Abstract
BACKGROUND AND AIMS:
Access to basic health needs remains a challenge for most of world’s population. In this study, we developed a care model for preventive and disease-specific health care for an extremely remote and marginalized population in Arunachal Pradesh, the northeasternmost state of India.
APPROACH AND RESULTS:
We performed patient screenings, performed interviews, and obtained blood samples in remote villages of Arunachal Pradesh through a tablet-based data collection application, which was later synced to a cloud database for storage. Positive cases of hepatitis B virus (HBV ) were confirmed and genotyped in our central laboratory. The blood tests performed included liver function tests, HBV serologies, and HBV genotyping. HBV vaccination was provided as appropriate. A total of 11,818 participants were interviewed, 11,572 samples collected, and 5,176 participants vaccinated from the 5 westernmost districts in Arunachal Pradesh. The overall hepatitis B surface antigen (HBsAg) prevalence was found to be 3.6% (n = 419). In total, 34.6% were hepatitis B e antigen positive (n = 145) and 25.5% had HBV DNA levels greater than 20,000 IU/mL (n = 107). Genotypic analysis showed that many patients were infected with HBV C/D recombinants. Certain tribes showed high seroprevalence, with rates of 9.8% and 6.3% in the Miji and Nishi tribes, respectively. The prevalence of HBsAg in individuals who reported medical injections was 3.5%, lower than the overall prevalence of HBV.
CONCLUSIONS:
Our unique, simplistic model of care was able to link a highly resource-limited population to screening, preventive vaccination, follow-up therapeutic care, and molecular epidemiology to define the migratory nature of the population and disease using an electronic platform. This model of care can be applied to other similar settings globally.
Balancing high quality care with cost-effective implementation strategies is challenging in all health care environments, particularly in resource-limited settings. We developed a model of care in the northeastern part of India, home to extremely marginalized populations with minimal infrastructure and used hepatitis B virus (HBV ) as a disease model to demonstrate our health care delivery. Mortality from hepatitis B is on the rise for a number of reasons, one being poor access to treatment,(1,2) which is even more amplified in resource-limited settings.(3) The availability of HBV therapeutics and combining treatment with prevention can dramatically reduce the morbidity and mortality of HBV globally(4,5) and is a necessary strategy to reduce the global burden of viral hepatitis.
Background
HBV is endemic in India, and estimates of its seroprevalence are around 3%.(6,7) Major modes of transmission include maternal-fetal spread in children and injection drug use in adults.(8) HBV vaccination is available in India and recommended in national guidelines, but incurred expenses by family members remains an issue.(9) Treatment for HBV for chronically infected patients is available, but many cannot afford its cost.(10,11) Moreover, HBV vaccination is one of the universal vaccines recommended and is shown to reduce HBV disease acquisition and increase prevention of liver cancer.(1,2) Despite the strong evidence supporting its use, HBV vaccination rates remain low in most remote places, including Arunachal Pradesh, the northeasternmost state in India.
Arunachal Pradesh has been an area of interest in chronic HBV because of published reports of hyperendemicity with rates approaching 21%.(12,13) However, the sampling that was done involved single tribes and was not representative of the population at large in northeast India. Hence, the epidemiology of HBV in this extremely marginalized region remains ill-defined. The major mode of transmission from these investigations is unknown because of the difficulty of conducting studies in this remote region and achieving adequate sampling. The uptake of preventive immunizations is not clear. One study reports a rate of 48%.(13) Another study demonstrates that many patients uninfected with HBV remain nonimmune to the virus(14) with no stable vaccination program in place. A combination of low uptake of HBV vaccination and hyperendemicity dictates an epidemiological catastrophe for explosive HBV infection and complications including liver cancer.
Presently, outpatient clinical departments in Arunachal Pradesh rarely provide HBV evaluation and treatment, despite prior reports of its large burden in the region.(16) Based on census data and population estimates,(17) the projected number of patients infected with HBV in Arunachal Pradesh may be well over 100,000, and there may be many more than that who are not immune to HBV despite their increased risk of acquiring it. HBV genotypes in the region are poorly understood because of minimal sampling. Data on regional HBV genotypes can further inform molecular epidemiology of the area and why high rates of HBV infection may be clustered in northeast India. Hepatocellular carcinoma has also been identified in the region, with HBV infection and alcohol consumption being major risk factors.(18) Chronic HBV has been well characterized to carry a long-term burden, including an increased risk of cirrhosis, liver failure, liver cancer, and death.(1).Investigations and implementation programs in Arunachal Pradesh are complicated by the extremely remote geographic terrain and lack of infrastructure.(19) Most programs are short-lived in nature, and many have trouble reaching a large population because of extensive mountainous terrain and poor geotechnical infrastructure separating villages, towns, and districts.(20)
Patients and Methods
To overcome the logistical challenges and small sampling size of prior interventions, we developed a remote outreach program with a unique data collection algorithm through a tablet application that was easily portable in the difficult geographical terrain. With a team of 2 field workers and a phlebotomist, field teams interviewed patients and collected blood samples from over 100 tribes and over 10,000 participants in the 5 westernmost districts of Arunachal Pradesh, covering an area of over 5,000 square miles. By this mechanism, HBV epidemiology in the region could be defined on a micro and macro level while concomitantly offering HBV screening and preventive strategies for all sampled areas. We also defined two cohorts: one that is HBV infected and requires longitudinal follow-up and another that is not HBV infected and requires vaccination. All participant information was stored in a cloud database for tracking and quality assurance. A subset of hepatitis B surface antigen (HBsAg)-positive samples were genotyped and sequenced to further understand circulating HBV genotypes in the region.
OPERATION DYNAMICS
A tablet application (Kiran app), web application (Kiran website), and database were created to facilitate and conduct an HBV screening and vaccination program in Arunachal Pradesh, India. The Kiran app was written in Android and developed by our software collaborators (CTIS, Rockville, MD). The web application was written in ASP.NET and hosted in the cloud on an Amazon Web Services server (Amazon, Seattle, WA). The database that stored all patient data was written in MySQL (Oracle, Redwood City, CA). Administrators monitored data for integrity. Technical support, database development, and maintenance were provided through CTIS.
Patient data collection through tablet and phlebotomy were done in West Kameng, East Kameng, Tawang, Lower Subansiri, and Papum Pare in Arunachal Pradesh, India. Our field headquarters was in Tezpur, Assam, and our central headquarters was in PG Hospital, Kolkata. Tablets and vacutainers for laboratory specimens were transported from the field headquarters to the field sites. Supplies always came from our central headquarters. Our aim was to screen as many participants as possible in 3 years.
FIELD ACTIVITIES
Participants were initially identified through targeting the geographical area of Western Arunachal Pradesh, encompassing the West Kameng, East Kameng, Tawang, Lower Subansiri, and Papum Pare districts. Each of these districts have their own respective villages, which we would enter and request an audience with the village leader. After explaining our objectives, we would request the village leader to announce to the village our desire to test for HBV and provide three-series vaccination for those eligible. Field testing was typically done in a central location easiest for participants to access, usually a Buddhist monastery or the village square. Participants would sign informed consent. In the case of children participating in the study, informed consent would be obtained from their parent on the child’s behalf. Regulatory approval was obtained from the Institutional Ethics Committee of the Directorate of Medical Education, Training & Research, Government of Arunachal Pradesh, Naharlagun.
Demographic data and patient questionnaire answers were entered into the tablet application during patient interviews by field workers at the various sites. Blood samples were collected for hepatitis serologies and biochemical testing. Tablets and laboratory specimens were transported back to our field headquarters in Tezpur, where demographic data was then uploaded from the tablets to the cloud. After processing patient blood samples in our clinical laboratory, results were entered by laboratory users directly into the web application, which saves and syncs its data to the cloud. Laboratory reports were also printed for each patient and packaged in a sealed envelope. A second field trip was then made to deliver results to each patient and offer longitudinal care for eligible participants. Physicians would accompany field teams to explain results to patients pending their availability.
SAMPLE PROCESSING
Samples were processed twice. In the field headquarters, gamma glutamyl transferase, aspartate aminotransferase, alanine aminotransferase (ALT), alkaline phosphatase, total bilirubin, direct bilirubin, albumin, hepatitis B surface antigen, hepatitis B core antibody, and hepatitis C antibody were completed. For HBV and hepatitis C virus (HCV) testing, HBV surface antigen, HBV core antibody, and HCV antibody were examined by commercially available kits. All of these tests were again repeated in the central headquarters to confirm accuracy. Additionally, hepatitis B viral load, genotyping, and sequencing were done for HBsAg-positive samples in our central headquarters (discussed in a separate section of the methods).
HBV VACCINATION
Separate field trips were made to offer HBV vaccination with Bevac-recombinant HBsAg (Biological E Ltd., Hyderabad, India). The first dose of vaccine was typically given about 6 months following the initial phlebotomy. Our typical approach for promulgation of vaccination to prevent infection was to approach village leaders and inform them regarding the safety and efficacy of the HBV vaccine. They would then relay the message to their communities who had a bond of trust with them, and the door would be open for us to offer vaccination to the community.
Initially, HBV vaccine was offered to a subset of eligible patients, consistent with international society guidelines. However, a stigma quickly developed for individuals who were anti-HBc and HBV viremic who were not receiving the vaccine, and they were socially isolated from their communities. Field teams quickly adjusted to offer HBV vaccination for all participants, whether or not it was medically indicated, which mitigated this issue.
HBV DNA ISOLATION AND QUANTIFICATION
HBV DNA was extracted from 200 μL serum of HBsAg-positive individuals using QIAamp DNA Mini Kit (Qiagen, Valencia, CA), and DNA was reconstituted in 50 μL of nuclease-free water. Viral load was measured by real-time PCR using SYBR Green PCR Master Mix (Applied Biosystems, CA) using primers F5 and R4 (Table 1). Lower limit of detection was <250 copies/mL.
TABLE1.
List of Primers Used for Amplification and Sequencing of HBV Genome
| Primer Name | Primer Sequences | Location (nt)* |
|---|---|---|
| HBVP1†,‡ (Sense) | 5′-CCGGAAAGCTTGAGCTCTTCTTTTTCACCTCTGCCTAATCA -3′ | 1821-1841 |
| HBVP2†,‡ (Antisense) | 5′-CCGGAAAGCTTGAGCTCTTCAAAAAGTTGCATGGTGCTGG -3′ | 1806-1825 |
| F10§,║ (Sense) | 5′ -GACCACCAAATGCCCCTATC -3′ | 2297-2317 |
| F3║ (Sense) | 5′-CGCCTCATTTTGTGGGTCAC-3′ | 2801-2820 |
| F4║ (Sense) | 5′-CTCAGGCCATGCAGTGGAA- 3′ | 3164-3182 |
| CF║ (Sense) | 5′-ACTGTTCAAGCCTCCAAGCT-3′ | 1861-1880 |
| R4║,¶ (Antisense) | 5′-AGAGGACAAACGGGCAACA-3′ | 462-480 |
| R2║ (Antisense) | 5′-AAATTACCACCCACCCAGG-3′ | 2109-2127 |
| R3║ (Antisense) | 5′-AACTGGAGCCACCAGCAG- 3′ | 57-74 |
| R5║ (Antisense) | 5′-AAAGCCCAAAAGACCCACAAT-3′ | 997-1017 |
| F5║,¶ (Sense) | 5′-GATGTGTCTGCGGCGTTTTA- 3′ | 376-395 |
| R9§,║ (Antisense) | 5′-TAGGAGTTCCGCAGTATGGA- 3′ | 1265-1284 |
| R10║ (Antisense) | 5′-CAGCCTCCTAGTACAAAGAC-3′ | 1761-1780 |
| F7║ (Sense) | 5′-TGTGCACTTCGCTTCACCTC-3′ | 1578-1597 |
nt positions are given according to HBV sequence with accession no. AF121242 obtained from GenBank.
Primers used for full-genome amplification of HBV isolates.
Günther et al.
Primers used for amplification of the complete surface region (2848–835 nt).
Primers used for sequencing of full-length as well as complete surface region.
Primers used for viral load determination by real-time PCR.
AMPLIFICATION OF HBV GENOME, SEQUENCING, AND PHYLOGENETIC ANALYSIS
The complete preS1/preS2/S region of HBV (2848–835 nt) was amplified from extracted viral DNA by PCR amplification using primers F10 and R9 (Table 1). Amplification of full-length genome of HBV (3.2 kb) of representative samples was performed by PCR using high-fidelity Taq DNA polymerase (Fermentas, Waltham, MA) and primer pair Hepatitis B Virus Primer (HBVP)1 and HBVP2(21) (Table 1). In the case of samples with low viral load, a second round of amplification with nested primer pairs was performed. All PCR products were purified by the QIAquick Gel Extraction Kit (Qiagen, Valencia, CA) and the nucleotide sequences were determined using a BigDye terminator cycle sequencing kit (Applied Biosystems, Foster City, CA) on an automated DNA sequencer ABI Prism 3130 (Thermo Fisher, Waltham, MA).
DNA sequence editing and analysis was performed using SeqScape v2.5 software (Applied Biosystems, Foster City, CA). To determine HBV genotype and subgenotype, preS/S sequences obtained in the study were compared with representative sequences of 10 genotypes (A-J) retrieved from GenBank (https://www.ncbi.nlm.nih.gov/genbank). Sequence alignments were carried out using CLUSTAL_X software, and phylogenetic trees were constructed by neighbor-joining method using Kimura 2 parameter model in MEGA software version 5 (www.megasoftware.net/). To confirm the reliability of phylogenetic tree analysis, bootstrap resampling and reconstruction were carried out 5,000 times.
INVESTIGATION OF RECOMBINATION EVENTS
The recombination in genome sequences of HBV and the localization of phylogenetic break points were determined by using a circular version of the jumping profile hidden Markov model (jpHMM) available online at http://jphmm.gobics.de/submission_hbv.htm that is specially designed to predict recombination involving circular genomes. In the jpHMM approach, the full-length HBV genome sequence that is to be searched for phylogenetic breakpoints was pasted or uploaded at the Gottingen Bioinformatics Computer Server, and the query sequence was aligned against a large set of HBV sequences of all genotypes. The output file contained a graphical representation of the predicted recombinant fragments within the HBV genome and also provided an interval estimate of the breakpoint, called the breakpoint interval.
NUCLEOTIDE SEQUENCE ACCESSION NUMBERS
The nucleotide sequences of 20 HBV isolates reported in this article are available in the DNA Data Bank of Japan/European Molecular Biology Laboratory/GenBank databases under accession numbers MN650068 to MN650087.
Results
A schematic of our clinical operations and workflow are shown in Fig. 1A,B, respectively. The tablet application (Kiran app) was created to facilitate HBV screening and vaccination in areas without electricity or internet infrastructure, which included the majority of our field sites. The web application was designed for use in Tezpur, which had stable internet access. Each patient had a unique barcode identifier that was generated from the web application and was used to mark blood samples.
FIG. 1.

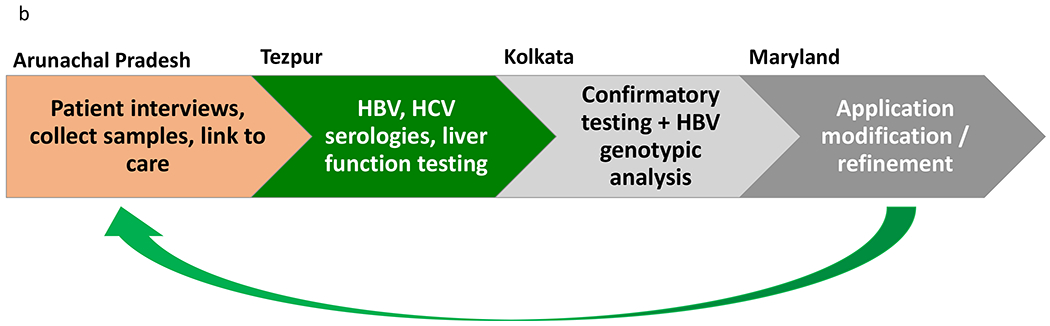
(A) Map of operation dynamics. Field teams, which typically consisted of two field workers and a phlebotomist, would travel to various field sites in Arunachal Pradesh. Application development, monitoring of data integrity, and technical support were provided from Maryland. Participant data was uploaded and maintained in the cloud through a secure server. (B) General workflow for field team operations in Arunachal Pradesh. After completing interviews with participants and collecting blood samples through tablet application, basic serologies and liver function testing were completed in Tezpur (field headquarters). Confirmatory testing was done in Kolkata (central headquarters) in addition to further analysis on HBsAg-positive samples. The field team would communicate with collaborators in Maryland for any required adjustments to the tablet application before embarking on their next field trip.
Screenshots of the Kiran app initial screens are shown in Fig. 2A. Demographic data and patient interviews were conducted on the screens shown in Fig. 2B. Phlebotomy and vaccination were completed at the end of the patient encounter (Fig. 2C). The full database could be viewed through the web application (Fig. 2D) on desktops at central offices in Kolkata and Baltimore. Additionally, laboratory reports were printed for patients through this application, which the field team would then deliver to the participants.
FIG. 2.

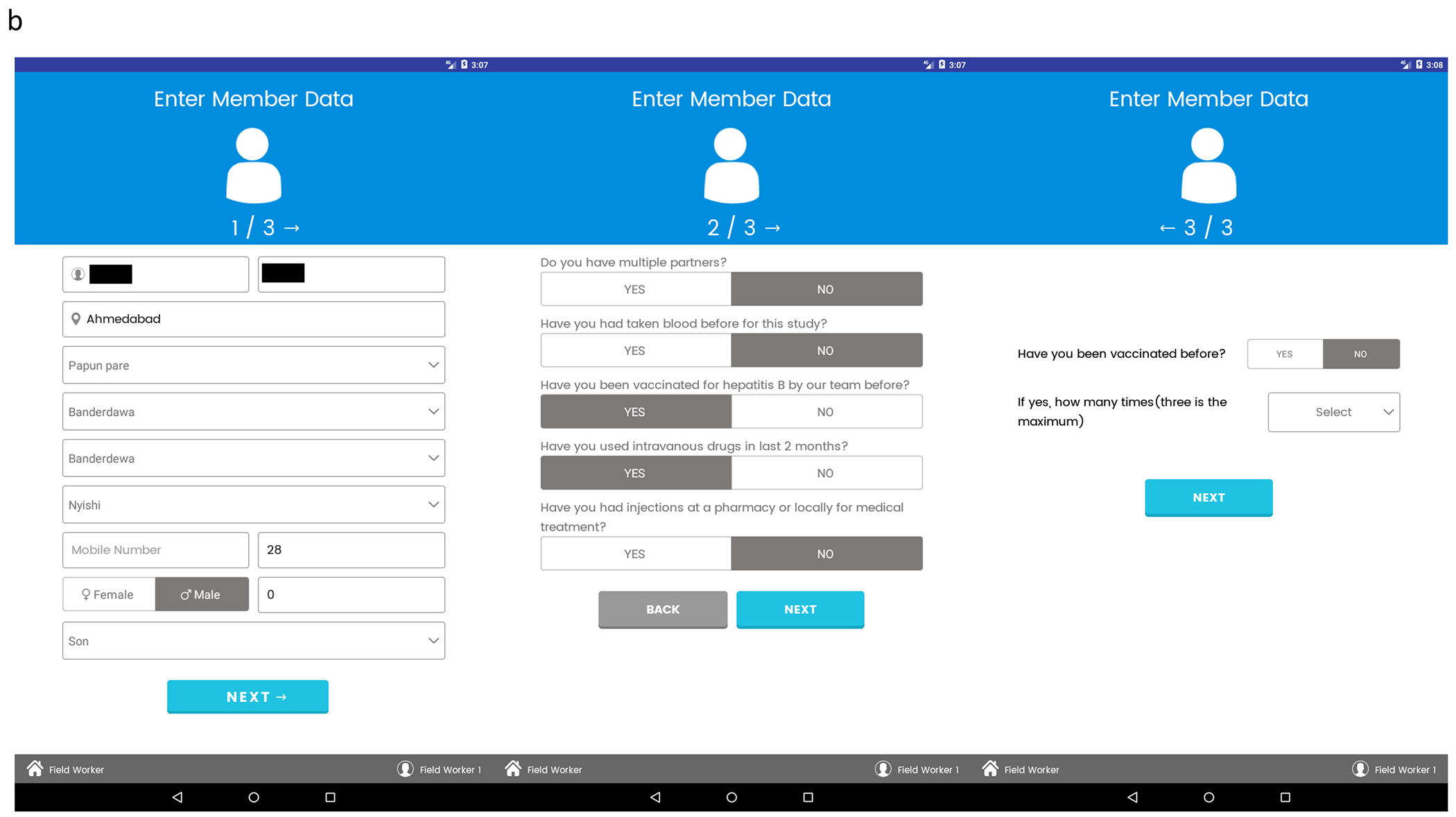
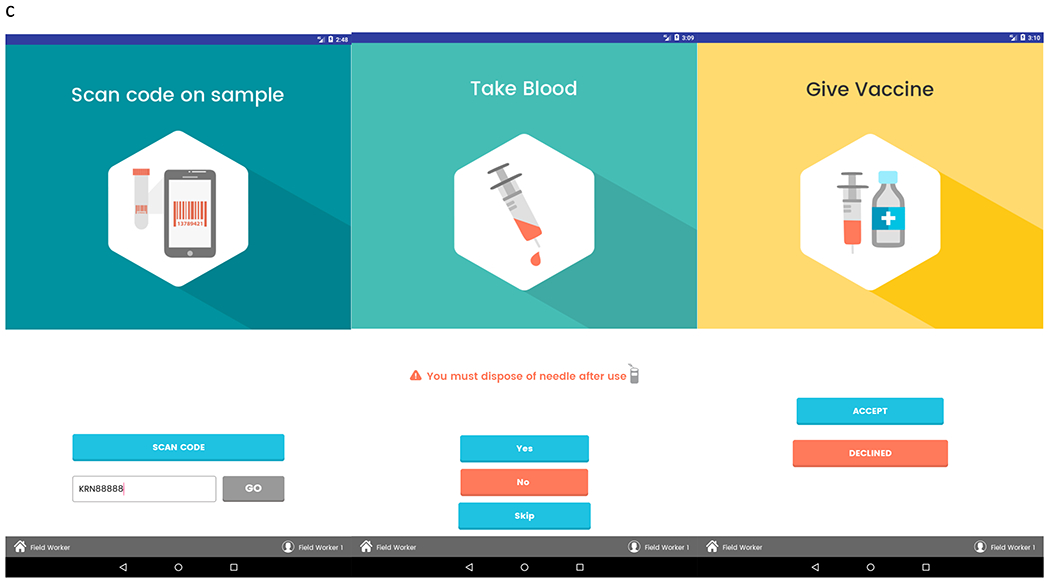

(A) Kiran app login screen, activity screen, and participant selection screen. (B) Field user can use these three screens to enter participant answers to questions about demographics and social history. (C) Each participant is identified by a unique barcode, which is scannable by the tablet and done before phlebotomy. Participant can opt out of phlebotomy if they are not interested or not able to have their blood drawn. When eligible, patient can receive HBV vaccination. (D) Web application dashboard. From field and central headquarters, user has the ability to view patient data, look at all microbiology reports, and generate new bar codes for future participants.
PARTICIPANT DEMOGRAPHICS
We interviewed a total of 11,818 patients, 5,564 (47.1%) of which were male. The median age was 27, and ages ranged from 1 to 87 years. If a participant was a child (less than 18 years of age), answers were provided by their parent. Questions regarding intravenous drug use, medical use of injections, and sexual partners were restricted to adult participants (18 years and above). Intravenous drug use with a recency of 2 months or less was reported in 4.4% of adults (n = 315), with a similar proportion using injections for medical purposes such as diabetes (n = 289, 4.0%). In total, 84.2% of adult participants reported multiple sexual partners (n = 6,031), and about 4.7% of all participants (adults and children) reported having previously received HBV vaccine (n = 550). We conducted our program in the western part of the state, with 66.8% (n = 7,891) of our attrition in West Kameng. We also frequented the East Kameng, Papum Pare, and Lower Subansiri districts (Table 2).
TABLE2.
Participant Demographics, Injection Practices, and Geographic Locations Where HBV Screening and Vaccination Was Performed
| Participant Demographics (n, %) | |||
|---|---|---|---|
| Total | 11,818 | ||
| Male | 5,564 (47.1) | ||
| Female | 5,903 (49.9) | ||
| Not provided | 351 (3.0) | ||
| Median age | 26.9 | ||
| Social History (n, %) | |||
| Adults (≥18 years, n = 7,159) | Yes | No | Not provided |
| IVDU in last 2 months | 315 (4.4) | 6,103 (85.2) | 741 (10.4) |
| Medical injections | 289 (4.0) | 6,124 (85.6) | 746 (10.4) |
| Multiple sexual partners | 6,031 (84.2) | 130 (1.8) | 998 (14.0) |
| All participants (n = 11,818) | |||
| Previous HBV vaccine | 550 (4.7) | 10,309 (87.2) | 959 (8.1) |
| District (n, %) | |||
| East Kameng | 2,858 (24.2) | ||
| West Kameng | 7,891 (66.8) | ||
| Tawang | 198 (1.7) | ||
| Other | 871 (7.4) | ||
Abbreviation: IVDU, intravenous drug use. Other = Papum Pare and Lower Subansiri districts.
HBV SEROPREVALENCE, CHARACTERISTICS, AND FOLLOW-UP CARE
Of the 11,818 participants interviewed, 11,572 opted to have their blood drawn for HBV testing. Of those 11,572 participants, 419 (3.6%) were HBsAg positive. Some participants who were infected with chronic HBV were found to have evidence of hepatocellular damage, with 75 (17.9%) having ALT greater than 40 units per liter. Of these 75 participants, 53 were men (70.7%), and the remaining 22 were women. Of the 75 participants with HBV infection and ALT > 40, 25 were children (33.3%). Of the 50 remaining adults, the majority (n = 45, 90%) were between the ages of 18 and 49. Of participants with HBsAg-positive serologies, 25.5% (n = 107) had HBV DNA levels greater than 20,000 IU/mL. In total, 145 participants who were HBsAg-positive (34.6%) were also positive for hepatitis B e antigen, showing evidence of active viral replication (Table 3). Core antibody positivity in the absence of HBsAg (HBsAg−, HBcAb+) was found in 39.6% (n = 4,581) of participants. Initially, HBV vaccination was offered to all patients who are HBsAg negative (because there was no HBsAb serology, we did not know which patients who were HBcAb+ had antibodies to HBsAg). However, to mitigate a rapidly evolving stigma, we ultimately offered vaccination to all participants, including those chronically infected. A total of 81.7% (n = 9,457) received the first dose of HBV vaccine, and to date 44.7% (n = 5,178) completed all 3 doses. We did not vaccinate household and sexual contacts of participants who were HBsAg-positive. A total of 2,064 participants (17.8%) refused HBV vaccination. The number of men (n = 943) and women (n = 1,026) refusing vaccination were comparable. Of the 2,064 participants refusing vaccination, 1,223 (59.3%) were adults. In total, 841 parents (40.7%) refused vaccination on behalf of their children.
TABLE3.
HBV Prevalence Characteristics and Vaccination Data
| HBV Prevalence Characteristics (n, %) | ||||
|---|---|---|---|---|
| Total tested for HBV | HBsAg+ | DNA > 10,000 copies/mL | ALT > 40 | HBeAg+ |
| 11,572 | 419 (3.6) | 133 (31.7) | 69 (16.5) | 145 (34.6) |
| HBV DNA (IU/mL) | Total (n, %) | |||
| <2,000 | 286 (68.3) | |||
| 2,000-20,000 | 24 (5.7) | |||
| >20,000 | 107 (25.5) | |||
| Not determined | 2 (0.5) | |||
| HBV Vaccination* | Total (n, %) | |||
| Dose 1 (0 months) | 9,253 (81.4) | |||
| Dose 2 (1 month) | 7,176 (63.1) | |||
| Dose 3 (6 months) | 5,176 (45.5) | |||
Because of evolving stigma and patients infected with HBV becoming socially isolated, HBV vaccination was offered to all patients, including those with HBV viremia.
Abbreviation: HBeAg+, hepatitis B eantigen positive.
Patients who were infected with HBV were spread across all age ranges, with a steep drop in patients over 60 years old (Table 4). No HBV infections were found in Tawang. A number of tribes were found to have seroprevalence rates over 3%, including the Miji (9.8%), Nyishi (6.3%), and Sartang (4.9%) tribes of West Kameng (Table 4). Each of these tribes had similar proportions of male and female individuals to the general population. Rates of intravenous drug use were low in all three tribes (between 0% and 1%). However, the proportions of participants with multiple sexual partners was noted to be higher than the general population (99.5%, 92.9%, and 98.9% in Miji, Nyishi, and Sartang tribes, respectively). HBV infection rates and clearance rates dramatically changed across villages, circles, and tribes, and significant differences were found across age groups, use of medical injections, and number of sexual partners (Table 5).
TABLE4.
Proportions of HBV Infection Across Different Age Groups (First Section) and in Major Districts (Second Section). From These Major Districts, a Number of Tribes Were Noted to Be Hyperendemic in West Kameng With High HBV Seroprevalence (Third Section)
| Age | HBsAg+ (n, %) |
|---|---|
| 0-19 | 156 (37.2) |
| 20-39 | 154 (36.8) |
| 40-59 | 88 (21.0) |
| >60 | 21 (5.0) |
| District | HBsAg+ (n, %) |
| East Kameng | 162 (38.7) |
| West Kameng | 239 (57.0) |
| Tawang | 0.0 (0.0) |
| Other* | 18 (4.3) |
| Hyperendemic Tribes in West Kameng | HBsAg+ (n, %) |
| Miji | 21 (9.8) |
| Nyishi | 146 (6.3) |
| Sartang | 19 (4.9) |
Other = Papum Pare and lower Subansiri districts.
TABLE5.
HBV Seroprevalence and HBV Clearance Rates Various Demographic Parameters as Well as Districts, Circles, and Villages
| HBV Status | sAg+cAb+ | sAg−cAb+ | sAg−cAb− | P Value |
|---|---|---|---|---|
| Total | 419 (4) | 4,581 (39) | 7,237 (57) | |
| Sex | ||||
| Male | 252 (60) | 2,204 (48) | 3,110 (48) | <0.01 |
| Female | 167 (40) | 2,373 (52) | 3,363 (52) | |
| Age group | ||||
| 0-19 | 156 (37) | 1,189 (26) | 3,749 (58) | <0.01 |
| 20-39 | 154 (37) | 1,654 (36) | 1,890 (30) | |
| 40-59 | 88 (21) | 1,336 (29) | 663 (10) | |
| ≥60 | 21 (5) | 399 (9) | 154 (2) | |
| Intravenous drug use | ||||
| Yes | 20 (5) | 181 (4) | 223 (4) | 0.11 |
| No | 380 (95) | 4,048 (96) | 5,987 (96) | |
| Use of medical injections | ||||
| Yes | 15 (4) | 54 (1) | 349 (6) | <0.01 |
| No | 385 (96) | 4,171 (99) | 5,860 (94) | |
| Number of partners | ||||
| 0 | 0 (0) | 0 (0) | 2 (0.03) | <0.01 |
| 1 | 8 (2) | 95 (2) | 33 (0.57) | |
| 2 | 373 (98) | 3,975 (98) | 5,816 (99.4) | |
| ALT | ||||
| >40 | 57 (14) | 271 (6) | 311 (5) | <0.01 |
| ≤40 | 360 (86) | 4,307 (94) | 6,131 (95) | |
| District | *Row percents | *Row percents | *Row percents | |
| East Kameng | 162 (5) | 1,656 (55) | 1,202 (40) | <0.01 |
| West Kameng | 239 (3) | 2,561 (32) | 5,330 (65) | |
| Tawang | 0 (0) | 32 (16) | 166 (84) | |
| Circle | *Row percents | *Row percents | *Row percents | |
| BDL | 2 (4) | 35 (71) | 12 (25) | |
| Bameng | 0 (0) | 26 (59) | 18 (41) | |
| Bana | 36 (7) | 360 (71) | 114 (22) | |
| Bhalukpo | 8 (2) | 163 (46) | 181 (52) | |
| Bomdila | 61 (3) | 498 (26) | 1,356 (71) | |
| Debeyar | 2 (4) | 41 (80) | 8 (16) | |
| Dirang | 71 (3) | 879 (41) | 1,186 (56) | |
| Jang | 0 (0) | 13 (21) | 50 (79) | |
| Lhou | 0 (0) | 9 (10) | 80 (90) | |
| PakkeKessang | 16 (6) | 160 (63) | 79 (31) | |
| Pijirang | 53 (8) | 424 (63) | 193 (29) | |
| Rupa | 71 (3) | 663 (24) | 2,016 (73) | |
| Seijosa | 19 (2) | 323 (34) | 605 (64) | |
| Seppa | 23 (8) | 179 (58) | 106 (34) | |
| Shergaon | 10 (2) | 150 (34) | 281 (64) | |
| Sinchung | 6 (2) | 73 (28) | 184 (70) | |
| TajaHapa | 13 (6) | 143 (62) | 75 (32) | |
| Tawang | 0 (0) | 10 (21) | 37 (79) | |
| Thembang | 10 (5) | 96 (44) | 111 (51) | |
| Village | *Row percentage | *Row percentage | *Row percentage | |
| 1 mile | 1 (3) | 12 (39) | 18 (58) | |
| 2 kilo | 0 (0) | 0 (0) | 58 (100) | |
| 6 kilo | 1 (5) | 19 (90) | 1 (5) | |
| 6 mile | 1 (3) | 4 (13) | 26 (84) | |
| 64 mile | 2 (5) | 8 (18) | 34 (77) | |
| 9th mile | 2 (6) | 14 (47) | 14 (47) | |
| A2 | 2 (1) | 63 (36) | 109 (63) | |
| Amar Basti | 2 (3) | 15 (21) | 54 (76) | |
| Ataro | 0 (0) | 26 (59) | 18 (41) | |
| Attaran | 3 (5) | 42 (70) | 15 (25) | |
| BDL | 2 (4) | 35 (71) | 12 (25) | |
| Bamsock | 0 (0) | 2 (10) | 18 (90) | |
| Bidum | 0 (0) | 7 (37) | 12 (63) | |
| Birpur | 4 (2) | 47 (27) | 122 (71) | |
| Bomdila | 8 (6) | 46 (34) | 80 (60) | |
| Chillipam | 0 (0) | 71 (37) | 122 (63) | |
| Craft Centre | 3 (8) | 26 (68) | 9 (24) | |
| Darbu | 1 (1) | 46 (61) | 29 (38) | |
| Darlong | 6 (3) | 38 (16) | 185 (81) | |
| Debgolo | 2 (13) | 8 (50) | 6 (37) | |
| Dikhing | 0 (0) | 5 (29) | 12 (71) | |
| Dikshi | 3 (7) | 14 (30) | 29 (63) | |
| Dirang | 18 (4) | 89 (20) | 332 (76) | |
| Don Bosco | 0 (0) | 1 (6) | 17 (94) | |
| Dukumpa | 0 (0) | 11 (27) | 30 (73) | |
| Elephant Flat | 1 (2) | 32 (60) | 20 (38) | |
Abbreviations: cAb, core antibody; sAg, surface antigen.
At the time of delivery of result reports (repeat visit by the field team), we approached participants who had ALT > 40 (n = 75) and HBV DNA > 2,000 IU/mL (n = 131, including 107 who were >20,000 IU/mL), irrespective of e antigen status. We offered them detailed evaluations, including an ultrasound examination at the linked hospital in Tezpur, assessment of treatment eligibility, and follow-up care. Transport to the hospital was arranged, and a hepatitis clinic was made operational twice a month in Tezpur. A hepatologist from the Indian Institute of Liver and Digestive Sciences visited this clinic twice a month. Arrangements also were made for task-shifting HBV treatment and follow-up to Tezpur physicians (general practitioners). Asian Pacific Association for the Study of the Liver (APASL) guidelines were used to determine eligibility for HBV therapy. A total of 34 patients met eligibility criteria and were started on tenofovir disoproxil fumarate. Therapy and transport were provided free of charge, and longitudinal follow-up was established. This clinic is still ongoing to date. Of the 34 patients initiated on therapy, 27 continue to be regularly seen and 7 have been lost to follow-up. The remaining participants with elevated HBV DNA who do not meet APASL criteria for therapy were offered HBV DNA and ALT testing every 6 months at the clinic. Follow-up of these participants has now been shifted to the National Viral Hepatitis Control Program in India, which is operational country-wide with several nodal centers.
HBV GENOTYPES
We amplified and sequenced the complete surface region (preS/S) of HBV genome from 45 patients who were infected with HBV. The sequence of two HBV isolates (KRN613 and KRN2697) harbored a major (approximately 900 bp) deletion in the surface region and was excluded from phylogenetic analysis. The remaining 43 sequences were compared with reference sequences obtained from GenBank representing each of the 10 HBV genotypes (A-J). The phylogenetic analysis indicated that 26 strains (60.4%) did not segregate with any known HBV genotype and formed a separate cluster. Out of the remaining 17 samples, 4 (9.3%) were distinctly clustered with the genotype D, 12 (27.9%) sequences were found to be genotype C, and one sequence was consistent with genotype I (2.3%). A phylogenetic tree of a representative sample of isolates is shown in Fig. 3A.
FIG. 3.
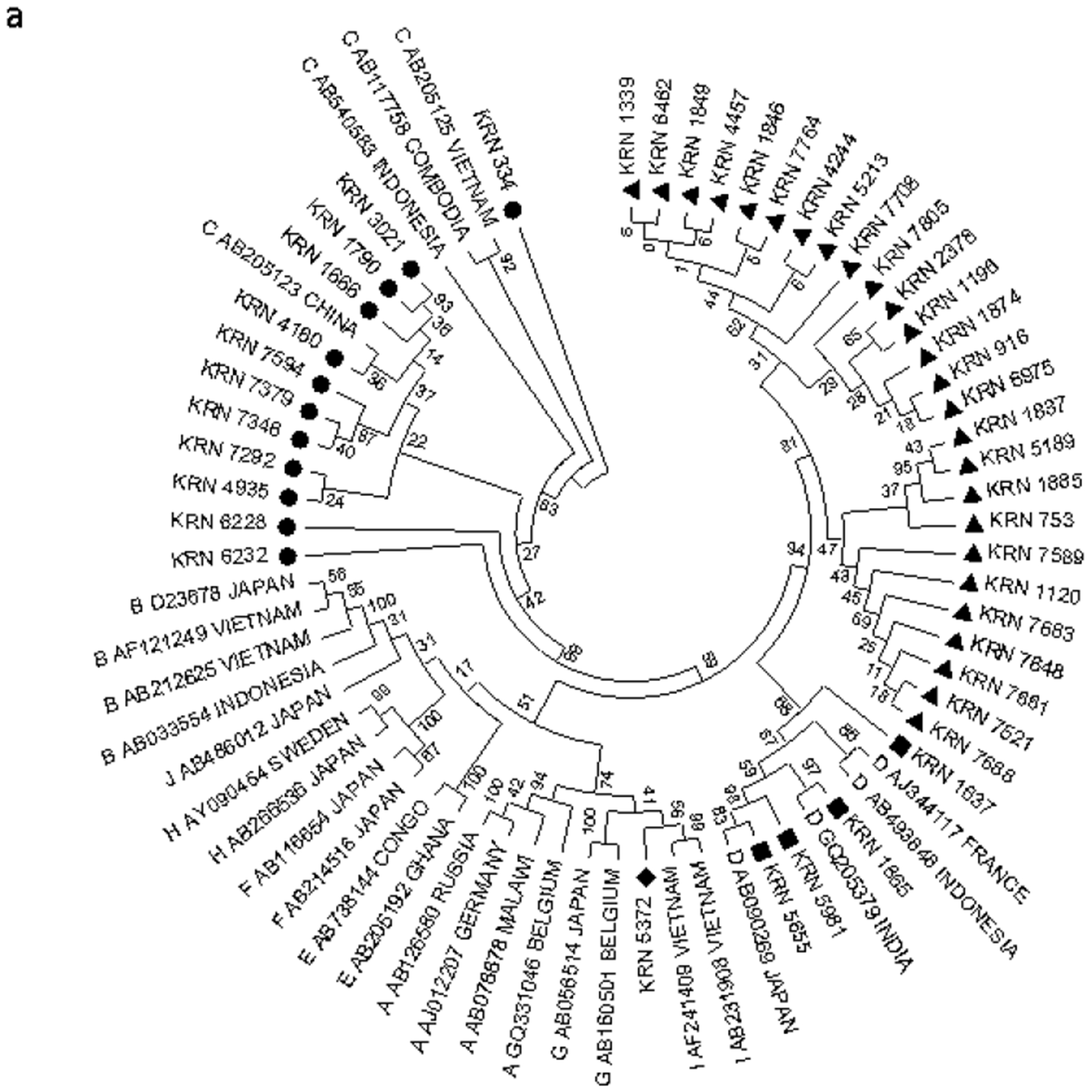
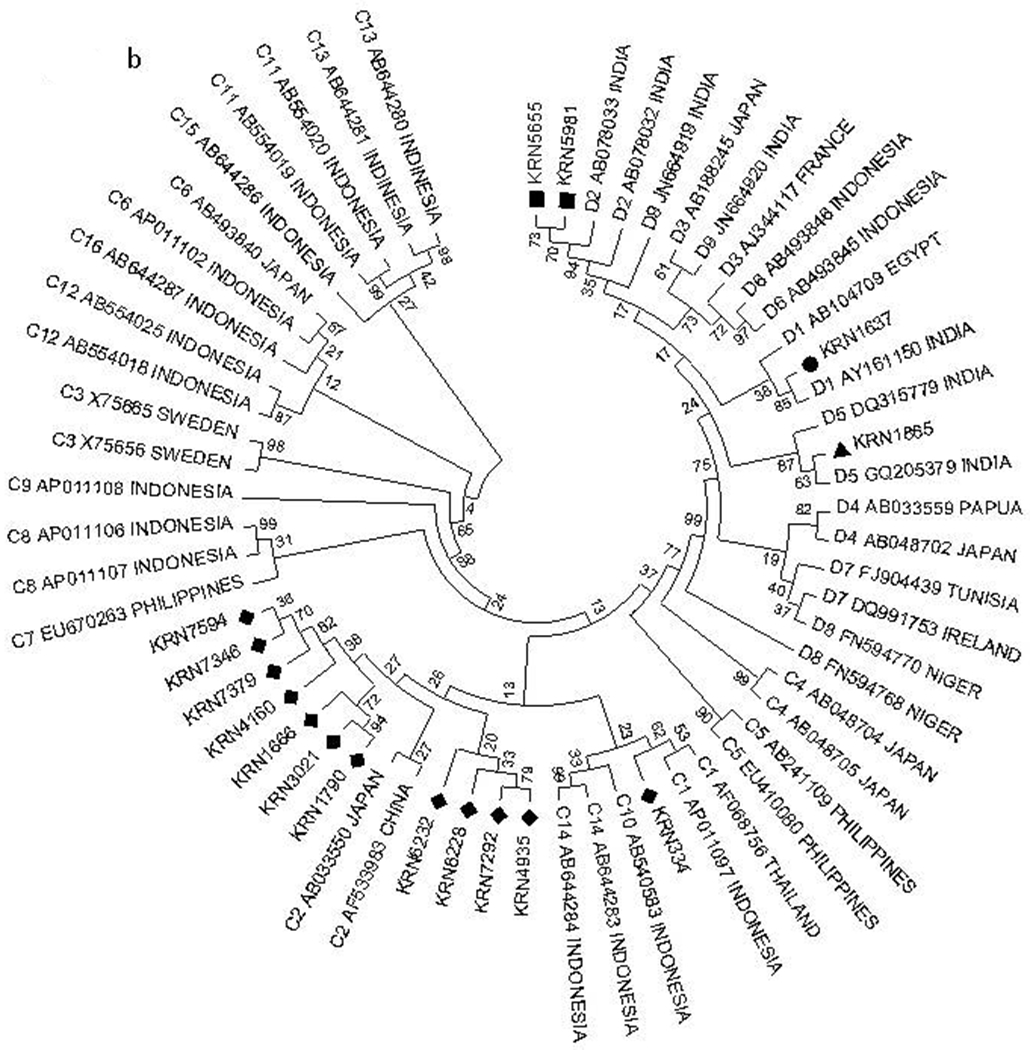
Phylogenetic analysis of HBV isolates for genotype and subgenotype determination. (A) Phylogenetic analysis of complete preS/S sequences of 43 HBV isolates, along with reference sequences of HBV strains belonging to different genotypes (A to J) derived from GenBank. (B) Phylogenetic analysis of complete preS/S sequences of 16 HBV isolates of genotypes C and D, along with reference sequences of HBV strains belonging to different subgenotypes (C and D) derived from GenBank. Phylogenetic tree was constructed using the Kimura 2 parameter model and neighbor-joining method by MEGA software version 5, and bootstrap resampling was carried out 5,000 times. The Tezpur sequences are indicated by respective isolate numbers beginning with “Kiran.”
To ascertain the subgenotypes of our isolates belonging to genotype C and D, their sequences were compared with representative sequences of different subgenotypes retrieved from GenBank. The subgenotypic analysis revealed that among the four HBV genotype D isolates, one sequence clustered with D1, two clustered with D2, and one clustered with D5. Among the 12 HBV genotype C isolates, one was of subgenotype C1 and 11 were subgenotyped as C2 (Fig. 3B).
IDENTIFICATION OF THE PUTATIVE RECOMBINATION SITES
Next, out of 26 HBV isolates that formed a separate cluster in the phylogenetic tree, we determined the full genome sequences of 20 isolates and explored possible recombination events in these strains. We used the online jpHMM tool to specify the recombination breakpoints in these HBV isolates. The analysis showed that these HBV isolates were C/D recombinants, with Fig. 4 depicting the jpHMM results of six representative isolates. In majority of the C/D recombinants, the 5′ termini of the breakpoints of recombination events were found to be located within 3,214 ± 16 nucleotides (nt), which lies within the preS2 region of the surface gene, whereas in one sequence (KRN753), the breakpoint was localized within 3,117 ± 6 nt. The 3′ termini of the breakpoints, in most of the recombinant sequences, were very closely located within 1,473.5 ± 10.5 nt, which coincides within the X-ORF and is very close to the DR2 region, whereas in KRN753 isolate, it was within 806 ± 27 nt, which was the 3′ end region of surface ORF of HBV (Fig. 4A,B). In these C/D recombinants, the region derived from genotype C included the major part of the hepatitis B x protein (HBx) gene, complete pre-core/core region, the N-terminus of HBV polymerase, and the preS1 region of surface gene, whereas the region derived from genotype D included the entire preS2/S region, C-terminus of viral polymerase, and N-terminus of HBx gene.
FIG. 4.

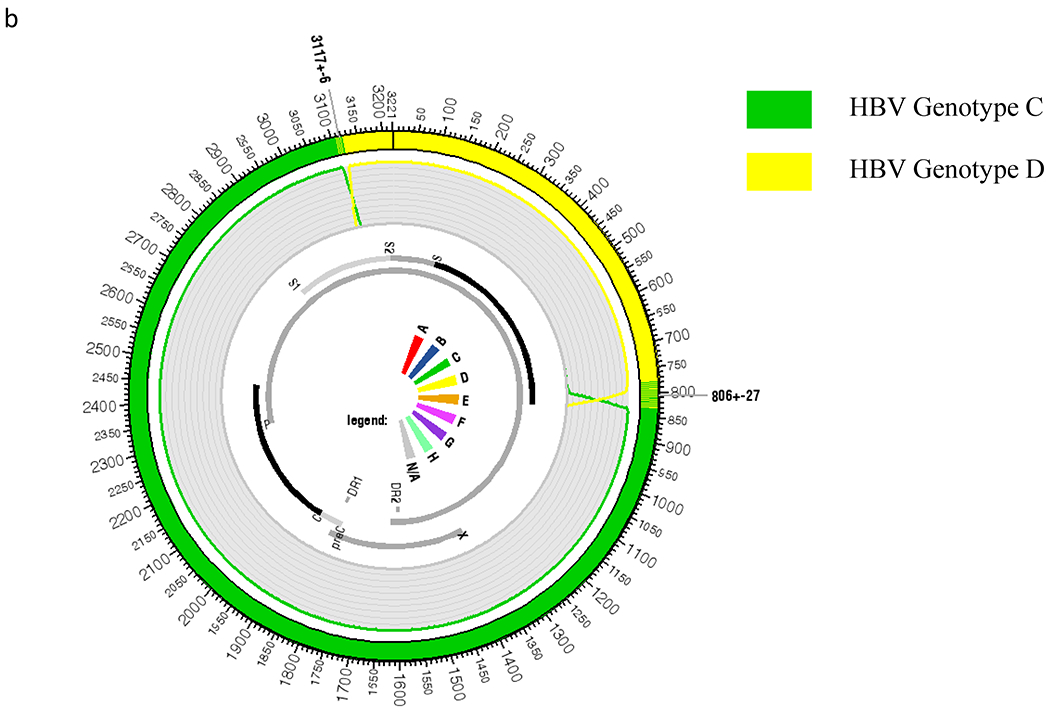
HBV genome recombination analysis using jpHMM approach. (A) Schematic diagram of jpHMM results of six representative isolates showing the circular HBV genome with alternate genotype D (yellow) and genotype C (green) regions. (B) Representative schematic and blown-up image of one circular HBV C/D recombinant genotype (KRN753). PreC, pre-core.
Discussion
Through the use of a cloud database and a remote data collection algorithm mastered by our field workers, we launched our health care delivery program across 7,731 square miles over 3 years and defined HBV epidemiology in a region with minimal health care infrastructure. Through one cohesive model, we were able to conduct screening, prevention, treatment, and research programs in parallel, providing high-quality services to marginalized populations while maintaining cost effectiveness and a feasible implementation strategy. We identified two cohorts: one with participants who were HBV infected and eligible for treatment and another with participants who were not infected with HBV and were eligible for vaccination. We offered vaccination with uptake at 81.7% for the first dose and 44.7% for all 3 doses. HBV seroprevalence rates in certain pockets of Arunachal Pradesh were over triple the 3% quoted rate for India’s general population. About 18% of participants who were HBV infected had evidence of liver injury, and about one third had evidence of active viral replication, findings that both demonstrate a necessity for HBV treatment centers in the area. About 5% had access to an HBV vaccine before our program, and that number has changed to over 80% after we offered HBV vaccination. Genotypic analysis revealed a number of circulating genotypes in Arunachal Pradesh, most notably C/D recombinant HBV, previously reported in the Tibet Autonomous Region.(22,23)
This is an HBV screening, vaccination, treatment, and research program of large scale in Arunachal Pradesh. Prior studies in the region enrolled less than 500 participants for HBV screening,(13) and a vaccination program enrolled 200 participants.(14) Prior studies have reported a similar high seroprevalence rate(13) and circulating genotypes A, C, D, and I.(12,15) Molecular epidemiological trends in Northeast India suggest that some circulating genotypes may be from migrant populations. Genotyping was done in one study in the upper Dibang valley in Arunachal Pradesh, toward the eastern part of the Indo-China border, and found varying genotypes A, C, and D among participants(15) However, these studies were also small in nature with suboptimal sampling. We report C/D recombinant HBV in Arunachal Pradesh. Similar C/D recombinant HBV has been reported only in Tibet. The introduction of these recombinant strains in Arunachal Pradesh is likely from Tibetan migration into the region.
There are a number of strengths to this study. We had a very large number of enrolled patients across several districts and over 100 different ethnic tribes, allowing us to sample a wide variety of patient populations in the region. Our outreach program utilizes software collection methods that provide follow-up identification of participants. We had an ability to collect data with sample identification on tablets that then synced to the cloud when Wi-Fi connection was available. We used molecular epidemiological tools to provide unique findings while simultaneously developing a longitudinal cohort to provide treatment and screening. We also provided large-scale vaccination effectively, and this model can be adapted for other resource-limited settings around the world. Current studies are ongoing in the Indian state of Manipur and Islamabad, Pakistan.
This study also had several limitations. We did not track the number of candidates for the study who declined participation and did not sign informed consent. Many patients wanted further explanation about their laboratory results, and there was not always a physician on hand to provide answers to their questions. We explained to the patients before enrollment that a physician would review their results and they would be provided with a paper report. However, participants often were interested in having a face-to-face conversation with a physician after review of their paper report. Future interventions may involve telemedicine support for patients to answer health concerns regarding vaccinations, diagnosis, and treatment. This will be important to maximize utility of our tablet application and ensure longitudinal follow-up.
Concluding Remarks
Our study demonstrates that a health care delivery model can be used to link and engage at-risk populations in preventive care and treatment. The key elements included the development of a tablet-based application, coordination of results, ensuring follow-up, and vaccination or treatment for those who required it. In addition, through molecular epidemiology, our study was able to demonstrate a migratory pattern of HBV and people to Arunachal Pradesh. The outcomes of our study show that this model is highly effective in reaching extremely remote populations and can be reproduced with modifications to other areas of the world.
Acknowledgments
Supported by a grant from the John C. Martin Foundation (2016-G05). Dr. Abutaleb was supported by a T32 Research Grant (DK067872-11) from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Potential conflict of interest: Dr. Kottilil advises and received grants from Merck. He received grants from Gilead and Arbutus.
Abbreviations:
- ALT
alanine aminotransferase
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- jpHMM
jumping profile hidden Markov model
- nt
nucleotide
REFERENCES
- 1).Tang LSY, Covert E, Wilson E, Kottilil S. Chronic hepatitis B infection: a review. JAMA 2018;319:1802–1813. [DOI] [PubMed] [Google Scholar]
- 2).World Health Organization. Combating hepatitis B and C to reach elimination by 2030. Geneva, Switzerland: World Health Organization; May 2016. [Google Scholar]
- 3).Mandeville KL, Krabshuis J, Ladep NG, Mulder CJ, Quigley EM, Khan SA. Gastroenterology in developing countries: issues and advances. World J Gastroenterol 2009;15:2839–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383–403. [DOI] [PubMed] [Google Scholar]
- 5).Scott N, Palmer A, Morgan C, Lesi O, Spearman CW, Sonderup M, et al. Cost-effectiveness of the controlled temperature chain for the hepatitis B virus birth dose vaccine in various global settings: a modelling study. Lancet Glob Health 2018;6:e659–e667. [DOI] [PubMed] [Google Scholar]
- 6).Arora A, Singh SP, Kumar A, Saraswat VA, Aggarwal R, Bangar M, et al. INASL position statements on prevention, diagnosis and management of hepatitis B virus infection in India: the Andaman statements. J Clin Exp Hepatol 2018;8:58–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;17:1546–1555. [DOI] [PubMed] [Google Scholar]
- 8).Lodha R, Jain Y, Anand K, Kabra SK, Pandav CS. Hepatitis B in India: a review of disease epidemiology. Indian Pediatr 2001;38:349–371. [PubMed] [Google Scholar]
- 9).Sokhey J, Jain DC, Harit AK, Dhariwal AC. Moderate immunization coverage levels in East Delhi: implications for disease control programmes and introduction of new vaccines. J Trop Pediatr 2001;47:199–203. [DOI] [PubMed] [Google Scholar]
- 10).Amarapurkar DN, Mada K, Kapoor D. Management of chronic hepatitis B Infection in India. J Assoc Physicians India 2015;63:43–52. [PubMed] [Google Scholar]
- 11).Lim SG, Amarapurkar DN, Chan HL, Crawford DH, Gane EJ, Han KH, et al. Reimbursement policies in the Asia-Pacific for chronic hepatitis B. Hepatol Int 2015;9:43–51. [DOI] [PubMed] [Google Scholar]
- 12).Haldipur BP, Walimbe AM, Arankalle VA. Circulation of genotype-I hepatitis B virus in the primitive tribes of Arunachal Pradesh in early sixties and molecular evolution of genotype-I. Infect Genet Evol 2014;27:366–374. [DOI] [PubMed] [Google Scholar]
- 13).Biswas D, Borkakoty BJ, Mahanta J, Jampa L, Deouri LC. Hyperendemic foci of hepatitis B infection in Arunachal Pradesh, India. J Assoc Physicians India 2007;55:701–704. [PubMed] [Google Scholar]
- 14).Borkakoty B, Mahanta J, Biswas D. HBV vaccination in hyperendemic remote tribal areas in India. Vaccine 2007;25:8347–8349. [DOI] [PubMed] [Google Scholar]
- 15).Borkakoty BJ, Mahanta J, Biswas D. Circulating genotypes of hepatitis B virus in Arunachal Pradesh. Indian J Med Res 2008;127:65–70. [PubMed] [Google Scholar]
- 16).Patra A Hepatitis B, C on the rise in northeast. https://timesofindia.indiatimes.com/city/guwahati/Hepatitis-B-C-on-the-rise-in-northeast/articleshow/21423715.cms. Published July 28, 2013. [Google Scholar]
- 17).Arunachal Pradesh Population 2011. https://www.census2011.co.in/census/state/arunachal+pradesh.html Accessed June 1, 2018.
- 18).Phukan RK, Borkakoty BJ, Phukan SK, Bhandari K, Mahanta J, Tawsik S, et al. Association of processed food, synergistic effect of alcohol and HBV with hepatocellular carcinoma in a high incidence region of India. Cancer Epidemiol 2018;53: 35–41. [DOI] [PubMed] [Google Scholar]
- 19).NDTV. Kiren Rijiju visits Arunachal Pradesh landslide victims, promises help. https://www.ndtv.com/india-news/kiren-rijiju-visits-arunachal-pradesh-landslide-victims-promises-help-1724792. Updated July 14, 2017. Accessed June 1, 2018.
- 20).Acharjee S Urban land use and geohazards in Itanagar, Arunachal Pradesh, India: the need for geotechnical intervention and geoethical policies in urban disaster resilience programmes in a changing climate. Geological Society 2015;419:63–68. [Google Scholar]
- 21).Günther S, Li BC, Miska S, Krüger DH, Meisel H, Will H. A novel method for efficient amplification of whole hepatitis B virus genomes permits rapid functional analysis and reveals deletion mutants in immunosuppressed patients. J Virol 1995;69: 5437–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Zhou B, Xiao L, Wang Z, Chang ET, Chen J, Hou J. Geographical and ethnic distribution of the HBV C/D recombinant on the Qinghai-Tibet Plateau. PLoS One 2011;11:e18708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Zhou B, Wang Z, Yang J, Sun J, Li H, Tanaka Y, et al. Novel evidence of HBV recombination in family cluster infections in Western China. PLoS One 2012;7:e38241. [DOI] [PMC free article] [PubMed] [Google Scholar]


