Abstract
Objectives and Methods: the current understanding of the interplay between cardiovascular (CV) risk and Covid-19 is grossly inadequate. CV risk-prediction models are used to identify and treat high risk populations and to communicate risk effectively. These tools are unexplored in Covid-19. The main objective is to evaluate the association between CV scoring systems and chest X ray (CXR) examination (in terms of severity of lung involvement) in 50 Italian Covid-19 patients. Results only the Framingham Risk Score (FRS) was applicable to all patients. The Atherosclerotic Cardiovascular Disease Score (ASCVD) was applicable to half. 62% of patients were classified as high risk according to FRS and 41% according to ASCVD. Patients who died had all a higher FRS compared to survivors. They were all hypertensive. FRS≥30 patients had a 9.7 higher probability of dying compared to patients with a lower FRS. We found a strong correlation between CXR severity and FRS and ASCVD (P < 0.001). High CV risk patients had consolidations more frequently. CXR severity was significantly associated with hypertension and diabetes. 71% of hypertensive patients’ CXR and 88% of diabetic patients’ CXR had consolidations. Patients with diabetes or hypertension had 8 times greater risk of having consolidations. Conclusions: High CV risk correlates with more severe CXR pattern and death. Diabetes and hypertension are associated with more severe CXR. FRS offers more predictive utility and fits best to our cohort. These findings may have implications for clinical practice and for the identification of high-risk groups to be targeted for the vaccine precedence.
Introduction
The Covid-19 pandemic emerged in Wuhan, China, and rapidly spread globally leading to an overwhelming pressure on healthcare systems that required substantial hygienic and containment measures.1 Progressive forms of Covid-19 are often characterized by hyperinflammation ("cytokine storm") with the development of an Acute Respiratory Distress Syndrome-like condition. Furthermore, reports of micro and macro thrombotic phenomena such as microangiopathy and pulmonary embolism are increasingly frequent.2 , 3 Clinical presentation of the disease ranges from asymptomatic to severe lung failure.1 , 2 Hospitalized patients were symptomatic, usually affected by interstitial pneumonia. Imaging is mandatory to investigate these patients at admission and chest X ray (CXR) is the widely used imaging technique.4
Many Covid-19 patients have underlying cardiovascular (CV) disease (CVD) or develop acute cardiac injury during the course of the illness.5 The impact of pre-existing CVD and new onset cardiac complications on clinical outcomes in these patients is under investigation.6
Patients with pre-existing cardiac disease, hypertension, diabetes and obesity are more likely to have a severe clinical course with a higher risk of mortality.2 , 7 , 8 In a meta-analysis including more than 40,000 patients, CVD was the third most common comorbidity in patients with Covid-19.9
In a meta-analysis including 76,638 patients, Matsushita and Colleagues explored pre-existing CVD and traditional CV risk factors as predictors of severe Covid-19 progression in patients. Their results suggest that hypertension, diabetes, and CVD are independently associated with severe Covid-19 and, together with age and male sex can be informative for predicting the risk of a more severe outcome.10
CV risk-prediction models are used in clinical practice to identify and treat populations, and to communicate risk effectively. Scoring systems are used to determine an individual's chance of developing CVD. These tools are unexplored in Covid-19 patients.
The Framingham Risk Score (FRS) is a gender-specific algorithm used to estimate the 10-year CV risk. It was first developed based on data obtained from the Framingham Heart Study.11 In order to assess the 10-year CVD risk, cerebrovascular events, peripheral artery disease and heart failure the original Framingham was updated and is now called Framingham 2008 Risk Score.12 Similarly, the ASCVD (atherosclerotic cardiovascular disease) risk score is a national guideline developed by the American College of Cardiology to estimate the 10-year primary risk of ASCVD among patients without pre-existing CVD who are between 40 and 79 years of age.13 The "ASCVD Plus" is the updated app version of the original ASCVD Risk Estimator functionality which was updated in 2016.
The main objective of this study is evaluating the association between CV scores (either FRS or ASCVD) and CXR examination (in term of severity of lung involvement) in Covid-19 patients derived from the Covid-19 Units of the University Hospital of Verona, Italy.
Methods
Ethical approval: all procedures have been conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The Ethical Committee of Verona and Rovigo (Protocol Number 2746CESC) approved the study.
Informed consent was obtained from all individual participants included in the study.
- Study type, setting, population and description of the protocol
The study started in fall 2020.
Study population: 50 Covid-19 patients admitted to the Covid sections of the Verona University Hospital.
Different CV scoring systems present different applicable age range for analysis. Considering all patients, we selected as eligible for this study patients aged between 40 years and 79 years old.
Exclusion Criteria: age less than 40 and more than 79 years, pregnancy.
Diagnostic Tests: Venous blood samples were collected from each subject for routine examination on admission: haemoglobin and white blood cells count, serum creatinine, sodium, potassium, urea, total cholesterol, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, triglycerides, glucose, CRP and D- dimer (measured with standard methods). Arterial blood samples were collected on admission and on discharge to test the PaO2/FiO2 ratio (P/F, the calculated ratio of arterial oxygen partial pressure to fractional inspired oxygen). Peripheral deep vein thrombosis was detected using a high-resolution B-mode ultrasound equipped with a 7.5-to 12-MHz probe. Electrocardiogram and CXR were assessed in all patients. Computed Tomography (CT) was performed only after clinical judgment to explore worsening patients.
CV scoring systems were applied to the patients according to their applicability (age-related limitations and CVD history).
Web-based programs or apps were used to calculate the 10-year percentage (%) of CV risk as different algorithms allows using the parameters indicated in Table 1 .
TABLE 1.
Parameters that are included or excluded by each CV risk score
| FRS | ASCVD plus | |
|---|---|---|
| Sex | ✓ | ✓ |
| Age | ✓ | ✓ |
| Ethnicity | ✓ | |
| Total cholesterol | ✓ | ✓ |
| HDL cholesterol | ✓ | ✓ |
| LDL cholesterol | ✓ | |
| SBP | ✓ | ✓ |
| DBP | ✓ | |
| History/medications for hypertension | ✓ | ✓ |
| Smoking habit | ✓ | ✓ |
| Diabetes | ✓ | ✓ |
| Statins treatment | ✓ | ✓ |
| Aspirin treatment | ✓ | |
| Known CVD | ✓ |
ASCVD, atherosclerotic cardiovascular disease; CVD, cardiovascular diseases; DBP, diastolic blood pressure; FRS, Framingham risk score 2008; HDL, high density lipoproteins; LDL, low density lipoproteins; SBP, SYSTOLIC BLOOD PRESSURE.
Days of hospitalization and death rate was evaluated as clinical outcome for the studied patients.
-Statistical Analysis: univariate associations between CV risk scores and the clinical outcomes were investigated using Fisher's Exact tests, Kruscal-Wallis test and Spearman's correlations, accordingly to the distributions of the variables. The associations between diabetes and hypertension with CXR severity were analysed using ordinal logistic regression models, using the CXR severity (0 = normal, 1 = interstitial signs, 2 = consolidations) as the dependent variable and diabetes/hypertension as the main independent variable; the models were adjusted for age and gender. Statistical analyses were performed with STATA 15 (Stata Corp. College Station, TX, USA).
Results
Baseline clinical characteristics, laboratory and instrumental data, treatment, respiratory status and clinical outcome of the 50 Covid-19 patients are described in Table 2 .
TABLE 2.
Characteristics of the Covid-19 patients (n = 50)
| Demographics | Covid-19 patients |
|---|---|
| Age (years) | 69 ± 10 |
| Hospitalisation period (days) | 15 ± 9 |
| Female gender (%) | 19 (38%) |
| Smoker (Current or Former) (%) | 4 (8%) |
| Comorbidities | |
| Diabetes (%) | 16 (32%) |
| Ischemic heart disease (%) | 9 (18%) |
| Cerebrovascular disease (%) | 11 (22%) |
| Hypertension (%) | 35 (70%) |
| Dislipidemia (%) | 19 (38%) |
| Obesity (%) | 9 (18%) |
| Cancer (%) | 2 (4%) |
| Chronic Obstructive Pulmonary Disease (%) | 4 (8%) |
| Previous medication | |
| Medications for hypertension (%) | 25 (50%) |
| Aspirin (%) | 14 (28%) |
| Statin (%) | 19 (38%) |
| In-hospital treatment | |
| Steroid (%) | 42 (84%) |
| Remdesivir (%) | 17 (34%) |
| Antibiotics (%) | 26 (52%) |
| Respiratory status | |
| Mechanical ventilation (%) | 17 (34%) |
| High flow oxygen (%) | 9 (18%) |
| Low flow oxygen (%) | 19 (38%) |
| Room air (%) | 5 (10%) |
| P/F | 200.5 ± 99.3 |
| Venous thromboembolism (%) | 3 (6%) |
| Chest X ray | |
| Normal (%) | 10 (20%) |
| Intersrtitial signs (%) | 10 (20%) |
| Consolidations (%) | 30 (60%) |
| Clinical Outcome | |
| Hospitalization period (days) for discharged patients | 16 ± 9 |
| Death (%) | 6 (12%) |
| Main Laboratory findings | |
| Hemoglobin, g/dL | 12.8 ± 2.1 |
| White blood cell count, × 109/L | 9.0 ± 3.8 |
| C-reactive protein, mg/L | 101.4 ± 81.2 |
| Procalcitonin, ng/mL | 0.7 ± 1.5 |
| Total cholesterol, mg/dL | 162.8 ± 34.9 |
| LDL cholesterol, mg/dL | 97.1 ± 35.2 |
| HDL cholesterol, mg/dL | 41.4 ± 10.2 |
| Triglycerides, mg/dL | 122.7 ± 66.4 |
| FRS Median value (IQR) | 28.95 (17.48 - 30.00) |
| ASCVD Plus Median value (IQR) | 15.50 (6.97 – 37.65) |
ASCVD, atherosclerotic cardiovascular disease; FRS, Framingham Risk Score 2008; HDL, high-density lipoproteins; LDL, low-density lipoproteins; P/F: PaO2/FiO2 ratio, the calculated ratio of arterial oxygen partial pressure to fractional inspired oxygen. Data are expressed as n (%) or mean ± standard deviation (SD).
The study population was predominantly male. The mean age was 69,4 (SD 9). The hospitalization period in days was 15.4 (SD 5). Six patients died (12%). The more frequent comorbidities were hypertension and diabetes. Patients were treated with Remdesivir (34%), but most of patients took steroids (84%) and experienced different types of oxygen supply, as depicted in Table 2.
CXR examinations showed a normal pattern in 10 patients (10%), an interstitial involvement in 10 patients (10%) and a consolidation pattern in the majority of the patients (60%).
Only Framingham 2008 was applicable to all 50 patients, while ASCVD plus was applicable to 32 of them. This fact was due to age-related limitations and CVD history, as explained in the Methods section. 62% of patients were classified as high risk according to Framingham 2008 and 41% according to ASCVD plus.
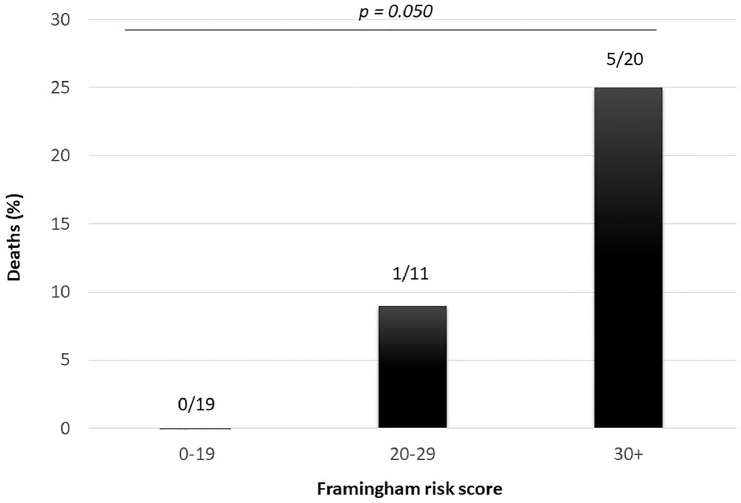
Overall, 6 patients died (12%), all of them were hypertensive. The risk of dying was greater in subjects with higher FRS (Fig 1 ); in univariate logistic analysis, patients with FRS≥30 had 9.7 higher risk of dying (Odds ratio: 9.7, 95%CI: 1.03-90.4, P = 0.047), compared to patients with a lower FRS (<30).
Fig. 1.
Percentage of deaths according to the Framingham risk score.
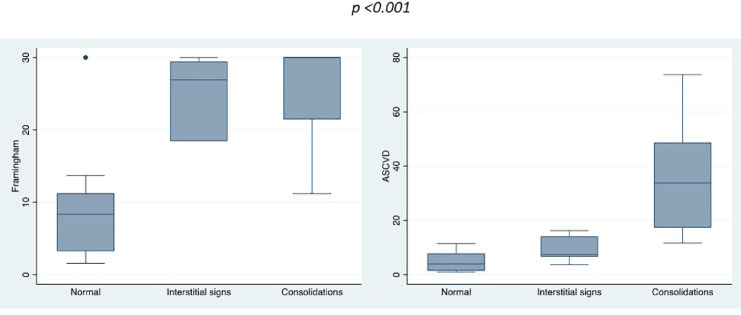
Ten patients (20%) had normal CXR, 10 (20%) had interstitial signs and 30 (60%) had consolidations. There was a strong correlation between CXR severity and both FRS and ASCVD plus (P < 0.001 both), and patients with CXR consolidation pattern had significantly greater CV risk than those with normal or intermediate patterns, as displayed in Figure 2 .
Fig 2.
Framingham 2008 (left) and ASCVD plus (right) scoring systems according to CXR severity at admission. (Color version of figure is available online.)
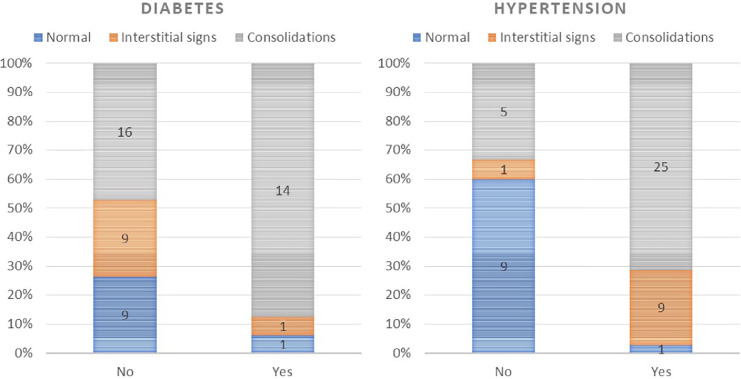
CXR severity was also significantly associated with hypertension and diabetes. 71% and 88% of patients with hypertension and diabetes, respectively, had a consolidation pattern, as compared with 33% and 47% of patients without (P < 0.001 and P = 0.038, respectively), as shown in Figure 3 .
Fig 3.
CXR severity in patients with diabetes (left) and hypertension (right). (Color version of figure is available online.)
After an adjustment to age and sex, these associations maintained their statistical significance, and patients with diabetes or hypertension had 7-folds greater risk of having a more severe CXR pattern (ORdiabetes: 7.95, 95%CIdiabetes: 1.41-44.8, P = 0.019; ORhypertension: 7.23, 95%CIhypertension: 1.74-30.0, P = 0.006).
No significant associations were found between parameters of the respiratory status and CV risk.
Of note, a possible connection was hinted between P/F and CV risk and P/F and CXR severity. In more detail, there was a moderate, but non-significant given the low number of observations, inverse correlation between P/F and FRS (Spearman's rho: -0.20, P = 0.161) and ASCVD plus (Spearman's rho: -0.25, P = 0.162).
Discussion
To our knowledge, up to now this is the first study that has evaluated the utility of CV scoring systems, in particular Framingham 2008 and ASCVD plus, in Covid-19 patients and in relation to their clinical status.
Our study shows that there is a strong significant correlation between CXR severity and both FRS and ASCVD plus.
This finding may be another little step to help physicians in the management of the disease. In fact, evaluating CXR severity next to CV score calculation could be a composed-parameter to be implicated in admission/discharge decision making, helping in patients’ management at emergency room hospital admission when the first CXR is performed.
CXR possesses great potential to facilitate the diagnosis and the management of patients with Covid-19 pneumonia. Recent published recommendations by the Fleischner and the European Society for Thoracic Imaging4 , 14 have also emphasized this approach. With appropriate use in a predefined patient population, conventional CXR imaging can provide critical information on the pulmonary status and facilitate management. Moreover, portable CXR machines can be easily deployed in the intensive/subintensive care units for disease monitoring and could even be applied through glass and/or smart glass doors in isolation rooms.15
For this reason, CXR instead of CT was extensively used as the focus of this study.
Recent reports suggest that the CXR16 as well as chest CT17 are able to predict whether the course of the disease will be moderate or severe in relation to the extent and morphology of pulmonary infiltrates on admission.
Covid-19 pandemic continues to spread. It now afflicts also developing countries that might not have broad access to CT imaging and CXR use has to be implemented.
For this reason, according to the importance of CXR in Covid-19, researchers are studying diagnostic support systems that could help Radiologists to detect pneumonia from CXR images.18
A further important result concerns comorbidities: diabetes and hypertension.
We found a strong correlation between these 2 diseases and the severity of CXR.
Patients who died had all higher FRS compared to survivors and they were all hypertensive.
Data from previous studies confirm that among patients with Covid-19 pneumonia, the commonest comorbidities in severe Covid-19 are hypertension and diabetes.19
Precise mechanisms by which hypertension and diabetes are “Covid-19 enhancers” are under investigation.20 The role of inflammation, oxidative stress, endothelial damage, microvascular damage and cytokine storm alterations may be the link.21 , 22 Nevertheless, these explanations are beyond the purpose of our study.
No correlations were found between the CV risk and the duration of hospitalization, nor for CV and parameters of the respiratory status.
The characteristics of our patients did not allow specific correlations with all CV scoring systems.
The identification of risk and prognostic factors for mortality rate are crucial for identifying patients’ outcome at an early stage in order to support clinical decision-making.23 In our population only FRS fitted all patients, followed by ASCVD (in 32 patients out of 50). This happens according to the Italian epidemiology of Covid-19 and hospitalized patients’ characteristics.24 A recent analysis by the Italian Institute of Health revealed that the overall-case fatality rate increased with aging in Italy; however, the fatality rate in Italy was remarkable higher compared to China (7.2% vs.2.3%) because of the higher death tolls in Italy among subjects aged > 70 years (12.8% vs. 8.0%) and among those older than 79 (20.2% vs. 14.8%). In Italy 23% of the population was aged > 65 years in 2019.25 Of note, aging represents one of the most detrimental risk factors for the CV system.26 This addresses the issue of cardiovascular disease burden in an older population as a critical parameter to be accounted for in the control of Covid-19 pandemics.
There are some limitations in our study. First, we evaluated the findings of only the baseline CXR, and so we could not assess the pattern of disease progression. However, the inclusion of a baseline CT or further CXR would not match well with the primary aim. Moreover, it is well known that both CXR and CT features of Covid-19 pneumonia depend on the duration of infection and they may be normal in the early phase of the disease.27
Secondly, as previously underlined, the characteristics of our patients did not allow specific correlations with all CV scoring systems. It should be possible with a larger sample. Nevertheless, this study may be the basis of future research especially in countries where the median age of hospital admission is lower.28 , 29 At that moment also comparisons between CV scoring systems will be possible and desirable in order to find the “one size fits all” for Covid-19 patients. In fact, not all CV risk prediction models can accurately identify higher risk individuals as previously largely discussed30, 31, 32 in other fields of research before Covid-19.
Conclusions
To conclude, high FRS and ASCVD correlate with more severe CXR pattern. Diabetes and hypertension are the 2 comorbidities associated with more severe CXR pattern. FRS offers more predictive utility and fits our cohort best. These findings may have implications for clinical practice and for the identification of high-risk groups to be targeted for the vaccine precedence.
Author Contributions
CM, SC, VR, AV, DG, conceived the study. GP and LC performed the statistical analysis, AS managed the database and organized patient recruitment. CM wrote the paper.
Funding
None.
Ethical Approval
All procedures are in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The Ethical Committee of Verona and Rovigo (Protocol Number 2746CESC) approved the study.
Informed Consent
Informed consent was obtained from all individuals participants included in the study.
Footnotes
Conflict of interest: Authors declare that they have no conflict of interest.
Declaration of interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klok FA, Kruip MJHA, van der Meer NJM. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.013. pii: S0049-3848(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner society. Radiology. 2020;296:172–180. doi: 10.1148/radiol.2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14:247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019. Novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323 doi: 10.1001/jama.2020.1585. 1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsushita K, Ding N, Kou M, et al. The relationship of COVID-19 severity with cardiovascular disease and its traditional risk factors: a systematic review and meta-analysis. Global Heart. 2020;15:64. doi: 10.5334/gh.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1997;18:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 12.D'Agostino RB, Vasan RS, Pencina MJ, Wolf PA. Cobain M General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 1998;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 13.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Revel MP, Parker AP, Prosch H, et al. COVID-19 patients and the radiology department—advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) Eur Radiol. 2020;30:4903–4909. doi: 10.1007/s00330-020-06865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rai A, MacGregor K, Hunt B, et al. Proof of concept: phantom study to ensure quality and safety of portable chest radiography through glass during the COVID-19 pandemic. Invest Radiol. 2020 doi: 10.1097/RLI.0000000000000716. Epub ahead of print. PMID: 32773486. [DOI] [PubMed] [Google Scholar]
- 16.Toussie D, Voutsinas N, Finkelstein M, et al. Clinical and chest radiography features determine patient outcomes in young and middle-aged adults withCOVID-19. Radiology. 2020;297:E197–E206. doi: 10.1148/radiol.2020201754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonardi A, Scipione R, Alfieri G, et al. Role of computed tomography in predicting critical disease in patients with covid-19 pneumonia: a retrospective study using a semiautomatic quantitative method. Eur J Radiol. 2020;130 doi: 10.1016/j.ejrad.2020.109202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fontanellaz M, Ebner L, Huber A, et al. A deep-learning diagnostic support system for the detection of COVID-19 using chest radiographs: a multireader validation study. Invest Radiol. 2020 doi: 10.1097/RLI.0000000000000748. Epub ahead of print. PMID: 33259441. [DOI] [PubMed] [Google Scholar]
- 19.Pititto BA. Ferreira SRG Diabetes and Covid-19: More than the sum of two morbidities. Rev Saude Publica. 2020;54:54. doi: 10.11606/s1518-8787.2020054002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Eng J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magadum A, Kishore R. Cardiovascular manifestations of COVID-19 infection. Cells. 2020;9:2508. doi: 10.3390/cells9112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cicco S, Cicco G, Racanelli V, Vacca A. Neutrophil extracellular traps (NETs) and damage-associated molecular patterns (DAMPs): two potential targets for COVID-19 treatment. Mediators Inflamm. 2020;2020:1–25. doi: 10.1155/2020/7527953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mozzini C, Girelli D. The role of neutrophil extracellular traps in Covid-19: only a hypothesis or a potential new field of research? Thromb Res. 2020;191:26–27. doi: 10.1016/j.thromres.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054–1106. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmieri L, Vanacore N, Donfrancesco C, et al. Italian National Institute of Health COVID-19 Mortality Group. Clinical characteristics of hospitalized individuals dying with COVID-19 by age group in Italy. J Gerontol A Biol Sci Med Sci. 2020 Sep 16;75(9):1796–1800. doi: 10.1093/gerona/glaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 27.Obas V, Vasan RS. The aging heart. Clin Sci. 2018;132:1367–1382. doi: 10.1042/CS20171156. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Dong C, Hu Y. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;296(2):E55–E64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernheim A, Mei X, Huang M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3) doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim K, Choi JW, Moon J, et al. Clinical Features of COVID-19 in Uzbekistan. J Korean Med Sci. 2020;23(35):e404. doi: 10.3346/jkms.2020.35.e404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owusu D, Kim L, O'Halloran A, et al. COVID-NET surveillance team. Characteristics of adults aged 18-49 years without underlying conditions hospitalized with laboratory-confirmed COVID-19 in the United States, COVID-NET - March-August 2020. Clin Infect Dis. 2020 3 doi: 10.1093/cid/ciaa1806. ciaa1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerra-Silva NM, Santucci FS, Moreira RC, et al. Coronary disease risk assessment in men: Comparison between ASCVD Risk versus Framingham. Int J Cardiol. 2017;228:481–487. doi: 10.1016/j.ijcard.2016.11.102. [DOI] [PubMed] [Google Scholar]