Abstract
Background
Previous investigations suggest the use of extract from the roots of Pelargonium sidoides (EPs 7630) for the therapy of uncomplicated rhinosinusitis. The aim of this prospective study was to compare the effects of herbal drug EPs 7630 and antibiotic roxithromycin on chemokine production in nasal mucosa and clinical parameters in patients with uncomplicated acute bacterial rhinosinusitis (ABRS).
Methods
Seventy‐eight ABRS patients were divided into 26 patients receiving EPs 7630 tablets, 3 × 20 mg/day per os (group 1), 26 patients receiving roxithromycin tablets, 2 × 150 mg/day per os (group 2), both for 10 days, and 26 patients who received no therapy (Control group). We measured chemokine levels in nasal secretions by flow cytometry and assessed clinical parameters on day 0 and day 10 of investigation.
Results
EPs 7630 increased concentrations of MCP‐1 (P = .001) and IP‐10 (P = .049) and decreased levels of MIP‐1α (P < .001), ENA‐78 (P < .001), and IL‐8 (P < .001). Roxithromycin increased levels of IP‐10 (P = .049) and decreased levels of MCP‐1 (P < .001), MIP‐1α (P < .016), ENA‐78 (P < .001), and IL‐8 (P < .001). Comparison of the non‐treated patients' group with groups 1 and 2 revealed significant improvement of all clinical parameters in treated patients (P < .001), but therapy with roxithromycin resulted in better improvement in nasal symptoms and endoscopic findings than therapy with EPs 7630.
Conclusion
Our results suggest the presence of similar modulatory effects of both therapies on production of chemokines that regulate the function of neutrophils and monocytes in nasal mucosa. Roxithromycin shows better clinical efficacy than EPs 7630 in patients with uncomplicated ABRS.
Level of Evidence
1b.
Keywords: antibiotic; cytokines; inflammation; nasal mucosa; plants, medicinal; sinusitis
Our results suggest that both Pelargonium sidoides extract (EPs 7630) and roxithromycin act as modulators of upper respiratory tract‐associated immunological responses.
These medications increase the levels of monocyte‐related and decrease the levels of neutrophil‐related chemokines in nasal secretions of patients with uncomplicated acute bacterial rhinosinusitis.
The patients treated by roxithromycin had better improvement of nasal symptoms and endoscopic findings than those treated by P. sidoides extract.

Abbreviations
- ENA‐78
epithelial‐neutrophil activating peptide
- GROα
growth‐regulated alpha protein precursor
- IL‐8
interleukin 8
- IP‐10
interferon gamma‐induced protein 10
- I‐TAC
interferon inducible T‐cell alpha chemoattractant
- MCP‐1
monocyte chemoattractant protein 1
- MIG
monokine induced by gamma interferon
- MIP‐1α
macrophage inflammatory protein 1 alpha
- MIP‐1β
macrophage inflammatory protein 1 beta
- MIP‐3α
macrophage inflammatory protein 3 alpha
- RANTES
regulated on activation, normal T‐cell expressed and secreted
- TARC
thymus and activation regulated chemokine
1. INTRODUCTION
Acute rhinosinusitis (ARS) is an inflammation which suddenly affects the mucosa of the nasal cavity and paranasal sinuses. Viral ARS usually passes for 10 days with or without symptomatic therapy, or can be complicated by secondary bacterial infection that requires antimicrobial therapy. 1 , 2 Symptoms of acute bacterial rhinosinusitis (ABRS) are caused by infection‐stimulated production of inflammatory mediators. 1 , 2 In most cases, the acute suppurative infection resolves promptly following a course of antibiotic therapy supplemented with adjuvant therapy of decongestants, antihistamines, and intranasal corticosteroid sprays. 3 , 4 However, the role of inflammatory mediators in ABRS is not explored in detail. Bacterial infection of the nasal mucosa stimulates the production and release of variety of cytokines and chemokines in the respiratory epithelial cells. 1 , 5 This bacterial infection induces multiple changes in the nasal mucosa, including infiltration and activation of various inflammatory cells, especially neutrophils and monocytes and defects in the host and adaptive immune defence functions. 1 , 5
Macrolide antibiotics, for example, erythromycin, clarithromycin, azithromycin and roxithromycin, are well‐known for decades as a good option in therapy of acute and chronic rhinosinusitis due to strong bacteriostatic and immunomodulatory effects. 1 , 4 , 5 They bind to the 50S subunits of the bacterial ribosome, inhibiting protein synthesis. 1 , 5
Herbal medicines have been used for centuries for therapy of acute upper airway infections. Preparation from the roots of Pelargonium sidoides was used for generations in South Africa for treatment of respiratory and gastrointestinal infections, due to its antiviral and antibacterial actions. 6 More than seven decades later, this polyphenol‐rich extract was finally developed in Germany with coding name EPs 7630. 6 According to the International Consensus Statement on Allergy & Rhinology: Rhinosinusitis and European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) 2020, the use of extract from Pelargonium sidoides is recommended as an option in therapy of ARS. 1 , 5 The immunomodulatory effects of this herbal drug are mediated mainly by stimulation of tumor necrosis factor alpha (TNF‐α), interferon beta (IFN‐β), IFN‐γ and interleukin‐10 (IL‐10) production and reducing production of IL‐6 and IL‐15 in human respiratory tract epithelial cells. 6 , 7 , 8 , 9 , 10 Intensity of inflammatory reaction during the ARS depends on action of chemokines, small cytokines that attract different inflammatory cells to the site of infection.
However, in vivo studies related to the effects of EPs 7630 on chemokine production in nasal mucosa of patients with ABRS were not previously conducted. This study is designed to compare the effects of therapy by EPs 7630 and macrolide antibiotic roxithromycin on chemokine production in nasal mucosa, as well as on clinical parameters of patients with mild‐to‐moderate ABRS.
2. PATIENTS AND METHODS
2.1. Study design and ethical considerations
This randomized, open label, noninferiority, prospective study was conducted from May 2019 to November 2020. The protocol for investigation is approved by the Institutional Review Board of the Military Medical Academy, Belgrade, Serbia (Ethics Committee Approval No. 05/2019) and this study is registered as a part of Projects of the Military Medical Academy, Belgrade, Serbia (MFVMA02/19‐21/) and the Ministry of Education, Science and Technology of the Republic of Serbia (No. III45005). Written informed consent was obtained from each patient.
2.2. Study participants
Seventy‐eight (n = 78) adult patients with mild‐to‐moderate ABRS were enrolled in this investigation. Diagnosis of ABRS was made according to the criteria of the updated Clinical Practice Guideline for adult sinusitis, published by American Academy of Otolaryngology Head & Neck Surgery. 2 Patients had diagnosis of ABRS if: (a) symptoms (nasal obstruction, anterior nasal secretion/postnasal drip, facial pain/pressure, and/or impaired or loss of the sense of smell) or signs of ARS (mucosal edema, mucopurulent secretion) persist without evidence of improvement for at least 10 days beyond the onset of upper respiratory symptoms or (b) symptoms or signs of ARS were worsen within first 10 days after an initial improvement. To confirm the diagnosis of ABRS, aspirate from middle meatus was taken from every patient and samples were cultivated on Blood Agar (HiMedia Laboratories, Mumbai, India).
Exclusion criteria were: <18 years or >65 years, nasal/paranasal sinus surgery within 6 months before study, systemic diseases (cystic fibrosis, Churg Strauss syndrome, Wegener's granulomatosis, etc.), symptomatic seasonal allergic rhinitis (after pollen exposure during the study), bronchial asthma, hypersensitivity to roxithromycin or to Pelargonium sidoides extract, patients taking anticoagulants and salicylates, patients with gastrointestinal and hepatobiliary diseases, the use of oral or topical antibiotics, antihistamines and corticosteroids within the 4 weeks before the start of the study, the use of mucolytic, decongestants and hypertonic seawater within the 7 days before the investigation, pregnancy, lactation, active cigarette smoking. Subjects were excluded if they had symptoms or signs of severe acute bacterial rhinosinusitis (fever > 38°C, persistent severe unilateral facial or tooth pain, facial swelling, profuse unilateral mucopurulent secretion and worsening of symptoms after initial improvement).
2.3. Randomization
The randomization was performed in accordance with the CONSORT statement. A hundred patients (n = 100) with mild‐to‐moderate ABRS, examined and treated in our ENT Department, were involved in the study. Seven (n = 7) refused to participate while 15 (n = 15) patients did not meet inclusion criteria. Seventy‐eight (n = 78) patients were thus recruited and assigned to the group 1 (n = 26), group 2 (n = 26), and control group (n = 26) by randomization. We used a simple computer‐generated randomization procedure to allocate the participants into groups. The participants were deemed eligible by the investigator who informed the nurse about the eligibility. The nurse than assigned the patient to group 1, 2 and control group using a computer‐generated random allocation. The study profile is presented in Figure 1.
FIGURE 1.
Randomization of study participants. One hundred patients (n = 100) with diagnosis of ABRS were selected to participate in the study. Five (n = 7) refused to participate and eleven (n = 15) patients did not meet inclusion criteria. Seventy‐eight (n = 78) patients were finally recruited and assigned by randomization to the group 1 (n = 26) and group 2 (n = 26) and control group (n = 26)
2.4. Treatment
The ABRS patients from group 1 received herbal drug EPs 7630 oral tablets 3 × 20 mg/day (Umckalor, Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany), 10 days in total. The patients from group 2 received roxithromycin oral tablets 2 × 150 mg/day (Roximisan, Slaviamed, Belgrade, Serbia), also for 10 days. The patients with control group did not receive any medication and all the patients with severe deterioration in nasal symptoms or endoscopic findings were excluded from further investigation. Both the investigators and the patients were aware of the drug being given or not given. The patients did not use other medications simultaneously with herbal drug/roxithromycin.
2.5. Detection of chemokines
Nasal fluid samples were obtained from all 78 ABRS patients, at the beginning of the study (day 0, visit 1) and again at day 10 (visit 2) after the start of study by absorption method. After the insertion of cotton wool stick (Institute for Virology, Vaccines and Sera, “Torlak,” Belgrade, Serbia) into the nasal middle meatus for 5 minutes, as previously described, 11 the stick watered with nasal fluid was put in a 2 mL tube, which contained 1 mL of transfer medium (two antibiotics and one antimycotic in phosphate‐buffered saline). It takes about 30 minutes for diffusion of mediators into the medium. After centrifugation of samples for 10 minutes and cell separation, the supernatants were frozen at −70°C, until mediator determination. The measurement of 13 chemokines (MCP‐1, RANTES, IP‐10, eotaxin, TARC, MIP‐1α, MIP‐1β, MIG, MIP‐3α, ENA‐78, GROα, I‐TAC and IL‐8) in nasal secretions of ABRS patients was done on a Flow Cytometer (NAVIOS, Beckmann Coulter, Brea, California), using human bead‐based multiple mediator detection commercial kit for chemokines (LEGEND plex, Bio Legend, San Diego, California). The levels of chemokines were expressed in picograms/milliliters (pg/mL). The sensitivities of detection, assay range and coefficients of variation for biochemical parameters are presented in Table 1.
TABLE 1.
Sensitivity of detection, assay range and coefficient of variation for investigated mediators
| Mediator | Sensitivity of detection (pg/mL) | Assay range (pg/mL) | Coefficient of variation (%) |
|---|---|---|---|
| MCP‐1 | 0.9 | 159.8‐3488.4 | 6 |
| RANTES | 4.3 | 188.2‐19 563.0 | 5 |
| IP‐10 | 1.1 | 37.3‐636.9 | 5 |
| Eotaxin | 1.4 | ND‐378.6 | 7 |
| TARC | 0.8 | 20.4‐151.3 | 4 |
| MIP‐1α | 2.1 | 7.0‐1999.7 | 4 |
| MIP‐1β | 1.4 | 6.1‐195.4 | 4 |
| MIG | 9.4 | ND‐ 420.8 | 9 |
| MIP‐3α | 2.5 | 6.7‐155.2 | 4 |
| ENA‐78 | 1.1 | 12.5‐935.4 | 7 |
| GROα | 6.7 | ND‐1550.9 | 3 |
| I‐TAC | 1.1 | 8.1‐139.1 | 6 |
| IL‐8 | 1.4 | 11.5‐7636.4 | 8 |
Abbreviation: ND, nondetectable.
2.6. Clinical evaluation
Total symptom score (TSS), the sum of intensities of 5 rhinosinusitis symptoms (nasal obstruction, rhinorrhea, postnasal drip, facial pain/pressure, loss of the sense of smell), as well as individual scores for each nasal symptom were assessed at the visit 1 and visit 2 by the same specialist, using a visual analogue scale (VAS) (0‐10 cm; 0 = absent, 10 = maximum intensity). Patients indicated their symptoms' score to be from 0 to 3 were diagnosed as “mild ARS.” Symptoms in the score range from 4 to 7 were diagnosed as “moderate ARS”, while the scores from 8 to 10 with fever of above 38°C for at least 3 days were diagnosed as “severe ARS.” The patients with severe disease were excluded from investigation. During the investigation, patients recorded their symptom scores and noted the use of medications on diary cards and the investigator recorded scores at the visit 2. The investigator evaluated compliance of the treatment by insight into the diary cards. TSS of control subjects was also assessed.
At visits 1 and 2, an experienced rhinologist evaluated the presence of mucosal edema and secretion in the middle meatus by use of nasal endoscopy (4 mm 0° endoscope, Karl Storz—Endoscope SE & Co, Tuttlingen, Germany). Four‐point scales were used for assessment of endoscopic findings, according to the Pfaar et al. 12 Mucosal edema scored from 0 (no edema) to 3 (severe edema); middle meatus secretion from 0 (none) to 3 (profuse). The maximum Total Endoscopic Score (TES) was 12, bilaterally. According to the current guidelines, radiological examinations (X‐ray, CT, MRI) were not used in the diagnostics of ABRS. 1 , 2
2.7. Safety
Reported adverse events were recorded throughout the study, with severity grades as mild, moderate and severe. At visit 2, nasal examination, laboratory tests and vital signs assessment were performed. All patients were aware of potential adverse effects of herbal medication or roxithromycin. Therefore, the development of any medical complications associated with progression of ABRS (orbital, endocranial or bone complications) was also recorded during the study.
2.8. Strength of the study and statistical analysis
We performed a power analysis with the use of the G*Power 3.1.9 programme (Heinrich Heine Univerität, Düsseldorf, Germany). For the effect size of 0.4 the type I error (α level) .05, the power of 80%, and the comparison of three groups with two measurements for each group, a total of 66 participants was calculated (22 per group). We calculated a drop‐out rate of 20% and therefore included 26 patients in each group. The parameters were expressed as mean ± SD. For comparison between the groups, the non‐parametric Kruskal‐Wallis test was used. If the test revealed significant difference among groups the Mann‐Whitney U test was further used to detect differences between groups. For paired comparisons in a group, we used the Wilcoxon's test. To calculate the relative changes of each parameter, we used the formula: posttherapeutic value—pretherapeutic value/pretherapeutic value. P values < .05 were considered significant. No adjustments for multiplicity were applied. The analysis was done by using the Statistical Package for the Social Sciences, version 15.0 software (SPSS Inc., Chicago, Illinois).
3. RESULTS
Baseline demographic characteristics of ABRS patients after randomization are presented in Table 2. Clinical parameters at visit 1 and visit 2 are presented in Table 3. We found no improvement in symptoms and endoscopic findings in non‐treated patients' group. After the both treatment modalities, we found significant improvement in all symptoms and endoscopic findings (P < .001). However, patients treated by roxithromycin had better relative improvement (P < .001) for all clinical parameters, except for rhinorrhea (P = .197) and postnasal drip score (P = .642) (Table 4).
TABLE 2.
Baseline demographic characteristics of ABRS patients, after randomization. They were divided into patients without treatment, patients treated by EPs 7630 and those treated by roxithromycin
| Parameter | Without treatment (n = 26) | EPs 7630 (n = 26) | Roxithromycin (n = 26) | P value |
|---|---|---|---|---|
| Male/female | 14/12 | 14/12 | 14/12 | 1.000 |
| Age (Mean ± SD [range]) |
43.5 ± 10.8 (19‐64) |
39.7 ± 12.8 (18‐62) |
42.3 ± 10.4 (18‐63) |
.372 |
TABLE 3.
Presentation of clinical parameters at visit 1 and visit 2
| Parameter | Controls (n = 26) Mean ± SD (range) | EPs 7630 (n = 26) |Mean ± SD (range) | Roxithromycin (n = 26) Mean ± SD (range) | P value Kruskal‐Wallis test |
|---|---|---|---|---|
| NO | ||||
| Visit 1 | 6.5 ± 0.6 (5‐7) | 6.5 ± 0.6 (5‐7) | 6.5 ± 0.6 (5‐7) | .975 |
| Visit 2 | 6.9 ± 0.3 (5‐7) | 3.1 ± 0.4 (2‐4) | 2.1 ± 0.7 (2‐3) | <.001 |
| RH | ||||
| Visit 1 | 6.4 ± 0.9 (4‐7) | 6.5 ± 0.6 (5‐7) | 6.6 ± 0.5 (6‐7) | .768 |
| Visit 2 | 6.9 ± 0.3 (6‐7) | 3.4 ± 0.8 (2‐5) | 3.5 ± 0.8 (2‐5) | <.001 |
| PD | ||||
| Visit 1 | 6.6 ± 0.6 (5‐7) | 6.4 ± 0.6 (5‐7) | 6.5 ± 0.6 (5‐7) | .624 |
| Visit 2 | 6.8 ± 0.4 (6‐7) | 3.0 ± 0.6 (2‐4) | 2.9 ± 0.7 (2‐5) | <.001 |
| FPP | ||||
| Visit 1 | 6.5 ± 0.8 (5‐7) | 6.5 ± 0.5 (6‐7) | 6.7 ± 0.7 (4‐7) | .147 |
| Visit 2 | 6.6 ± 0.6 (6‐7) | 3.0 ± 0.3 (2‐4) | 2.1 ± 0.6 (2‐4) | <.001 |
| LSS | ||||
| Visit 1 | 6.4 ± 0.8 (5‐7) | 6.6 ± 0.6 (5‐7) | 6.4 ± 0.6 (5‐7) | .384 |
| Visit 2 | 6.8 ± 0.5 (6‐7) | 3.1 ± 0.5 (2‐3) | 2.0 ± 0.6 (1‐3) | <.001 |
| TSS | ||||
| Visit 1 | 32.3 ± 2.0 (29‐35) | 32.5 ± 1.6 (28‐35) | 32.7 ± 1.8 (29‐35) | .701 |
| Visit 2 | 34.0 ± 1.0 (32‐35) | 15.9 ± 1.2 (12‐20) | 12.3 ± 2.0 (10‐15) | <.001 |
| TES | ||||
| Visit 1 | 10.6 ± 1.0 (9‐12) | 10.9 ± 0.9 (9‐12) | 10.4 ± 0.8 (9‐12) | .085 |
| Visit 2 | 11.8 ± 0.4 (11‐12) | 5.9 ± 0.7 (5‐7) | 3.8 ± 0.7 (3‐5) | <.001 |
| ME | ||||
| Visit 1 | 5.3 ± 0.8 (4‐6) | 5.8 ± 0.4 (5‐6) | 5.6 ± 0.5 (5‐6) | .046 |
| Visit 2 | 5.8 ± 0.4 (5‐6) | 2.9 ± 0.4 (2‐4) | 2.1 ± 0.5 (2‐3) | <.001 |
| MS | ||||
| Visit 1 | 5.3 ± 0.8 (4‐6) | 5.2 ± 0.7 (4‐6) | 4.8 ± 0.7 (4‐6) | .102 |
| Visit 2 | 5.9 ± 0.3 (4‐6) | 3.0 ± 0.6 (2‐4) | 1.8 ± 0.5 (1‐3) | <.001 |
Abbreviations: FPP, facial pain/pressure; LSS, loss of sense of smell; ME, mucosal edema; MS, mucopurulent secretion; NO, nasal obstruction; PD, postnasal drip; RH, rhinorrhea; TES, total endoscopic score; TSS, total symptom score.
TABLE 4.
Comparison of relative improvement of clinical parameters after two different treatment regimens (Mann‐Whitney U test)
| Clinical parameters | Control group** (%) | EPs 7630 (%) | Roxithromycin (%) | P value EPs 7630/Roxithromycin |
|---|---|---|---|---|
| Nasal obstruction | 7.1 ± 13.4 | −51.2 ± 11.9 | −66.3 ± 7.5 | <.001 |
| Rhinorrhea | 10.2 ± 19.3 | −48.7 ± 10.7 | −52.7 ± 13.7 | .197 |
| Postnasal drip | 4.2 ± 14.7 | −52.4 ± 9.6 | −56.3 ± 9.1 | .642 |
| Facial pain/pressure | 4.2 ± 17.7 | −53.9 ± 8.3 | −67.7 ± 4.9 | <.001 |
| Loss of the sense of smell | 7.9 ± 17.6 | −49.9 ± 9.0 | −69.7 ± 7.2 | <.001 |
| Total symptom score | 5.3 ± 6.7 | −51.4 ± 5.5 | −62.7 ± 3.7 | <.001 |
| Total endoscopic score | 11.6 ± 9.5 | −44.2 ± 8.0 | −64.7 ± 5.5 | <.001 |
| Mucosal edema | 11.9 ± 17.9 | −49.7 ± 10.3 | −63.1 ± 6.2 | <.001 |
| Mucopurulent secretion | 14.9 ± 18.7 | −36.7 ± 14.4 | −66.2 ± 10.3 | <.001 |
Comparison of the control group with EPs® 7630 or Roxithromycin revealed P < .001 for all parameters.
Chemokine concentrations in nasal secretions at visit 1 and visit 2 are presented in Table 5. The inflammatory mediator levels in nontreated patients are increased at visit 2, except for RANTES and GROα. After the therapy by EPs 7630, the concentrations of MCP‐1 and IP‐10 were significantly higher (P = .001; P = .049, respectively) and levels of MIP‐1α, ENA‐78, and IL‐8 were significantly lower (P < .001; P < .001; P < .001, respectively). After the roxithromycin therapy, we found increased concentration of IP‐10 (P = .049) and decreased levels of MCP‐1, MIP‐1α, ENA‐78, and IL‐8 (P < .001; P = .016; P < .001; P < .001, respectively). The concentration of MIP‐1β increase after the therapy by herbal drug and decrease after the roxithromycin therapy, but it was a difference that was close to a level of statistical significance (P = .057) (Table 5).
TABLE 5.
Presentation of chemokine concentrations at visit 1 and visit 2
| Parameter a | Without treatment (n = 26) Mean ± SD (range) | EPs 7630 (n = 26) Mean ± SD (range) | Roxithromycin (n = 26) Mean ± SD (range) | P value Kruskal‐Wallis test |
|---|---|---|---|---|
| MCP1 | ||||
| Visit 1 | 347.8 ± 277.4 | 465.3 ± 314.1 | 493.1 ± 328.4 | .015 |
| Visit 2 | 414.8 ± 314.2 | 598.3 ± 327.3 | 311.8 ± 205.6 | <.001 |
| RANTES | ||||
| Visit 1 | 990.3 ± 1138.9 | 938.9 ± 1107.7 | 884.5 ± 1102.2 | .935 |
| Visit 2 | 604.4 ± 409.8 | 902.8 ± 1038.9 | 824.2 ± 984.7 | .911 |
| IP‐10 | ||||
| Visit 1 | 128.4 ± 78.4 | 199.8 ± 155.4 | 208.8 ± 148.9 | .160 |
| Visit 2 | 296.0 ± 636.0 | 307.6 ± 179.4 | 289.9 ± 161.1 | .049 |
| Eotaxin | ||||
| Visit 1 | 13.0 ± 12.1 | 23.1 ± 37.8 | 31.9 ± 44.1 | .667 |
| Visit 2 | 14.7 ± 13.6 | 26.5 ± 50.0 | 19.6 ± 18.4 | .587 |
| TARC | ||||
| Visit 1 | 8.7 ± 14.9 | 10.9 ± 17.4 | 6.6 ± 14.0 | .840 |
| Visit 2 | 101 ± 18.2 | 10.8 ± 19.5 | 4.3 ± 11.9 | .885 |
| MIP 1α | ||||
| Visit 1 | 504.6 ± 381.7 | 703.6 ± 265.6 | 622.8 ± 369.7 | .008 |
| Visit 2 | 668.3 ± 433.1 | 375.5 ± 166.8 | 451.8 ± 258.8 | .016 |
| MIP 1β | ||||
| Visit 1 | 49.3 ± 53.9 | 62.9 ± 31.4 | 64.5 ± 32.7 | .007 |
| Visit 2 | 63.0 ± 57.4 | 73.0 ± 32.0 | 56.1 ± 24.4 | .057 |
| MIG | ||||
| Visit 1 | 1.1 ± 3.4 | 0.5 ± 1.5 | 0.6 ± 1.7 | .860 |
| Visit 2 | 1.7 ± 4.2 | 0.6 ± 1.7 | 0.8 ± 2.0 | .542 |
| MIP 3α | ||||
| Visit 1 | 33.8 ± 34.9 | 56.6 ± 29.1 | 58.4 ± 30.9 | <.001 |
| Visit 2 | 40.1 ± 35.5 | 55.8 ± 26.9 | 55.6 ± 26.9 | .008 |
| ENA‐78 | ||||
| Visit 1 | 207.2 ± 159.5 | 335.6 ± 150.8 | 322.8 ± 145.7 | .008 |
| Visit 2 | 558.8 ± 376.6 | 142.4 ± 98.8 | 232.4 ± 131.8 | <.001 |
| GROα | ||||
| Visit 1 | 44.5 ± 51.5 | 153.1 ± 243.3 | 158.9 ± 233.3 | .620 |
| Visit 2 | 42.9 ± 63.7 | 162.1 ± 114.4 | 102.7 ± 154.1 | .506 |
| I‐TAC | ||||
| Visit 1 | 11.4 ± 19.7 | 20.3 ± 33.4 | 25.2 ± 43.7 | .590 |
| Visit 2 | 13.6 ± 23.3 | 17.4 ± 32.0 | 26.0 ± 51.1 | .883 |
| IL‐8 | ||||
| Visit 1 | 596.5 ± 621.7 | 589.8 ± 518.7 | 640.3 ± 539.6 | .597 |
| Visit 2 | 862.5 ± 618.9 | 224.5 ± 157.6 | 415.5 ± 392.3 | <.001 |
Concentrations of inflammatory mediators in nasal secretions are expressed in pg/mL.
Regarding the relative changes of chemokine levels, treatment by EPs 7630 resulted in bigger increase of IP‐10 (P < .001) and bigger decrease of MIP‐1α, ENA‐78, and IL‐8 (P < .001; P < .001; P < .037, respectively) (Figure 2).
FIGURE 2.
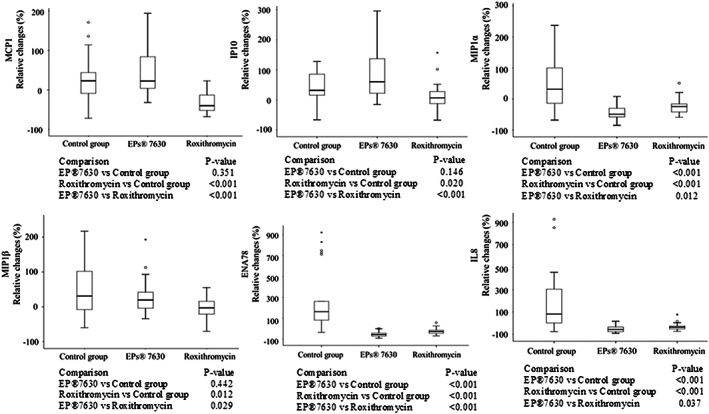
Relative changes in nasal secretion concentrations of chemokines MCP‐1, IP‐10, MIP‐1α, MIP‐1β, ENA‐78, and IL‐8 between visit 1 and visit 2
No adverse events were noted during the therapy by EPs 7630 and roxithromycin.
4. DISCUSSION
Inflammatory mediator‐related investigations in patients with ABRS have rarely been performed and our study is the first one which evaluated the chemokine production by nasal mucosa during the therapy with Pelargonium sidoides extract. According to previous investigations, contents of nasal secretions reflects the inflammatory status of the nasal mucosa and evolution of mucosal disease. 14 , 15 , 16 Nasal epithelial cells elicit their own repertoire of immune responses and actively prevent pathogens from damaging the airway. Upon viral and bacterial infection, nasal epithelium release not only anti‐microbial surfactants and mucus to delay pathogen transmission in the airway, but also secrete various cytokines and chemokines to drive immune responses against invading pathogens. 1 , 2 , 3 , 4 , 5 Bacterial infection of respiratory epithelium leads to an increased production of cytokines (IFN‐β, IFN‐γ, IL‐1β, TNF‐α, and IL‐6) and chemokines (IL‐8, IP‐10, I‐TAC, etc.). 1 , 5 , 13 , 14 , 15
Many different mechanisms of macrolide antibiotics immunomodulatory effects have been described throughout the literature. Effects have been reported on airway secretions, inflammation, and direct effect on bacteria. The many different actions of macrolide antibiotics on cellular function, including airway epithelial cells, neutrophils, eosinophils, lymphocytes, monocytes, macrophages, fibroblasts, and vascular endothelial cells have been described. 16 The epithelial cells demonstrated a decrease in mucin secretion and expression of inflammatory cytokines after the therapy by roxithromycin. 17 Relaxation in smooth muscle cells, inhibition of IL‐8 production and release, and decreases in fibroblast growth factors are also seen with macrolide use in in vitro studies. 5 , 16 , 17
Herbal drug EPs 7630 showed many actions against viral and bacterial infections. It increases ciliary beat frequency (CBF) of an adherent monolayer culture of human nasal epithelial cells. 6 This drug demonstrates effects against influenza and parainfluenza virus, respiratory syncytial virus, and, especially, human coronavirus by inhibitory action of herbal bioflavonoids and polyphenols against enzyme neuraminidase, very important in viral replication 6 , 7 In a controlled randomized study, Bachert et al. 8 found EPs 7630 to be well tolerated and superior in efficacy compared to placebo in the treatment of ARS of bacterial origin. Significant and clinically relevant benefits of treatment by this herbal medicine were already evident after 7 days of treatment. 8 EPs 7630 has direct effect against a spectrum of Gram‐positive and Gram‐negative bacteria by stimulating non‐specific immune response. 8 , 9 This mode of actions includes inhibition of bacterial adhesion to epithelial cells, stimulation of phagocytosis, nitric oxide (NO) release, and oxidative burst. 8 , 9 Immunomodulatory actions of this drug are also interesting. A large body of evidence indicates that induction of non‐specific host defence mechanisms against a number of pathogens, especially bacteria and viruses, is related to the production of IFN‐β and IFN‐γ. Previous in vitro investigations demonstrated an up‐regulation of these cytokines, as well as TNF‐α after the stimulation of human macrophages, lymphocytes and epithelial cells with Pelargonium sidoides extract. 6 , 7 , 8 , 9
We found significant improvement for all symptoms and endoscopic findings in our patients after the use of this herbal medicine. However, these clinical effects were better in patients treated by roxithromycin, except for the rhinorrhea and postnasal drip. This finding suggests that roxithromycin and EPs 7630 almost equally suppress the production and stimulate the elimination of mucus. In non‐treated patients from the control group, we found no improvement in clinical parameters, suggesting the conclusion that in ABRS patients we cannot expect the spontaneous resolution of symptoms and local findings. Therefore, the concentrations of almost all inflammatory mediators in patients from the control group found to be increased during the 10 days. On the other hand, our results suggest that therapy of ABRS patients by EPs 7630 stimulates MCP‐1 and IP‐10 and inhibits MIP‐1α, ENA‐78, and IL‐8 production in the nasal and sinus mucosa. On the other hand, we found that therapy by roxithromycin significantly increased levels of IP‐10 and decreased levels of MCP‐1, MIP‐1α, ENA‐78, and IL‐8 in nasal secretions. The concentration of MIP‐1β increase after the therapy by herbal drug and decrease after the roxithromycin therapy, but it was a difference that was close to a level of statistical significance. MCP‐1 is secreted by monocytes and macrophages and this chemokine exhibits chemotactic activity for monocytes, basophils and eosinophils, but it does not attract neutrophils. 18 IP‐10 is secreted by several cell types (monocytes, endothelial cells, and fibroblasts) in response to IFN‐γ action. This mediator has been attributed to several roles, such as chemoattraction for monocytes, macrophages, T‐cells and natural killer cells, all very important in defence against pathogens. 18 Both MCP‐1 and IP‐10 have functions mainly connected to monocyte actions. 18 On the other hand, ENA‐78 and IL‐8 are chemokines related to function of neutrophils and they are produced by nasal epithelial cells, monocytes and macrophages following stimulation of these cells with pro‐inflammatory cytokines IL‐1β and TNF‐α. 19 These chemokines stimulate the chemotaxis of neutrophils to the site of inflammation caused by bacterial infection. 19 , 20 MIP‐1α and MIP‐1β are strong pro‐inflammatory chemokines, well known as chemoattractants for neutrophils, monocytes, as well as eosinophils in patients with acute and chronic upper airway inflammations. 21 With respect to different effects on MCP‐1 and IL‐1β production in nasal mucosa, our results suggest the presence of similar modulatory actions of two drugs on monocyte and neutrophil functions in ABRS patients. Although neutrophils have protective functions against bacterial infections, a recent in vitro study, conducted by Kao et al. 22 demonstrated that serine proteases, enzymes derived from neutrophils showed detrimental effects on the mucosal epithelial barrier integrity with increased permeability, allowing for potential bacterial infection. Accordingly, we speculate that reduction of neutrophil chemokines by Pelargonium sidoides and roxithromycin leads to reduced attraction of neutrophils and production of neutrophil proteases, resulting in better protection and stabilization of nasal respiratory epithelium.
According to our results, none of the patients reported adverse events. However, there have been reports of allergic reactions, liver toxicity and hemorrhage after treatment with Pelargonium sidoides extract. 23 , 24 There is a theoretical risk of interactions with anticoagulants such as warfarin, and antiplatelet drugs such as aspirin. There are also cautions against use of the tested substance by patients with serious liver diseases or during pregnancy. 23 , 24 Allergic reactions and gastrointestinal symptoms are main side effects after the use of macrolide antibiotics. 5 , 17
This study has some limitations. It was an open label study based on the assessment of symptoms and endoscopic findings. Therefore, due to our financial limitations, we performed only biochemical analyses of chemokine profile in nasal fluid, but not a polymerase chain reaction (PCR) analysis of nasal and sinus mucosa regarding the capacity for production of these chemokines.
5. CONCLUSION
According to our results, Pelargonium sidoides extract and roxithromycin have similar modulatory effects on activation and migration of monocytes and neutrophils to the site of acute inflammation. Both drugs significantly improve symptoms and endoscopic findings in patients with uncomplicated ABRS. However, roxithromycin provides better clinical improvement relative to EPs 7630, except for postnasal drip and rhinorrhea as there was a non‐statistically significant difference.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This investigation was conducted as a part of scientific project of the Military Medical Academy Faculty of Medicine, Belgrade, Serbia (MFVMA02/19‐21/). Dr. Aleksandra Barać received support for research from the Project of Ministry of Education, Science and Technology of the Republic of Serbia (No. III45005).
Perić A, Vezmar Kovačević S, Barać A, Perić AV, Vojvodić D. Effects of Pelargonium sidoides extract vs roxithromycin on chemokine levels in nasal secretions of patients with uncomplicated acute rhinosinusitis. Laryngoscope Investigative Otolaryngology. 2021;6:25–33. 10.1002/lio2.514
Funding information Ministry of Education, Science and Technology of the Republic of Serbia, Grant/Award Number: III45005
BIBLIOGRAPHY
- 1. Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy & rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(1):S22‐S209. [DOI] [PubMed] [Google Scholar]
- 2. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2):S1‐S39. [DOI] [PubMed] [Google Scholar]
- 3. Berger G, Kattan A, Bernheim J, Ophir D, Finkelstein Y. Acute sinusitis: a histopathological and immunohistochemical study. Laryngoscope. 2000;110(12):2089‐2094. [DOI] [PubMed] [Google Scholar]
- 4. Park JJ, Bachert C, Dazert S, Kostev K, Seidel DU. Current healthcare pathways in the treatment of rhinosinusitis in Germany. Acta Otolaryngol. 2018;138(12):1086‐1091. [DOI] [PubMed] [Google Scholar]
- 5. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(suppl S29):1‐464. [DOI] [PubMed] [Google Scholar]
- 6. Roth M, Fang L, Stolz D, Tamm M. Pelargonium sidoides radix extract EPs 7630 reduces rhinovirus infection trough modulation of viral binding proteins on human bronchial epithelial cells. PLoS ONE. 2019;14:e0210702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kolodziej H, Kiderlen AF. In vitro evaluation of antibacterial and immunomodulatory activities of Pelargonium reniforme, Pelargonium sidoides and the related herbal drug preparation EPs® 7630. Phytomedicine. 2007;14(suppl 6):18‐26. [DOI] [PubMed] [Google Scholar]
- 8. Bachert C, Schapowal A, Funk P, Kieser M. Treatment of acute rhinosinusitis with the preparation from Pelargonium sidoides EPs 7630: a randomized, double‐blind, placebo‐controlled trial. Rhinology. 2009;47(1):51‐58. [PubMed] [Google Scholar]
- 9. Kolodziej H. Antimicrobial, antiviral and immunomodulatory activity studies of Pelargonium sidoides (EPs® 7630) in the context of health promotion. Pharmaceuticals. 2011;4(10):1295‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luna LA Jr, Bachi ALL, Novaes e Brito RR, et al. Immune response induced by Pelargonium sidoides extract in serum and nasal mucosa of athletes after exhaustive exercise: modulation of secretory IgA, IL‐6 and IL‐15. Phytomedicine. 2011;18(4):303‐308. [DOI] [PubMed] [Google Scholar]
- 11. Perić A, Sotirović J, Špadijer‐Mirković C, Matković‐Jožin S, Perić AV, Vojvodić D. Nonselective chemokine levels in nasal secretions of patients with perennial nonallergic and allergic rhinitis. Int Forum Allergy Rhinol. 2016;6(4):392‐397. [DOI] [PubMed] [Google Scholar]
- 12. Pfaar O, Mullol J, Anders C, Hörmann K, Klimek L. Cyclamen europaeum nasal spray, a novel phytotherapeutic product for the management of acute rhinosinusitis: a randomized double‐blind, placebo‐controlled trial. Rhinology. 2012;50(1):37‐44. [DOI] [PubMed] [Google Scholar]
- 13. Rudack C, Stoll W, Bachert C. Cytokines in nasal polyposis, acute and chronic sinusitis. Am J Rhinol. 1998;12(6):383‐388. [DOI] [PubMed] [Google Scholar]
- 14. Riechelmann H, Deutschle T, Rozsasi A, Keck T, Polzehl D, Bürner H. Nasal biomarker profiles in acute and chronic rhinosinusitis. Clin Exp Allergy. 2005;35(9):1186‐1191. [DOI] [PubMed] [Google Scholar]
- 15. Scheckenbach K, Wagenmann M. Cytokine patterns and endotypes in acute and chronic rhinosinusitis. Curr Allergy Asthma Rep. 2016;16(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruh C, Banjade R, Mandadi S, Marr C, Sumon Z, Crane JK. Immunomodulatory effects of antimicrobial drugs. Immunol Invest. 2017;46(8):847‐863. [DOI] [PubMed] [Google Scholar]
- 17. Mandal R, Patel N, Ferguson BJ. Role of antibiotics in sinusitis. Curr Opin Infect Dis. 2012;25(2):183‐192. [DOI] [PubMed] [Google Scholar]
- 18. Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN‐gamma‐inducible protein 10 (IP‐10, CXCL10)‐deficient mice reveal a role for IP‐10 in effector T‐cell generation and trafficking. J Immunol. 2002;168(7):3195‐3204. [DOI] [PubMed] [Google Scholar]
- 19. Chang MS, McNinck J, Basu R, Simonet S. Cloning and characterization of the human neutrophil‐activity peptide (ENA‐78) gene. J Biol Chem. 1994;269(41):25277‐25282. [PubMed] [Google Scholar]
- 20. Dorresteijn PM, Muller D, Xie Y, Zhang Z, Barrett BR. Validation of the nasal mucus index, a novel measurement of acute respiratory infection severity. Am J Rhinol Allergy. 2016;30(5):324‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein‐1. Cytokine Growth Factor Rev. 2002;13(6):455‐481. [DOI] [PubMed] [Google Scholar]
- 22. Kao SS, Ramezanpour M, Bassiouni A, Wormald PJ, Psaltis AJ, Vreugde S. The effect of neutrophil serine proteases on human nasal epithelial cell barrier function. Int Forum Allergy Rhinol. 2019;9(10):1220‐1226. [DOI] [PubMed] [Google Scholar]
- 23. De Boer HJ, Hagemann U, Bate J, Meyboom RH. Allergic reactions to medicines derived from Pelargonium species . Drug Saf. 2007;30(8):677‐680. [DOI] [PubMed] [Google Scholar]
- 24. Timmer A, Günther J, Motschall E, Rücker G, Antes G, Kern WV. Pelargonium sidoides extract for treating acute respiratory tract infections. Cochrane Database Syst Rev. 2013;10:CD006323. [DOI] [PMC free article] [PubMed] [Google Scholar]


