Abstract
Healthcare-associated infections (HAI) are generally preventable causes of increased cost, morbidity, and mortality. Further, HAI carry penalties in the era of hospital value-based care. However, very little is known about the incidence and outcomes of HAI among patients hospitalized with common cardiovascular conditions. Using a national database, we identified adults aged ≥18 years hospitalized with 5 common cardiovascular conditions, including heart failure, acute myocardial infarction, coronary artery bypass grafting, cardiogenic shock, and atrial fibrillation or flutter. We assessed for temporal trends in incidence, cost, length of stay (LOS), and mortality associated with ventilator-associated pneumonia, catheter-associated urinary tract infections, central line-associated bloodstream infection, and Clostridium difficile infections. Between 2008 and 2015, we identified 159,021 hospitalizations ≥1 HAI (49.6% heart failure, 20.4% acute myocardial infarction, 10.5% coronary artery bypass grafting, 18.6% cardiogenic shock, and 11.9% atrial fibrillation or flutter). Clostridium difficile infections (75.4%) were the most common followed by catheter-associated urinary tract infections (15.1%), ventilator-associated pneumonia (7.9%), and central line-associated bloodstream infection (3.1%). Nearly half of the patients (46.3%) with HAI required discharge to a skilled care facility compared with 15.7% of patients who did not. After propensity matching, HAI remained associated with an increased LOS (4.9 vs 9.6 days, p <0.0001), total hospital charges ($79,227 vs $50,699, p <0.0001), and in-hospital mortality (13% vs 10.4%, p <0.0001) compared with patients who did not acquire a HAI. In conclusion, patients with cardiovascular disease acquiring a HAI had substantially higher costs, LOS, and mortality.
Cardiovascular disease remains the most common cause of death in the United States (US) and continues to represent a considerable burden on the healthcare system.1,2 Currently, cardiovascular admissions represent roughly 25% of the 20 most expensive inpatient conditions.3 A substantial portion of these healthcare resources are spent on inpatient admissions.4–6 As the population continues to age and co-morbidities increase, these costs are likely to increase.1 One factor associated with increased costs is healthcare-associated infections (HAI), which are potentially preventable.7 However, the extent of this problem and the association of HAI with clinical outcomes and utilization for patients admitted with common cardiovascular conditions are unknown. Using the National Inpatient Sample (NIS), we sought to define the magnitude of HAI by assessing for national trends in cost, length of stay, and mortality for those hospitalized for heart failure, cardiogenic shock, atrial fibrillation/flutter (AF), acute myocardial infarction (AMI), and coronary artery bypass grafting (CABG).
Methods
Details on the NIS database have been previously well described.8 It contains a 20% sample of all inpatient hospitalizations (excluding observation status and psychiatric hospitals), and captures procedures and diagnoses by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding. In addition to patient demographics, patient co-morbidities, in-hospital outcomes, hospital characteristics, insurance status, and cost are available.
In our study, we used data for the years 2008 through September 2015, which includes a design change to the NIS.9 Before 2012, the NIS included all inpatient discharges from a random 20% of inpatient hospitals. After 2012, the NIS database included 20% sampling of inpatient discharges from all US hospitals. A new set of weights (“trendwt”) was developed to allow for patient-level trend analysis for years 1993 to 2011.8,10,11 Our analyses continued until September 2015 when the NIS went to ICD-10-CM coding. October to December 2015 was ICD-10-CM-coded data, and given the challenges with cross walking ICD-9-CM and ICD-10-CM, these months were excluded. The Institutional Review Board at Yale University and Ohio State University considered this study exempt from formal review because the NIS is a public database without patient identifiers.
Before designing this study, we identified the common admission diagnoses which spanned the realms of cardiac surgery, general and critical care cardiology. The diagnoses thus identified were heart failure, AF, AMI, cardiogenic shock, and CABG.12–15 We used ICD-9-CM codes to identify all hospitalized adults aged ≥18 years with a primary diagnosis of HF (ICD-9-CM 425,428, 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, or 404.93), AF (ICD-9-CM 427.31 or 427.32), or AMI (ICD-9-CM 410). Additionally, adults with diagnosis of cardiogenic shock (DX1 to DX30 in NIS, ICD-9-CM 785.51) or procedural ICD-9-CM code of CABG (PR1 to PR15 in NIS, ICD-9-CM 36.1 or 36.2) were included. In this cohort of 5 common cardiovascular admissions, we identified hospitalizations with ventilator-associated pneumonia (VAP), central line-associated bloodstream infection (CLABSI), catheter-associated urinary tract infection (CAUTI), and Clostridium difficile infection (CDI) using ICD-9 CM code 997.31, 996.64, 999.32, and 008.45, respectively. If 1 or more of these infections occurred, it was defined as a HAI hospitalization.
Consistent with previous studies,16 to better evaluate conditions where the cohort was not defined by the primary diagnosis, we used DXCCS1 to identify cardiac discharge diagnosis of coronary artery disease (101), valvular heart disease (96), peripheral vascular disease (114 to 119), arrhythmias (106, 107), hypertension (98, 99, 183, 249), and ischemic stroke (109 to 113). ICD-9 CM codes 427.41 and 437.5 were utilized to identify the diagnosis of out-of-hospital cardiac arrest. ICD-9-CM procedure codes or PRCCS codes were used to identify procedures (eTable 1 in the Supplement). Patient co-morbidities were determined using Elixhauser-based co-morbidity measures derived from ICD-9-CM coding.17
We assessed the trends in hospital costs and charges, length of stay, in-hospital mortality, and discharge disposition for patients acquiring a HAI. Hospital charges were multiplied with cost-to-charge ratios and wage index to calculate total hospitalization costs and then adjusted for inflation in 2015 US dollars.18,19 Finally, we assessed for associations between HAI and procedures traditionally considered cardiac and noncardiac.
We used recommended analyses for survey data, including specific statements (eg, SURVEYFREQ, SURVEY-MEANS) to obtain descriptive statistics. National estimates were acquired using patient and hospital-specific discharge weights. The DOMAIN method was used to ensure estimated statistics and measures of variance were accurate.20,21 Continuous variables were assessed using survey-specific linear regression, and the Cochrane Armitage test was used for trends across categorical variables.
HAI incidence was calculated and presented per 100,000 hospitalizations for each diagnosis. Next, since there is a difference in baseline risk and disease severity in HAI versus non-HAI patient populations, we used a propensity score-matched design for survey data to account for patient-level and hospital-level variables (eTable 2 in the Supplement). We used an unfitted multivariable logistic regression model to determine each patient’s propensity of acquiring a HAI. Per survey data recommendations, NIS weights were used in the propensity estimation model.22 Even though rates of urinary catheter use and total parenteral nutrition was likely under-reported, they were included in this model to account for infection risk. The propensity score, generated by logistic regression, represents the relation between multiple characteristics and the dependent variable as a single characteristic. The propensity score thus obtained (between 0 and 1) is utilized by an 81 Digit Match algorithm which matches a case to control at the eighth decimal point followed by seventh decimal point followed by sixth decimal point and so on using a greedy matching algorithm.23 We then matched 2 non-HAI:1 HAI (2:1 match). Next, we examined trends in all the outcomes as described above over time. Specifically, for Figure 2, insurance type was not used in the propensity model described above (c-statistic = 0.8). Additionally, for Figure 3, procedures were not included in the model (2008 to 2009, 2011 to 2012, and 2014 to 2015 c-statistic = 0.74, 0.75, and 0.74, respectively) since they are considered “in-hospital events” which would likely increase mortality. Charges and length of stay were log-transformed (natural log) because they were not normally distributed, and geometric mean was presented.24,25 A value of 0.0001 was imputed for length of stay of 0 days to avoid negative log values. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, North Carolina).
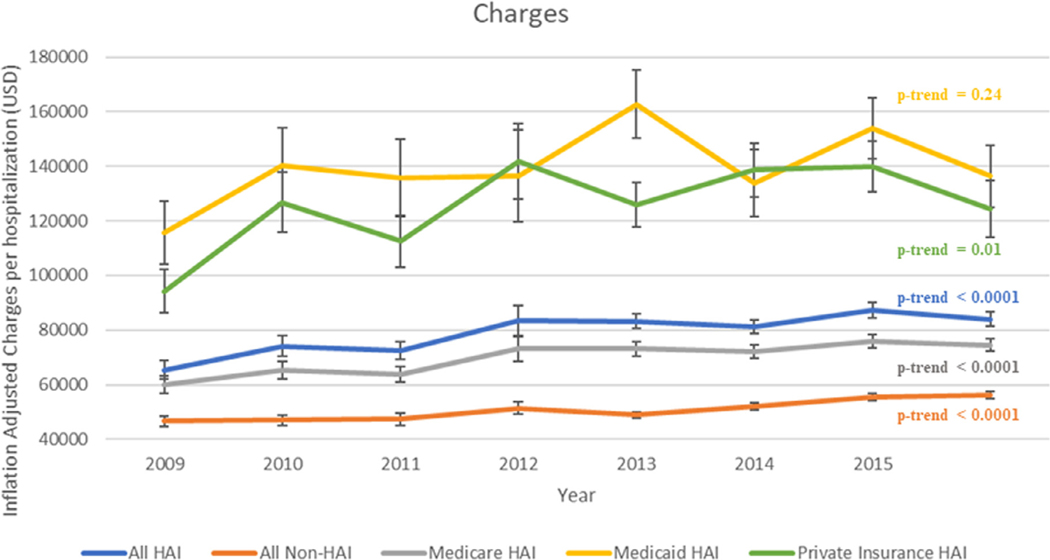
Figure 2.
Trends in hospital charges by insurance type in a fully propensity/risk adjusted model.
p-trends presented of geometric means after log transforming the values.
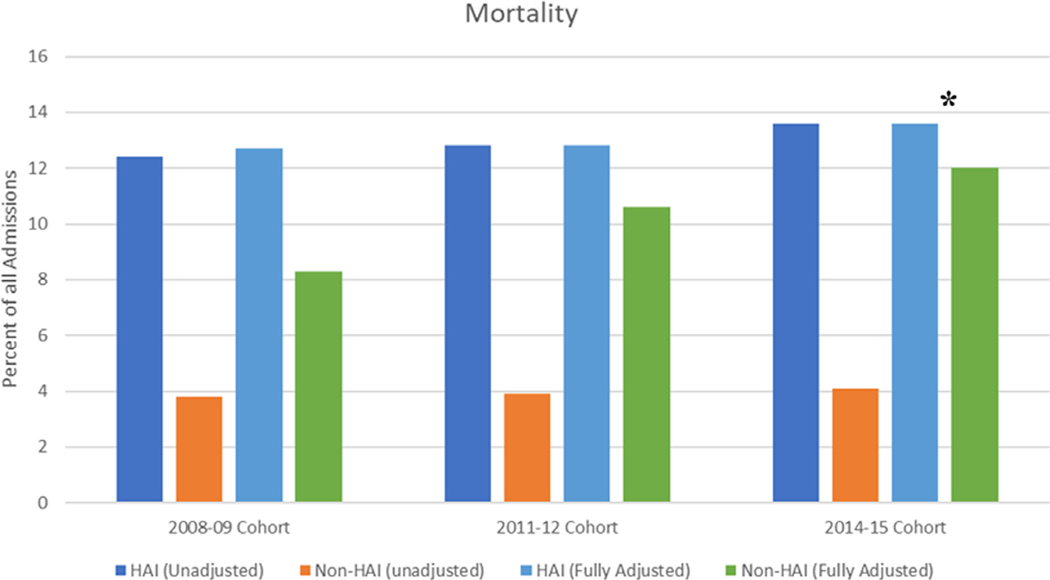
Figure 3.
In-hospital mortality in our unadjusted and fully propensity/risk adjusted model for 3 different time periods
All p values <0.0001 except * = 0.001, odds ratio (confidence interval) for adjusted models 2008 to 2009 cohort, 2011 to 2012 cohort, and 2014 to 2015 cohort 1.6 (1.4 to 1.8), 1.2 (1.1 to 1.3), and 1.2 (1.1 to 1.3), respectively.
Results
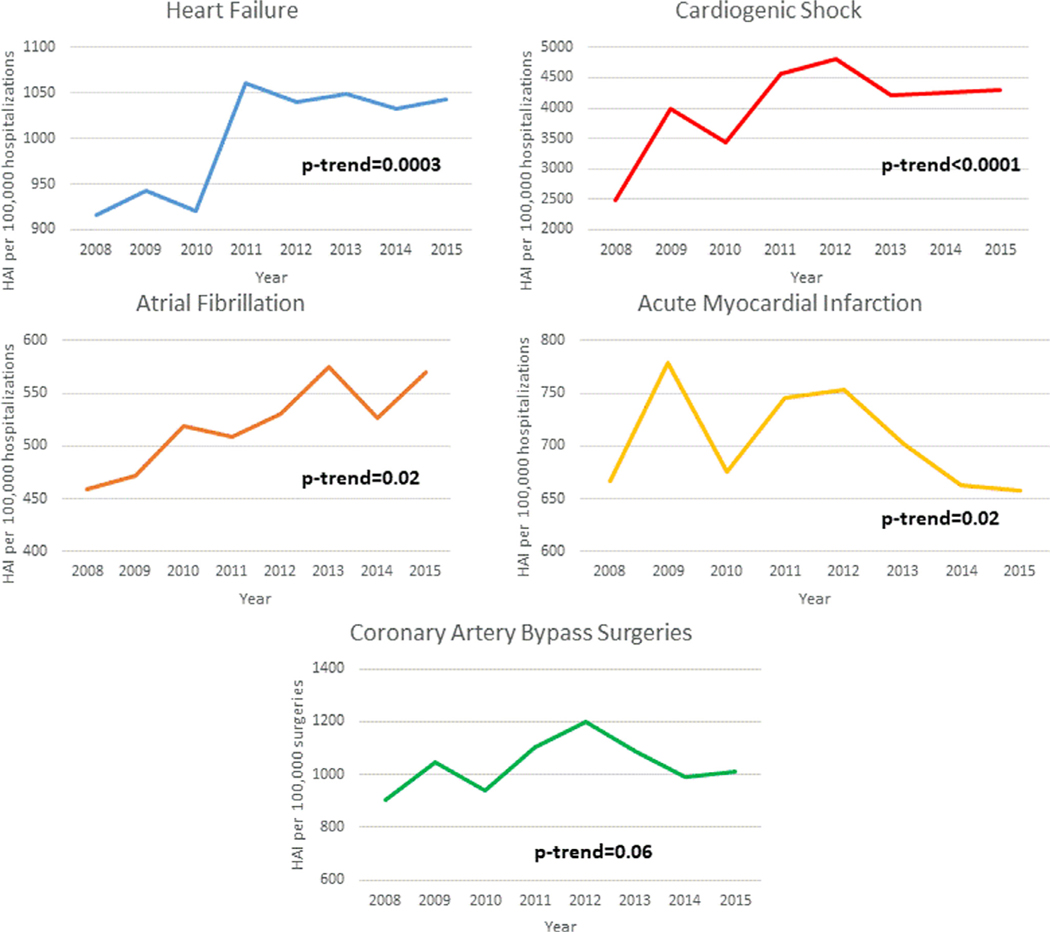
From 2008 to 2015, the study included 17,889,852 hospitalizations with 44.3% heart failure, 4.2% cardiogenic shock, 20.5% AF, 26.2% AMI, and 9.4% CABG. Of these, we identified 158,971 hospitalizations from NIS with at least 1 HAI. Overall, the most common HAI was CDI (75.4%) followed by CAUTI (15.1%), VAP (7.9%), and CLABSI (3.1%) (eTable 3 in the Supplement). Of the 5 included admission diagnoses, cardiogenic shock (4%) was most commonly associated with HAIs. Since 2008, there was a trend toward increased HAIs for patients hospitalized for heart failure (ptrend = 0.0003), cardiogenic shock (ptrend <0.0001), and atrial fibrillation/flutter (p = 0.02; Figure 1). However, the overall incidence of HAI remained unchanged for patients admitted for AMI and CABG.
Figure 1.
Incidence per 100,000 hospitalizations.
Patient demographics and hospital characteristics among hospitalizations with HAI over time are shown in Table 1. There were no differences in the occurrence of HAIs based upon gender or race/ethnicity. Over the study period, infection events increased in patients in the lowest income quartile while decreasing in the highest income quartile (p = 0.0016). We found that cardiovascular discharge diagnoses decreased, whereas noncardiovascular diagnoses, with respiratory and infectious diagnoses, increasing from 5.6% to 14.3% (p <0.0001). Similarly, noncardiovascular co-morbidities, including anemia, chronic liver and kidney disease, thyroid disease, fluid/electrolyte disorders, and substance abuse increased in frequency for patients acquiring a HAI (p <0.0001). Demographic and hospital characteristics stratified by each of the 5 diagnoses (eTable 4) and HAI versus non-HAI (eTable 5) are found in the Supplement.
Table 1.
Patient-level characteristics for hospitalizations with HAI by calendar year
| HAI (n= 159,021) | 2008 (n = 18,564) | 2009 (n = 20,215) | 2010 (n = 18,267) | 2011 (n = 21,655) | 2012 (n = 21,320) | 2013 (n = 20,975) | 2014 (n = 21,085) | 2015* (n = 16,940) | p value |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) (mean ± SE) | 76.2 ± 0.29 | 74.5 ± 0.35 | 74.8 ± 0.35 | 73.9 ± 0.36 | 72.6 ± 0.26 | 73.1 ± 0.24 | 71.8 ± 0.26 | 72 ± 0.27 | <0.0001 |
| 18–39 years | 1.0% | 1.4% | 1.8% | 1.7% | 1.9% | 1.8% | 2.5% | 2.3% | <0.0001 |
| 40–59 years | 9.1% | 11.8% | 11.2% | 13.0% | 14.9% | 13.6% | 15.3% | 14.6% | |
| 60–79 years | 42.7% | 45.5 | 44.4% | 45.4% | 46.5% | 46.7% | 48.8% | 50.2% | |
| ≥80 years | 47.2% | 41.2% | 42.6% | 40.0% | 36.7% | 38.0% | 33.3% | 32.9% | |
| ≥65 years | 83.1% | 78.7% | 78.8% | 76.8% | 74.7% | 76.0% | 72.9% | 73.6% | <0.0001 |
| Women | 54.6% | 51.6% | 53.7% | 52.9% | 51.9% | 51.9% | 50.3% | 49.6% | 0.0032 |
| Race | 0.34 | ||||||||
| White | 77.2% | 78.0% | 76.7% | 75.2% | 73.6% | 75.4% | 73.2% | 72.8% | |
| Black | 11.1% | 11.0% | 13.1% | 12.6% | 14.0% | 13.1% | 14.4% | 15.5% | |
| Hispanic | 6.2% | 6.0% | 5.6% | 7.0% | 6.7% | 6.8% | 6.7% | 6.7% | |
| Other | 5.6% | 5.0% | 4.6% | 5.3% | 5.7% | 4.7% | 5.8% | 4.9% | |
| Income quartiles† | 0.0016 | ||||||||
| 0–25 | 26% | 25.2% | 25.3% | 26.8% | 29.8% | 27.4% | 28.9% | 31.1% | |
| 26–50 | 26% | 24.3% | 24.8% | 23.3% | 22.6% | 25.8% | 27.5% | 25.3% | |
| 51–75 | 23.5% | 25.3% | 25.4% | 26.6% | 24.3% | 23.6% | 23.0% | 23.4% | |
| 76–100 | 24.5% | 25.2% | 24.5% | 23.3% | 23.4% | 23.2% | 20.7% | 20.1% | |
| Discharge diagnoses | |||||||||
| Coronary artery disease | 5.5% | 5.5% | 4.4% | 4.5% | 4.4% | 3.7% | 3.5% | 3.6% | <0.0001 |
| Acute coronary syndrome | 22.1% | 23.0% | 21.1% | 20.0% | 20.9% | 19.8% | 18.7% | 17.9% | <0.0001 |
| Heart failure | 47.4% | 49.5% | 50.7% | 49.5% | 46.6% | 48.3% | 48.6% | 48.3% | 0.0038 |
| Cardiac arrest‡ | 0.1% | 0.2% | 0.2% | 0.5% | 0.2% | 0.5% | 0.7% | 0.4% | <0.0001 |
| Valvular heart disease | 2.0% | 2.6% | 1.9% | 2.4% | 2.4% | 2.4% | 1.9% | 2.1% | 0.64 |
| Peripheral vascular disease | 0.3% | 0.6% | 0.5% | 0.5% | 0.7% | 0.5% | 0.5% | 0.5% | 0.33 |
| Arrhythmia | 11.7% | 11.4% | 13.3% | 12.3% | 12.9% | 13.9% | 12.6% | 12.6% | 0.03 |
| Hypertension | 3.3% | 3.0% | 3.8% | 4.2% | 4.1% | 4.4% | 5.3% | 5.4% | <0.0001 |
| Stroke | 0.05% | 0.2% | 0.2% | 0.2% | 0.3% | 0.1% | 0.3% | 0.2% | 0.16 |
| Respiratory diagnosis | 0.9% | 0.8% | 0.9% | 1.3% | 1.3% | 1.2% | 1.7% | 1.3% | 0.004 |
| Infectious diagnosis | 2.3% | 2.9% | 3.3% | 4.9% | 5.1% | 5.4% | 6.1% | 7.1% | <0.0001 |
| Co-morbidities | |||||||||
| Cardiomyopathy | 19.5% | 25.0% | 24.9% | 24.5% | 24.6% | 25.7% | 28.1% | 28.4% | <0.0001 |
| Peripheral vascular disease | 13.5% | 13.6% | 14.0% | 16.7% | 16.2% | 16.3% | 16.2% | 16.7% | <0.0001 |
| Valvular heart disease | 2.3% | 3.4% | 3.3% | 3.7% | 3.5% | 4.4% | 3.3% | 4.3% | 0.0002 |
| Hypertension | 57.9% | 59.6% | 61.6% | 63.4% | 64.0% | 64.9% | 65.4% | 65.2% | <0.0001 |
| Diabetes m\mellitus | 34.5% | 36.1% | 35.5% | 39.6% | 40.8% | 40.3% | 40.3% | 41.7% | <0.0001 |
| Obesity | 7.6% | 9.8% | 10.1% | 14.0% | 16.3% | 16.2% | 18.3% | 18.7% | <0.0001 |
| Smoker | 7.0% | 13.1% | 15.6% | 17.6% | 17.9% | 18.8% | 23.5% | 26.7% | <0.0001 |
| Weight loss | 12.6% | 15.9% | 16.0% | 19.0% | 18.4% | 17.8% | 18.6% | 18.7% | <0.0001 |
| AIDS | 0.2% | 0.4% | 0.3% | 0.2% | 0.5% | 0.2% | 0.5% | 0.4% | 0.09 |
| Anemia | 30.5% | 33.5% | 35.6% | 37.5% | 37.4% | 37.1% | 36.7% | 38.0% | <0.0001 |
| Arthritis and collagen vascular disease | 2.7% | 2.4% | 3.2% | 4.0% | 3.3% | 3.5% | 4.1% | 4.3% | <0.0001 |
| Cancer | 5.7% | 5.4% | 5.5% | 5.8% | 6.2% | 5.7% | 5.7% | 6.1% | 0.74 |
| Chronic liver disease | 2.3% | 2.4% | 2.6% | 2.9% | 3.6% | 3.6% | 5.2% | 5.1% | <0.0001 |
| Chronic renal disease | 35.9% | 37.9% | 40.2% | 42.5% | 43.9% | 45.7% | 47.1% | 47.5% | <0.0001 |
| Chronic lung disease | 30.5% | 31.2% | 31.5% | 32.3% | 31.9% | 31.6% | 31.4% | 31.3% | 0.88 |
| Hypothyroidism | 13.1% | 13.8% | 14.9% | 16.3% | 16.7% | 17.2% | 17.8% | 17.1% | <0.0001 |
| Psychiatric | 10.8% | 11.1% | 12.3% | 14.2% | 13.4% | 14.9% | 15.2% | 15.1% | <0.0001 |
| Fluid/electrolyte disorder | 43.7% | 49.3% | 49.1% | 53.6% | 55.2% | 56.1% | 60.0% | 61.3% | <0.0001 |
| Coagulation disorder | 8.7% | 12.6% | 12.0% | 14.6% | 15.4% | 14.4% | 16.0% | 16.5% | <0.0001 |
| Substance abuse | 2.6% | 2.7% | 2.7% | 3.6% | 4.0% | 4.7% | 5.6% | 5.5% | <0.0001 |
| Hospital characteristics | |||||||||
| Teaching hospital | 50.9% | 53.0% | 50.7% | 54.9% | 58.7% | 57.8% | 68.8% | 70.2% | <0.0001 |
| Bed size | <0.0001 | ||||||||
| Small | 11.6% | 9.6% | 9.4% | 9.0% | 10.2% | 10.9% | 15.2% | 14.5% | |
| Medium | 22.2% | 20.4% | 22.2% | 22.1% | 23.7% | 23.8% | 25.8% | 27.0% | |
| Large | 66.2% | 70.0% | 68.5% | 68.9% | 66.1% | 65.3% | 59.0% | 58.4% | |
| Region | 0.92 | ||||||||
| Northeast | 26.2% | 24.0% | 24% | 24.8% | 23.5% | 23.1% | 21.2% | 19.7% | |
| Midwest | 26.4% | 25.3% | 24.8% | 22.9% | 24.0% | 22.6% | 22.0% | 23.8% | |
| South | 31.8% | 33.7% | 33.7% | 34.3% | 35.6% | 36.5% | 38.1% | 37.3% | |
| West | 15.6% | 17.0% | 17.5% | 18.1% | 17.0% | 17.9% | 17.8% | 19.2% |
Only data until September 2015 presented.
Median household income quartiles based on patient zip code.
Out-of-hospital.
During the study period, there was a stable incidence of HAIs in patients undergoing procedures that have traditionally been considered cardiac and an increase in HAIs in “non-traditional” cardiovascular procedures. Patients undergoing intra-aortic balloon pump, coronary angiography with or without coronary intervention, ablation, pacemaker placement, and pulmonary artery catheters, showed a stable incidence of HAI (p >0.05 for all; eTable 6 in the Supplement). Both percutaneous left ventricular assist devices and extracorporeal membrane oxygenation showed an increased association with HAI. In contrast, many noncardiac procedures, such as invasive and noninvasive mechanical ventilation, hemodialysis, and bronchoscopy, were associated with a temporal increase in HAIs (p <0.0001 for all).
Nearly 85% of HAI hospitalizations were billed to either Medicare or Medicaid. Since 2008, Medicare as the payment source has decreased from 81.3% to 76.7% of HAI stays. Alternatively, there has been in increase in Medicaid charges from 4.3% to 7.7% (Table 2). In 2008, the mean total hospital charge (±SE) for a hospitalization with a HAI increased from $63,075 (±$2,715) to $85,372 (±2657) (p <0.0001). In our full-adjusted model, excluding insurance type, hospitalizations associated with at least 1 HAI showed a greater increase in charges compared with non-HAI hospitalizations ($18,564 in HAI vs $9,712 per HAI vs non-HAI hospitalization in the 7 years considered, p <0.0001; Figure 2). All insurance types showed an increase in total charges with exception of Medicaid (ptrend = 0.24), which was associated with the highest charges throughout the study period.
Table 2.
Cost and outcomes for hospitalizations with HAI by calendar year
| HAI (n = 159,021) | 2008 (n = 18,564) | 2009 (n = 20,215) | 2010 (n = 18,267) | 2011 (n = 21,655) | 2012 (n = 21,320) | 2013 (n = 20,975) | 2014 (n = 21,085) | 2015* (n = 16,940) | p value |
|---|---|---|---|---|---|---|---|---|---|
| Payment source | 0.0008 | ||||||||
| Medicare | 81.3% | 77.7% | 78.2% | 79.0% | 77.9% | 79.3% | 76.4% | 76.7% | |
| Medicaid | 4.3% | 6.2% | 6.3% | 5.6% | 6.3% | 6.5% | 7.6% | 7.7% | |
| Private | 11.7% | 12.5% | 12.0% | 11.9% | 12.8% | 11.2% | 13.0% | 12.5% | |
| Self-Pay | 1.1% | 2.0% | 1.6% | 1.6% | 1.2% | 1.5% | 1.4% | 1.1% | |
| Others | 1.6% | 1.6% | 1.3% | 1.3% | 1.8% | 1.5% | 1.7% | 2.0% | |
| Outcomes | |||||||||
| Length of stay (mean ± SE, d) | 9.4 ± 0.3 | 10.1 ± 0.3 | 9.2 ± 0.3 | 9.6 ± 0.4 | 9.8 ± 0.2 | 9.4 ± 0.2 | 9.6 ± 0.2 | 9.4 ± 0.2 | 0.59 |
| Total hospital charges (mean ± SE, US$) | 63,075 ± 2,715 | 75,073 ± 3,762 | 70,503 ± 2,732 | 81,902 ± 4,669 | 82,805 ± 2,617 | 80,864 ± 2,554 | 87,393 ± 2,759 | 85,372 ± 2,657 | <0.0001 |
| Total hospital costs (mean ± SE, US$) | 22,164 ± 812 | 24,512 ± 1,106 | 22,499 ± 778 | 24,957 ± 1,641 | 24,819 ± 781 | 23,441 ± 727 | 24,565 ± 766 | 23,229 ± 720 | 0.4 |
| Unadjusted mortality | 11.7% | 13.2% | 12.3% | 12.5% | 13.1% | 12.7% | 12.9% | 14.6% | 0.14 |
| Disposition | 0.0002 | ||||||||
| Home | 18.5% | 16.9% | 18.1% | 17.4% | 19.0% | 19.1% | 18.2% | 18.9% | |
| Short-term hospital | 3.3% | 2.6% | 3.0% | 3.0% | 3.2% | 3.2% | 3.4% | 3.2% | |
| Skilled care facility | 48.3% | 47.9% | 46.4% | 48.4% | 45.6% | 46.1% | 44.9% | 42.9% | |
| Home health care | 18% | 19.2% | 19.7% | 18.4% | 18.6% | 18.5% | 20.2% | 19.4% | |
| Against medical advice | 0.2% | 0.2% | 0.2% | 0.3% | 0.4% | 0.3% | 0.4% | 0.9% | |
| Unknown | 0.1% | 0.2% | 0.2% | 0.3% | 0.1% | 0.2% | 0.1% | 0.1% |
Only data until September 2015 presented.
In unadjusted analyses, hospitalizations associated with at least 1 HAI spent a mean of 6.6 days longer in the hospital were charged $42,470 more, and had an 8.9% increased risk of in-hospital mortality (p <0.0001 for all; eTable 4 in the Supplement). Figure 3 shows the in-hospital mortality for unadjusted and propensity score-matched analyses from 3 different time cohorts (2008 to 2009, 2011 to 2012, and 2014 to 2015). Over the study period, in-hospital mortality continued to increase in both unadjusted and matched cohorts. In our modern cohort (2014 to 2015), hospitalizations associated with at least 1 HAI had an in-hospital mortality of 13.6% compared with 12.0% for non-HAI hospital stays (p = 0.001). Supplemental Figures 1A and 1B show the unadjusted mortality stratified by HAI type and cardiovascular condition. Length of stay was higher in those with HAI (9.6 vs 4.9 days, p <0.0001), but remained similar over time (eFigure 2 in the Supplement). Less than 20% of patients with a HAI were discharged home while over 40% required discharge to a skilled nursing facility (eFigure 3 in the Supplement). Comparatively, only 15.7% of non-HAI patients required discharge to a skilled nursing facility.
Discussion
This national study of 5 common cardiovascular conditions demonstrates that the occurrence of even 1 HAI was associated with a substantial increase in cost, length of stay, and in-hospital mortality. Compared with patients not acquiring a HAI, we found that patients who develop 1 of these generally preventable infections were associated with an absolute 8.9% increase in in-hospital mortality. Mean charges were 2.3 times higher and the length of stay was 6.6 days longer. Further, nearly 45% hospitalizations associated with a hospital infection required discharge to a skilled nursing facility, incurring additional costs both to the patient and the healthcare system.
To our knowledge, this is the first nationwide study of these 5 specific cardiovascular diagnoses detailing associations between cost, length of stay, and mortality associated with HAIs. Although it is intuitive that infectious complications would portend poorer outcomes, the scope and magnitude of this problem has not been well described. Specifically, information on HAIs in patients admitted with cardiovascular disease is sparse. In our study, each of the 5 diagnoses had substantially different rates of each infection. For instance, patients with cardiogenic shock had a greater incidence of VAP, which is most likely due to higher rates of mechanical ventilation.26 Although broad HAI data are available,7,27,28 diverse patient populations have varying exposures (procedures, devices, catheters, etc.), which requires identification of at-risk populations, as well as tailored interventions to prevent or reduce complications.
In 2000, the Institute of Medicine released To Err Is Human, which estimated that 44,000 to 98,000 patients died yearly due to their exposure to the healthcare system.29 Since then, there has been a greater focus on HAI prevention, as well as the creation of several surveillance programs, including the NHSN, the Medicare Patient Safety Monitoring System (MPSMS), and several state-level programs. However, no single surveillance system can collect all infectious complications for all inpatient hospitalizations in the United States. Each has limitations partially driven by HAI definition, sampling, and the infections reported.28
A recent analysis from the Emerging Infectious Program found a HAI prevalence of 3.2% in a random sample of 12,299 patients from 199 hospitals.30 In contrast, our incidence levels were substantially lower, which likely reflects differences in HAI identification and our reliance on ICD-9-CM codes. Among those identified as having a HAI, we found a trend toward an increased incidence of HAI for patients admitted with HF, AF, and cardiogenic shock. The etiology for the increase in these patient populations is unclear. One possible explanation could be the temporal increase in noncardiac co-morbidities and a greater exposure to noncardiac procedures, which increases opportunity for infectious complication. Another possibility could be an increase in ICD-9-CM coding intensity.
Over the study period, device-associated infection (CLABSI and CAUTI) estimates remained generally unchanged, which may reflect the greater emphasis on device-associated infection prevention.28 Alternatively, we found an increase in CDI, which was the most common infectious complication in our cohort. In a recent multistate review of HAIs, sponsored by the Centers for Disease Control and Prevention, Magill et al similarly found fewer device-associated infections and a larger proportion of CDI than expected.28 Although this could potentially be due to the CDI definition used or an increased detection with more sensitive assays, these results highlight the increasing need for CDI prevention strategies.
There are several limitations of our study that warrant consideration. First, there are inherent limitations with administrative claim data, including the observational nature of our study and the inability to definitively determine causality between HAI and the outcomes studied. Second, due to under-reporting, our choice of ICD-9-CM codes used to determine HAIs likely underestimated our reported incidence.31 However, the hospitalizations we identified with HAIs likely represent true events, given the known penalties with HAI, and therefore, an accurate representation of outcomes. Although the incidence may be low, there is minimal reason to believe that properly coded HAIs represent a different population than those not coded properly. Third, it is possible that the longer length of stays associated with HAIs could reflect a greater exposure or opportunity for infectious complications as opposed to HAIs extending the hospitalization. It is also possible that the higher mortality in the HAI population was due to a selection bias of a sicker population with more co-morbidities than our non-HAI comparison group. We attempted to address this by using robust propensity matching, which resulted in suitable discrimination (model C statistic, 0.80) and persistence of our findings. However, there is likely residual confounding. Finally, with the exception on C. difficile, we were unable to identify specific infectious species.
Patients hospitalized for 5 common cardiovascular conditions, who acquired at least 1 HAI, had substantially higher costs, length of stay, and mortality compared with those without a HAI. Among patients admitted with a primary cardiovascular etiology, there has been a temporal increase in infectious complications for patients with noncardiac co-morbidities and those requiring traditionally noncardiac procedures. Further research and awareness are needed to address disease-specific susceptibilities, in order to prevent these avoidable causes of morbidity and mortality.
Supplementary Material
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2019.06.029.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Naghavi M, Mokdad AH, Murray CJL. Trends and patterns of geographic variation in cardiovascular mortality among US counties, 1980–2014. JAMA 2017;317:1976–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer, 2013: HCUP Statistical brief #204. May 2016. Agency for Healthcare Research and Quality, Rockville, MD. [PubMed] [Google Scholar]
- 4.Naccarelli GV, Johnston SS, Lin J, Patel PP, Schulman KL. Cost burden of cardiovascular hospitalization and mortality in ATHENA-like patients with atrial fibrillation/atrial flutter in the United States. Clin Cardiol 2010;33:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugiyama T, Hasegawa K, Kobayashi Y, Takahashi O, Fukui T, Tsugawa Y. Differential time trends of outcomes and costs of care for acute myocardial infarction hospitalizations by ST elevation and type of intervention in the United States, 2001–2011. J Am Heart Assoc 2015;4:e001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whellan DJ, Greiner MA, Schulman KA, Curtis LH. Costs of inpatient care among Medicare beneficiaries with heart failure, 2001 to 2004. Circ Cardiovasc Qual Outcomes 2010;3:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013;173:2039–2046. [DOI] [PubMed] [Google Scholar]
- 8.Overview of the National (Nationwide) Inpatient Sample (NIS), 2018. https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed June 25, 2018.
- 9.Houchens RL, Ross DN, Elixhauser A, Jiang J. Nationwide Inpatient Sample Redesign: Final Report. 2014. https://www.hcup-us.ahrq.gov/reports/methods/2014-04.pdf. Accessed June 25, 2018.
- 10.Trend Weights for HCUP NIS Data, 2015. https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. Accessed June 25, 2018.
- 11.Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to methodological standards in research using the national inpatient sample. JAMA 2017;318: 2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K, Parikh V, Rathod A, Badheka AO, Lafferty J, Kowalski M, Mehta JL, Mitrani RD, Viles-Gonzalez JF, Paydak H. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation 2014;129:2371–2379. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Dharmarajan K, Wang Y, Krumholz HM. National trends in heart failure hospital stay rates, 2001 to 2009. J Am Coll Cardiol 2013;61:1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, Fonarow GC. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc 2014;3:e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rathore SS, Epstein AJ, Volpp KG, Krumholz HM. Hospital coronary artery bypass graft surgery volume and patient mortality, 1998–2000. Ann Surg 2004;239:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwok CS, Rao SV, Potts JE, Kontopantelis E, Rashid M, Kinnaird T, Curzen N, Nolan J, Bagur R, Mamas MA. Burden of 30-day readmissions after percutaneous coronary intervention in 833,344 patients in the United States: predictors, causes, and cost: insights from the nationwide readmission database. JACC Cardiovasc Interv 2018;11: 665–674. [DOI] [PubMed] [Google Scholar]
- 17.Healthcare Cost & Utilization Project. Overview of Disease Severity Measures Disseminated with the Nationwide Inpatient Sample (NIS) and Kids’ Inpatient Database (KID). Rockville, MD, 2005. https://www.hcup-us.ahrq.gov/db/nation/nis/OverviewofSeveritySystems.pdf. Accessed June 25, 2018. [Google Scholar]
- 18.Healthcare Cost & Utilization Project. Cost-to-Charge Ratio Files. 2017. https://www.hcup-us.ahrq.gov/db/state/CCR_NIS_UserGuide_2001-2015.pdf. Accessed June 25, 2018.
- 19.Ailawadhi S, Frank RD, Sharma M, Menghani R, Temkit M, Paulus S, Khera N, Hashmi S, Advani P, Swaika A, Paulus A, Aslam N, Sher T, Roy V, Colon-Otero G, Chanan-Khan A. Trends in multiple myeloma presentation, management, cost of care, and outcomes in the Medicare population: a comprehensive look at racial disparities. Cancer 2018;124:1710–1721. [DOI] [PubMed] [Google Scholar]
- 20.Houchens R, Ross D, Elixhauser A. Final report on calculating National Inpatient Sample (NIS) variances for data years 2012 and later HCUP methods series report # 2015–09. ONLINE, 2015. [Google Scholar]
- 21.Houchens R, Elixhauser A. Final report on calculating Nationwide Inpatient Sample (NIS) variances for data years 2011 and earlier HCUP method series report # 2003–02. ONLINE, 2015. [Google Scholar]
- 22.Dugoff EH, Schuler M, Stuart EA. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res 2014;49:284–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons LS. Performing a 1: N case-control match on propensity score. In: Proceedings of the 29th Annual SAS Users Group International Conference SAS Institute; 2004:165–129. [Google Scholar]
- 24.Bland JM, Altman DG. Transformations, means, and confidence intervals. BMJ 1996;312:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert BD, Horstman JM. Captain’s LOG: Taking Command of SAS® Logarithm Functions, 2014. https://www.mwsug.org/proceedings/2014/RF/MWSUG-2014-RF06.pdf. Accessed June 25, 2018.
- 26.Alviar CL, Miller PE, McAreavey D, Katz JN, Lee B, Moriyama B, Soble J, van Diepen S, Solomon MA, Morrow DA, ACC Critical Care Cardiology Working Group. Positive pressure ventilation in the cardiac intensive care unit. J Am Coll Cardiol 2018;72: 1532–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Vital signs: central line-associated blood stream infections−United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep 2011;60:243–248. [PubMed] [Google Scholar]
- 28.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014;370:1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Io Medicine. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 30.Magill SS, O’Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, Wilson LE, Kainer MA, Lynfield R, Greissman S, Ray SM, Beldavs Z, Gross C, Bamberg W, Sievers M, Concannon C, Buhr N, Warnke L, Maloney M, Ocampo V, Brooks J, Oyewumi T, Sharmin S, Richards K, Rainbow J, Samper M, Hancock EB, Leaptrot D, Scalise E, Badrun F, Phelps R, Edwards JR, Emerging Infections Program Hospital Prevalence Survey Team. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med 2018;379:1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metersky ML, Wang Y, Klompas M, Eckenrode S, Bakullari A, Eldridge N. Trend in ventilator-associated pneumonia rates between 2005 and 2013. JAMA 2016;316:2427–2429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.