Abstract
Alpha1-antitrypsin deficiency (AATD) is an autosomal codominant disease, and different genetic variants are known, some of which very rare. Usual pulmonary manifestations include emphysema, bronchiectasis and asthma. Pulmonary fibrosis is uncommon. We describe a case of a 64 year old man with an inaugural diagnosis of cirrhosis and lung fibrosis, without emphysema or bronchiectasis, associated with AATD. Further investigation identified a rare variant in heterozigosity (MMPalermo), usually associated with liver disease. Concomitantly, he had a secondary iron overload, and in the course of the investigation, a type 2 diabetes mellitus installed. The association between AATD and pulmonary fibrosis is rare, however it has been identified in a few studies and case reports, questioning the role of AAT in pulmonary fibrosis. (Sarcoidosis Vasc Diffuse Lung Dis 2020; 37 (4): e2020019)
Keywords: Alpha1-antitrypsin deficiency, pulmonary fibrosis, liver diseases
Introduction
Alpha-1 antitrypsin (AAT) deficiency is a rare, underdiagnosed inherited autosomal codominant condition, caused by mutations in the SERPINA1 gene, resulting in a reduction and/or dysfunction of circulating AAT. Several variants are known. Normal individuals are described as MM, and disease is most frequently seen when S and/or Z alleles are present (1).
Alpha-1 antitrypsin deficiency (AATD) increases the risk of lung and liver disease, especially when the Z allele occurs. The first is consequential to low AAT and usually manifests as early onset chronic obstructive lung disease (COPD), panlobar emphysema and bronchiectasis (1). Pulmonary fibrosis is uncommon. The latter is caused by the accumulation of AAT polymers, and not by its scarcity (1). Therefore only some variants may promote liver disease, such as the Z, SIiyama, MDuarte and MMalton but also S, MPalermo, MNichinan and MWurzburg.
Clinical case
We present a case of a 64 year old male, former smoker of 15 pack-years with past medical history of malaria and obesity, admitted at outpatient clinic for suspicion of chronic liver disease and hemochromatosis. A diagnosis of cirrhosis (Child-Pugh B7 and MELD-Na 14, and signs of hypertensive gastropathy) was established.
Although there was a known alcohol consumption of approximately 90gr/alcohol/day, an extended etiological study was preformed, identifying an AAT value of 70,9 mg/dL (nephelometry), and an iron overload (total iron [Fe] of 204ug/dL; ferritin of 1078ng/ml and transferrin saturation of 99%), without primary hemochromatosis (heterozigosity for the H63D gene). Immune, copper and virology tests were negative.
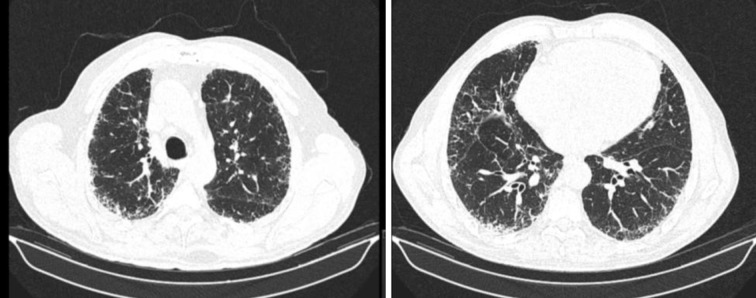
Simultaneously, the patient complained of fatigue, cough and exertion dyspnoea, without relevant findings on physical examination. Further investigation revealed a pattern of probable UIP and no emphysema in high-resolution computed tomography (HRCT) (Figure 1). Bronchoscopy was unremarkable, with no microbiological agents identified, and a bronchoalveolar lavage (BAL) showing increased cellularity, with 94,0% macrophages, 5,0% lymphocytes, 1,0% neutrophils, and 0% mast cells and eosinophils. Lung function tests (LFT) were normal [post-BD FEV1/FVC 87,8; post-BD FEV1 98,4%pred; post-BD FVC 87,1%pred; TLC 89,7%pred and RV 113,9%pred], except for a moderately decreased diffusing capacity for CO (DLCO) [49% predicted]. Arterial blood gas showed hypoxemia of 68mmHg and the six-minute walking test showed a 10% decrease in peripheral oxygen saturation. No occupational risk exposures were described, and autoantibodies screening and rheumatologic evaluation were negative.
Fig. 1.
Chest high resolution computed tomography, showing subpleural reticulation, scarce traction bronchiectasis, without predominantly basal distribution. Defined as a probable usual interstitial pneumonia (UIP) pattern.
Multiplex polymerase chain reaction combined with restriction fragment length polymorphism (2) detected a heterozygous MPalermo allele (M1MPalermo).
During the investigation, the patient presented hyperglycaemias above 200mg/dL and a glycated haemoglobin of 13.6%, hence diagnosing type-2 diabetes mellitus.
Thus the final diagnoses of concomitant type-2 diabetes, alcoholic and AATD cirrhosis, secondary iron overload, and idiopathic pulmonary fibrosis (IPF) were established. For the first, blood glucose control was achieved with long-acting insulin, for the second, alcohol abstinence was implemented, and for the latter pirfenidone was started as well as portable oxygen concentrator.
The patient’s two daughters were tested, showing normal AAT levels.
At 6-months the patient remains stable, without hospital or emergency admissions.
Discussion
We report a case of liver cirrhosis and lung fibrosis associated with AATD, the latter representing a rare finding in this disease. Regarding the pulmonary fibrosis, AATD increases neutrophil elastase activity, promoting the destruction of lung structures such as matrix components and alveoli (3). Emphysema and bronchiectasis are the usual pulmonary disorders associated with AATD. Our patient presented pulmonary fibrosis with a radiologic pattern of probable UIP.
Our patient also presented a mutation of the H63D gene. Sangiuolo et al (4). compared the BAL of IPF patients with non-IPF patients, and demonstrated that iron-dependent oxygen radical generation was increased in IPF. Interestingly they also showed that the H63D HFE allele was significantly more frequent in IPF patients than in controls. Further studies are necessary to validate the hypothesis of iron-dependent oxidant generation association with the carriage of HFE allelic variants in IPF.
We performed a literature review to understand the rarity of this variant, its clinical significance, to understand whether AATD is associated with lung fibrosis and if the concomitant diagnosis of both conditions is usual, or not.
The patient’s AATD was associated to a heterozygote MPalermo allele (MMPalermo), with a p.Phe52del mutation (NM_000295.5(SERPINA1):c.221_223TCT[2] (p.Phe76del)). This allele is uncommon and shares similar characteristics to the MMalton allele, both characterized by a p.Phe52del (NM_000295.5(SERPINA1):c.221_223TCT[2] (p.Phe76del)) mutation, however MPalermo is linked to M1Val213 allele, and the other to M2 basic allele (5).
Both variants share similar pathologic mechanisms, producing a poorly formed protein with loop-sheet polymers, which becomes sequestered in the endoplasmic reticulum of hepatocytes, resulting in low AAT serum concentration (6). The intracellular accumulation and reduced secretion of the protein increases the risk of hepatic disease, even in heterozigosity (5). This, associated with alcohol intake, may explain our patient’s severe liver disease.
A Portuguese study on rare AAT variants, published by a national reference laboratory identified, between 2009 and 2013, twelve different rare variants among 51 patients, of which MPalermo was present in eight (15.7%) (5).
Ferrarotti et al. published an analysis of the Italian Registry for Severe AATD database over a 98-month span. From a total of 2922 subjects, rare alleles were identified in 37 patients with intermediate to severe AATD, of which 21 were heterozygote with a normal M allele. These showed a mean AAT level of 61 mg/dL, similar to our patient (7).
Calabró et al. reported a case of a ZZ patient with AATD and a pulmonary presentation of UIP pattern, without emphysema or bronchiectasis, and without liver involvement. It was also described how certain molecules (TNF-α, IL-1, metalloproteinases and cathepsins) overexpressed in IPF patients’ BAL are also affected by AAT (3).
Michalski et al. conducted a study to determine whether AAT phenotypes were related to pulmonary fibrosis in rheumatoid arthritis (RA) and systemic sclerosis (SSc) patients. They concluded that RA patients with M1M2 phenotype were more likely to develop pulmonary fibrosis. The same was not true for SSc patients (8).
In AATD, neutrophil elastase degradation is markedly reduced, leading to an increase of its activity against lung matrix components and alveolar structures, resulting in the usual panlobar emphysema presentation (3). Neutrophil elastase activity is also increased in IPF, which is not surprising considering the usual high BAL neutrophil count in IPF patients (9,10).
Certain mediators overexpressed in IPF patients’ BAL (TNF-α, IL-1, metalloproteinases and cathepsins) are also affected by AAT (3), and AAT is responsible for the elimination of reactive oxygen species produced by neutrophils via the NADPH oxidase enzyme as well (3). MUC5B promoter polymorphisms, a known genetic risk factor for IPF, lead to mucus hypersecretion and mucociliary dysfunction. This and other mucin genes are also upregulated by neutrophil elastase (11). Neutrophil elastase may also induce fibroblast proliferation and myofibroblast differentiation (12). All these mechanisms may indicate an increased tissue remodelling, which is characteristic of diseases such as IPF.
On a similar fashion, in AATD, the reduced AAT serum levels and consequential low inhibition of neutrophil elastase may result in similar lung damage, such as fibrosis.
Finally, an expert panel from Italy recently published a commentary on the ERS AATD statement, about respiratory disorders other than emphysema caused by AATD. The evidence is greater in bronchiectasis and asthma; however a brief reference to pulmonary fibrosis states its rarity in this context (13).
Regarding our patient’s cirrhosis, he presented only a heterozygote mutation of H63D therefore not fulfilling diagnostic criteria for primary hemochromatosis. He presented a non-inherited form of the disease, defined as secondary iron overload or secondary hemochromatosis, where hepcidin deficiency is consequential to conditions such as erythropoiesis disorders, chronic liver disease, metabolic syndrome or excessive alcohol intake (14). The clinical significance of H63D heterozigosity is controversial (15), since it occurs in about 13,6% of European population (4). Some authors claim it increases susceptibility to cirrhosis in alcohol liver disease and in viral hepatitis (4).
Cirrhosis aetiology, in our patient, was most likely the result of both moderate alcohol consumption and a rare AAT variant causing its accumulation in the hepatocytes.
Conclusion
We present a case of cirrhosis and pulmonary fibrosis, in a patient with past history of smoking, excessive alcohol intake and a rare genomic variant resulting in an AATD. This rare variant is associated with liver disease, and although in heterozigosity, combined with the alcohol consumption, is the likely cause of cirrhosis. Pulmonary fibrosis is a rare pattern for AATD, with few documented cases. More studies are required to understand whether AATD plays a role in pulmonary fibrosis physiopathology, or if the development of an independent lung disease such as IPF is coincidental.
References
- 1.Lopes AP, Mineiro MA, Costa F, et al. Portuguese consensus document for the management of alpha-1-antitrypsin deficiency. Pulmonology. 2018;24:1–21. doi: 10.1016/j.pulmoe.2018.09.004. doi:10.1016/j.pulmoe.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Meira L, Boaventura R, Seixas S, Sucena M. Alpha-1 Antitrypsin Deficiency Detection in a Portuguese Population. COPD J Chronic Obstr Pulm Dis. 2018;15(1):4–9. doi: 10.1080/15412555.2017.1414779. doi:10.1080/15412555.2017.1414779. [DOI] [PubMed] [Google Scholar]
- 3.Calabrò AG, Torricelli E, Rosi E, et al. SM Gr up SM Journal of Case Reports Idiopathic Pulmonary Fibrosis Associated with Alpha1-Antitrypsin Deficiency : Concomitant Finding or Real Association. 2017;3(8):1–3. [Google Scholar]
- 4.Sangiuolo F, Puxeddu E, Pezzuto G, et al. HFE gene variants and iron-induced oxygen radical generation in idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(2):483–490. doi: 10.1183/09031936.00104814. doi:10.1183/09031936.00104814. [DOI] [PubMed] [Google Scholar]
- 5.Silva D, Oliveira MJ, Guimarães M, Lima R, Gomes S, Seixas S. Alpha-1-antitrypsin (SERPINA1) mutation spectrum: Three novel variants and haplotype characterization of rare deficiency alleles identified in Portugal. Respir Med. 2016;116:8–18. doi: 10.1016/j.rmed.2016.05.002. doi:10.1016/j.rmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Joly P, Guillaud O, Hervieu V, Francina A, Mornex JF, Chapuis-Cellier C. Clinical heterogeneity and potential high pathogenicity of the Mmalton Alpha 1 antitrypsin allele at the homozygous, compound heterozygous and heterozygous states. Orphanet J Rare Dis. 2015;10(1):1–7. doi: 10.1186/s13023-015-0350-6. doi:10.1186/s13023-015-0350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrarotti I, Baccheschi J, Zorzetto M, et al. Prevalence and phenotype of subjects carrying rare variants in the Italian registry for alpha1-antitrypsin deficiency. J Med Genet. 2005;42(3):282–287. doi: 10.1136/jmg.2004.023903. doi:10.1136/jmg.2004.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michalski JP, McCombs CC, Scopelitis E, Biundo JJ, Medsger TA. Alpha1-antitrypsin phenotypes, including M subtypes, in pulmonary disease associated with rheumatoid arthritis and systemic sclerosis. Arthritis Rheum. 1986;29(5):586–591. doi: 10.1002/art.1780290502. doi:10.1002/art.1780290502. [DOI] [PubMed] [Google Scholar]
- 9.Kristensen JH, Karsdal MA, Sand JMB, et al. Serological assessment of neutrophil elastase activity on elastin during lung ECM remodeling. BMC Pulm Med. 2015;15(1):1–7. doi: 10.1186/s12890-015-0048-5. doi:10.1186/s12890-015-0048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugino K, Nakamura Y, Muramatsu Y, Hata Y, Shibuya K, Homma S. Analysis of blood neutrophil elastase, glutathione levels and pathological findings in postoperative acute exacerbation of idiopathic pulmonary fibrosis associated with lung cancer: Two case reports. Mol Clin Oncol. 2016;5(4):402–406. doi: 10.3892/mco.2016.993. doi:10.3892/mco.2016.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt AMD, Glasgow AMA, Humphreys H, Greene CM. Alpha-1 antitrypsin—a target for microRNA-based therapeutic development for cystic fibrosis. Int J Mol Sci. 2020;21(3):1–19. doi: 10.3390/ijms21030836. doi:10.3390/ijms21030836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregory AD, Kliment CR, Metz HE, et al. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. J Leukoc Biol. 2015;98(2):143–152. doi: 10.1189/jlb.3HI1014-493R. doi:10.1189/jlb.3hi1014-493r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gramegna A, Aliberti S, Confalonieri M, et al. Alpha-1 antitrypsin deficiency as a common treatable mechanism in chronic respiratory disorders and for conditions different from pulmonary emphysema? A commentary on the new European Respiratory Society statement 11 Medical and Health Sciences 1102 Cardi. Multidiscip Respir Med. 2018;13(1):1–10. doi: 10.1186/s40248-018-0153-4. doi:10.1186/s40248-018-0153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kowdley K V, Brown KE, Ahn J, Methodologist FG. ACG Clinical Guideline : Hereditary Hemochromatosis. 2019;114(August):1202–1218. doi: 10.14309/ajg.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 15.Gurrin LC, Bertalli NA, Dalton GW, et al. HFE C282Y/H63D compound heterozygotes are at low risk of hemochromatosis-related morbidity. Hepatology. 2009;50(1):94–101. doi: 10.1002/hep.22972. doi:10.1002/hep.22972. [DOI] [PMC free article] [PubMed] [Google Scholar]