death, are not negligible (10-12).
Transbronchial Lung Cryobiopsy (TBLC) have been introduced since 2009 by Babiak et al. as an alternative to SLB in the diagnostic approach to DPLD (13). A growing body of evidence suggests the utility of TBLC in the diagnostic algorithm of ILD as it allows, compared to transbronchial lung biopsy with conventional forceps, a better identification of complex histological patterns and can provide information which seems to have a clinical impact on the MDT discussion similar to that provided by SLB (14-22). Additionally, if performed correctly, it appears to have a better safety profile than SLB (15, 16, 23-25).
Although several series evaluating TBLC diagnostic yield contain patients with smoking-related ILDs, the role of TBLC in smoking-related ILDs is still a topic under discussion.
Concerning the regular use of TBLC in MDT diagnostic evaluation, the aim of this study was to find its diagnostic accuracy and safety in patients with suspected smoking-related ILDs, based on clinical, radiological and BAL features.
Materials and Methods
Design
We conducted a retrospective review of the medical records of patients undergoing TBLC from September 2014 to December 2019 at the bronchoscopy unit of the pulmonology department at Centro Hospitalar São João (Porto, Portugal). The ethics committee approved this study.
Patients
All the patients had a prior evaluation at the ILD outpatient clinic where a detailed history, complete physical examination, in addition to laboratory tests, lung function, thoracic HRCT were taken. The patients’ electronic medical records were assessed retrospectively, and the following data were collected: demographic data, drug and occupational history, thoracic HRCT, BAL results, procedure details and complications and pathology reports.
The diagnosis of smoking-related ILDs was based on a multimodality approach that combined clinical, radiological and BAL features. Over the period examined, all patients with suspected smoking-related ILDs were discussed on a MDT meeting, composed by clinicians, radiologists and pathologists with a long time experience on ILD. They were proposed for TBLC only when clinical, radiological findings and BAL features did not conclude a final diagnosis and a biopsy was deemed useful for a diagnosis. After TBLC procedure, there were a second MDT meeting where clinical information, radiological features and biopsy results were then reviewed and a multidisciplinary diagnosis was made, with cryobiopsy considered diagnostic if additional evaluation was considered to be unnecessary.
Procedure
The TBLC procedure was performed according to 2018 expert statement (26). All TBLC were performed by a senior bronchoscopist. As previously described by Almeida et al. (24), it was used a combination of rigid bronchoscopy (tracheoscope 14mm, Karl Storz, Germany) and flexible bronchoscopy (Olympus BF-XT40, Europe) under general anesthesia with manual jet ventilation (working pressure of approximately 2 bar). A 2.4 mm cryoprobe (ERBE, Germany) was introduced through the working channel of the flexible bronchoscope. Biopsies were taken under fluoroscopic guidance from an optimal distance between the probe and the thoracic wall of 10 mm. Biopsy sites were selected based on HRCT abnormalities evaluated in MDT discussion. Once brought into position, the probe was cooled to −85°C with nitrogen oxide for approximately 5–6s, thus freezing the lung tissue in contact with the probe. The frozen specimen attached to the tip of the probe was removed by pulling out the cryoprobe together with the bronchoscope. In all procedures, a Fogarty balloon was always routinely used to prevent severe bleeding. When bleeding occurred, the Fogarty balloon was deflated only after cessation of bleeding and before any additional biopsies were performed. The samples, still attached to the probe, were first inserted in saline and then in formalin. Following the procedure, patients were extubated and kept under observation. After 3h, a chest X-ray was performed to exclude pneumothorax.
Written informed consent was obtained before TBLC from all patients.
Complications
Pneumothorax was described according to observation measures or chest tube insertion requirements. Endobronchial bleeding was defined using the British Thoracic Society system as mild bleeding (requiring suction to clear but no other endoscopic procedures), moderate bleeding (requiring endoscopic procedures like bronchial occlusion-collapse and/or instillation of ice-cold saline), and severe bleeding (causing hemodynamic or respiratory instability, requiring tamponade or other surgical interventions, transfusions or admission to the intensive care unit).
Clinical, Radiological, and Functional Assessment and BAL
The HRCT pattern was classified as micronodular, ground-glass opacity, reticulation and emphysema according to the glossary of the Fleischner Society (27). Pulmonary function tests were performed in accordance with the standard recommendations of the American Thoracic Society (ATS) and European Respiratory Society (ERS) and findings categorized as normal, obstructive, restrictive, or mixed abnormalities (28). BAL was performed following the recommendations of the ERS (29). BAL cellular patterns were classified as lymphocytic (>15%), neutrophilic (>3%), and eosinophilic (>1%).
Pathologic assessment
The sample tissue was formalin-fixed for at least 6h and at most 24h before paraffin embedding. Sections measuring 3μm were stained with hematoxylin–eosin. In all cases serial cuts were made at three levels, with additional use of special stains or immunohistochemical stains when necessary. All samples were measured under the optical microscope and the area of each fragment evaluated. The presence of pleural tissue was recorded. The artifact areas were included in the measurement, however when the fragments were only pleura or adipose tissue they were not measured.
The biopsy was considered adequate if at least one of the fragments consisted of alveolated lung parenchyma. The diagnosis was made based on the same criteria used for SLB.
Statistical Analysis
Descriptive statistics were used to analyze patient characteristics. Normally distributed continuous data were described as means and SD. The categorical variables were reported in percentages of total subjects. The data were analyzed using SPSS software (version 25; IB, Armonk, NY USA).
Results
Forty-five patients were included in the study cohort with a mean age of 53.9 years (range, 31-74 years [SD, 9.1]). Of these, 25 were men (55.6%) and all had a history of smoking. Pulmonary function testing revealed ventilatory abnormalities in 36.6% of subjects. Diffuse lung capacity for carbon monoxide (DLCO) was impaired in 87.8% of subjects, in whom the mean percentage of DLCO was 52% of predicted (SD, 10). Mean PaO2 was 79 mmHg (SD 10). The most frequent HRCT pattern was ground glass opacity present in 42 patients. A BAL with total and differential cell counts was performed in 37 patients (82.2%) and eosinophilia was the most frequent feature (table 1).
Table 1.
Demographic and clinical characteristics of patients.
| Variable (n = 45) | No. |
| Age at diagnosis, mean (SD), y | 53.9 (9.1) |
| Sex, male (%) | 25 (55.6) |
| Smoking habits, % | |
| Smoker | 94.8 |
| Ex-Smoker | 15.2 |
| Lung function pattern, % | |
| Normal | 63.4 |
| Obstructive | 29.3 |
| Restrictive | 7.3 |
| Diffuse lung capacity for carbon monoxide | |
| Decrease, % | 87.8 |
| Percentage of predicted, mean (SD) | 52 ± 10 |
| Normal, % | 12.2 |
| Percentage of predicted, mean (SD) | 82 ± 13 |
| PaO2, mean (SD), mmHg | 79 (10) |
| Bronchoalveolar lavage cellular pattern, (n = 37), No., % | |
| Normal | 9 (24.3) |
| Eosinophilic | 14 (37.8) |
| Eosinophilic and Neutrophilic | 10 (27.1) |
| Neutrophilic | 4 (10.8) |
| Predominant high-resolution CT pattern, (n = 45), No., % | |
| Ground-glass | 27 (60.0) |
| Ground-glass + Emphysema | 9 (20.0) |
| Ground-glass + Micronodularity | 4 (8.9) |
| Micronodularity | 2 (4.5) |
| Cystic | 1 (2.2) |
| Ground-glass + Cystic | 1 (2.2) |
| Ground-glass + Reticulation | 1 (2.2) |
| No. of specimens per procedure (n = 45), median (range, SD) | 3 (1-5, 1) |
| Diameter (n = 45), mean (range, SD) mm | 5.2 (3-16, 2.0) |
| Area (n = 45), mean (range, SD) mm² | 17.5 (5-35, 7.3) |
| No. of pleura per patient (%) | 16 (36) |
| No. of Pneumothorax (%) | 7 (15) |
| No. of Moderate Bleeding (%) | 1 (2) |
TBLC was performed in different segments of the same lobe in 38 patients and in two lobes in 7 cases. The median number of samples per procedure was three (range, 1-5). All the samples were considered adequate. The mean sample length was 5.2 mm (range, 3–16 mm [SD, 2.0]), and the mean area was 17.5 mm2 (range, 5-35 mm [SD, 7.3]). Histologic slides were available for review by two lung pathologists before the MDT discussion. The presence of pleura was observed in 16 patients (36%).
The most frequent histopathologic pattern found was DIP (33 patients), followed by SRIF (7 patients), RB-ILD (1 patient) and PLCH (1 patient). In one patient the histopathologic pattern was suggestive of interstitial pneumonia with unspecific features, and in another it was suggestive of pneumoconiosis. One patient had normal lung parenchyma in cryobiopsy (Figure 1).
Figure 1.
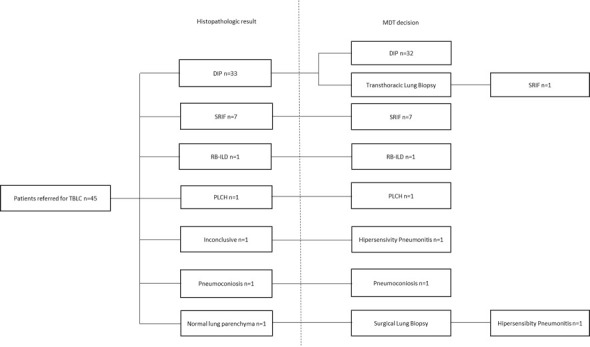
Diagrammatic representation of the diagnostic performance of patients with suspected Smoking Related ILD. DIP (Desquamative Interstitial Pneumonia), ILD (Insterstitial Lung Diseases), MDT (Multidisciplinary Team), PLCH (Pulmonary Langerhans Cell Histiocytosis), RB-ILD (Respiratory Bronchiolitis - Interstitial Lung Disease), SRIF (Smoking-Related Interstitial Fibrosis), TBLC (Transbronchial Lung Cryobiopsy).
In the MDT, correlation of clinical, radiological and histopathologic findings yielded a definite diagnosis in 43 patients. Forty-one patients had a diagnosis of smoking-related ILD. One patient had a working diagnosis of Hypersensitivity Pneumonitis and another one the diagnosis of Pneumoconiosis.
There was a patient whose thoracic HRCT showed images of diffuse ground-glass, with centrilobular nodules and cystic lesions. The cryobiopsy histopathologic features were suggestive of DIP. Due to the discrepancy between radiological and histopathologic features, a CT transthoracic lung biopsy was performed that showed SRIF features, which was in accordance with CT imaging.
The patient which cryobiopsy revealed normal lung parenchyma was submitted to surgical lung biopsy that was suggestive of Hypersensitivity Pneumonitis and was treated adequately.
Of the patients with a diagnosis of smoking related ILD, 10 were lost for follow up, since they had been followed in a different institution. Of the 32 patients in our ILD’s outpatient clinic, all of them were advised to participate in smoking cessation counselling and 10 patients initiated corticosteroid therapy. The mean time of follow up were 30 months (range, 9 - 69). To the date of submission, no patients were referred to lung transplant.
Complications
Pneumothorax was the most common complication, observed in 7 patients (15%). Of these, 5 patients had pleura in the histologic sample. None of them had concomitant emphysema. In 4 cases it was necessary to insert a chest drain; the remaining cases improved spontaneously, without intervention. Mean prolonged hospital stay due to this complication was about 2.8 ± 2 days. There were several cases of mild bleeding, but these were not documented as we considered them to be standard events for this procedure. One patient had moderate bleeding, that motivated a hospital stay of 2 days. None of these events was reported as life-threatening. No acute exacerbation of the underlying condition was observed.
Discussion/Conclusion
We found that TBLC proved to be an accurate and safe procedure for the diagnosis of smoking-related ILDs. A specific pathological diagnosis was achieved in 43 of the 45 patients and a definitive MDT diagnosis was confirmed in 43 of 45 patients (95.5%). The concordance between pathologists’ impression and MDT diagnosis was of 93.3%. Only two patients were submitted to other diagnostic techniques (transthoracic lung biopsy and SLB) after TBLC, due to discrepancy between radiological and histopathologic findings.
A consensus clinical diagnosis reached by a MDT discussion is currently the gold standard when establishing a diagnosis of DPLD (7-9). This approach has significantly improved diagnostic accuracy, overall agreement, and diagnostic confidence (7, 8). When smoking-related ILDs are suspected, the information recorded (clinical scenario, lung function, BAL data and HRCT scan features) must be thought-fully reviewed and analyzed (2, 3). More invasive procedures are considered only when these investigations are considered to be insufficient to provide a confident diagnosis (2). The role of SLB is well established in DPLD, and in most cases, a confident diagnosis was reached in more than 90% of patients (29-31). However, numerous studies have reported the risks of a SLB in ILDs. More recent reviews, albeit heterogeneous in their study composition, report a 30-day mortality of around 2% (10-12). To the best of our knowledge, there aren’t any studies specifically dedicated to the SLB on smoking-related ILDs.
The introduction of TBLC as a promising and safer alternative to SLB is generating considerable interest in the pulmonary community. Indications for TBLC in diagnostic algorithm of DPLD are currently under evaluation, and a standardization of this technique is imminent. Most published data on clinical usefulness of TBLC to date are on fibrosing DPLD, namely in Usual Interstitial Pneumonia (UIP) / Idiopathic Pulmonary Fibrosis (IPF). In this context, this technique has been reported to be significantly accurate (14, 16). Less high-quality evidence is available for other patterns such as Non-Specific Interstitial Pneumonia or DIP. However, the combination of morphological information provided by TBLC and BAL profiles (e.g., an increase of “smoker” macrophages and eosinophils in patients with DIP or the presence of granulomatous-like nodules composed mainly of histiocytes with a positive stain for CD1a antigen in patients with PLCH) along with clinical profile and HRCT features might be diagnostic and avoid SLB (25-26, 33).
Few data are available on the specific role of TBLC for the diagnosis of smoking-related ILDs. There is a small case series reporting only five cases of DIP diagnosed through TBLC (27). The largest series of patients (699 patients) with suspected DPLD that were submitted to TBLC reported 36 cases of DIP/RB-ILD and 7 cases of PLCH, after an MDT discussion (28). To the best of our knowledge, this is the first study dedicated specifically to the role of TBLC in patients with suspected smoking-related ILDs.
In our study, the diagnosis based on MDT discussion was reliable with high confidence in 43 of 45 patients (95.5%). Only two patients were submitted to other diagnostic techniques. One patient with a suspected SRIF/PLCH (a history of cigarette smoke with a HRCT revealing ground glass and thin-walled cysts) with a histology of DIP and CD1a stain negative was submitted to Transthoracic Lung Biopsy, due to discrepancy between radiological and histopathologic diagnosis. Other patient with a suspected DIP (smoking history and GGOs on HRCT) with a cryobiopsy with normal lung parenchyma, was submitted to SLB that was suggestive of Hypersensitivity Pneumonia. The diagnostic yield of TBLC in our study is higher than that previously reported in overall DPLD series. Despite methodological differences, diagnostic yield in previous studies, varied from 51% to 98%, with a pooled estimate of 79% (95% CI, 65-93) (32-33). One possible explanation is that in contrast with UIP, DIP and SRIF have a uniform involvement (3-6). On the other hand, in case of PLCH a specific histological diagnosis can be made instead of an identification of a pattern of injury (e.g., UIP), increasing the diagnostic yield of a TBLC sample (3).
Complications of TBLC were detected in 17% of patients, which is consistent with the literature. Pneumothorax was the most common complication observed in our series, occurred in 15% of cases (in 4 cases it was necessary to insert a chest drain). Reported incidence rates vary considerably (0 to 30%), possibly due to differences in procedure or in types of disorders (16, 32-33). This complication rate could be related to our approach, as we usually take samples from a distance of 10mm from the pleura, or it might also be explained by the presence of associated emphysema in smoking-related ILDs. Bleeding is a relatively frequent event in TBLC, but the use of prophylactic placement of a bronchial blocker allows for immediate tamponade without further positioning maneuver (25, 33). Only one patient (2%) experienced moderate bleeding, which was managed by standard flexible bronchoscope techniques (e.g., scope tamponade, iced saline). No severe bleeding was observed in our cohort. Bleeding rates for moderate/severe bleeding associated with TBLC vary considerably in the literature, with rates ranging from 0% to 78%. This wide range can probably be explained by the use of different approaches and classification systems (32, 33). None of the events that occurred in our cohort were considered life-threatening.
Our study is limited by its retrospective design. Moreover, it is the result of the experience of only one centre, so data inevitably reflects its specific clinical and technical methodology. On the other hand, it contains a small number of patients, due to the fact that smoking-related ILDs encloses a group of rare diseases and a significant number of cases are diagnosed without histology requirement. Additionally, some of these patients are heavy smokers with poor lung function and other comorbidities that contraindicates invasive procedures. However, the availability of TBLC with lower morbidity compared to SLB may extend the indications for lung biopsy.
As a conclusion, our single-center cohort demonstrated that TBLC has a meaningful diagnostic value (95.5%) in the context of an MDT approach of smoking-related ILDs and should be considered a reliable diagnostic tool in this particular scenario.
Statement of Ethics:
The ethics committee of Centro Hospitalar Universitário de São João in Porto approved this study.
Written informed consent was obtained before TBLC from all patients.
Disclosure Statement:
The authors have no conflicts of interest to declare.
Funding Sources:
The authors declare that no funding was received for this paper.
Author Contributions:
M. B. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. M. B. contributed to the conception and design; collection, analysis, and interpretation of data; drafting and critical revision of the article. H. N. B. contributed to the analysis and interpretation of data. S. G. contributed to the analysis and interpretation of data. P. C. M. contributed to the analysis and interpretation of data. N. M. contributed to the analysis and interpretation of data. C. S. M. contributed to the analysis and interpretation of data. J. M. P. contributed to the analysis and interpretation of data. A. M. Substantial contribution to conception and design, analysis and interpretation of data, drafting the article, and finalizing the version to be published. All authors approved the final article.
References
- 1.Margaritopoulos GA, Harari S, Caminati A, Antoniou KM. Smoking-related idiopathic interstitial pneumonia: A review. Respirology. 2016;21:57–64. doi: 10.1111/resp.12576. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias: An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A., Cherian SV, Vassallo R, Yi ES, Ryu JH. Current Concepts in Pathogenesis, Diagnosis, and Management of Smoking-Related Interstitial Lung Diseases. Chest. 2018;154(2):394–408. doi: 10.1016/j.chest.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Katzenstein AL. Smoking-related interstitial fibrosis (SRIF), pathogenesis and treatment of usual interstitial pneumonia (UIP), and transbronchial biopsy in UIP. Mod Pathol. 2012;25(suppl1):S68–S78. doi: 10.1038/modpathol.2011.154. [DOI] [PubMed] [Google Scholar]
- 5.Konopka KE, Myers JL. A review of smoking-related interstitial fibrosis, respiratory bronchiolitis, and desquamative interstitial pneumonia: overlapping histology and confusing terminology. Arch Pathol Lab Med. 2018;142(10):1177–1118. doi: 10.5858/arpa.2018-0240-RA. [DOI] [PubMed] [Google Scholar]
- 6.Sousa C, Rodrigues M, Carvalho A, Viamonte B, Cunha R, Guimarães S, et al. Diffuse smoking-related lung diseases: insights from a radiologic-pathologic correlation. Insights into Imaging. 2019;10:73. doi: 10.1186/s13244-019-0765-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh SLF, Wells AU, Desai SR, Poletti V, Piciucchi S, Dubini A, et al. Multicentre evaluation of multidisciplinary team meeting agreement on diagnosis in diffuse parenchymal lung disease: a case-cohort study. Lancet Respir Med. 2016;4:557–565. doi: 10.1016/S2213-2600(16)30033-9. [DOI] [PubMed] [Google Scholar]
- 8.Walsh SLF. Multidisciplinary evaluation of interstitial lung diseases: current insights: Number 1 in the Series “Radiology” Edited by Nicola Sverzellati and Sujal Desai. Eur Respir Rev. 2017 May 17;26(144) doi: 10.1183/16000617.0002-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biglia C, Ghaye B, Reychler G, Koenig S, Yildiz H, Lacroix V, et al. Multidisciplinary management of interstitial lung diseases: A real-life study. Sarcoidosis VDLD. 2019 Jun; 6;36(2):108–15. doi: 10.36141/svdld.v36i2.8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen W, Meyer KC. Surgical lung biopsy for the diagnosis of interstitial lung disease: a review of the literature and recommendations for optimizing safety and efficacy. Sarcoidosis VDLD. 2013;30:3–16. [PubMed] [Google Scholar]
- 11.Hutchinson JP, Fogarty AW, McKeever TM, Hubbard RB. In-Hospital Mortality after Surgical Lung Biopsy for Interstitial Lung Disease in the United States. 2000 to 2011. Am J Respir Crit Care Med. 2016;193(10):1161–1167. doi: 10.1164/rccm.201508-1632OC. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JH, Shapera S, To T, Marras TK, Gershon A, Dell S. Procedure volume and mortality after surgical lung biopsy in interstitial lung disease. Eur Respir J. 2019 Feb 21;53(2) doi: 10.1183/13993003.01164-2018. [DOI] [PubMed] [Google Scholar]
- 13.Babiak A, Hetzel J, Krishna G, Fritz P, Moeller P, Balli T, et al. Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration. 2009;78(2):203–8. doi: 10.1159/000203987. [DOI] [PubMed] [Google Scholar]
- 14.Casoni GL, Tomassetti S, Cavazza A, Colby TV, Dubini A, Ryu JH, et al. Transbronchial Lung Cryobiopsy in the Diagnosis of Fibrotic Interstitial Lung Diseases. PLoS One. 2014 Feb 28;9(2):e86716. doi: 10.1371/journal.pone.0086716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagmeyer L, Theegarten D, Wohlschläger J, Treml M, Matthes S, Priegnitz C, et al. The role of transbronchial cryobiopsy and surgical lung biopsy in the diagnostic algorithm of interstitial lung disease. Clin Respir J. 2016;10:589–95. doi: 10.1111/crj.12261. [DOI] [PubMed] [Google Scholar]
- 16.Ravaglia C, Bonifazi M, Wells AU, Tomassetti S, Gurioli C, Piciucchi S, et al. Safety and Diagnostic Yield of Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Diseases: A Comparative Study versus Video-Assisted Thoracoscopic Lung Biopsy and a Systematic Review of the Literature. Respiration. 2016;91:215–27. doi: 10.1159/000444089. [DOI] [PubMed] [Google Scholar]
- 17.Poletti V, Ravaglia C, Gurioli C, Piciucchi S, Dubini A, Cavazza A, et al. Invasive diagnostic techniques in idiopathic interstitial pneumonias. Respirology. 2016;21:44–50. doi: 10.1111/resp.12694. [DOI] [PubMed] [Google Scholar]
- 18.Tomassetti S, Wells AU, Costabel U, Cavazza A, Colby TV, Rossi G, et al. Bronchoscopic Lung Cryobiopsy Increases Diagnostic Confidence in the Multidisciplinary Diagnosis of Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2016;193:745–52. doi: 10.1164/rccm.201504-0711OC. [DOI] [PubMed] [Google Scholar]
- 19.Ravaglia C, Wells AU, Tomassetti S, Dubini A, Cavazza A, Piciucchi S, et al. Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Disease: Comparison between Biopsy from 1 Segment and Biopsy from 2 Segments - Diagnostic Yield and Complications. Respiration. 2017;93:285–92. doi: 10.1159/000456671. [DOI] [PubMed] [Google Scholar]
- 20.Lentz RJ, Argento AC, Colby TV, Rickman OB, Maldonado F. Transbronchial cryobiopsy for diffuse parenchymal lung disease: a state-of-the-art review of procedural techniques, current evidence, and future challenges. J Thorac Dis. 2017;9:2186–2203. doi: 10.21037/jtd.2017.06.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romagnoli M, Colby TV, Berthet JP, Gamez AS, Mallet JP, Serre I, et al. Poor concordance between sequential transbronchial lung cryobiopsy and surgical lung biopsy in the diagnosis of diffuse interstitial lung diseases. Am J Respir Crit Care Med. 2019;199(10):1249–1256. doi: 10.1164/rccm.201810-1947OC. [DOI] [PubMed] [Google Scholar]
- 22.Troy LK, Grainge C, Corte TJ, Williamson JP, Vallely MP, Cooper WA, et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med. 2020 Feb;8(2):171–181. doi: 10.1016/S2213-2600(19)30342-X. [DOI] [PubMed] [Google Scholar]
- 23.Linhas R, Marçôa R, Oliveira A, Almeida J, Neves S, Campainha S. Transbronchial lung cryobiopsy: associated complications. Rev Port Pneumol (2006) 2017 Nov - Dec;23(6):331–337. doi: 10.1016/j.rppnen.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Almeida LM, Lima B, Mota PC, et al. Learning curve for transbronchial lung cryobiopsy in diffuse lung disease. Pulmonology. 2018;24(1):23–31. doi: 10.1016/j.rppnen.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Hetzel J, Maldonado F, Ravaglia C, Wells AU, Colby TV, Tomassetti S, et al. Transbronchial Cryobiopsies for the Diagnosis of Diffuse Parenchymal Lung Diseases: Expert Statement from the Cryobiopsy Working Group on Safety and Utility and a Call for Standardization of the Procedure. Respiration. 2018;95(3):188–200. doi: 10.1159/000484055. [DOI] [PubMed] [Google Scholar]
- 26.Colella S, Haentschel M, Shah P, Poletti V, Hetzel J. Transbronchial Lung Cryobiopsy in Interstitial Lung Diseases: Best Practice. Respiration. 2018;95:383–391. doi: 10.1159/000488910. [DOI] [PubMed] [Google Scholar]
- 27.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 28.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 29.Klech H, Pohl W. Technical recommendations and guidelines for bronchoalveolar lavage (BAL). Report of the European Society of Pneumology Task Group. Eur Respir J. 1989;2:561–585. [PubMed] [Google Scholar]
- 27.Dias C, Mota P, Neves I, Guimarães S, Souto Moura C, Morais A. Transbronchial cryobiopsy in the diagnosis of desquamative interstitial pneumonia. Rev Port Pneumol (2006) 2016 Sep-Oct;22(5):288–90. doi: 10.1016/j.rppnen.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Ravaglia C, Wells AU, Tomassetti S, Gurioli C, Gurioli C, Dubini A, et al. Diagnostic yield and risk/benefit analysis of trans-bronchial lung cryobiopsy in diffuse parenchymal lung diseases: a large cohort of 699 patients. BMC Pulm Med. 2019 Jan 16;19(1):16. doi: 10.1186/s12890-019-0780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah SS, Tsang V, Goldstraw P. Open lung biopsy: a safe, reliable and accurate method for diagnosis in diffuse lung disease. Respiration. 1992;59(4):243–6. doi: 10.1159/000196066. [DOI] [PubMed] [Google Scholar]
- 30.Morell F, Reyes L, Doménech G, Gracia J, Majó J, Ferrer J. Diagnoses and diagnostic procedures in 500 consecutive patients with clinical suspicion of interstitial lung disease. Arch Bronconeumol. 2008 Apr;44(4):185–91. [PubMed] [Google Scholar]
- 31.Rotolo N, Imperatori A, Dominioni L, Facchini A, Conti V, Castiglioni M, et al. Efficacy and safety of surgical lung biopsy for interstitial disease. Experience of 161 consecutive patients from a single institution in Italy. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:251–258. [PubMed] [Google Scholar]
- 32.Jonhanson KA, Marcoux VS, Ronksley PE, Ryerson CJ. Diagnostic yield of transbronchial lung cryobiopsy for interstitial lung diseases, a systematic review and metaanalyses. Ann Am Thorac Soc. 2016;13:828–38. doi: 10.1513/AnnalsATS.201606-461SR. [DOI] [PubMed] [Google Scholar]
- 33.Maldonado F, Danoff SK, Wells AU, Colby TV, Ryu JH, Liberman M, et al. Transbronchial Cryobiopsy for the Diagnosis of Interstitial Lung Diseases CHEST Guideline and Expert Panel Report. Chest. 2019 Nov 27 doi: 10.1016/j.chest.2019.10.048. doi: 10.1016/j.chest.2019.10.048. [DOI] [PubMed] [Google Scholar]


