Methanomassiliicoccales are less-known members of the human gut archaeome. Members of this order use methylated amines, including trimethylamine, in methane production.
KEYWORDS: Methanomassiliicoccales, archaea, comparative genomics, human gut, metagenomes, microbiome
ABSTRACT
Archaea of the order Methanomassiliicoccales use methylated amines such as trimethylamine as the substrates for methanogenesis. They form two large phylogenetic clades and reside in diverse environments, from soil to the human gut. Two genera, one from each clade, inhabit the human gut: Methanomassiliicoccus, which has one cultured representative, and “Candidatus Methanomethylophilus,” which has none. Questions remain regarding their distribution across biomes and human populations, their association with other taxa in the gut, and whether host genetics correlate with their abundance. To gain insight into the Methanomassiliicoccales clade, particularly its human-associated members, we performed a genomic comparison of 72 Methanomassiliicoccales genomes and assessed their presence in metagenomes derived from the human gut (n = 4,472, representing 22 populations), nonhuman animal gut (n = 145), and nonhost environments (n = 160). Our analyses showed that all taxa are generalists; they were detected in animal gut and environmental samples. We confirmed two large clades, one enriched in the gut and the other enriched in the environment, with notable exceptions. Genomic adaptations to the gut include genome reduction and genes involved in the shikimate pathway and bile resistance. Genomic adaptations differed by clade, not habitat preference, indicating convergent evolution between the clades. In the human gut, the relative abundance of Methanomassiliicoccales spp. correlated with trimethylamine-producing bacteria and was unrelated to host genotype. Our results shed light on the microbial ecology of this group and may help guide Methanomassiliicoccales-based strategies for trimethylamine mitigation in cardiovascular disease.
IMPORTANCE Methanomassiliicoccales are less-known members of the human gut archaeome. Members of this order use methylated amines, including trimethylamine, in methane production. This group has only one cultured representative; how its members adapted to inhabit the mammalian gut and how they interact with other microbes is largely unknown. Using bioinformatics methods applied to DNA from a wide range of samples, we profiled the abundances of these Archaea spp. in environmental and host-associated microbial communities. We observed two groups of Methanomassiliicoccales, one largely host associated and one largely found in environmental samples, with some exceptions. When host associated, these Archaea have smaller genomes and possess genes related to bile resistance and aromatic amino acid precursors. We did not detect Methanomassiliicoccales in all human populations tested, but when present, they were correlated with bacteria known to produce trimethylamine. Due to their metabolism of trimethylamine, these intriguing Archaea may form the basis of novel therapies for cardiovascular disease.
INTRODUCTION
Archaea spp. generally make up a tenth or less of the biomass of the human gut microbiota; however, they are widely prevalent and occupy a unique metabolic niche, utilizing byproducts of bacterial metabolism as the substrates for methanogenesis (1). Members of Methanobacteriales are the dominant species of the human gut archaeome (1, 2). These include Methanobrevibacter smithii, which uses CO2, formate and H2 as the substrates for methane production (3), and Methanosphaera stadtmanae, which consumes methanol and H2 (4). Through methanogenesis, Archaea decrease partial pressures of H2, potentially increasing the energetic efficiency of primary fermenters and the production of short-chain fatty acids (5).
A second archaeal lineage, the order Methanomassiliicoccales, is also found within the human gut, yet its members are less well characterized than those of Methanobacteriales. Members of this order, including human-derived Methanomassiliicoccus luminyensis, “Candidatus Methanomassiliicoccus intestinalis,” and “Candidatus Methanomethylophilus alvus,” perform H2-dependent methylotrophic methanogenesis for their sole energy source (6–8). Their genomes encode several methyltransferases and associated proteins that reduce methylamines, methanol, and methylated sulfides to methane (9). Studies based on 16S rRNA and mcrA gene diversity analysis indicate that the order Methanomassiliicoccales is made up of two large clades, which mostly group species that have either a free-living (FL) or host-associated (HA) lifestyle (10, 11). Based on analyses of the genomes from three human-derived species from both clades, Borrel et al. (9) suggested that each clade colonized the mammalian gut independently. Members of the HA clade, including the human-associated “Ca. M. alvus,” might be expected to show adaptations similar to those of other methanogens from the gut microbiota (12, 13). How members of the FL clade, including the human-associated M. luminyensis and “Ca. M. intestinalis,” have converged on the gut niche remains to be explored.
A better understanding of the ecology of Methanomassiliicoccales may be of interest to human health, as they can utilize mono-, di-, and trimethylamine (TMA) as the substrates for methanogenesis in the gut (14). TMA, a byproduct of bacterial metabolism of carnitine, choline, and other compounds, is transformed in the liver into trimethylamine N-oxide (TMAO) (15). Circulating TMAO inhibits cholesterol transport and promotes its accumulation in macrophages, inducing the formation of atherosclerotic plaques (16). Decreasing TMA levels in the gut and reducing circulating TMAO levels have been proposed as a therapeutic strategy for cardiovascular disease (17). One way to use the gut microbiome to this end would be to boost levels of Methanomassiliicoccales (18). To accomplish this goal requires a deeper understanding of its ecology.
Here, we conducted a comparative analysis of 71 Methanomassiliicoccales genomes, together with an additional metagenome-assembled genome (MAG) corresponding to a strain of “Ca. M. alvus” that we retrieved by metagenome assembly of gut samples from subjects of the United Kingdom Adult Twin Registry (TwinsUK) cohort (19). We used 305 publicly available metagenomes to assess the prevalence of taxa across various habitat types. While the two large clades grouping host-associated (HA) and free-living (FL) taxa are generally enriched in host-associated and environmental metagenomes, a few exceptions stand out. Our results showed that the repertoire of adhesion proteins encoded by the genomes of taxa from each clade tended to differ. Genes involved in bile resistance and the shikimate pathway are likely involved in the adaptation to the gut environment of members of the HA clade, but not for the FL clade. Thus, gut-adapted members converged on life in the gut using different genomic adaptations. Methanomassiliicoccales genera present in the human gut positively correlate with TMA-producing bacteria.
RESULTS
Genome-based phylogeny confirms two large Methanomassiliicoccales clades.
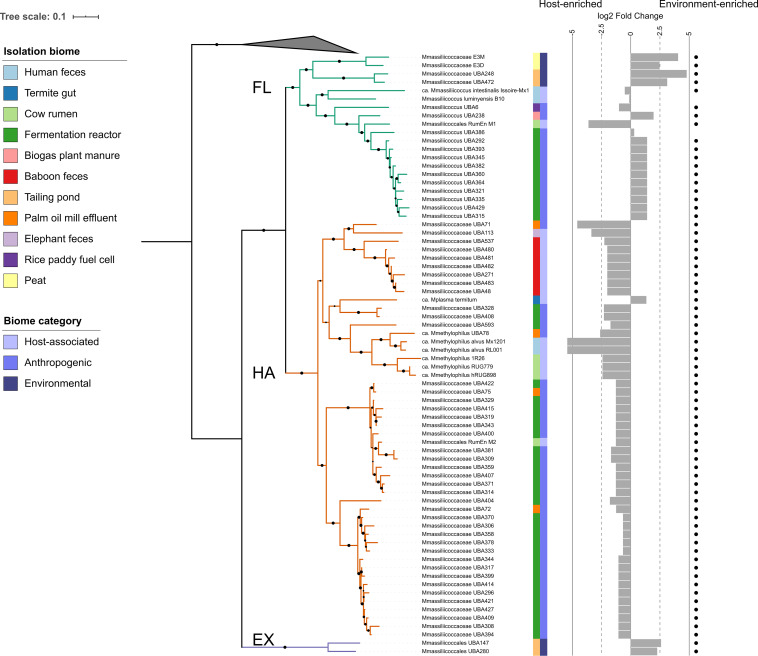
Based on whole-genome phylogenetic analysis, the order Methanomassiliicoccales forms two clades with robust support (Fig. 1). This phylogeny is in agreement with previously reported phylogenies based on 16S rRNA and mcrA genes (10, 20, 21). A third distal clade was formed by two closely related MAGs generated in a recent massive metagenome assembly effort (22), which we labeled external (EX) (Fig. 1). We use the terminology of Borrel et al. (23), as follows: the clade including Methanomassiliicoccus is labeled free living (FL), and the clade containing “Candidatus Methanomethylophilus” is labeled host associated (HA).
FIG 1.
The order Methanomassiliicoccales forms two large clades that loosely follow the source of isolation. Maximum-likelihood phylogeny of concatenated single-copy marker genes. The gray triangle corresponds to Thermoplasma acidophilum, Picrophilus oshimae, Ferroplasma acidarmanus, Acidiplasma aeolicum, and Cuniculiplasma divulgatum, which are outgroup taxa from class Thermoplasmata. Black circles indicate bootstrap values of >80 (of 1,000 bootstrap permutations), branch color represents the clade, and the scale bar represents the number of amino acid substitutions per site. Colored strips show the source of isolation of each of the included genomes and the general category to which the source belongs. Bar plots show the genome abundance enrichment in gut metagenome samples compared to environmental samples, calculated using DESeq2; dots indicate taxa with significant enrichment in either host or environmental biomes (adj. P < 0.05). Mmassiliicoccaceae, Methanomassiliicocceae; Mmassiliicoccus, Methanomassiliicoccus; Mplasma, Methanoplasma; Mmethylophilus, Methanomethylophilus; Mmassiliicoccales, Methanomassiliicoccales.
As observed previously (10), the reported source of the genomes was not always consistent with the clade in which it was grouped. For instance, while publicly available genomes originally retrieved from human, baboon, elephant, and cow gastrointestinal tracts were related to “Candidatus Methanomethylophilus” (HA), this clade also contained MAGs derived from digestors and reactors (Fig. 1) reportedly not treating animal waste (see Table S1A in the supplemental material). Moreover, MAGs retrieved from pit mud of solid-state fermentation reactors used for the production of Chinese liquor were present in both the HA and FL clades (Table S1A). Similarly, “Ca. M. intestinalis” Issoire-Mx1, M. luminyensis B10, and Methanomassiliicoccales archaeon RumEn M1, all retrieved from mammal hosts, grouped in the FL clade.
(A) NCBI assembly accession number, genome characteristics, study information, and source of isolation of 71 publicly available genomes from the order Methanomassiliicoccales retrieved from NCBI in June 2018, plus the “Ca. M. alvus” metagenome-assembled genome (MAG) reported here. Study accession and title of UBA genomes obtained from supplementary tables of Parks et al., 2017 (https://doi.org//10.1038/s41564-017-0012-7) (22), otherwise, obtained from the NCBI BioProject database. (B) SRA and MGnify accession information of publicly available metagenome samples from gastrointestinal and environmental biomes. (C) SRA, study, and country information of publicly available human gut metagenome samples. (D) Prevalence and mean abundance of “Candidatus Methanomethylophilus” and Methanomassiliicoccus taxa across multiple human populations. (E) InterPro, eggNOG, and Prokka annotations of gene clusters significantly enriched in clade FL compared to clade HA. (F) Coabundance and cooccurrence measures of positively associated taxa of the human gut microbiota with “Ca. Methanomethylophilus” and Methanomassiliicoccus. For each of the two Methanomassiliicoccales genera, the observed cooccurrences, expected cooccurrences, adjusted P values of cooccurrence, and association of abundances across samples (rho) are provided. (G) Intraclass correlation coefficients (ICC) and adjusted P values of relative abundances of Methanomassiliicoccus and other control taxa in monozygotic and dizygotic twins. Download Table S1, XLSX file, 0.2 MB (238KB, xlsx) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Abundance of Methanomassiliicoccales clades differs in gastrointestinal and environmental samples.
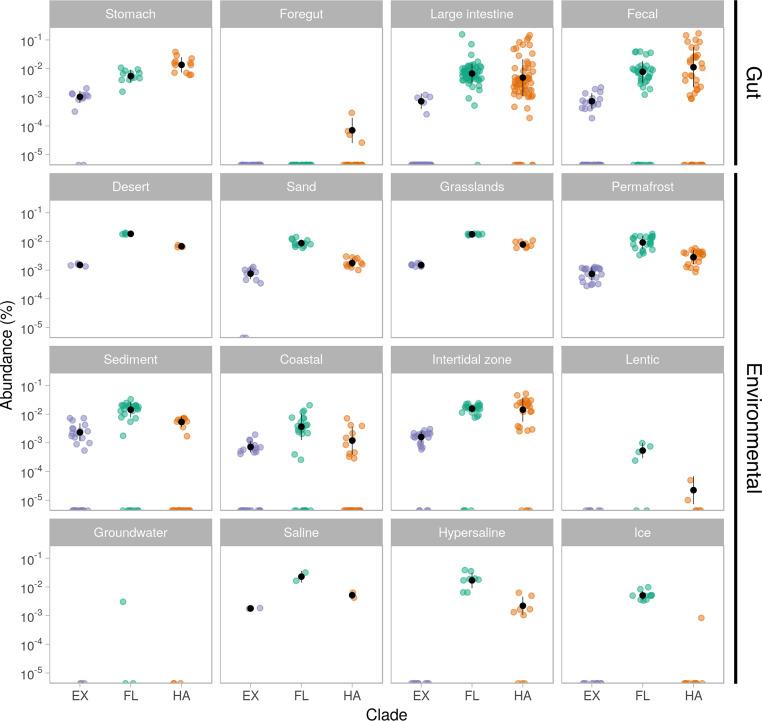
We assessed the abundance of species-level representative Methanomassiliicoccales taxa in publicly available metagenomes that included 145 samples from gastrointestinal tracts of nonhuman animals, such as cats, pigs, elks, cows, mice, white-throated woodrats, trout, chickens, and geese, and 160 environmental samples from sediment, ice, and diverse water and soil sources (Table S1B).
Taxa from all three clades were detected in a wide range of metagenomes from environmental and gut origin. We observed differences in environmental preference by clade. Abundance of taxa from clade EX was highest in environmental metagenomes (0.001% ± 0.0012%) (Fig. 1). These were also detected in gut samples (0.0002% ± 0.0005%), albeit with a very low abundance, and in in fecal (0.0003% ± 0.0005), large intestine (0.0001% ± 0.0002%), and stomach (0.0009% ± 0.0006%) metagenomes (Fig. 2). Given their low abundances, further analysis is focused on the FL and HA clades.
FIG 2.
Methanomassiliicoccales clades are widespread but not abundant across a range of environments and animal hosts. Combined abundance of representative genomes of the EX (purple), FL (green), and HA (orange) clades in metagenome samples from diverse biomes, as follows: stomach (n = 12), foregut (n = 23), large intestine (n = 66), fecal (n = 44), desert (n = 4), sand (n = 12), grasslands (n = 8), permafrost (n = 22), sediment (n = 31), coastal (n = 28), intertidal zone (n = 25), lentic (n = 6), groundwater (n = 3), saline (n = 2), hypersaline (n = 9), and ice (n = 10). Abundances were calculated for individual genomes using KrakenUniq and aggregated by clade. The y axis is in logarithmic scale; black points indicate mean relative abundance in percentage, and black bars indicate standard deviation.
The aggregated abundance of clades FL and HA differed across biomes (Fig. 2). In agreement with their names, HA clade members were more abundant in host-associated samples, and FL in non-host-associated samples (Fig. 2). The prevalence and abundance of Methanomassiliicoccales taxa varied across animal hosts, yet the overall abundance patterns were consistent across hosts and sample types (see Fig. S2 in the supplemental material).
The combined abundance of members of clade FL was higher in samples from environmental biomes (0.01% ± 0.008%), although nonzero abundances were observed in digestive system metagenomes (0.008% ± 0.015%), with some samples containing levels comparable to that of clade HA (Fig. 2).
The mean abundance of clade HA in aggregate was higher in metagenomes from gut samples (0.014% ± 0.03%) compared to those from environmental biomes (0.004% ± 0.008%). However, among the environmental biomes, nonzero abundances of clade HA were detected in freshwater (0.002% ± 0.003%), marine (0.006% ± 0.011%), saline and alkaline (0.002% ± 0.002%), and soil (0.004% ± 0.003%) samples.
We further validated the differences in clade abundances across biomes by generating a dendrogram of Methanomassiliicoccales taxa using the DESeq2-based log fold change of individual taxa on gut versus environmental biomes (i.e., the effect size of the test as a measure of enrichment on a given environment). We then compared the structure of this dendrogram with that of the phylogenomic tree and found that they were positively correlated (cophenetic correlation = 0.67; P < 0.01).
Overall, we observed a low abundance of individual Methanomassiliicoccales taxa across all samples, ranging from 0 to 0.15% (Fig. 2 and Fig. S1). Their prevalence across hosts differed; they were prevalent in animals such as elks, pigs, poultry, and cattle, while in others, such as trout and geese, they were largely absent (see Fig. S3 in the supplemental material). The enrichment analysis of individual taxa from clade FL from diverse biomes showed that while most were significantly enriched in environmental metagenomes (adjusted [adj.] P < 0.1), some taxa showed the opposite enrichment. M. luminyensis and Methanomassiliicoccus sp. UBA386 were not significantly enriched in gut or environmental biomes. “Ca. M. intestinalis” Issoire-Mx1, Methanomassiliicoccales archaeon RumEn M1, and Methanomassiliicoccus sp. UBA6 were significantly enriched in gut biomes (Fig. 1), although they were also present in multiple environmental biomes (fig. S1).
Methanomassiliicoccales taxa from all clades are widespread but not abundant across a range of environments and animal hosts. The abundances of members of the free-living (FL) and host-associated (HA) clades are comparable within similar biomes, particularly in animal-derived metagenomes. Abundance of each representative genome on diverse metagenome and environmental metagenome samples colored by clade (green, FL; orange, HA; purple, external [EX]). Abundances calculated for individual genomes using KrakenUniq and aggregated by clade. Note that the y axis is in logarithmic scale and each plot has a different scale. Black points indicate mean relative abundance in percentage, and black bars indicate standard deviation. Metagenome samples from stomach (n = 12), foregut (n = 23), large intestine (n = 66), fecal (n = 44), desert (n = 4), sand (n = 12), grasslands (n = 8), permafrost (n = 22), sediment (n = 31), coastal (n = 28), intertidal zone (n = 25), lentic (n = 6), groundwater (n = 3), saline (n = 2), hypersaline (n = 9), and ice (n = 10) biomes. Download FIG S1, TIF file, 2.0 MB (2MB, tif) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Abundance of Methanomassiliicoccales clades varies across animal hosts and sample types. Samples from the same host but different sampling sites show consistent patterns. Metagenome samples from cat (n = 30), pig (n = 30), rainbow trout (n = 23), mouse (n = 16), poultry (n = 14), goose (n = 12), cattle (n = 8), white-throated woodrat (n = 8), and Eurasian elk (n = 3). See Fig. S1 for details. Download FIG S2, TIF file, 0.5 MB (541.3KB, tif) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Animal hosts show different patterns of prevalence of individual Methanomassiliicoccales taxa. cat (n = 30), pig (n = 30), rainbow trout (n = 23), mouse (n = 16), poultry (n = 14), goose (n = 12), cattle (n = 8), white-throated woodrat (n = 8), and Eurasian elk (n = 3). See Fig. S1 for details. Download FIG S3, TIF file, 2.2 MB (2.2MB, tif) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
When assessed on a per-taxon basis, the vast majority of clade HA taxa were significantly enriched in gut samples (adj. P < 0.1), with the exception of “Candidatus Methanoplasma termitum.” which was highly abundant in soil samples from grasslands and water samples from intertidal zones (Fig. 1).
Genome characteristics and core genes functions differ between Methanomassiliicoccales clades.
Given the tendency of clades FL and HA to be enriched in environmental or animal metagenomes, respectively, we searched for genes and genome features linked to putative adaptations of Methanomassiliicoccales to an animal gut. For this, we compared 72 genomes from Methanomassiliicoccales taxa retrieved from humans, nonhuman animals, and environmental sources.
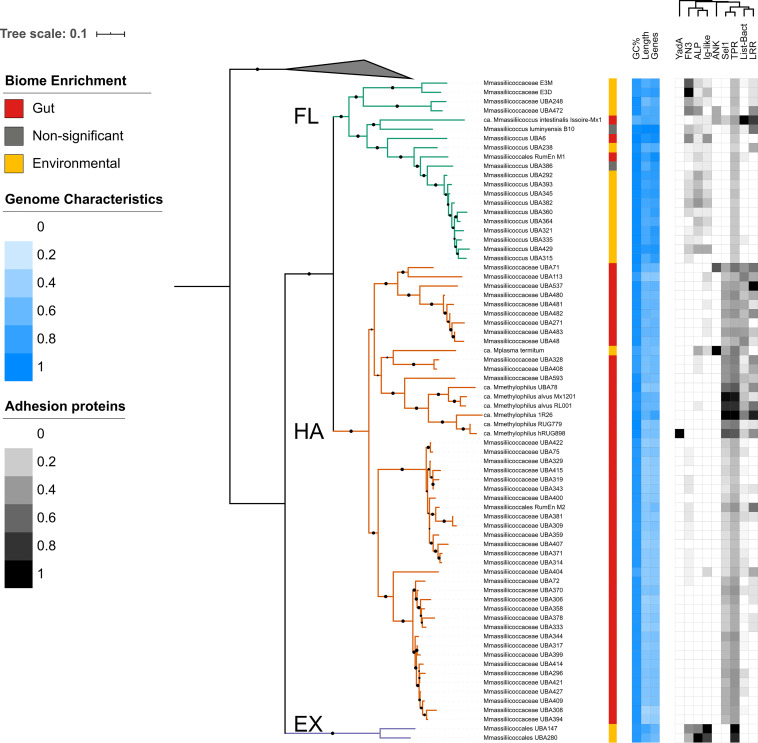
We observed that genomes were more similar to others closely located on the phylogeny in terms of genome GC content, genome length and total gene count (local indicator of phylogenetic association [LIPA] adj. P < 0.01 in all cases) (Fig. 3). To determine whether these features differed between clades, while accounting for the autocorrelation due to evolutionary history, we performed a phylogenetic analysis of variance (ANOVA). Clade FL taxa had significantly larger genomes (mean ± standard deviation [SD], 1,985.1 ± 245.1 kb) than either clade HA (1,318.3 ± 187.3 kb) or clade EX (1,872.2 ± 173.8 kb) (phylogenetic ANOVA adj. P = 0.028). In accordance with this, clade FL also had the highest gene count (FL, 2,153.1 ± 233.7 genes; HA, 1,377.7 ± 187.7 genes; EX, 1,567.0 ± 90.5 genes; adj. P = 0.025). While this was nonsignificant, clades HA and EX taxa tended to have a lower GC content than clade FL taxa (FL, 59.1% ± 4.8%; HA, 55.8% ± 2.8%; EX, 54.4% ± 0.5%; adj. P = 0.6).
FIG 3.
Genome characteristics and adhesion proteins of Methanomassiliicoccales reflect division of the order into clades. Note that members of clade FL not enriched in environmental biomes resemble those of clade HA. The phylogeny is the same as that shown in Fig. 1. The colored strip summarizes the biome enrichment analysis. Heatmaps show genome features, including genome GC content (“GC%,” range, 41.26% to 62.74%), genome length (“Length,” 969.311 bp to 2.620.233 bp), and number of predicted genes (“Genes,” 1,057 to 2,607) (blue scale), or a repertoire of eukaryote-like proteins: YadA-like domain (YadA; 0, 1), fibronectin type III (FN3; 0, 20) domains, bacterial Ig-like domains (Ig-like; 0, 12), ankyrin repeats (ANK; 0, 3), Sel1-containing proteins (Sel1; 0, 29), tetratricopeptide repeats (TPR; 7, 40), Listeria-Bacteroides repeat-containing proteins (List-Bact; 0, 26), and leucine-rich repeats (LRR; 0, 9) (gray scale shows columns ordered by hierarchical clustering); and adhesion-like proteins (ALP; 0, 12). On both heatmaps, the color intensity of each feature is relative to the maximum value of each category.
To compare gene presence and absence across clades, we performed a pangenome analysis. After identification of orthologous gene clusters based on sequence similarity using panX software, we obtained 13,695 clusters, of which 7,312 were present at least once in clade FL, 6,592 in clade HA, and 1,833 in clade EX. A large proportion of gene clusters were of unknown function according to the clusters of orthologous genes (COG) functional classification (38.4% ± 4.3%); gene clusters of unknown function tended to be detected in one or two genomes (see Fig. S4A in the supplemental material).
Small clusters of unknown function dominate the pangenome of the order Methanomassiliicoccales. Gene cluster frequency spectrum of the order Methanomassiliicoccales separated by (A) unknown or (B) known function; the x axis represents the number of genomes that have at least one gene in a given cluster, and the y axis is the frequency of clusters of a given size. Note the difference in scale of the y axis between panels A and B. (C) Fraction of gene clusters belonging to each clusters of orthologous genes (COG) category per clade. Core clusters were defined as being present in ≥80% of genomes of a clade; for the complete order (i.e., all included Methanomassiliicoccales taxa without grouping them by clade), gene clusters were present in ≥80% of the included genomes and in at least one member of each clade. The proportion of clusters of unknown functions in the core genome of each clade was large and varied between clades, ranging from 23.0% in clade HA to 38.5% in clade EX. The proportion of unknown clusters was lowest in the complete taxonomic order, where it only accounted for 14.7% of gene clusters. COG functional classification descriptions by groups, as follows. Information storage and processing: B, chromatin structure and dynamic; J, translation, ribosomal structure, and biogenesis; K, transcription; L, replication, recombination, and repair. Cellular processes and signaling: D, cell cycle control, cell division, and chromosome partitioning; M, cell wall/membrane/envelope biogenesis; N, cell motility; O, posttranslational modification, protein turnover, and chaperone; T, signal transduction mechanisms; U, intracellular trafficking, secretion, and vesicular transport; V, defense mechanisms; Z, cytoskeleton. Metabolism: C, energy production and conversion; E, amino acid transport and metabolism; F, nucleotide transport and metabolism; G carbohydrate transport and metabolism; H, coenzyme transport and metabolism; I, lipid transport and metabolism; P, inorganic ion transport and metabolism; Q, secondary metabolites biosynthesis, transport, and catabolism. Poorly characterized: X, no annotation retrieved; S, function unknown. Download FIG S4, TIF file, 0.5 MB (530.7KB, tif) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
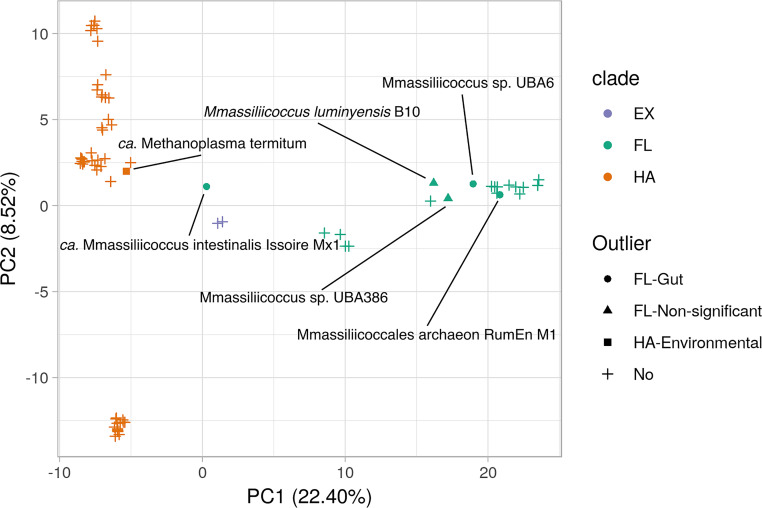
Principal component analysis (PCA) of gene cluster presence/absence differentiated clades along principal component 1 (PC1) (Fig. 4). We defined outlier taxa as FL taxa enriched in gut biomes (Methanomassiliicoccales archaeon RumEn M1, Methanomassiliicoccus sp. UBA6, “Ca. M. intestinalis” Issoire-Mx1, M. luminyensis B10, and Methanomassiliicoccus sp. UBA386) and the HA taxon enriched in non-host biomes (“Ca. M. termitum”). Outliers mostly clustered with their close relatives, not with the taxa enriched in the same biome (Fig. 4), with the exception of “Ca. M. intestinalis” Issoire-Mx1, which did not cluster with either clade.
FIG 4.
Ordination of gene content of Methanomassiliicoccales group taxa by phylogenetic clade rather than by biome enrichment. Principal-component analysis of the gene cluster presence of taxa from clades FL (green), HA (orange), and EX (purple). Highlighted points correspond to outliers, namely, taxa either not significantly enriched in environmental or gut biomes or with enrichment opposite to expectation given their clade.
Gene clusters enriched in clade HA evidence adaptation to the gut environment.
Because of the small number of genomes that cluster within clade EX, and because these are largely absent from animal-associated samples, subsequent analyses focus on comparisons between clades FL and HA.
To identify gene clusters potentially involved in the adaptation of members of Clade HA to a host environment, we compared the gene cluster content between clades. The gene cluster frequency spectrum shows many clusters present in few genomes; 7,990 (58.3%) gene clusters were singletons, and 2,002 (14.6%) were doubletons (Fig. S4A and B). After removing rare gene clusters by filtering those with near-zero variance, we included 2,937 clusters, which we then used to perform in phylogenetic ANOVA. Results reveal 14 gene clusters significantly enriched in HA compared to FL (adj. P < 0.1 in all cases) (Table 1). Three gene clusters are involved in detoxification and xenobiotic metabolism, namely, those encoding bile acid: sodium symporter, bleomycin resistance protein and HAD superfamily hydrolase. Two clusters are related to shikimate or chorismate metabolism, namely, those encoding chorismate mutase II and prephenate dehydratase. Other annotated clusters include the small unit of exonuclease VII, Holliday junction resolvase Hjc, nitrogen regulatory protein PII, xylose isomerase-like protein, and metal-binding domain containing protein; four had poor or no annotation (Table 1). Similar results were obtained when we performed this analysis without outlier taxa and when biome enrichment was used as independent variable (not shown), further indicating that genomic adaptations differ by clade, not habitat preference. Likewise, 89 clusters were enriched in clade FL compared to HA; these are presented in Table S1E.
TABLE 1.
InterPro, eggNOG, and Prokka annotations of gene clusters significantly enriched in clade HA compared to clade FL
| InterPro accession no. | InterPro annotation | NOG accession no. | COG categorya | Prokka gene name | Prokka annotation |
|---|---|---|---|---|---|
| IPR002657 | Bile acid:sodium symporter/arsenical resistance protein Acr3 | COG0385@NOG | S | Hypothetical protein | |
| IPR029068 | Glyoxalase/bleomycin resistance protein/dihydroxybiphenyl dioxygenase | - | X | Hypothetical protein | |
| IPR006357 | HAD superfamily hydrolase, subfamily IIA | COG0647@NOG | G | gph | Glyceraldehyde 3-phosphate phosphatase |
| IPR002701 | Chorismate mutase II, prokaryotic-type | COG1605@NOG | E | aroQ | Chorismate mutase |
| IPR001086 | Prephenate dehydratase | COG0077@NOG | E | pheA | Prephenate dehydratase |
| IPR003761 | Exonuclease VII, small subunit | COG1722@NOG | L | xseB | Exodeoxyribonuclease 7 small subunit |
| IPR002732 | Holliday junction resolvase Hjc | COG1591@NOG | L | rutD | Putative aminoacrylate hydrolase RutD |
| IPR015867 | Nitrogen regulatory protein PII/ATP phosphoribosyltransferase, C-terminal | COG3323@NOG | S | Hypothetical protein | |
| IPR013022 | Xylose isomerase-like, TIM barrel domain | 11IHC@NOG | L | Hypothetical protein | |
| IPR019271 | Protein of unknown function DUF2284, metal-binding | 11RTN@NOG | S | Hypothetical protein |
COG functional classification descriptions: E, amino acid transport and metabolism; L, replication, recombination, and repair; G, carbohydrate transport and metabolism; S, function unknown; X, no annotation retrieved.
Genomic adaptations to the gut of members of the FL clade.
To determine whether outlier taxa belonging to clade FL had similar adaptations to the gut to those of members of clade HA, we explored gene clusters that were present in these outliers and in clade HA but were rare in other members of clade FL. We selected gene clusters present in the core genome of clade HA (i.e., present in at least 40 taxa or 80% of this clade, see Text S1 in the supplemental material) and present in less than half of the FL taxa. A total of 15 gene clusters were obtained, most of them encoded by only one of the outlier taxa. Two gene clusters, ferrous iron transport proteins A and B (InterPro accession numbers IPR030389 and IPR007167), were present in three of the outliers (M. luminyensis, “Ca. M. intestinalis” Issoire-Mx1, and Methanomassiliicoccales archaeon RumEn M1). Other clusters detected in more than one outlier included an uncharacterized membrane protein (InterPro number IPR005182, in Methanomassiliicoccus sp. UBA6 and Methanomassiliicoccales archaeon RumEn M1), a putative nickel-responsive regulator (InterPro number IPR014864, in M. luminyensis B10 and Methanomassiliicoccus sp. UBA386), and an ABC transporter (InterPro number IPR037294, in M. luminyensis B10 and Methanomassiliicoccus sp. UBA386). The remaining gene clusters, detected once, corresponded to transcriptional regulators or proteins of unknown function.
Supplementary methods contain detailed description of the tests carried in the present work and the versions of the software and R packages used. Supplementary results contain description of the retrieval of a genome from “Candidatus Methanomethylophilus alvus” by metagenomic assembly; comparison of core gene functions between Methanomassiliicoccales clades; and assessment of Methanomassiliicoccales with methanol producers in the human gut. Download Text S1, DOCX file, 0.02 MB (23.5KB, docx) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The repertoire of adhesion proteins tended to differ between clades HA and FL.
We compared between FL and HA clades two large groups of membrane proteins involved in adhesion, namely eukaryote-like proteins (ELPs), a series of protein families involved in microbial adherence to the host (24), and adhesin-like proteins (ALPs), a class of proteins hypothesized to be involved in the microbe-microbe interactions of Methanobacteriales in the gut (13). We aggregated the counts of gene clusters annotated as the ALP and ELP classes and performed phylogenetic ANOVA. This analysis showed a trend toward differing repertoires of adhesion proteins by clade (Fig. 3), although we did not observe significant differences in the frequency of these factors (adj. P > 0.1 in all cases). Taxa from clade HA tended to have higher mean counts of tetratricopeptide repeats (TPR) (mean ± SD counts: HA, 16.30 ± 6.56, and FL. 9.55 ± 1.70), Sel1-containing repeats (Sel1) (HA, 9.32 ± 5.69, and FL, 0.35 ± 1.35), Listeria-Bacteroides repeats (List-Bact) (HA, 3.68 ± 3.76, and FL, 1.65 ± 5.78), and leucine-rich repeats (LRR) (HA, 1.5 ± 2.15, and FL, 1.1 ± 2.02) than FL taxa. Conversely, adhesin-like proteins (ALPs) (FL, 2.25 ± 1.48, and HA, 0.14 ± 0.61), Ig-like domains (FL, 1.55 ± 1.32, and HA, 0.20 ± 0.53) and fibronectin type III (FN3) domains (FL, 4.55 ± 5.09, and HA, 0.32 ± 0.62) tended to be more abundant in the genomes of members of clade FL. We did not detect invasion protein B (IalB) in any of the analyzed genomes.
Hierarchical clustering based on the presence or absence of adhesion factors largely grouped Methanomassiliicoccales taxa by clade (see Fig. S5 in the supplemental material). Additionally, all adhesion factors, with the exceptions of ankyrin repeats (ANK) and the Yersinia adhesin A-like domain (YadA), showed a significant phylogenetic signal (adj. P < 0.05 in all cases), further highlighting that closely related taxa had similar counts.
The set of adhesion proteins tends to differ by clade. The tanglegram compares a dendrogram of adhesion genes calculated using hierarchical clustering of Jaccard index matrix (left) versus the maximum-likelihood phylogeny (right). Tip labels colored by clade: HA, orange; FL, green; EX, purple. Download FIG S5, TIF file, 0.9 MB (920KB, tif) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Interestingly, outlier taxa from clade FL had gene counts of several of the adhesion factors higher than the mean of their own clade and more characteristic of clade HA. In some cases, the gene counts were higher than the mean for clade HA. These included Listeria-Bacteroides repeats (gene cluster counts: M. luminyensis, 2; “Ca. M. intestinalis” Issoire-Mx1, 26; Methanomassiliicoccales archaeon RumEn M1, 2), Sel1 repeats (M. luminyensis, 1; “Ca. M. intestinalis” Issoire-Mx1, 6), and leucine-rich repeats (M. luminyensis, 5; “Ca. M. intestinalis” Issoire-Mx1, 7).
Methanomassiliicoccales taxa cooccur with each other, with other Archaea, and with TMA-producing bacteria in the human gut.
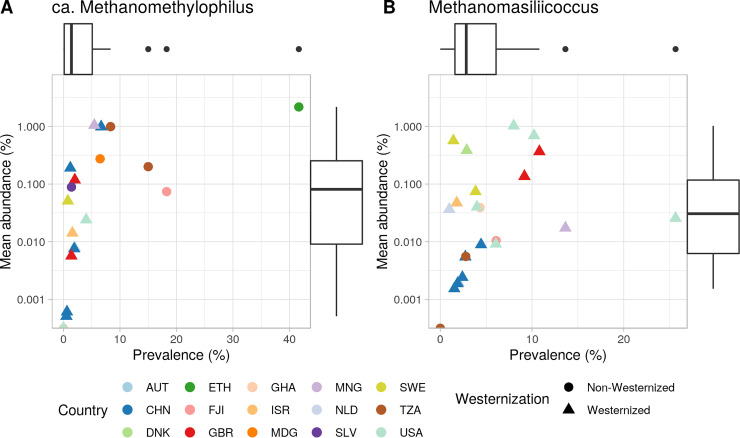
We characterized the distribution of Methanomassiliicoccales spp. across a collection of human gut metagenomes derived from 34 studies. Together, the combined 4,472 samples represented people from 22 countries, resulting in 35 unique data sets (i.e., study-country combinations). Across the whole set, we detected just two genera, Methanomassiliicoccus (clade FL) and “Ca. Methanomethylophilus” (clade HA), both rare members of the human gut microbiota (Fig. 5). “Ca. Methanomethylophilus” was detectable in 19 out of 35 data sets; in these 19 data sets, it had a prevalence ranging from 0.5% to 41.7%, and mean abundance ranged from 4.8 × 10−6% to 2.2 × 10−2%. Similarly, Methanomassiliicoccus was detectable in 22 of the 35 data sets; in the 22 data sets, it had a prevalence range of 1% to 25.7% and a mean abundance range of 1.5 × 10−5% to 1.0 × 10−2% (Table S1D).
FIG 5.
Methanomassiliicoccales are rare members of the human gut microbiota. Scatterplots of the genera (A) “Ca. Methanomethylophilus” and (B) Methanomassiliicoccus show that their prevalence and mean abundance is low across most studies and populations (35 data sets) with subjects (n = 4,472) from Austria (AUT), China (CHN), Denmark (DNK), Ethiopia (ETH), Fijo (FJI), Great Britain (GBR), Ghana (GHA), Israel (ISR), Madagascar (MDG), Mongolia (MNG), The Netherlands (NDL), El Salvador (SLV), Sweden (SWE), Tanzania (TZA), and the United States (USA).
We tested associations of these two genera with age, sex, and Westernization status of the subjects using linear mixed models that included the data set and country as random effects. Subjects from non-Westernized countries had a significantly higher prevalence of “Ca. Methanomethylophilus” (mean prevalence ± SD: non-Westernized = 8.9% ± 28.5%, Westernized = 1.1% ± 10.3%; adj. P = 0.002). Westernized individuals were more likely to harbor higher prevalences of Methanomassiliicoccus, although differences were not significant (non-Westernized = 3.9% ± 19.4%, Westernized, 5.0% ± 21.7%; adj. P > 0.1). The age and sex of the individuals did not explain variance in the prevalence or abundance of either genus (adj. P > 0.1 in all cases).
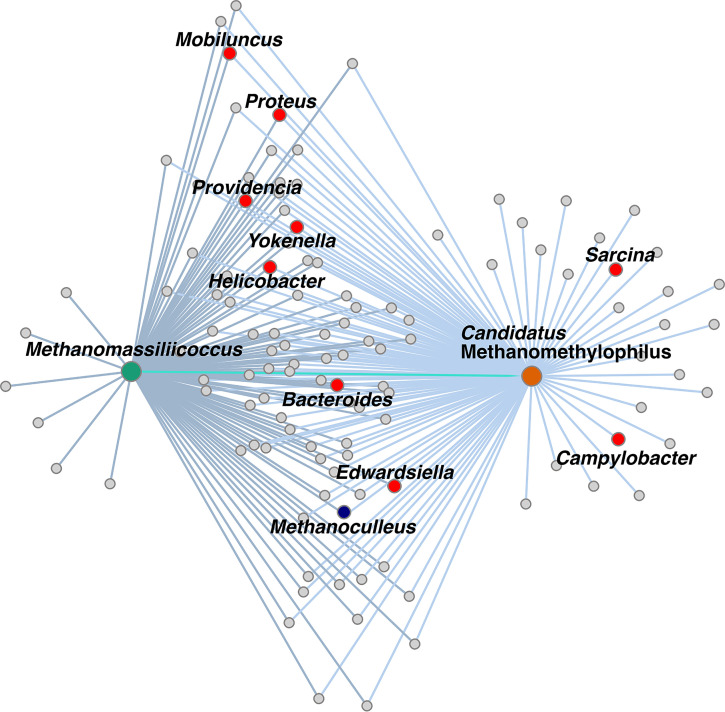
To identify other microbial taxa positively associated with members of Methanomassiliicoccales in the human gut, we calculated a network of positively associated microorganisms (i.e., coabundant taxa) across samples (rho > 0.1 in all cases) (25). In addition, we determined which taxa were present with members of Methanomassiliicoccales at a greater prevalence than that expected by chance (i.e., cooccurring taxa) relative to a permuted null model (26). Results showed that both “Ca. Methanomethylophilus” and Methanomassiliicoccus were part of the same coabundance network, together with a third archaeal genus, Methanoculleus (order Methanomicrobiales). We did not find evidence of positive or negative abundance associations of either Methanomassiliicoccales genus with Methanobrevibacter. Coocurrence analysis showed a random association pattern between these taxa (P > 0.05 for both “Ca. Methanomethylophilus” and Methanomassiliicoccus), indicating that their ecological niches do not overlap that of Methanobrevibacter.
Analysis of the combined network of “Ca. Methanomethylophilus” and Methanomassiliicoccus revealed a large overlap between taxa associated with either genus (Fig. 6 and Table S1F): out of 119 taxa in the network, 86 (72.3%) were associated with both. Moreover, 51 taxa (42.9%) also had a significant positive cooccurrence pattern with both genera (adj. P < 0.05 in all cases). Most bacterial members of this network had low relative abundances; only Bacteroides and Parabacteroides had a mean relative abundance above 1% (range, 22.7% to 0.0005%). Interestingly, they included taxa that can potentially produce TMA, since their genomes contain genes encoding enzymes involved in its synthesis; these taxa included Bacteroides, Campylobacter, Yokenella, Mobiluncus, Proteus, Providencia, and Edwardsiella (27).
FIG 6.
Coabundance networks of Methanomassiliicoccus (green node, dark edges) and “Ca. Methanomethylophilus” (orange node, light edges) in the human gut largely overlap. Both Methanomassiliicoccales genera are significantly coabundant (cyan edge). Their abundances are also coordinated with those of another archaeon (blue node) and TMA-producing bacterial taxa (red nodes).
Abundance of Methanomassiliicoccales species is not concordant in monozygotic or dizygotic human twins.
To evaluate whether host genetics influences the abundance of Methanomassiliicoccales in the human gut, we compared the intraclass correlation coefficient (ICC) of their abundances at the genus level using a set of 153 monozygotic (MZ) and 200 dizygotic (DZ) twin pairs from the TwinsUK cohort. As a control, we first compared the mean ICC across all taxa between MZ and DZ twins and found that ICCMZ (0.1) was significantly higher than ICCDZ (0.03) (P < 0.01). In addition, we assessed the ICC values of bacterial (Christensenella, Faecalibacterium, and Bifidobacterium) and archaeal (Methanobrevibacter) genera, and consistently found a higher correlation for MZ compared to DZ twins (Table S1G). We were only able to assess ICC values of Methanomassiliicoccus, as it was the only Methanomassiliicoccales taxon detected in the twins with a prevalence (8.64%) above the 5% cutoff (see Materials and Methods). We did not detect a significant concordance between the abundances of Methanomassiliicoccus in MZ (ICCMZ = 0.004; adj. P = 0.59) or in DZ twins (ICCDZ = 0.017; adj. P = 0.71). Given the low abundance of Methanomassiliicoccales taxa, we performed a sensitivity analysis using samples with a high sequencing depth (>12 million reads/sample); however, we did not observe differences in the abundance and prevalence of the Methanomassiliicoccales genera or in the ICC estimates (data not shown).
DISCUSSION
While the source of the members of the Methanomassiliicoccales has been noted in previous surveys of single markers such as 16S rRNA and mcrA genes (10, 11), here, we searched metagenomes from host-associated and environmental samples for their relative abundances. Overall, the HA taxa were enriched in host-associated samples and the FL taxa were enriched in environmental samples; intriguingly, all taxa, regardless of clade, were detected in both biomes. This suggests that members of the order Methanomassiliicoccales are generalists with an overall habitat preference according to clade, although there were some exceptions to the general pattern. We show that members of Methanomassiliicoccales use many of the same adaptations to the gut as other methanogens. These adaptations include genome reduction and genes involved in the shikimate pathway and bile resistance. In addition, gut-enriched taxa tend to have a distinct repertoire of genes encoding adhesion factors. We observed that potential adaptations to the gut differed by clade, not preferred habitat, indicating convergence on a shared niche through different genomic solutions. In the human gut, Methanomassiliicoccales taxa correlated with TMA-producing bacteria, rather than host genetics or other host factors.
For members of the HA clade, adaptations to life in the gut included an enrichment of genes involved in bile acid transport, efflux pumps, and hydrolases, which play a role in tolerance to these compounds in the gastrointestinal tract (28). This adaptation is also shared with other members of the gut microbiota, including Methanobacteriales taxa; Methanobrevibacter smithii and Methanosphaera stadtmanae are resistant to bile salts (3, 4). Other gene clusters with known functions enriched in clade HA are involved in metabolism of shikimate and chorismate. The shikimate pathway is involved in the synthesis of aromatic amino acids in plants and microbes, but it is absent in mammals. Shikimate metabolism is carried out by archaeal (29) and bacterial (30, 31) members of the animal gut microbiota and was reported as one of the most conserved metabolic modules in a large-scale gene catalogue from the human gut (32). In turn, aromatic amino acids can be transformed by the gut microbiota into active metabolites, which are involved in diverse physiological processes (33) and conditions such as cardiovascular disease (34). Indeed, plasma concentrations of microbial derivatives of tryptophan have even been shown to negatively correlate with atherosclerosis (35). It remains to be elucidated whether Methanomassiliicoccales are involved in human health through the metabolism of aromatic amino acids and associated compounds.
We observed that each clade tended to encode different adhesion factors, although without statistical significance. These factors are involved in the maintenance of syntrophic relationships of the methanogens with bacterial (12, 36) or eukaryotic (37) microorganisms. Two groups of adhesion factors, proteins containing Sel1 domains and Listeria-Bacteroides repeats, have been previously studied in Methanomassiliicoccales taxa retrieved from the gut (9, 23). Our assessment of these factors in the broader context of the order Methanomassiliicoccales showed that these two groups are more likely to be higher in clade HA than clade FL taxa, with the exception of the outlier taxa. Indeed, the repertoire of ELPs and ALPs was similar between species inhabiting the gut, regardless of their clade. This emphasizes the potential involvement of these proteins in the adaptation to the intestinal environment, although the exact mechanisms are yet to be elucidated.
In contrast, members of clade FL appear to be generalists that colonized the animal gut independently from the HA clade. It has been previously noted that M. luminyensis, an outlier from clade FL, could have a facultative association to the animal gut. It possesses genes involved in nitrogen fixation, oxidative stress (9), and mercury methylation (23), which are common in soil microorganisms but rare in members of the gut microbiota (38). In accordance with this, we observed that members of clade FL are widespread and abundant in soil, water, and gut metagenomes, with a preference for environmental biomes. Similarities in ELP content between gut-dwelling taxa from both clades indicate that interaction with the host or other members of the gut microbiota might be a key factor in the adaptation of these methanogens.
Analysis of the gene content of outlier taxa from clade FL showed that they tended to be more similar to members of their own clade than to taxa from clade HA, with the exception of “Ca. M. intestinalis” Issoire-Mx1, which was distinct from either clade FL and HA. In addition, there was little overlap in gene clusters commonly observed in clade HA and outlier taxa from clade FL, with the exception of the adhesion factors discussed above. These observations support the hypothesis that colonization of animal guts by members of Methanomassiliicoccales occurred in two independent events (9, 23), and suggests that there is not one solution to life in the gut for these archaea, as members from two clades seem to have solved the problem with a different set of adaptations.
Characterization of the abundance of Methanomassiliicoccales taxa across human populations showed members of this group are rare in the microbiota of healthy adults. We did not detect them in all the studied populations, and, when detected, they had low prevalence and abundance. Our extensive analysis of human gut samples corroborates estimates of Methanomassiliicoccales prevalence (up to 11%) (23, 39, 40) and mean abundance (below 1%) (23, 41). Differences in Methanomassiliicoccales carriage between Westernized and non-Westernized populations remain to be explained, and may be due to diet. While Westernized diets are richer in TMA precursors than non-Western diets (42), intake varies across populations (43).
Our analysis allowed us to assess whether Archaea in the human gut are mutually exclusive. We observed positive correlations of “Ca. Methanomethylophilus” and Methanomassiliicoccus with each other and with Methanoculleus, another rare archaeal member of the gut microbiota (45). We did not find evidence of association between members of Methanomassiliicoccales and Methanobrevibacter, positive or otherwise, confirming the previous report that these methanogens are not mutually exclusive (39); abundance of H2 in the gut, together with differences in other substrate utilization, might result in nonoverlapping niches (46).
While genus Methanobrevibacter was consistently found to have a moderate heritability in the TwinsUK (19, 39, 47) and other cohorts (48, 49), this was not the case for members of Methanomassiliicoccales. Similarly to humans, methane production (50) and abundance of Methanobrevibacter (51) are also heritable in bovine cattle, but Methanomassiliicoccales taxa are not (51). Thus, host genetics might be linked to particular taxa and methanogenesis pathways, not to all Archaea or to methane production as a whole.
Genera “Ca. Methanomethylophilus” and Methanomassiliicoccus cooccur with TMA-producing bacteria (27), further supporting their potential use as a way of targeting intestinal TMA (52). The exact nature of the ecological relationships each of these taxa establishes with other members of the microbiome remains to be elucidated. In a facilitation scenario between the methanogens and H2 and TMA producers, freely available TMA and H2 required for methylotrophic methanogenesis could be utilized by Methanomassiliicoccales taxa (53) without cost to the producer. Alternatively, the methanogens could establish syntrophic interactions with other microorganisms, whereby the consumption of these metabolites is also beneficial to the producer (53).
The present study extends our understanding of the order Methanomassiliicoccales by revealing genomic adaptations to life in the gut by members of both clades that make up this group. Furthermore, the positive correlation between the relative abundances of these TMA-utilizing Archaea with TMA-producing bacteria in the gut is a first step toward understanding how they may be harnessed for therapeutic management of gut TMA levels in the context of cardiovascular disease.
MATERIALS AND METHODS
For a detailed description of the methods, see Text S1 in the supplemental material.
Genome annotation and phylogenomic tree reconstruction.
We used 71 substantially complete genomes (completeness, ≥70%) with low contamination (contamination, <5%) retrieved from the NCBI Assembly database (https://www.ncbi.nlm.nih.gov/assembly), plus an additional high-quality metagenome-assembled genome (MAG) corresponding to “Candidatus Methanomethylophilus alvus.” Gene calling was performed using Prokka (54). A maximum-likelihood phylogenomic tree was constructed using PhyloPhlAn (55) with the 72 Methanomassiliicoccales genomes plus members of the order Thermoplasmatales as an outgroup. We used interactive Tree Of Life (iTOL) (56) to visualize the tree.
Abundance of Methanomassiliicoccales in environmental and animal gastrointestinal metagenomes.
We retrieved 305 publicly available gastrointestinal and environmental metagenome samples (57) (see Table S1B in the supplemental material). To avoid multiple mapping of reads, we dereplicated the 72 genomes at a species level (95% average nucleotide identity [ANI]) using dRep (58), resulting in 29 representative genomes. We quantified the abundance of dereplicated Methanomassiliicoccales genomes in the metagenomes using KrakenUniq (59). We estimated the enrichment of each representative Methanomassiliicoccales taxon in host or environmental metagenomes using DESeq2 with the Wald test (60) on sequence counts and classifying metagenome samples as either host derived or environmental.
Comparative genomics.
We grouped the predicted genes into gene clusters using panX (61) and used InterProScan (62) and eggNOG-mapper (63) for annotation. Phylogenetic signal of genome characteristics and gene cluster presence was tested using the phylosignal R package with the local indicator of phylogenetic association (LIPA) (64). The R package micropan (65) was used to create a pangenome principal-component analysis (PCA). We performed phylogenetic ANOVA using the R package phytools (66) to determine clusters enriched in clades FL or HA. We adjusted P values for multiple comparisons with the Benjamini-Hochberg method. Due to the exploratory nature of this work, tests were considered significant if they had an adjusted P value (adj. P) of <0.1; a false-discovery-rate-adjusted P value cutoff of 0.1 implies that 10% of significant tests will result in false-positives. In cases where adjusting P values was not necessary, raw P values are provided.
We assessed the presence of eukaryote-like proteins (ELPs) (24) by combining the counts of gene clusters classified as Sel1-containing proteins (Sel1), Listeria-Bacteroides repeat-containing proteins (List-Bact), tetratricopeptide repeats (TPR), ankyrin repeats (ANK), leucine-rich repeats (LRR), fibronectin type III (FN3) domains, laminin G domains, bacterial Ig-like domains, Yersinia adhesin A-like domain (YadA), TadE-like domain, or invasion protein B (IalB). Likewise, we characterized the presence of parallel beta-helix-repeat-containing proteins, also known as adhesin-like proteins (ALPs).
Characterization of Methanomassiliicoccales distribution across human populations.
We retrieved and quality controlled 4,472 publicly available human gut metagenomes from 34 independent studies (Table S1C). Reads were classified using Kraken (67) and Bracken (68) with custom databases (69). Taxa with <100 reads in a given sample were considered absent. To determine the cooccurrence patterns of Methanomassiliicoccales in the human gut, we used the cooccur package (26); to determine their coabundance patterns, we calculated the proportionality of taxa abundance (rho) with the propr R package (70). The lme4 and lmerTest R packages (71) were used to fit linear mixed effects models to test differences of Methanomassiliicoccales genera abundance by Westernization status, age, and gender. We employed binomial linear mixed models to test differences in genera prevalence. Lists of potential TMA (27, 72, 73) and methanol (74) (see Text S1 in the supplemental materials) producers were compiled from the available literature.
Heritability of Methanomassiliicoccales taxa was assessed by comparing relative abundances of taxa within 153 monozygotic (MZ) and 200 dizygotic (DZ) twin pairs from the United Kingdom Adult Twin Registry (TwinsUK) (19, 39, 75). Absolute read counts were transformed using the Yeo-Johnson transformation and adjusted by body mass index (BMI), sex, and sequencing depth (19, 39). We calculated the intraclass correlation coefficient (ICC) in MZ and DZ twins with the irr R package, and adjusted P values using the Benjamini-Hochberg method. We compared the mean ICC across all taxa between MZ and DZ twins using the Mann-Whitney test and by assessing the ICC of taxa previously reported as heritable (Methanobrevibacter, Faecalibacterium, Christensenella, and Bifidobacterium) (39, 48).
Data availability.
The raw sequence data are available from the European Nucleotide Archive under study accession number PRJEB40256. Jupyter notebooks are available at https://github.com/leylabmpi/Methanomassilii. The “Candidatus Methanomethylophilus” MAG generated here can be found at http://ftp.tue.mpg.de/ebio/projects/Mmassilii/.
ACKNOWLEDGMENTS
This work was supported by the Max Planck Society. The study also received support from the National Institute for Health Research (NIHR) BioResource Clinical Research Facility and Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. We thank EMBO and the organizers and participants of the Bioinformatics and Genome Analyses course held at the Fondazione Edmund Mach in San Michele all’Adige, Italy, for sponsoring the attendance of J.D.L.C.-Z. and for their feedback.
We are also grateful to Daphne Welter, Jessica Sutter, and Albane Ruaud for the fruitful discussions and comments.
We declare no competing interests.
REFERENCES
- 1.Borrel G, Brugère J-F, Gribaldo S, Schmitz RA, Moissl-Eichinger C. 2020. The host-associated archaeome. Nat Rev Microbiol 18:622–636. doi: 10.1038/s41579-020-0407-y. [DOI] [PubMed] [Google Scholar]
- 2.Moissl-Eichinger C, Pausan M, Taffner J, Berg G, Bang C, Schmitz RA. 2018. Archaea are interactive components of complex microbiomes. Trends Microbiol 26:70–85. doi: 10.1016/j.tim.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Miller TL, Wolin MJ. 1982. Enumeration of Methanobrevibacter smithii in human feces. Arch Microbiol 131:14–18. doi: 10.1007/BF00451492. [DOI] [PubMed] [Google Scholar]
- 4.Miller TL, Wolin MJ. 1985. Methanosphaera stadtmaniae gen. nov., sp. nov.: a species that forms methane by reducing methanol with hydrogen. Arch Microbiol 141:116–122. doi: 10.1007/BF00423270. [DOI] [PubMed] [Google Scholar]
- 5.Horz H-P, Conrads G. 2010. The discussion goes on: what is the role of Euryarchaeota in humans? Archaea 2010:967271. doi: 10.1155/2010/967271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrel G, Harris HMB, Tottey W, Mihajlovski A, Parisot N, Peyretaillade E, Peyret P, Gribaldo S, O’Toole PW, Brugère J-F. 2012. Genome sequence of “Candidatus Methanomethylophilus alvus” Mx1201, a methanogenic archaeon from the human gut belonging to a seventh order of methanogens. J Bacteriol 194:6944–6945. doi: 10.1128/JB.01867-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrel G, Harris HMB, Parisot N, Gaci N, Tottey W, Mihajlovski A, Deane J, Gribaldo S, Bardot O, Peyretaillade E, Peyret P, O’Toole PW, Brugère J-F. 2013. Genome sequence of “Candidatus Methanomassiliicoccus intestinalis” Issoire-Mx1, a third thermoplasmatales-related methanogenic archaeon from human feces. Genome Announc 1:e00453-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dridi B, Fardeau M-L, Ollivier B, Raoult D, Drancourt M. 2012. Methanomassiliicoccus luminyensis gen. nov., sp. nov., a methanogenic archaeon isolated from human faeces. Int J Syst Evol Microbiol 62:1902–1907. doi: 10.1099/ijs.0.033712-0. [DOI] [PubMed] [Google Scholar]
- 9.Borrel G, Parisot N, Harris HMB, Peyretaillade E, Gaci N, Tottey W, Bardot O, Raymann K, Gribaldo S, Peyret P, O’Toole PW, Brugère J-F. 2014. Comparative genomics highlights the unique biology of Methanomassiliicoccales, a Thermoplasmatales-related seventh order of methanogenic archaea that encodes pyrrolysine. BMC Genomics 15:679. doi: 10.1186/1471-2164-15-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Söllinger A, Schwab C, Weinmaier T, Loy A, Tveit AT, Schleper C, Urich T. 2016. Phylogenetic and genomic analysis of Methanomassiliicoccales in wetlands and animal intestinal tracts reveals clade-specific habitat preferences. FEMS Microbiol Ecol 92:fiv149. doi: 10.1093/femsec/fiv149. [DOI] [PubMed] [Google Scholar]
- 11.Speth DR, Orphan VJ. 2018. Metabolic marker gene mining provides insight in global diversity and, coupled with targeted genome reconstruction, sheds further light on metabolic potential of the. PeerJ 6:e5614. doi: 10.7717/peerj.5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuel BS, Hansen EE, Manchester JK, Coutinho PM, Henrissat B, Fulton R, Latreille P, Kim K, Wilson RK, Gordon JI. 2007. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc Natl Acad Sci U S A 104:10643–10648. doi: 10.1073/pnas.0704189104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen EE, Lozupone CA, Rey FE, Wu M, Guruge JL, Narra A, Goodfellow J, Zaneveld JR, McDonald DT, Goodrich JA, Heath AC, Knight R, Gordon JI. 2011. Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proc Natl Acad Sci U S A 108 Suppl 1:4599–4606. doi: 10.1073/pnas.1000071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Söllinger A, Urich T. 2019. Methylotrophic methanogens everywhere—physiology and ecology of novel players in global methane cycling. Biochem Soc Trans 47:1895–1907. doi: 10.1042/BST20180565. [DOI] [PubMed] [Google Scholar]
- 15.Brown JM, Hazen SL. 2018. Microbial modulation of cardiovascular disease. Nat Rev Microbiol 16:171–181. doi: 10.1038/nrmicro.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng J, Yang C, Wang B, Zhang X, Hu T, Gu Y, Li J. 2018. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed Pharmacother 97:941–947. doi: 10.1016/j.biopha.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. 2015. Non-lethal Inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brugère J-F, Borrel G, Gaci N, Tottey W, O’Toole PW, Malpuech-Brugère C. 2014. Archaebiotics: proposed therapeutic use of archaea to prevent trimethylaminuria and cardiovascular disease. Gut Microbes 5:5–10. doi: 10.4161/gmic.26749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie H, Guo R, Zhong H, Feng Q, Lan Z, Qin B, Ward KJ, Jackson MA, Xia Y, Chen X, Chen B, Xia H, Xu C, Li F, Xu X, Al-Aama JY, Yang H, Wang J, Kristiansen K, Wang J, Steves CJ, Bell JT, Li J, Spector TD, Jia H. 2016. Shotgun metagenomics of 250 adult twins reveals genetic and environmental impacts on the gut microbiome. Cell Syst 3:572–584.e3. doi: 10.1016/j.cels.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul K, Nonoh JO, Mikulski L, Brune A. 2012. “Methanoplasmatales,” Thermoplasmatales-related archaea in termite guts and other environments, are the seventh order of methanogens. Appl Environ Microbiol 78:8245–8253. doi: 10.1128/AEM.02193-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borrel G, O’Toole PW, Harris HMB, Peyret P, Brugère J-F, Gribaldo S. 2013. Phylogenomic data support a seventh order of methylotrophic methanogens and provide insights into the evolution of methanogenesis. Genome Biol Evol 5:1769–1780. doi: 10.1093/gbe/evt128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parks DH, Rinke C, Chuvochina M, Chaumeil P-A, Woodcroft BJ, Evans PN, Hugenholtz P, Tyson GW. 2017. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat Microbiol 2:1533–1542. doi: 10.1038/s41564-017-0012-7. [DOI] [PubMed] [Google Scholar]
- 23.Borrel G, McCann A, Deane J, Neto MC, Lynch DB, Brugère J-F, O'Toole PW. 2017. Genomics and metagenomics of trimethylamine-utilizing Archaea in the human gut microbiome. ISME J 11:2059–2074. doi: 10.1038/ismej.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alex A, Antunes A. 2018. Genus-wide comparison of Pseudovibrio bacterial genomes reveal diverse adaptations to different marine invertebrate hosts. PLoS One 13:e0194368. doi: 10.1371/journal.pone.0194368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinn TP, Erb I, Richardson MF, Crowley TM. 2018. Understanding sequencing data as compositions: an outlook and review. Bioinformatics 34:2870–2878. doi: 10.1093/bioinformatics/bty175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffith DM, Veech JA, Marsh CJ. 2016. cooccur: probabilistic species co-occurrence analysis in R. J Stat Softw 69:1–17. [Google Scholar]
- 27.Fennema D, Phillips IR, Shephard EA. 2016. Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab Dispos 44:1839–1850. doi: 10.1124/dmd.116.070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begley M, Gahan CGM, Hill C. 2005. The interaction between bacteria and bile. FEMS Microbiol Rev 29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Hovey R, Lentes S, Ehrenreich A, Salmon K, Saba K, Gottschalk G, Gunsalus RP, Deppenmeier U. 2005. DNA microarray analysis of Methanosarcina mazei Gö1 reveals adaptation to different methanogenic substrates. Mol Genet Genomics 273:225–239. doi: 10.1007/s00438-005-1126-9. [DOI] [PubMed] [Google Scholar]
- 30.Kamke J, Kittelmann S, Soni P, Li Y, Tavendale M, Ganesh S, Janssen PH, Shi W, Froula J, Rubin EM, Attwood GT. 2016. Rumen metagenome and metatranscriptome analyses of low methane yield sheep reveals a Sharpea-enriched microbiome characterised by lactic acid formation and utilisation. Microbiome 4:56. doi: 10.1186/s40168-016-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. 2013. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol 24:160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Almeida A, Nayfach S, Boland M, Strozzi F, Beracochea M, Shi ZJ, Pollard KS, Sakharova E, Parks DH, Hugenholtz P, Segata N, Kyrpides NC, Finn RD. 2020. A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat Biotechnol 490:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin R, Liu W, Piao M, Zhu H. 2017. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 49:2083–2090. doi: 10.1007/s00726-017-2493-3. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Hou Y, Wang G, Zheng X, Hao H. 2020. Gut microbial metabolites of aromatic amino acids as signals in host-microbe interplay. Trends Endocrinol Metab 31:818–834. doi: 10.1016/j.tem.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Cason CA, Dolan KT, Sharma G, Tao M, Kulkarni R, Helenowski IB, Doane BM, Avram MJ, McDermott MM, Chang EB, Ozaki CK, Ho KJ. 2018. Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J Vasc Surg 68:1552–1562.e7. doi: 10.1016/j.jvs.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruaud A, Esquivel-Elizondo S, de la Cuesta-Zuluaga J, Waters JL, Angenent LT, Youngblut ND, Ley RE. 2020. Syntrophy via interspecies H2 transfer between and underlies their global cooccurrence in the human gut. mBio 11:e03235-19. doi: 10.1128/mBio.03235-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng F, Kittelmann S, Patchett ML, Attwood GT, Janssen PH, Rakonjac J, Gagic D. 2016. An adhesin from hydrogen-utilizing rumen methanogen Methanobrevibacter ruminantium M1 binds a broad range of hydrogen-producing microorganisms. Environ Microbiol 18:3010–3021. doi: 10.1111/1462-2920.13155. [DOI] [PubMed] [Google Scholar]
- 38.Podar M, Gilmour CC, Brandt CC, Soren A, Brown SD, Crable BR, Palumbo AV, Somenahally AC, Elias DA. 2015. Global prevalence and distribution of genes and microorganisms involved in mercury methylation. Sci Adv 1:e1500675. doi: 10.1126/sciadv.1500675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE. 2014. Human genetics shape the gut microbiome. Cell 159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dridi B, Henry M, Richet H, Raoult D, Drancourt M. 2012. Age-related prevalence of Methanomassiliicoccus luminyensis in the human gut microbiome. APMIS 120:773–777. doi: 10.1111/j.1600-0463.2012.02899.x. [DOI] [PubMed] [Google Scholar]
- 41.Vanderhaeghen S, Lacroix C, Schwab C. 2015. Methanogen communities in stools of humans of different age and health status and co-occurrence with bacteria. FEMS Microbiol Lett 362:fnv092. doi: 10.1093/femsle/fnv092. [DOI] [PubMed] [Google Scholar]
- 42.Zeisel SH, Mar M-H, Howe JC, Holden JM. 2003. Concentrations of choline-containing compounds and betaine in common foods. J Nutr 133:1302–1307. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 43.Wiedeman AM, Barr SI, Green TJ, Xu Z, Innis SM, Kitts DD. 2018. Dietary choline intake: current state of knowledge across the life cycle. Nutrients 10:1513. doi: 10.3390/nu10101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reference deleted.
- 45.Horz H-P. 2015. Archaeal lineages within the human microbiome: absent, rare or elusive? Life (Basel) 5:1333–1345. doi: 10.3390/life5021333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldewert C, Lang K, Brune A. 2020. The hydrogen threshold of obligately methyl-reducing methanogens. FEMS Microbiol Lett 367:fnaa137. doi: 10.1093/femsle/fnaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, Spector TD, Bell JT, Clark AG, Ley RE. 2016. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe 19:731–743. doi: 10.1016/j.chom.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodrich JK, Davenport ER, Clark AG, Ley RE. 2017. The relationship between the human genome and microbiome comes into view. Annu Rev Genet 51:413–433. doi: 10.1146/annurev-genet-110711-155532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurilshikov A, Medina-Gomez C, Bacigalupe R, Radjabzadeh D, Wang J, Demirkan A, Le Roy CI, Raygoza Garay JA, Finnicum CT, Liu X, Zhernakova DV, Bonder MJ, Hansen TH, Frost F, Rühlemann MC, Turpin W, Moon J-Y, Kim H-N, Lüll K, Barkan E, Shah SA, Fornage M, Szopinska-Tokov J, Wallen ZD, Borisevich D, Agreus L, Andreasson A, Bang C, Bedrani L, Bell JT, Bisgaard H, Boehnke M, Boomsma DI, Burk RD, Claringbould A, Croitoru K, Davies GE, van Duijn CM, Duijts L, Falony G, Fu J, van der Graaf A, Hansen T, Homuth G, Hughes DA, Ijzerman RG, Jackson MA, Jaddoe VWV, Joossens M, Jørgensen T, Keszthelyi D, Knight R, Laakso M, Laudes M, et al. 2020. Genetics of human gut microbiome composition. bioRxiv 10.1101/2020.06.26.173724. [DOI]
- 50.Roehe R, Dewhurst RJ, Duthie C-A, Rooke JA, McKain N, Ross DW, Hyslop JJ, Waterhouse A, Freeman TC, Watson M, Wallace RJ. 2016. Bovine host genetic variation influences rumen microbial methane production with best selection criterion for low methane emitting and efficiently feed converting hosts based on metagenomic gene abundance. PLoS Genet 12:e1005846. doi: 10.1371/journal.pgen.1005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Difford GF, Plichta DR, Løvendahl P, Lassen J, Noel SJ, Højberg O, Wright A-DG, Zhu Z, Kristensen L, Nielsen HB, Guldbrandtsen B, Sahana G. 2018. Host genetics and the rumen microbiome jointly associate with methane emissions in dairy cows. PLoS Genet 14:e1007580. doi: 10.1371/journal.pgen.1007580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hania WB, Ballet N, Vandeckerkove P, Ollivier B, O’Toole PW, Brugère J-F. 2017. Archaebiotics: archaea as pharmabiotics for treating chronic disease in humans? p 42–62. In Sghaier H, Najjari A, Ghedira K (ed), Archaea: new biocatalysts, novel pharmaceuticals and various biotechnological applications. InTech Open, London, UK. [Google Scholar]
- 53.Douglas AE. 2020. The microbial exometabolome: ecological resource and architect of microbial communities. Philos Trans R Soc Lond B Biol Sci 375:20190250. doi: 10.1098/rstb.2019.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 55.Segata N, Börnigen D, Morgan XC, Huttenhower C. 2013. PhyloPhlAn is a new method for improved phylogenetic and taxonomic placement of microbes. Nat Commun 4:2304. doi: 10.1038/ncomms3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Letunic I, Bork P. 2016. Interactive Tree Of Life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell AL, Scheremetjew M, Denise H, Potter S, Tarkowska A, Qureshi M, Salazar GA, Pesseat S, Boland MA, Hunter FMI, Ten Hoopen P, Alako B, Amid C, Wilkinson DJ, Curtis TP, Cochrane G, Finn RD. 2018. EBI metagenomics in 2017: enriching the analysis of microbial communities, from sequence reads to assemblies. Nucleic Acids Res 46:D726–D735. doi: 10.1093/nar/gkx967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olm MR, Brown CT, Brooks B, Banfield JF. 2017. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J 11:2864–2868. doi: 10.1038/ismej.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Breitwieser FP, Baker DN, Salzberg SL. 2018. KrakenUniq: confident and fast metagenomics classification using unique k-mer counts. Genome Biol 19:198. doi: 10.1186/s13059-018-1568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ding W, Baumdicker F, Neher RA. 2018. panX: pan-genome analysis and exploration. Nucleic Acids Res 46:e5. doi: 10.1093/nar/gkx977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finn RD, Attwood TK, Babbitt PC, Bateman A, Bork P, Bridge AJ, Chang H-Y, Dosztányi Z, El-Gebali S, Fraser M, Gough J, Haft D, Holliday GL, Huang H, Huang X, Letunic I, Lopez R, Lu S, Marchler-Bauer A, Mi H, Mistry J, Natale DA, Necci M, Nuka G, Orengo CA, Park Y, Pesseat S, Piovesan D, Potter SC, Rawlings ND, Redaschi N, Richardson L, Rivoire C, Sangrador-Vegas A, Sigrist C, Sillitoe I, Smithers B, Squizzato S, Sutton G, Thanki N, Thomas PD, Tosatto SCE, Wu CH, Xenarios I, Yeh L-S, Young S-Y, Mitchell AL. 2017. InterPro in 2017-beyond protein family and domain annotations. Nucleic Acids Res 45:D190–D199. doi: 10.1093/nar/gkw1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huerta-Cepas J, Forslund K, Coelho LP, Szklarczyk D, Jensen LJ, von Mering C, Bork P. 2017. Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol Biol Evol 34:2115–2122. doi: 10.1093/molbev/msx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keck F, Rimet F, Bouchez A, Franc A. 2016. phylosignal: an R package to measure, test, and explore the phylogenetic signal. Ecol Evol 6:2774–2780. doi: 10.1002/ece3.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snipen L, Liland KH. 2015. micropan: an R-package for microbial pan-genomics. BMC Bioinformatics 16:79. doi: 10.1186/s12859-015-0517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. doi: 10.1111/j.2041-210X.2011.00169.x. [DOI] [Google Scholar]
- 67.Wood DE, Lu J, Langmead B. 2019. Improved metagenomic analysis with Kraken 2. Genome Biol 20:257. doi: 10.1186/s13059-019-1891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu J, Breitwieser FP, Thielen P, Salzberg SL. 2017. Bracken: estimating species abundance in metagenomics data. PeerJ Computer Science 3:e104. doi: 10.7717/peerj-cs.104. [DOI] [Google Scholar]
- 69.de la Cuesta-Zuluaga J, Ley RE, Youngblut ND. 2020. Struo: a pipeline for building custom databases for common metagenome profilers. Bioinformatics 36:2314–2315. doi: 10.1093/bioinformatics/btz899. [DOI] [PubMed] [Google Scholar]
- 70.Quinn TP, Richardson MF, Lovell D, Crowley TM. 2017. propr: an R-package for identifying proportionally abundant features using compositional data analysis. Sci Rep 7:16252. doi: 10.1038/s41598-017-16520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. [Google Scholar]
- 72.Rath S, Heidrich B, Pieper DH, Vital M. 2017. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome 5:54. doi: 10.1186/s40168-017-0271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rath S, Rud T, Pieper DH, Vital M. 2019. Potential TMA-producing bacteria are ubiquitously found in Mammalia. Front Microbiol 10:2966. doi: 10.3389/fmicb.2019.02966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dorokhov YL, Shindyapina AV, Sheshukova EV, Komarova TV. 2015. Metabolic methanol: molecular pathways and physiological roles. Physiol Rev 95:603–644. doi: 10.1152/physrev.00034.2014. [DOI] [PubMed] [Google Scholar]
- 75.Visconti A, Le Roy CI, Rosa F, Rossi N, Martin TC, Mohney RP, Li W, de Rinaldis E, Bell JT, Venter JC, Nelson KE, Spector TD, Falchi M. 2019. Interplay between the human gut microbiome and host metabolism. Nat Commun 10:4505. doi: 10.1038/s41467-019-12476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) NCBI assembly accession number, genome characteristics, study information, and source of isolation of 71 publicly available genomes from the order Methanomassiliicoccales retrieved from NCBI in June 2018, plus the “Ca. M. alvus” metagenome-assembled genome (MAG) reported here. Study accession and title of UBA genomes obtained from supplementary tables of Parks et al., 2017 (https://doi.org//10.1038/s41564-017-0012-7) (22), otherwise, obtained from the NCBI BioProject database. (B) SRA and MGnify accession information of publicly available metagenome samples from gastrointestinal and environmental biomes. (C) SRA, study, and country information of publicly available human gut metagenome samples. (D) Prevalence and mean abundance of “Candidatus Methanomethylophilus” and Methanomassiliicoccus taxa across multiple human populations. (E) InterPro, eggNOG, and Prokka annotations of gene clusters significantly enriched in clade FL compared to clade HA. (F) Coabundance and cooccurrence measures of positively associated taxa of the human gut microbiota with “Ca. Methanomethylophilus” and Methanomassiliicoccus. For each of the two Methanomassiliicoccales genera, the observed cooccurrences, expected cooccurrences, adjusted P values of cooccurrence, and association of abundances across samples (rho) are provided. (G) Intraclass correlation coefficients (ICC) and adjusted P values of relative abundances of Methanomassiliicoccus and other control taxa in monozygotic and dizygotic twins. Download Table S1, XLSX file, 0.2 MB (238KB, xlsx) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Methanomassiliicoccales taxa from all clades are widespread but not abundant across a range of environments and animal hosts. The abundances of members of the free-living (FL) and host-associated (HA) clades are comparable within similar biomes, particularly in animal-derived metagenomes. Abundance of each representative genome on diverse metagenome and environmental metagenome samples colored by clade (green, FL; orange, HA; purple, external [EX]). Abundances calculated for individual genomes using KrakenUniq and aggregated by clade. Note that the y axis is in logarithmic scale and each plot has a different scale. Black points indicate mean relative abundance in percentage, and black bars indicate standard deviation. Metagenome samples from stomach (n = 12), foregut (n = 23), large intestine (n = 66), fecal (n = 44), desert (n = 4), sand (n = 12), grasslands (n = 8), permafrost (n = 22), sediment (n = 31), coastal (n = 28), intertidal zone (n = 25), lentic (n = 6), groundwater (n = 3), saline (n = 2), hypersaline (n = 9), and ice (n = 10) biomes. Download FIG S1, TIF file, 2.0 MB (2MB, tif) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Abundance of Methanomassiliicoccales clades varies across animal hosts and sample types. Samples from the same host but different sampling sites show consistent patterns. Metagenome samples from cat (n = 30), pig (n = 30), rainbow trout (n = 23), mouse (n = 16), poultry (n = 14), goose (n = 12), cattle (n = 8), white-throated woodrat (n = 8), and Eurasian elk (n = 3). See Fig. S1 for details. Download FIG S2, TIF file, 0.5 MB (541.3KB, tif) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Animal hosts show different patterns of prevalence of individual Methanomassiliicoccales taxa. cat (n = 30), pig (n = 30), rainbow trout (n = 23), mouse (n = 16), poultry (n = 14), goose (n = 12), cattle (n = 8), white-throated woodrat (n = 8), and Eurasian elk (n = 3). See Fig. S1 for details. Download FIG S3, TIF file, 2.2 MB (2.2MB, tif) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Small clusters of unknown function dominate the pangenome of the order Methanomassiliicoccales. Gene cluster frequency spectrum of the order Methanomassiliicoccales separated by (A) unknown or (B) known function; the x axis represents the number of genomes that have at least one gene in a given cluster, and the y axis is the frequency of clusters of a given size. Note the difference in scale of the y axis between panels A and B. (C) Fraction of gene clusters belonging to each clusters of orthologous genes (COG) category per clade. Core clusters were defined as being present in ≥80% of genomes of a clade; for the complete order (i.e., all included Methanomassiliicoccales taxa without grouping them by clade), gene clusters were present in ≥80% of the included genomes and in at least one member of each clade. The proportion of clusters of unknown functions in the core genome of each clade was large and varied between clades, ranging from 23.0% in clade HA to 38.5% in clade EX. The proportion of unknown clusters was lowest in the complete taxonomic order, where it only accounted for 14.7% of gene clusters. COG functional classification descriptions by groups, as follows. Information storage and processing: B, chromatin structure and dynamic; J, translation, ribosomal structure, and biogenesis; K, transcription; L, replication, recombination, and repair. Cellular processes and signaling: D, cell cycle control, cell division, and chromosome partitioning; M, cell wall/membrane/envelope biogenesis; N, cell motility; O, posttranslational modification, protein turnover, and chaperone; T, signal transduction mechanisms; U, intracellular trafficking, secretion, and vesicular transport; V, defense mechanisms; Z, cytoskeleton. Metabolism: C, energy production and conversion; E, amino acid transport and metabolism; F, nucleotide transport and metabolism; G carbohydrate transport and metabolism; H, coenzyme transport and metabolism; I, lipid transport and metabolism; P, inorganic ion transport and metabolism; Q, secondary metabolites biosynthesis, transport, and catabolism. Poorly characterized: X, no annotation retrieved; S, function unknown. Download FIG S4, TIF file, 0.5 MB (530.7KB, tif) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Supplementary methods contain detailed description of the tests carried in the present work and the versions of the software and R packages used. Supplementary results contain description of the retrieval of a genome from “Candidatus Methanomethylophilus alvus” by metagenomic assembly; comparison of core gene functions between Methanomassiliicoccales clades; and assessment of Methanomassiliicoccales with methanol producers in the human gut. Download Text S1, DOCX file, 0.02 MB (23.5KB, docx) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The set of adhesion proteins tends to differ by clade. The tanglegram compares a dendrogram of adhesion genes calculated using hierarchical clustering of Jaccard index matrix (left) versus the maximum-likelihood phylogeny (right). Tip labels colored by clade: HA, orange; FL, green; EX, purple. Download FIG S5, TIF file, 0.9 MB (920KB, tif) .
Copyright © 2021 de la Cuesta-Zuluaga et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
The raw sequence data are available from the European Nucleotide Archive under study accession number PRJEB40256. Jupyter notebooks are available at https://github.com/leylabmpi/Methanomassilii. The “Candidatus Methanomethylophilus” MAG generated here can be found at http://ftp.tue.mpg.de/ebio/projects/Mmassilii/.