Abstract
Objectives
Under usual circumstances, sleep timing is strongly influenced by societal imperatives. The sweeping whole-of-society measures introduced in response to the COVID-19 pandemic may represent a unique opportunity to examine the impact of large-scale changes in work practices on sleep timing. As such, we examined the impact of the travel restrictions and work from home orders imposed in Ireland in March 2020 on sleep timing and quality.
Methods
We utilized a cross-sectional survey deployed shortly after the imposition of restrictions which assessed current and retrospective ratings of sleep timing and quality; the final response set analysed was from 797 adults. Participants completed the ultra-short Munich Chronotype Questionnaire, the Pittsburg Sleep Quality Index, and answered questions pertaining to work status such as working from home during the period of restrictions.
Results and conclusion
There was a significant shift to later sleep start and end times, as well as delayed time of midsleep on both work and free days, during the period of restrictions. Sleep duration was longer for work days, while free day sleep duration was shorter and there was a reduction in social jetlag during the restrictions. Those who worked from home during restrictions had longer sleep duration on work day and had a significantly larger difference in sleep end on work day than “essential” workers who continued to attend their normal place of work.
Keywords: Circadian, Chronotype, Social jetlag, Pandemic, COVID-19
1. Introduction
Following the establishment of community transmission of the SARS-CoV2 virus and increasing rates of the disease COVID-19 in Ireland, on March 12th 2020 the Government of Ireland announced the implementation of a broad range of societal-level non-pharmacological steps to mitigate the national epidemic. These steps included the closure of schools and child-care facilities and the discontinuation of on-campus university tuition, and were augmented on the 27th March 2020 with the introduction of more radical “stay-at-home” measures, requiring home working except for designated key workers and the suspension of all non-essential travel (Government of Ireland, n.d.) [1]. These measures remained in force until May 5th 2020 when a phased relaxation of restrictions was initiated. Various data indicate that social and travel restrictions implemented in response to COVID-19 resulted in a remarkable decrease of human mobility across the globe [2,3]. For the vast majority of non-essential workers in Ireland, travel restrictions resulted in a requirement to work from home, and students were tutored at home by their care-givers and/or virtual tuition. As such, commuting was not required for the majority of workers and students, and there may have been an increase in flexibility for scheduled work and study duties throughout the day; clearly, there are potential implications for the timing and quality of sleep that may arise from such radical re-arrangements of daily schedules.
Under usual circumstances, almost 80% of participants in Europe reported using an alarm clock on workdays [4], indicating widespread sleep loss resulting from the conflict between the circadian clock (the endogenous near 24 h timekeeping system that imposes a temporal architecture on physiology and partially dictates sleep time) and socially-required waking times to meet school or work obligations. This conflict between internal biological time and social schedules is termed social jetlag, and is formally described as the discrepancy in the timing of midsleep (the midpoint between sleep start and wakening) between work and free days [5]. The timing of midsleep on free days, when corrected for sleep debt accumulated during work days, is believed to indicate the phase of entrainment of the circadian clock (the timing of a circadian event relative to the 24-h day [6]. As such, midsleep on “free” days may be used as an indicator of chronotype (individuals’ tendency towards earlier or later sleep/wake cycles), as inter-individual difference in circadian processes manifest themselves in sleep/wake timing differences [6,7]. School and work schedules tend to be early in industrialised societies leading to greater conflict and discrepancy between work and free day sleep for those with a predisposition for later sleep start and wake times [8].
The radical societal-level mitigation measures introduced in Ireland and other countries during the COVID-19 pandemic represents a unique “natural experiment” through which to examine the effects of work schedules on the timing of sleep/wake activity. In the current study we sought to examine these effects by assessing sleep/wake timings before and during restrictions, the relationship between changes in key sleep parameters following imposition of the restrictions and the impact of essential worker status during restrictions on sleep timing and duration.
2. Materials and methods
2.1. Recruitment
As part of a study examining attitudes to the transition into daylight savings time, we had intended to assess sleep/wake timing and sleep quality briefly after the transition to Irish summer time (29th March, 2020). Given that this was shortly after the introduction of the COVID-19 mitigation measures, we amended our survey to ask participants to rate their sleep/wake timing before and after the introduction of such measures (which were characterized as before and during “lockdown” in the survey). Adults resident in Ireland were invited to take part in an on-line survey, via the Qualtrics survey platform (an on-line research services provider, www.qualtrics.com). The survey went online on 11th April 2020 and concluded on 4th June 2020 (when travel was still restricted to within five kilometers and non-essential workers and students were still required to work and study from home). Recruitment was via the Brainstorm blog of the national broadcaster (Radio Television Eireann), personal contacts, social media posts, and recruitment via Qualtrics research services. Ethical approval was granted by the Research Ethics Committee of Maynooth University and informed consent was indicated by all participants whose data was included in the analysis.
2.2. Questionnaires
The ultra-short version of the Munich Chronotype Questionnaire (μMCTQ [9]); was used to assess sleep/wake timing and calculate midsleep timings and social jetlag. The μMCTQ was modified so that participants were asked to indicate their sleep and wake time for work day and free day before and during restrictions. Social jetlag was calculated as the absolute difference between midsleep on work day and free day, both before and during restrictions. A“free” day is any day when sleep start (the night before the free day) and sleep end (in the morning of the free day) times are not dictated by work or school commitments and time schedules. As per standard protocol for calculating mid-sleep values for free days, a sleep correction was applied to free day midsleep timing (MSFsc) for participants who had longer sleep duration on free day; these participants slept longer on free day to compensate for sleep debt accumulated over the week, which was adjusted for to reflect their true free day midsleep timing as accurately as possible ((MSFsc = MSF– (SDf-SDweek)/2); [6]. Key sleep parameters, such as sleep start and end and duration were also calculated for both before and during restrictions. To recognise the potential reduction in accuracy associated with retrospective application of the μMCTQ for self-reporting of sleep/wake timings before restrictions, we rounded the timings up or down to the nearest 15-min intervals, and for consistency the same rounding was applied to the timings reported for during restrictions. The Pittsburgh Sleep Quality Index [10] was also administered to assess sleep quality during restrictions, but for the brevity of survey, participants were not asked to complete the PSQI retrospectively. Instead we requested participants to rate their sleep quality before and during restrictions with two questions, on a simple single item scale of zero to ten, with higher scores indicating better sleep. Further demographic information was also obtained, including age, sex, employment status before the restrictions, shiftworker status and essential worker status during the period of restrictions.
2.3. Statistical analyses
Statistical analysis was conducted in SPSS (IBM Corporation) and Jamovi (version 1.2). Paired sample t-tests or Wilcoxon Signed Rank tests were used to compare mean values of midsleep, social jetlag and sleep debt before and during restrictions, depending on whether the dependent variable was normally distributed. Chi-square tests for independence were used for relationships between categorical variables. Correlation analysis was conducted with either Pearson's product moment correlation or with Spearman's Rho, depending on the distributions of the variables of interest. Between-groups multivariate analysis of covariance (MANCOVA), controlling for age and sex, was conducted to examine the effects of essential services work status on the change in sleep timing and duration, and social jetlag. For paired tests p < 0.05 was taken as indicating a statistically significant effect and a more stringent value of p < 0.01 was applied for the MANCOVA, as the assumption of equality of variance was not fulfilled with this sample. Effect sizes were calculated as eta squared for t tests and partial eta squared for the MANOVA, and interpreted according to Cohen's guidelines [11].
3. Results
3.1. Demographics
Table 1 shows the demographic profile of the study sample (mean age = 40.2, SD = 12.7). Following the exclusion of shift workers, a total of 797 participants were included. Participants were asked if they are working in essential services during restrictions and 80% indicated they were not, whilst 17% identified as essential workers and continued to attend their place of work during the restrictions (information on work status was missing for 3% of participants).
Table 1.
Demographic constitution of the study sample.
| N = 797 | ||
|---|---|---|
| Mean age = 40.2 (SD = 12.7) | ||
| n | % | |
| Gender | ||
| Female (mean age = 40.0, SD = 12.2) | 497 | 62.4 |
| Male (mean age = 40.5, SD = 13.6) | 298 | 37.3 |
| Non-Binary (mean age = 21.0, SD = 2.8) | 2 | 0.3 |
| Age Groups | ||
| 18-25 | 109 | 14 |
| 26-35 | 195 | 25 |
| 36-45 | 217 | 27 |
| 46-55 | 176 | 22 |
| 56-65 | 76 | 10 |
| >65 | 24 | 3 |
| Work status | ||
| Essential services | 132 | 17 |
| Non-essential services | 641 | 80 |
| Information missing | 24 | 3 |
3.2. Sleep timing before and during restrictions
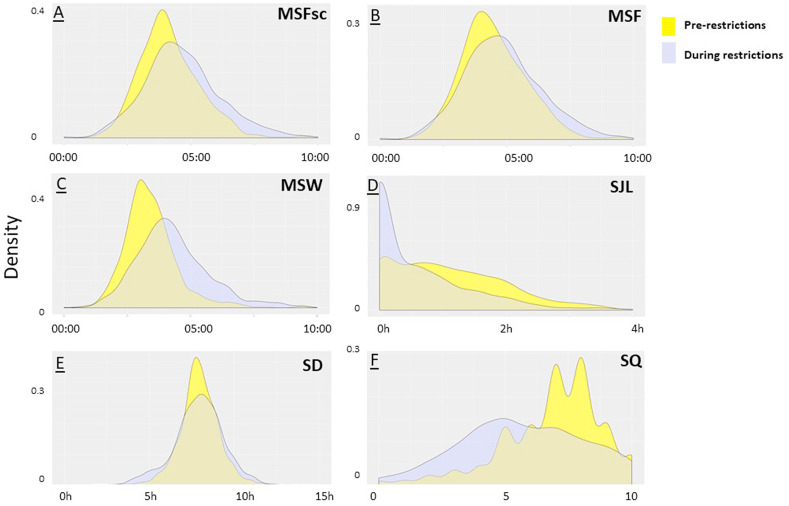
The introduction of restrictions was associated with changes in the timing of midsleep on work and free days and in social jetlag (Table 2 and Fig. 1 A–D). Midsleep on work day was significantly later following the introduction of restrictions (03:25 vs 04:31; p < 0.001; Fig. 1C). A smaller change of 31 min was observed in midsleep on free day during the restrictions (04:08 vs 04:39; p < 0.001; Fig. 1A and B). Social jetlag decreased by 29 min following the imposition of restrictions (1.10 h vs 0.62 h; p < 0.001). There was a decrease in number of participants with midsleep on work days in the early hours of the morning (midnight-2am and 2am–4am), and an increase in later midsleep (from 4am to 6am and later than 6am; (p < 0.001)). Similarly, on free day, there was a decrease in number of participants with early mid-sleep between 2am and 4am, but there was an increase in mid-sleep between 4am and 6am and after 6am (p < 0.001). Notably the percentage of participants reporting no social jetlag increased from 18% before restrictions to 45% during restrictions (p < 0.001). The proportion of participants who reported using an alarm clock on work day fell from 80% before restrictions to 38% during restrictions (p < 0.001).
Table 2.
Sleep timing and duration, social jetlag and sleep quality before and during restrictions.
| Before Restrictions | During Restrictions | |
|---|---|---|
| Alarm clock use (p < 0.001) | ||
| Yes | 639 (80%) | 306 (38%) |
| No | 162 (20%) | 495 (62%) |
| Midsleep work day (p < 0.001) | ||
| Midnight-2am | 32 (4%) | 22 (3%) |
| 2am–4am | 566 (71%) | 313 (39%) |
| 4am–6am | 178 (22%) | 359 (45%) |
| Later than 6am | 25 (3%) | 107 (13%) |
| Midsleep free day (p < 0.001) | ||
| Midnight-2am | 13 (2%) | 20 (2%) |
| 2am–4am | 371 (46%) | 234 (29%) |
| 4am–6am | 354 (44%) | 398 (50%) |
| Later than 6am | 63 (8%) | 149 (19%) |
| Social Jetlag (p < 0.001) | ||
| No SJL | 148 (18%) | 361 (45%) |
| Less than 1 h | 236 (30%) | 223 (28%) |
| 1–2 h | 310 (39%) | 168 (21%) |
| 2–3 h | 82 (10%) | 33 (4%) |
| More than 3 h | 25 (3%) | 16 (2%) |
| PSQI | ||
| Good sleeper | – | 205 (27%) |
| Poor sleeper | – | 549 (73%) |
| Sleep start work day (p < 0.001) | 23:35 (SE = 0:04) | 00:23 (SE = 0:05) |
| Sleep end work day (p < 0.001) | 07:15 (SE = 0:04) | 08:11 (SE = 0:06) |
| Sleep start free day (p < 0.001) | 00:13 (SE = 0:05) | 00:44 (SE = 0:06) |
| Sleep end free day (p < 0.001) | 08:46 (SE = 0:06) | 08:56 (SE = 0:07) |
| Sleep duration work day (p = 0.012) | 7 h 40 mins (SE = 0:04) | 7 h 48 mins (SE = 0:06) |
| Sleep duration free day (p < 0.001) | 8 h 33 mins (SE = 0:05) | 8 h 11 mins (SE = 0:06) |
| Social Jetlag (p < 0.001) | 1 h 6min (SE = 0:02) | 36 min (SE = 0:01) |
| Sleep Debt (p < 0.001) | 34 min (SE = 0:01) | 18min (SE = 0:01) |
Fig. 1.
Density plots showing the distribution of (A) midsleep free days sleep corrected (MSFsc), (B) midsleep free days not sleep corrected (MSF), (C) midsleep work days (MSW), (D) social jetlag (SJL), (E) average weekly sleep duration (SD) and (F) subjective single-item sleep quality rating (SQ).
During restrictions, work day sleep timing shifted closer to sleep timing on free days, resulting in a number of changes in key sleep parameters and impacting sleep debt and social jetlag (Table 2). Both sleep start and sleep end on work and free days during restrictions were later than before restrictions. Restrictions were also associated with changes in differences in sleep end time between work and free days: before restrictions, the mean sleep end time on free day was 1 h 31 min later than on work day, but during restrictions this difference was only 45 min (p < 0.001). Similarly sleep start time difference between work and free days reduced during restrictions, from a mean of 38 min before to 21 min during restrictions (p < 0.001). Consequently, the amount of sleep debt accumulated in the working week reduced during restrictions, reflected in a reduction of the difference between MSf and MSfsc (mean of 21 min before restrictions and 11 min during restrictions; p < 0.001). However, the average sleep duration for work days was only 8 min longer than before restrictions (p = 0.012) and free day sleep duration was shorter by a mean of 22 min during restrictions (p < 0.001). This result indicates that for a 5 work day/2 free day week the total change in sleep duration over the work week (5 × 8min = 40min) is balanced by shorter weekend sleep (2 × 22min = 44min). The mean difference in sleep duration between work and free day was smaller during restrictions than before, 53 min before and 23 min during restrictions (P < 0.001).
3.3. Relationship between changes in sleep parameters following imposition of restrictions
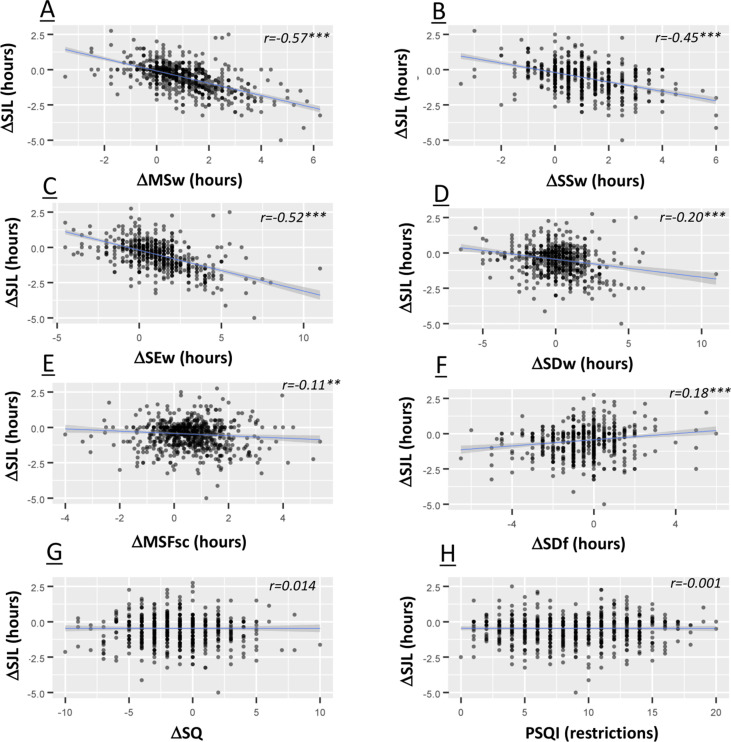
Next, we examined the relationship of the changes in the various sleep parameters following imposition of societal restrictions (Fig. 2 ). To do this we calculated the change (Δ) for each participant for each parameter examined, representing the change in that parameter following the introduction of restrictions. The Δ social jetlag was strongly associated with Δ midsleep work day (r = −0.57, p < 0.001; indicating an association between later midsleep on work days during restrictions and decreased social jetlag), Δ sleep start work day (r = −0.45, p < 0.001; indicating an association between a move towards later sleep start times on work days during restrictions and decreased social jetlag) and Δ sleep end work day (r = −0.52, p < 0.001; indicating an association between a move to later wake times on work days during restrictions and decreased social jetlag). Δ social jetlag was more weakly associated with Δ midsleep on free days (r = −0.10, p < 0.01; indicating that later midsleep on frees during restrictions is associated with less social jetlag, and as such the decrease in social jetlag during restrictions is somewhat moderated by later midsleep on free days). Δ social jetlag was also weakly associated with Δ sleep duration work day (r = −0.18, p < 0.001; lengthening sleep duration on work days during restrictions associated with decreases in social jetlag), Δ sleep duration free day (r = 0.19, p < 0.001; indicating that longer sleep duration on free days during restrictions was associated with increases in social jetlag), and Δ sleep end free day (r = 0.18, p < 0.01; indicating that later sleep end time on free days during restrictions was associated with increased social jetlag). Δ social jetlag was not associated with changes in free day sleep start. These data would suggest that the reduction observed in social jetlag is primarily driven by sleep timing changes on work days following the introduction of restrictions.
Fig. 2.
Scatterplots illustrating the correlations between the change (Δ) in social jetlag following the imposition of restrictions and Δ in other parameters of sleep timing. The shaded area is the 95% confidence interval of the linear fit. Reported r values are from Spearman's Rho statistics. ∗∗ denoted P < 0.01, ∗∗∗P < 0.001.
3.4. Sleep quality
Participants were asked to rate their sleep quality before and during restrictions on a zero to ten scale, with higher scores indicating better sleep quality. This rating declined after the imposition of restrictions (6.87 ± 0.07 to 5.69 ± 0.09, p < 0.001, Table 2 and Fig. 1F). Further, the number of participants categorized according to a sleep quality score of 5 or less increased from 22% to 49% during restrictions (Table 2). 73% of the participants who completed the PSQI reported poor quality sleep during the restrictions (indicated by PSQI global scores of >5; Table 2). The ten-point scale item scale rating of sleep quality during restrictions had a strong negative correlation with global PSQI score (r = −0.764, p < 0.001), suggesting that the single item rating was a reasonable indicator for comparison of sleep quality before and during restrictions. PSQI score associated with Δ midsleep work day (r = 0.12, p < 0.001), Δ midsleep free day (r = 0.08, p < 0.05), Δ sleep start work day (r = 0.30, p < 0.001) and Δ sleep start free day (r = 0.26, p < 0.001). There was also no significant association between Δ social jetlag and PSQI scores.
3.5. Impact of working status during restrictions on sleep timing and duration
During the COVID-19 restrictions, workers designated as essential workers continued to attend their normal place of work, whilst all other workers were required to work from home. In our sample, 17% of respondents self-reported as being essential workers (Table 1). We undertook an analysis to examine if there were differential effects of the COVID-19 restrictions on changes in sleep parameters in essential workers compared to those who were required to work from home (Table 3 ). A between-groups multivariate analysis of variance (MANCOVA) was conducted to examine the effects of essential services work on Δ sleep start and end, Δ midsleep, Δ sleep duration and Δ social jetlag. Essential worker status had a significant but small effect on Δ midsleep work day (F 2, 796 = 11.9, F 1, 771 = 23.00 p < 0.001, partial eta2 = 0.029), Δ social jetlag (F 2, 796 = 10.9, F 1, 771 = 21.98 p < 0.001, partial eta2 = 0.028), Δ sleep duration work day (F 2, 796 = 8.4, F 1, 771 = 16.11 p < 0.001, partial eta2 = 0.021) and Δ sleep end on work day (F 2, 796 = 15.5, F 1, 771 = 30.06 p < 0.001, partial eta2 = 0.038). For all of these measures, the change in sleep timing associated with the imposition of restrictions was lower for essential workers compared to those who were working from home. There were no significant differences between essential and non-essential workers in the changes in sleep start or end times or sleep duration on free days.
Table 3.
Difference in sleep timing between essential and nonessential workers.
| Δ during-to-before restrictions (Mean ± SEM) | Essential Worker |
p | Partial Eta2 | |
|---|---|---|---|---|
| No | Yes | |||
| Δ Midsleep work day (mins) | +58 (±3) | +28 (±3) | <0.001 | 0.029 |
| Δ Midsleep free day (mins) | +34 (±3) | +16 (±5) | 0.002 | 0.013 |
| Δ Social jetlag (mins) | −32 (±2) | −10 (±4) | <0.001 | 0.028 |
| Δ Sleep duration work day (mins) | +14 (±4) | −21 (±7) | <0.001 | 0.021 |
| Δ Sleep duration free day (mins) | −22 (±4) | −5 (±8) | 0.917 | 0.000 |
| Δ Sleep start work day (mins) | +51 (±3) | +38 (±6) | 0.035 | 0.006 |
| Δ Sleep end work day (mins) | +64 (±4) | +17 (±6) | <0.001 | 0.038 |
| Δ Sleep start free day (mins) | +34 (±4) | +27 (±6) | 0.231 | 0.002 |
| Δ Sleep end free day (mins) | +12 (±3) | +4 (±6) | 0.241 | 0.002 |
4. Discussion
This study highlights the strong influence of work day schedules on sleep/wake timing, and how changes to sleep timing on work days could reduce social jetlag. Our study, along with other sleep studies conducted during the first wave of the COVID-19 pandemic [12,13] illustrate, through the prism of the unique natural experiment of the radical societal COVID-19 mitigation steps imposed, the profound influence of work and social schedules on the timing of sleep behavior. The most marked changes were associated with working days, and included later timing of midsleep, later wake times and markedly decreased use of alarm clocks. Changes on free days were lesser, although we report a later time of midsleep on free days during restrictions. These changes resulted in reductions in social jetlag, which may represent a beneficial outcome as social jetlag is associated with a number of adverse health including metabolic disorders [14], cognitive and affective impairments [15], and lower academic achievement and quality of life [16]. However, in our study as well as in that of Blume and colleagues [12], decreased social jetlag and longer sleep duration during COVID-19 restrictions were not associated with better subjective sleep quality. Indeed, some studies have reported high prevalence of insomnia and daytime sleepiness during COVID-19 restrictions [17], suggesting that there are other significant COVID-19-related factors that may override any benefits derived from changes in sleep timing and reductions in social jetlag. For example, increased anxiety levels have been previously reported during epidemics and pandemics as a result of heightened fear of contagion and possible death, loss of contact with loved ones and financial worries [18,19]. Similarly, during the COVID-19 pandemic high prevalence of anxiety and worries have been noted [20,21]. Given the intimate relationship between sleep quality and affect [22], it is therefore not surprising that potential benefits from the reduction of social jetlag does not translate into increases in subjective sleep quality. Further, a recent study from our group recently reported only weak association between social jetlag and subjective sleep quality in a normative population [23]. However, we did not record affective status in the current study, and as such we cannot directly test the relationships between changes in sleep timing, sleep quality and affect.
Clearly the impact of the COVID-19 restrictions on sleep behaviors may not be homogenous across the population and may be moderated by factors such as household composition and family structure, socioeconomic status, age and caregiving duties [24]. In our study, we specifically examined one important such variable, the requirement to continue to attend one's normal place of work rather than working from home. Such essential workers included those working in healthcare and those working in essential retail services and accounted for 17% of the study population. As expected, these essential workers experienced less changes to their sleep timing and a significantly smaller reduction in social jetlag compared to those workers who were required to work from home. This finding further reinforces the importance of working and commuting schedules for determining sleep timing in our society.
There are a number of important caveats and limitations to consider in the interpretation of the current study. Firstly, all measures were based on subjective measures, and the assessment of measures prior to the imposition of restrictions was by necessity retrospective. This clearly introduces the risk of recall bias, although given the nature of the sudden onset of the COVID-19 crisis in Ireland in Spring 2020, a prospective approach was not feasible. Further, to increase participant completion rate we relied on a single-item rating scale for sleep quality before and during restrictions, although this score did correlate strongly with PSQI score during restrictions. Secondly, the sample was collected by convenience, and should not be treated as nationally-representative. A majority of the respondents were females, and the mean age of the sample was 40. Females and older participants may have had a different experience of the restrictions and working from home, have experienced different caregiving or other family duties such as supervising home schooling. Thirdly, as noted earlier we did not record information on mental and physical health. Fourthly we did not record information on pre-pandemic commuting times; previous work has indicated an inverse linear relationship between commuting times and sleep duration [25]; it may be that those with the longest pre-pandemic commute experienced the greatest changes in sleep timing following the imposition of restrictions. Finally, it is not clear if the current findings generalize to countries other than Ireland, as there has been considerable variance in the nature of COVID-19 mitigations imposed in different countries.
5. Conclusion
The imposition of radical societal restrictions during the first phase of the COVID-19 presented a unique opportunity to examine how radical and widespread changes in work practices impact on the timing of sleep. Our study reports that the imposition of restrictions in Ireland resulted in significant changes in sleep timing on work days and decreases in social jetlag. However, benefits arising from these may be offset by other factors in the context of COVID-19, such as increases in psychological distress. As the COVID-19 pandemic continues to evolve, prospective approaches might be gainfully employed to examine the impacts of future tightening or loosening of societal restrictions.
Acknowledgements
We acknowledge scholarship support to SR from the John and Pat Hume Scholarship fund of Maynooth University
Footnotes
SR and AC jointly conceived the study, analysed the data, and wrote the manuscript.
The authors declare no potential or actual conflicts of interest.
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2021.02.024
Conflict of interest
The following is the Supplementary data to this article:
References
- 1.Government of Ireland, Department of health. (n. d). From https://www.gov.ie/en/publication/cf9b0d-new-public-health-measures-effective-now-to-prevent-further-spread-o/?referrer=/en/publication/539d23-stay-at-home-the-latest-public-health-measures-to-prevent-the-spread/.
- 2.Bonaccorsi G., Pierri F., Cinelli M., et al. Economic and social consequences of human mobility restrictions under COVID-19. Proc Natl Acad Sci Unit States Am. 2020;117(27):15530–15535. doi: 10.1073/pnas.2007658117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadjidemetriou G.M., Sasidharan M., Kouyialis G., et al. The impact of government measures and human mobility trend on COVID-19 related deaths in the UK. Transport Res Interdiscip Perspect. 2020;6:100167. doi: 10.1016/j.trip.2020.100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roenneberg T., Allebrandt K.V., Merrow M., et al. Social jetlag and obesity. Curr Biol. 2012;22(10):939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 5.Roenneberg T., Pilz L.K., Zerbini G., et al. Chronotype and social jetlag: a (self-) critical review. Biology. 2019;8(3):54. doi: 10.3390/biology8030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roenneberg T., Merrow M. Entrainment of the human circadian clock. Cold Spring Harbor Symp Quant Biol. 2007;72:293–299. doi: 10.1101/sqb.2007.72.043. [DOI] [PubMed] [Google Scholar]
- 7.Adan A., Archer S.N., Hidalgo M.P., et al. Circadian typology: a comprehensive review. Chronobiol Int. 2012;29(9):1153–1175. doi: 10.3109/07420528.2012.719971. [DOI] [PubMed] [Google Scholar]
- 8.Raman S., Coogan A.N. vol. 30. Elsevier; 2019. Closing the loop between circadian rhythms, sleep, and attention deficit hyperactivity disorder; pp. 707–716. (Handbook of behavioral neuroscience). [Google Scholar]
- 9.Ghotbi N., Pilz L.K., Winnebeck E.C., et al. The μMCTQ: an ultra-short version of the Munich ChronoType Questionnaire. J Biol Rhythm. 2020;35(1):98–110. doi: 10.1177/0748730419886986. [DOI] [PubMed] [Google Scholar]
- 10.Buysse D.J., Reynolds C.F., 3rd, Monk T.H., et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J. Erlbaum; Hillsdale, MI, USA: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 12.Blume C., Schmidt M.H., Cajochen C. Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr Biol. 2020;30(14):R795–R797. doi: 10.1016/j.cub.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright K.P., Jr., Linton S.K., Withrow D., et al. Sleep in university students prior to and during COVID-19 Stay-at-Home orders. Curr Biol. 2020;30(14):R797–R798. doi: 10.1016/j.cub.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly R.M., Healy U., Sreenan S., et al. Clocks in the clinic: circadian rhythms in health and disease. Postgrad Med. 2018;94(1117):653–658. doi: 10.1136/postgradmedj-2018-135719. [DOI] [PubMed] [Google Scholar]
- 15.McGowan N.M., Voinescu B.I., Coogan A.N. Sleep quality, chronotype and social jetlag differentially associate with symptoms of attention deficit hyperactivity disorder in adults. Chronobiol Int. 2016;33(10):1433–1443. doi: 10.1080/07420528.2016.1208214. [DOI] [PubMed] [Google Scholar]
- 16.Chang S.J., Jang S.J. Social jetlag and quality of life among nursing students: a cross-sectional study. J Adv Nurs. 2019;75(7):1418–1426. doi: 10.1111/jan.13857. [DOI] [PubMed] [Google Scholar]
- 17.Janati Idrissi A., Lamkaddem A., Benouajjit A., et al. Sleep quality and mental health in the context of COVID-19 pandemic and lockdown in Morocco. Sleep Med. 2020;74:248–253. doi: 10.1016/j.sleep.2020.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks S.K., Webster R.K., Smith L.E., et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taha S., Matheson K., Cronin T., et al. Intolerance of uncertainty, appraisals, coping, and anxiety: the case of the 2009 H1N1 pandemic. Br J Health Psychol. 2014;19(3):592–605. doi: 10.1111/bjhp.12058. [DOI] [PubMed] [Google Scholar]
- 20.Jungmann S.M., Witthöft M. Health anxiety, cyberchondria, and coping in the current COVID-19 pandemic: which factors are related to coronavirus anxiety? J Anxiety Disord. 2020;73:102239. doi: 10.1016/j.janxdis.2020.102239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lades L.K., Laffan K., Daly M., et al. Daily emotional well-being during the COVID-19 pandemic. Br J Health Psychol. 2020 doi: 10.1111/bjhp.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konjarski M., Murray G., Lee V.V., et al. Reciprocal relationships between daily sleep and mood: a systematic review of naturalistic prospective studies. Sleep Med Rev. 2018;42:47–58. doi: 10.1016/j.smrv.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Raman S., Coogan A.N. A cross-sectional study of the associations between chronotype, social jetlag and subjective sleep quality in healthy adults. Clocks & Sleep. 2019;2(1):1–6. doi: 10.3390/clockssleep2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usher K., Jackson D., Durkin J., et al. Pandemic-related behaviours and psychological outcomes; A rapid literature review to explain COVID-19 behaviours. Int J Ment Health Nurs. 2020 doi: 10.1111/inm.12790. [DOI] [PubMed] [Google Scholar]
- 25.Petrov M.E., Weng J., Reid K.J., et al. Commuting and sleep: results from the hispanic community health study/study of Latinos Sueño ancillary study. Am J Prev Med. 2018;54(3):e49–e57. doi: 10.1016/j.amepre.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.