Abstract
Purpose of Review:
In recent decades, food allergy has become an increasing concern for families, clinicians, and policymakers. This review aims to summarize what is currently known about the epidemiology and population-level burden of IgE-mediated food allergy, including its effects on quality of life.
Recent Findings:
Prevalence surveys, healthcare utilization data and findings from longitudinal cohort studies across the globe indicate that food allergy imposes a growing societal burden. Worryingly, recent data indicate that food allergies may be more prevalent among adult populations than previously acknowledged, with many reported cases of adult-onset allergies.
Summary:
While it remains unclear how much of the current population-level burden of disease results from true, IgE-mediated allergy, as much epidemiological data does not incorporate clinical confirmation of disease prevalence—it is clear that affected individuals suffer substantial impairments in their quality of life and incur substantial economic costs—beyond the physical health burden imposed by anaphylaxis.
Keywords: Food Allergy Epidemiology, Burden of disease
INTRODUCTION
In recent decades, food allergy has become an increasing concern for families, clinicians, and policymakers around the world. It is a key component of the “atopic march”—the natural history of allergic disease manifestations that often progresses from allergic sensitization early in infancy, to atopic dermatitis, food allergy, asthma and allergic rhinitis. While its burden is heterogenous, food allergy affects people of all ages, races/ethnicities and socioeconomic strata—and therefore has become a public health issue of global importance. This review summarizes what is known about the current epidemiology and population-level burden of Immunoglobulin E (IgE)-mediated food allergy, including economic burden and the effects on quality of life.
Prevalence of Food Allergy
In the US, a population-based cross-sectional prevalence survey of over 50,000 households published in 2018 estimated that IgE-mediated food allergy is likely to affect approximately 1 in 10 adults (1) and 1 in 12 children. (2) Altogether, these data indicate that over 10% of the US population is likely to suffer from at least one IgE-mediated food allergy—with even more individuals reporting current food allergy in the absence of convincingly IgE-mediated symptoms. For example, in this survey, nearly 1 in 5 adults (19%) reported that they had at least one current food allergy and over 11% of children had parent-reported food allergy. However, individuals reporting symptomatology determined to be more consistent with other non-IgE conditions (e.g. oral allergy syndrome, intolerance) did not meet the stringent criteria used to ascertain “convincing” food allergy in this study. It is important to note that many of these individuals who did not report convincingly IgE-mediated symptomatology nevertheless strictly avoid their suspected allergens and therefore are likely to suffer impaired quality of life and incur additional economic burden associated with chronic food allergy management.
Crucially, while self-selection bias is a concern in self/parent-report prevalence questionnaires such as this—the prevalence point estimates from this survey were gleaned from the nationally-representative AmeriSpeak Panel—hosted by NORC at the University of Chicago, whose participants reported prevalence rates of other atopic conditions (i.e. asthma (3), eczema(4)) consistent with previous US population-based epidemiological studies, suggesting that atopic individuals were unlikely to be substantially over-represented in the resulting estimates.
While the aforementioned food allergy prevalence estimates may appear remarkably high, they are consistent with previous population-based survey research. A 2011 pediatric food allergy prevalence study, which utilized a similar survey sampling design and questionnaire structure, reported that 8% of US children had a convincingly IgE-mediated current food allergy based on symptom report. (5) However, the criteria used to define convincing food allergy in this previous pediatric survey were less stringent than those used in the 2018 study, as they did not exclude patients with suspected oral allergy syndrome (i.e. those reporting only non-systemic oropharyngeal reaction symptoms to common OAS triggers). Consequently, while the prevalence estimates are not directly comparable, they nevertheless suggest that US pediatric food allergy prevalence rates are unlikely to have decreased in the last decade. Other studies have also concluded that food allergy prevalence has risen substantially in recent decades. For estimates from the National Health and Nutrition Examination Survey (NHANES) from 2007-2010 suggested that 6.5% of children were reported to have a food allergy. (6) This same study found that 10% of US adults reported a current food allergy, while estimates from the 2010 FDA Food Safety Survey data, estimated reported adult food allergy prevalence at 13% and physician-diagnosed adult food allergy prevalence at 6.5%. (7) A previous US cross-sectional, random digit dial telephone survey assessing self-reported peanut, tree nut, and sesame allergy, suggested that peanut allergy prevalence among children was 1.4% in 2008, a marked increase from 1997 (0.4%) and 2002 (0.8%) (8). Tree nut allergy prevalence was estimated to be 1.1% in 2008, compared to 0.5% in 2002 and 0.2% in 1997 (8), also indicative of a substantial increase in food allergy prevalence.
It is important to acknowledge that, even if repeated cross-sectional prevalence surveys were to use identical criteria at each time point, temporal trends in food allergy prevalence are undoubtedly influenced to some degree by increasing awareness of food allergy among both patients and physicians. Furthermore, previous studies indicate that self-/parent-proxy report instruments may substantially over-estimate true food allergy prevalence, (9) underscoring the need for future epidemiological work incorporating clinical confirmation of participant-reported symptoms—ideally via oral food challenge. However, such studies are costly, logistically challenging in a population-based context and riskier for patients than traditional survey-based approaches, leading to concerns about the generalizability of resulting data owing to non-representative samples.
One study that has surmounted the aforementioned barriers and provides unique insights into the true prevalence of IgE-mediated food allergies is the HealthNuts cohort study, currently underway in the Australian state of Victoria. This study estimated oral food challenge-proven food allergy prevalence to be over 10% in a population-based cohort of 12 month-old infants.
Specifically, prevalence of egg, peanut and sesame seed allergy were estimated to be 8.8%, 3%, and 0.8% respectively, while cow’s milk allergy prevalence was 2.7% - although this was not challenge confirmed, but based on skin prick testing and convincing clinical history.(10) A more recent study, which followed-up the cohort at 4 years estimated challenge-confirmed prevalence of egg, peanut, and sesame allergy to be 1.2%, 1.9% and 0.4%. (11) It is notable that these 4 year estimates are comparable with the corresponding point prevalence estimates of convincingly IgE-mediated egg (1.3%), peanut (2.1%), and sesame (0.2%) allergy reported in the latest crosssectional pediatric US food allergy survey among 3-5 year olds. (2) Other recent epidemiological studies incorporating oral food challenges within the first two years of life have come out of the nine country EuroPrevall birth cohort, which reported a challenge-confirmed cow’s milk allergy prevalence estimate of 0.6% (12) and a challenge confirmed hen’s egg allergy prevalence estimate of 1.2%. (13) An Irish birth cohort also reported 1.8% prevalence of peanut allergy and 2.9% prevalence of egg allergy within the first two years of life.(14) See Figure 1 for available estimates of pediatric food allergy prevalence around the world.
Figure 1.
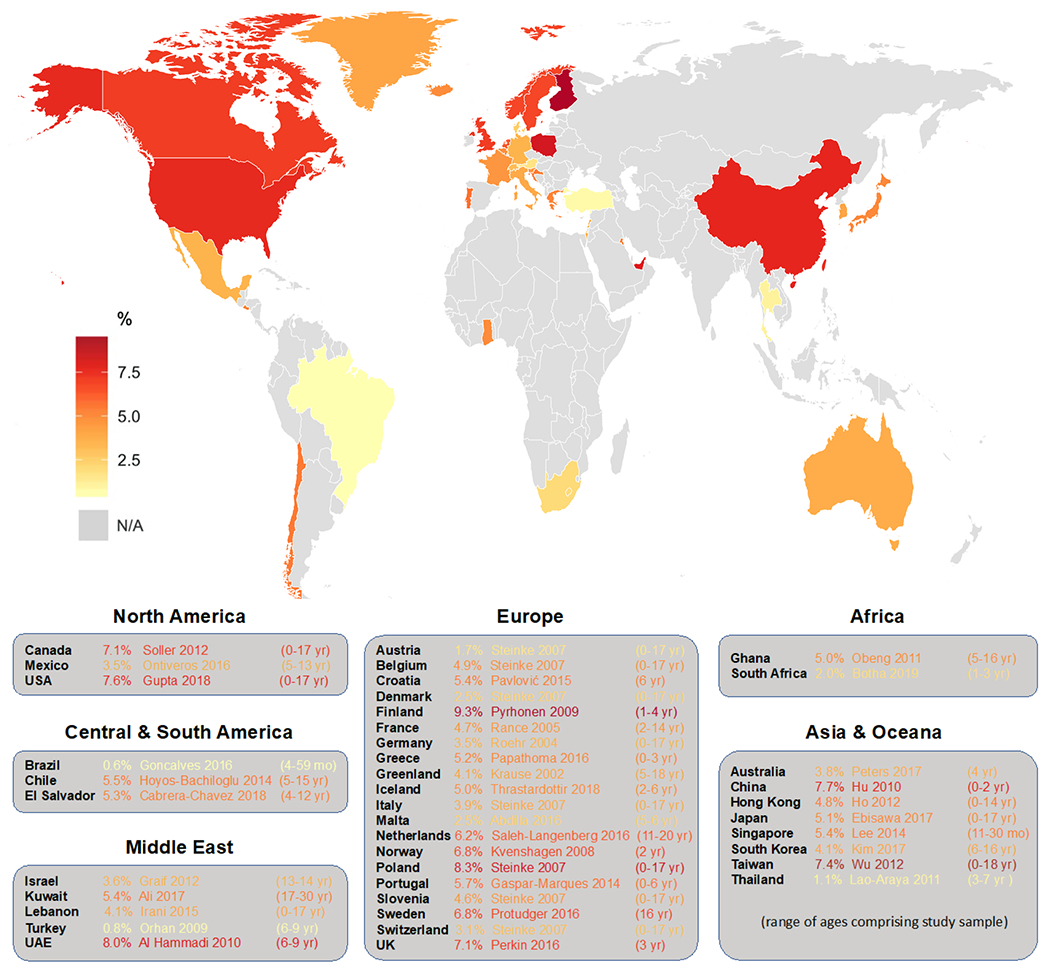
Population-based estimates of current pediatric food allergy prevalence in countries around the world
While the aforementioned cohort studies reporting challenge-confirmed food allergy rates provide valuable insights into the burden of disease among infants and young children—little work to date has systematically characterized the prevalence of food allergy among older around the world children, adolescents, and adults using similarly rigorous clinical diagnostic approaches. This is important since, while HealthNuts found that nearly two-thirds of peanut, egg, and sesame allergy cases resolved between 1 and 4 years (49)—consistent with previous data indicating that 20% of peanut (50) and 80% of egg allergies are likely to resolve naturally (51)—much remains unknown about the natural history of food allergies. Recent data from the unselected Isle of Wight birth cohort (52)—which has been followed prospectively for 18 years with low participant attrition and mobility—has observed a pattern of resolution of transient food allergies during early childhood followed by an increase in food allergen sensitization and new food allergy onset during teenage years. This resulted in elevated prevalence rates at 18 years relative to mid-childhood. These patterns are somewhat consistent with recent cross-sectional prevalence survey findings in the US, which reported higher food allergy prevalence rates among young adults than among pediatric samples, as well as high rates of adult-onset food allergy. (1)
Racial Differences in Food Allergy Burden
Like other atopic conditions in the US, food allergies appear to be particularly prevalent among African-American children relative to their White counterparts. (2) Recent data also indicate that White adults have lower rates of food allergy relative to their Black, Hispanic, Asian, and multiracial counterparts and prevalence rates appear similar among adults in the highest and lowest income strata. (1) These epidemiological findings of racial differences are mostly consistent with previous literature, (53) such as the random digit dial telephone survey research by Sicherer et al that demonstrated Black respondents reported higher rates of seafood allergy than other racial/ethnic groups. (54) Similar differences were reported by Luccioli et al in 2008 whose analysis of data from the 2005-2007 Infant Feeding Practices Study II concluded there were more Black children with reported, probable food allergy than White or Hispanic children (12.5% vs 5.6% vs 5.1%, respectively). (55) Data from the National Health Interview Survey (NHIS) between 1997-2007 also indicated a greater increase in parent-reported rates of food allergy among Black children relative to other races. (56)
Moreover, a study of urban tertiary care hospital networks in Chicago and Cincinnati reported higher rates of food-induced anaphylaxis and related emergency department visits among African-American and Hispanic pediatric food allergy patients, relative to their non-Hispanic White counterparts. (57) Recent national US survey data also indicate that Black adult food allergy patients report higher rates of severe reactions, as well as higher rates of food allergy-related emergency department visits than White adults, even after adjusting for comorbid atopy and a large number of other relevant sociodemographic factors (i.e. household income, educational attainment, whether the food allergy was physician-diagnosed, and whether the patient was prescribed epinephrine). (1) Pediatric data from the same nationally-representative survey also found elevated rates of food allergy-related emergency department visits among Black and Hispanic children adjusting for a similar set of covariates. (2) Taken together, these data highlight the importance of better understanding and addressing root causes of these apparent racial differences in food allergy prevalence, and possibly severity.
Racial Differences in Food Sensitization
Furthermore, numerous studies have found that Black/African-American children have substantially higher rates of food-allergic sensitization—including to peanut, (58) milk, egg, and shrimp. (56, 59) The 2005-2006 nationally representative NHANES study reported that food sensitization prevalence among Black individuals (regardless of age) was 27% compared to 13.8% for White and 21.2% for Hispanic individuals. The estimated clinical food allergy rates were 5.9%, 1.9%, and 2.7%, respectively. (59) Recent work from the Detroit-area WHEALS birth cohort study has found that these higher rates of food-allergic sensitization among African-American children may not always translate into higher rates of food allergy compared to non-African-American children. (60) This has important implications for epidemiological studies relying on specific IgE levels for food allergy diagnosis and underlines the importance of clinical history and food challenges (where appropriate) to confirm diagnoses—to help ensure unbiased evaluation. (61) A longitudinal analysis concluding that no population-level increases in food-allergic sensitization were observed among US children between (NHANES) Ill (1988–1994) and NHANES 2005–2006 further highlights the divergence between rates of food-allergic sensitization and survey-based prevalence rates in population-based studies. (62)
Food Allergy Severity, Anaphylaxis, and Healthcare Utilization
The apparent rise in food allergy prevalence among adolescents and young adults is particularly concerning given that these age groups appear disproportionately affected by food-induced anaphylaxis, including fatal reactions. (63) However, despite well-publicized fatalities resulting from food allergy, fatal food-induced anaphylactic reactions are exceedingly rare, particularly among infants and young children. (64) More generally, recent US population-weighted survey data indicate that approximately 1 in 10 convincingly food-allergic individuals report receiving treatment for a food-allergic reaction in the emergency department within the past 12 months. Over 1 in 3 report that they have received food allergy treatment in the emergency department at some point in their lifetime. (1, 2) While reaction severity is difficult to characterize via patient-report, approximately half of children and adults with convincingly IgE-mediated food allergy report a history of at least one severe reaction—characterized by multiple serious acute symptoms (i.e. hives, vomiting, wheeze) reported within multiple organ systems (i.e. skin, gastrointestinal, respiratory, cardiovascular). In this US population-based study, rates of severe reactions varied substantially by food allergen, with peanut-, tree-nut, shellfish and/or fin fish-allergic children and adults more likely to report a severe reaction than their counterparts with other allergies. (1, 2) Moreover, among these same food-allergic individuals, 1 in 5 report having treated a food-allergic reaction with an epinephrine auto-injector with the highest rates of epinephrine auto-injector (EAI) use reported among food-allergic adolescents and young adults.
Numerous recent analyses of healthcare utilization data are consistent with European, Australian, and US survey research findings indicating rising food allergy burden. Rates of hospitalizations due to food-induced anaphylaxis doubled in Britain between 1998 and 2012—although fatality rates remained constant. (65) A doubling of hospitalizations for allergic reactions was also observed among Finnish and Swedish children aged 0-19 from 1999 to 2011. (66) Meanwhile, in Australia between 1994-1995 and 2004-2005 rates of hospitalizations for food-induced anaphylaxis increased an average of 13.2% per year, and over 5-fold among infants/children aged 0-4 years old. (67) More recent data found that food-related anaphylaxis hospitalization rates rose further beginning in 2005 reaching 8.2 cases per 100,000 persons/year by 2012. (68) Furthermore, A study observing emergency department visits and hospital admissions in the US State of Illinois due to anaphylaxis, estimated a 29.1% annual increase of emergency department and hospital admissions among children from 6.3 per 100,000 children in 2008 to 17.2 per 100,000 children in 2012 (69). These 2012 estimates are substantially higher than previous estimates of 10.5 per 100,000 person/year for all-cause anaphylaxis among pediatric patients treated from 1991-1997 by a large health maintenance organization in the state of Washington. (70)
Cross-sectional data indicating rising food allergy prevalence in the United States were recently corroborated by a longitudinal analysis of national health insurance claims data, (71) which demonstrated the incidence of peanut allergy increased steadily from 2001 to 2006 and is estimated to continue increasing through 2020. Another analysis of national claims data published in a white paper by FAIR Health found that from 2007-2016 the percent of claim lines with diagnoses of anaphylactic food reactions rose 377%. (72) The most common eliciting allergic foods were peanut and tree nut, which is consistent with other US studies showing these as among the most frequent causative foods of severe allergic reactions and emergency department visits. (2, 73)
Urban vs Rural Differences
Numerous studies have reported systematic differences in food allergy burden between urban and rural communities, with higher prevalence rates reported in urban areas than rural areas, even after accounting for differences in racial and socioeconomic composition. (74) A recent analysis of the Healthcare Cost and Utilization Project data for New York and Florida from 2009-2014 reported that emergency department admission rates due to food-induced anaphylaxis were 12.3/100,000 in an urban setting vs. 4.6 in rural settings. Such urban/rural differences have also been shown outside of the US context. A recent high-quality unselected cohort study found higher rates of both sensitization and allergy to food among rural-dwelling South Africans relative to urban-dwelling South Africans. (75) Remarkably, in contrast to the research hypothesis, this study found similar rates of food sensitization and allergy among urban South Africans of White, Mixed, and Black race—indicating that environmental influences may be more important in determining atopy risk than ethnicity, at least in this South African cohort. Such putative environmental influences include, but are not limited to: differences in aeroallergen exposure, proximity to farm and domestic animals, helminth parasite infestation, ultraviolet light exposure, environmental tobacco smoke/air pollution exposure, probiotic use and/or other factors which influence the composition of the skin and/or gut microbiome, breastfeeding practices, as well as frequency, timing, and composition of allergenic food and fiber consumption.
Effects of Migration
Previous literature has suggested that as highly populous South and East Asian countries increasingly urbanize and/or adopt lifestyles more akin to those of Western and/or industrialized countries, their burden of allergic disease is likely to increase (76) —absent large-scale implementation of effective allergy prevention interventions. In fact, data from two Chinese regional cross-sectional food allergy prevalence surveys administered in 1999 and 2009, which incorporated confirmatory oral food challenge, indicated that food allergy prevalence approximately doubled over a ten year period from 3.3% to 7.7% of 0-24 month old infants in Chongqing—a highly urbanized city of roughly 30 million people (42). Another source of such worries is recent data suggesting high rates of food-allergic sensitization among South Asians. (77) While this sensitization may not manifest as food allergy in their home country, evidence shows that the children of South Asian immigrants to countries with high allergic disease prevalence have significantly elevated allergy risk relative to their peers with native born parents. (78) For example, in the HealthNuts study, peanut allergy and sensitization were roughly three times as prevalent among infants whose parents were born in East Asia relative to infants with Australian-born parents. (79) Importantly, this difference was only partially attenuated after accounting for differences in food introduction practices and key household-level characteristics. Since studies also indicate that first generation immigrants first experience lower rates of allergy than their native-born peers, (80, 81) and then gradually acquire the sensitization and allergic disease profiles of their host country over time—this suggests that gene-environment interactions are likely to underlie the etiology of food-allergic disease in these populations. This also suggests that as global migration continues to urbanized areas where food allergy prevalence appears highest, (74, 82) that the global burden of food allergy is likely to further increase.
Psycho-Social Burden of Food Allergy
At the population-level, given the relatively low fatality rates associated with the condition (63) and general lack of symptoms in the absence of allergenic food exposure—food allergies impose significant burden on the quality of life of affected patients and their families. Previous studies have indicated that patients with a greater number of food allergies reported lower quality of life compared to their counterparts with fewer food allergies— likely owing to the fact that a greater degree of vigilance is required for allergen avoidance. (83–85) Among food allergic-children, those with milk and egg allergy have reported lower quality of life compared to children with easily avoidable allergens such as peanut and tree nut. (86, 87) Relatedly, individuals with milk or egg allergy who are able to tolerate baked milk/egg products have been found to report better quality of life, owing to fewer dietary restrictions. Impaired food allergy-specific quality of life has also been reported to be worse among children and caregivers for those with a history of severe food allergic reactions, more symptoms during a previously reported food allergic reaction, and any previous epinephrine use. (83, 85, 88) Similarly, individuals who believe themselves more likely to experience potentially life-threatening anaphylaxis report worse quality of life than their counterparts who are less concerned about potential fatality. Due to the ubiquity of food, management of food allergies remains a daily challenge for many patients, as they seek to strike the appropriate balance between vigilance, preparedness, and stress management. Previous research describes how the stress of daily food allergy management compounded by the dearth of effective treatment options impacts family relationships and often limits social activities, ultimately contributing to impaired food allergy-related quality of life. (89)
Economic Burden of Food Allergy
Besides its effects on physical and psychological health, food allergy has also been found to impose substantial economic burden on the individual and societal levels. In a 2013 study by Gupta et al, the annual economic cost of food allergy was estimated to be $24.8 billion, roughly $4,184 per year per child. (90) Annual direct medical costs including hospitalizations, clinician visits, and emergency department visits were $4.3 billion. Additionally, annual out-of-pocket costs related to food allergy were $5.5 billion and annual opportunity costs such as a caregiver leaving/changing jobs to care for their food allergic child was estimated to be $14.2 billion. (90) A follow-up study identified significant socioeconomic disparities in the economic burden of childhood food allergy, with children in the lowest income stratum spending more than twice as much as those in other income groups on food allergy-related emergency department and hospitalization costs. (91) On the other hand, greater household income was associated with greater spending on out-of-pocket medication costs (e.g. for epinephrine auto-injectors). A 2019 systematic review of eleven studies addressing the economic burden of food allergy concluded that household-level lost opportunity costs were the primary driver of food allergy-related economic burden—although further work is needed to better characterize the particular time and lost labor costs associated with food allergy. (92)
CONCLUSIONS
In sum, recent data suggest that food allergy affects a growing number of infants, children and adults around the world. While it has traditionally been conceptualized as a largely pediatric condition, recent data indicate that an even greater number of adults are impacted. Overall population-level burden of food allergy is likely to increase in the absence of rapid deployment of effective prevention interventions. In light of global trends of increased migration and urbanization, recent evidence suggesting that migrants from the world’s most populous regions (i.e. South and East Asia) may be predisposed to food allergy when exposed to a constellation of environmental factors associated with residence in major urban centers is particularly troubling. Unfortunately, it remains unclear how much of the current population-level burden of disease results from true, IgE-mediated allergy—since much epidemiological data to date has not incorporated clinical confirmation of disease prevalence. Nevertheless, affected individuals suffer substantial impairments in quality of life, utilize increasing amounts of healthcare services, and incur substantial economic costs—beyond the physical health burden imposed by anaphylaxis.
REFERENCES
- 1.Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, et al. Prevalence and Severity of Food Allergies Among US AdultsPrevalence and Severity of Food Allergies Among US AdultsPrevalence and Severity of Food Allergies Among US Adults. JAMA Network Open. 2019;2(1):e185630–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, et al. The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics. 2018;142(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevention CfDCa. 2017. National Health Interview Survey (NHIS) Data: Centers for Disease Control and Prevention; 2017 [Available from: https://www.cdc.gov/asthma/nhis/2017/table2-1.htm.
- 4.Hanifin JM, Reed ML. A population-based survey of eczema prevalence in the United States. Dermatitis : contact, atopic, occupational, drug. 2007;18(2):82–91. [DOI] [PubMed] [Google Scholar]
- 5.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The Prevalence, Severity, and Distribution of Childhood Food Allergy in the United States. Pediatrics. 2011;128(1):e9–e17. [DOI] [PubMed] [Google Scholar]
- 6.McGowan EC, Keet CA. Prevalence of self-reported food allergy in the National Health and Nutrition Examination Survey (NHANES) 2007-2010. The Journal of allergy and clinical immunology. 2013;132(5):1216–9.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verrill L, Bruns R, Luccioli S. Prevalence of self-reported food allergy in U.S. adults: 2001, 2006, and 2010. Allergy Asthma Proc. 2015;36(6):458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. The Journal of allergy and clinical immunology. 2010;125(6):1322–6. [DOI] [PubMed] [Google Scholar]
- 9.Woods RK, Stoney RM, Raven J, Walters EH, Abramson M, Thien FC. Reported adverse food reactions overestimate true food allergy in the community. European journal of clinical nutrition. 2002;56(1):31–6. [DOI] [PubMed] [Google Scholar]
- 10.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. The Journal of allergy and clinical immunology. 2011;127(3):668-76.e1-2. [DOI] [PubMed] [Google Scholar]
- 11.Peters RL, Koplin JJ, Gurrin LC, Dharmage SC, Wake M, Ponsonby AL, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. The Journal of allergy and clinical immunology. 2017;140(1):145–53.e8. [DOI] [PubMed] [Google Scholar]
- 12.Schoemaker AA, Sprikkelman AB, Grimshaw KE, Roberts G, Grabenhenrich L, Rosenfeld L, et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children--EuroPrevall birth cohort. Allergy. 2015;70(8):963–72. [DOI] [PubMed] [Google Scholar]
- 13.Xepapadaki P, Fiocchi A, Grabenhenrich L, Roberts G, Grimshaw KE, Fiandor A, et al. Incidence and natural history of hen’s egg allergy in the first 2 years of life-the EuroPrevall birth cohort study. Allergy. 2016;71(3):350–7. [DOI] [PubMed] [Google Scholar]
- 14.Kelleher MM, Dunn-Galvin A, Gray C, Murray DM, Kiely M, Kenny L, et al. Skin barrier impairment at birth predicts food allergy at 2 years of age. The Journal of allergy and clinical immunology. 2016;137(4):1111–6.e8. [DOI] [PubMed] [Google Scholar]
- 15.Soller L, Ben-Shoshan M, Harrington DW, Fragapane J, Joseph L, St-Pierre Y, et al. Overall Prevalence Of Self-reported Food Allergy In Canada. Journal of Allergy and Clinical Immunology. 2012;129(2):AB234. [DOI] [PubMed] [Google Scholar]
- 16.Ontiveros N, Valdez-Meza EE, Vergara-Jimenez MJ, Canizalez-Roman A, Borzutzky A, Cabrera-Chavez F. Parent-reported prevalence of food allergy in Mexican schoolchildren: A population-based study. Allergologia et immunopathologia. 2016;44(6):563–70. [DOI] [PubMed] [Google Scholar]
- 17.Gonzales-González VA, Díaz AM, Fernández K, Rivera MF. Prevalence of food allergens sensitization and food allergies in a group of allergic Honduran children. Allergy Asthma Clin Immunol. 2018;14:23-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachiloglu R, Ivanovic-Zuvic D, Alvarez J, Linn K, Thone N, de los Angeles Paul M, et al. Prevalence of parent-reported immediate hypersensitivity food allergy in Chilean school-aged children. Allergologia et immunopathologia. 2014;42(6):527–32. [DOI] [PubMed] [Google Scholar]
- 19.Goncalves LC, Guimaraes TC, Silva RM, Cheik MF, de Ramos Napolis AC, Barbosa ESG, et al. Prevalence of food allergy in infants and pre-schoolers in Brazil. Allergologia et immunopathologia. 2016;44(6):497–503. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hammadi S, Al-Maskari F, Bernsen R. Prevalence of food allergy among children in Al-Ain city, United Arab Emirates. International archives of allergy and immunology. 2010;151(4):336–42. [DOI] [PubMed] [Google Scholar]
- 21.Ali F A Survey of Self-Reported Food Allergy and Food-Related Anaphylaxis among Young Adult Students at Kuwait University, Kuwait. Medical principles and practice : international journal of the Kuwait University, Health Science Centre. 2017;26(3):229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irani C, Maalouly G. Prevalence of Self-Reported Food Allergy in Lebanon: A Middle-Eastern Taste. Int Sch Res Notices. 2015;2015:639796-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graif Y, German L, Livne I, Shohat T. Association of food allergy with asthma severity and atopic diseases in Jewish and Arab adolescents. Acta paediatrica (Oslo, Norway : 1992). 2012;101(10):1083–8. [DOI] [PubMed] [Google Scholar]
- 24.Orhan F, Karakas T, Cakir M, Aksoy A, Baki A, Gedik Y. Prevalence of immunoglobulin E-mediated food allergy in 6-9-year-old urban schoolchildren in the eastern Black Sea region of Turkey. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2009;39(7):1027–35. [DOI] [PubMed] [Google Scholar]
- 25.Caffarelli C, Coscia A, Ridolo E, Povesi Dascola C, Gelmett C, Raggi V, et al. Parents’ estimate of food allergy prevalence and management in Italian school-aged children. Pediatrics international : official journal of the Japan Pediatric Society. 2011;53(4):505–10. [DOI] [PubMed] [Google Scholar]
- 26.Steinke M, Fiocchi A, Kirchlechner V, Ballmer-Weber B, Brockow K, Hischenhuber C, et al. Perceived food allergy in children in 10 European nations. A randomised telephone survey. International archives of allergy and immunology. 2007;143(4):290–5. [DOI] [PubMed] [Google Scholar]
- 27.Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. The New England journal of medicine. 2016;374(18):1733–43. [DOI] [PubMed] [Google Scholar]
- 28.Protudjer JLP, Vetander M, Kull I, Hedlin G, van Hage M, Wickman M, et al. Food-Related Symptoms and Food Allergy in Swedish Children from Early Life to Adolescence. PLoS One. 2016;11(11):e0166347–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kvenshagen B, Halvorsen R, Jacobsen M. Is there an increased frequency of food allergy in children delivered by caesarean section compared to those delivered vaginally? Acta paediatrica (Oslo, Norway : 1992). 2009;98(2):324–7. [DOI] [PubMed] [Google Scholar]
- 30.Rance F, Grandmottet X, Grandjean H. Prevalence and main characteristics of schoolchildren diagnosed with food allergies in France. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2005;35(2):167–72. [DOI] [PubMed] [Google Scholar]
- 31.Saleh-Langenberg J, Bootsma GM, van Ginkel CD, Kollen BJ, Flokstra-de Blok BM, Dubois AE. The prevalence of food allergy and epinephrine auto-injectors in Dutch food-allergic adolescents. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2016;27(7):755–9. [DOI] [PubMed] [Google Scholar]
- 32.Gaspar-Marques J, Carreiro-Martins P, Papoila AL, Caires I, Pedro C, Araujo-Martins J, et al. Food allergy and anaphylaxis in infants and preschool-age children. Clinical pediatrics. 2014;53(7):652–7. [DOI] [PubMed] [Google Scholar]
- 33.Baricic TV, Catipovic M, Cetinic EL, Krmek V, Horvat I. Parental Perception, Prevalence and Primary Care Physicians’ Knowledge on Childhood Food Allergy in Croatia. Children (Basel, Switzerland). 2015;2(3):305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papathoma E, Triga M, Fouzas S, Dimitriou G. Cesarean section delivery and development of food allergy and atopic dermatitis in early childhood. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2016;27(4):419–24. [DOI] [PubMed] [Google Scholar]
- 35.Thrastardottir AR, Thordardottir FR, Torfadottir J. [Protocols Related to Food Allergies and Intolerances in Preschools in Reykjavik, Iceland]. Laeknabladid. 2018;104(1):11–7. [DOI] [PubMed] [Google Scholar]
- 36.Roehr CC, Edenharter G, Reimann S, Ehlers I, Worm M, Zuberbier T, et al. Food allergy and non-allergic food hypersensitivity in children and adolescents. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2004;34(10):1534–41. [DOI] [PubMed] [Google Scholar]
- 37.Krause TG, Koch A, Poulsen LK, Kristensen B, Olsen OR, Melbye M. Atopic sensitization among children in an Arctic environment. Clinical & Experimental Allergy. 2002;32(3):367–72. [DOI] [PubMed] [Google Scholar]
- 38.Pyrhönen K, Hiltunen L, Kaila M, Näyhä S, Läärä E. Heredity of food allergies in an unselected child population: An epidemiological survey from Finland. Pediatric Allergy and Immunology. 2011;22(1pt2):e124–e32. [DOI] [PubMed] [Google Scholar]
- 39.Obeng BB, Amoah AS, Larbi IA, Yazdanbakhsh M, van Ree R, Boakye DA, et al. Food allergy in Ghanaian schoolchildren: data on sensitization and reported food allergy. International archives of allergy and immunology. 2011;155(1):63–73. [DOI] [PubMed] [Google Scholar]
- 40.Basera W, Botha M, Gray CL, Lunjani N, Watkins AS, Venter C, et al. The South African Food Sensitisation and Food Allergy population-based study of IgE-mediated food allergy: validity, safety, and acceptability. Ann Allergy Asthma Immunol. 2015;115(2):113–9. [DOI] [PubMed] [Google Scholar]
- 41.Kung S-J, Steenhoff AP, Gray C. Food Allergy in Africa: Myth or Reality? Clinical Reviews in Allergy & Immunology. 2014;46(3):241–9. [DOI] [PubMed] [Google Scholar]
- 42.Hu Y, Chen J, Li H. Comparison of food allergy prevalence among Chinese infants in Chongqing, 2009 versus 1999. Pediatrics International. 2010;52(5):820–4. [DOI] [PubMed] [Google Scholar]
- 43.Lee AJ, Shek LP-C. Food allergy in Singapore: opening a new chapter. Singapore Med J. 2014;55(5):244–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Chang E, Han Y, Ahn K, Lee SI. The incidence and risk factors of immediate type food allergy during the first year of life in Korean infants: a birth cohort study. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2011;22(7):715–9. [DOI] [PubMed] [Google Scholar]
- 45.Noda R Prevalence of food allergy in nursery school (nationwide survey). Jpn J Food Allergy. 2010:5–9. [Google Scholar]
- 46.Ho MH, Lee SL, Wong WH, Ip P, Lau YL. Prevalence of self-reported food allergy in Hong Kong children and teens--a population survey. Asian Pacific journal of allergy and immunology. 2012;30(4):275–84. [PubMed] [Google Scholar]
- 47.Wu TC, Tsai TC, Huang CF, Chang FY, Lin CC, Huang IF, et al. Prevalence of food allergy in Taiwan: a questionnaire-based survey. Internal medicine journal. 2012;42(12):1310–5. [DOI] [PubMed] [Google Scholar]
- 48.Lao-araya M, Trakultivakorn M. Prevalence of food allergy among preschool children in northern Thailand. Pediatrics international : official journal of the Japan Pediatric Society. 2012;54(2):238–43. [DOI] [PubMed] [Google Scholar]
- 49.Peters RL, Koplin JJ, Gurrin LC, Dharmage SC, Wake M, Ponsonby A-L, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. Journal of Allergy and Clinical Immunology. 2017;140(1):145–53.e8. [DOI] [PubMed] [Google Scholar]
- 50.Ho MHK, Wong WHS, Heine RG, Hosking CS, Hill DJ, Allen KJ. Early clinical predictors of remission of peanut allergy in children. Journal of Allergy and Clinical Immunology. 2008;121(3):731–6. [DOI] [PubMed] [Google Scholar]
- 51.Boyano-Martínez T, García-Ara C, Díaz-Pena JM, Martín-Esteban M. Prediction of tolerance on the basis of quantification of egg white-specific IgE antibodies in children with egg allergy. Journal of Allergy and Clinical Immunology. 2002;110(2):304–9. [DOI] [PubMed] [Google Scholar]
- 52.Venkataraman D, Erlewyn-Lajeunesse M, Kurukulaaratchy RJ, Potter S, Roberts G, Matthews S, et al. Prevalence and longitudinal trends of food allergy during childhood and adolescence: Results of the Isle of Wight Birth Cohort study. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2018;48(4):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor-Black S, Wang J. The prevalence and characteristics of food allergy in urban minority children. Ann Allergy Asthma Immunol. 2012;109(6):431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. Journal of Allergy and Clinical Immunology. 2004;114(1):159–65. [DOI] [PubMed] [Google Scholar]
- 55.Luccioli S, Ross M, Labiner-Wolfe J, Fein SB. Maternally reported food allergies and other food-related health problems in infants: characteristics and associated factors. Pediatrics. 2008;122 Suppl 2:S105–12. [DOI] [PubMed] [Google Scholar]
- 56.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124(6):1549–55. [DOI] [PubMed] [Google Scholar]
- 57.Mahdavinia M, Fox SR, Smith BM, James C, Palmisano EL, Mohammed A, et al. Racial Differences in Food Allergy Phenotype and Health Care Utilization among US Children. The journal of allergy and clinical immunology In practice. 2017;5(2):352–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sicherer SH, Wood RA, Stablein D, Lindblad R, Burks AW, Liu AH, et al. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. The Journal of allergy and clinical immunology. 2010;126(6):1191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. The Journal of allergy and clinical immunology. 2010;126(4):798–806.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joseph CL, Zoratti EM, Ownby DR, Havstad S, Nicholas C, Nageotte C, et al. Exploring racial differences in IgE-mediated food allergy in the WHEALS birth cohort. Ann Allergy Asthma Immunol. 2016;116(3):219–24.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keet CA, Wood RA, Matsui EC. Limitations of reliance on specific IgE for epidemiologic surveillance of food allergy. The Journal of allergy and clinical immunology. 2012;130(5):1207–9.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGowan EC, Peng RD, Salo PM, Zeldin DC, Keet CA. Changes in Food-Specific IgE Over Time in the National Health and Nutrition Examination Survey (NHANES). The journal of allergy and clinical immunology In practice. 2016;4(4):713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turner PJ, Jerschow E, Umasunthar T, Lin R, Campbell DE, Boyle RJ. Fatal Anaphylaxis: Mortality Rate and Risk Factors. The journal of allergy and clinical immunology In practice. 2017;5(5):1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Umasunthar T, Leonardi-Bee J, Hodes M, Turner PJ, Gore C, Habibi P, et al. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2013;43(12):1333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turner PJ, Gowland MH, Sharma V, Ierodiakonou D, Harper N, Garcez T, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992-2012. The Journal of allergy and clinical immunology. 2015;135(4):956–63.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kivisto JE, Protudjer JL, Karjalainen J, Wickman M, Bergstrom A, Mattila VM. Hospitalizations due to allergic reactions in Finnish and Swedish children during 1999-2011. Allergy. 2016;71(5):677–83. [DOI] [PubMed] [Google Scholar]
- 67.Poulos LM, Waters AM, Correll PK, Loblay RH, Marks GB. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993-1994 to 2004-2005. The Journal of allergy and clinical immunology. 2007;120(4):878–84. [DOI] [PubMed] [Google Scholar]
- 68.Mullins RJ, Dear KB, Tang ML. Time trends in Australian hospital anaphylaxis admissions in 1998-1999 to 2011-2012. The Journal of allergy and clinical immunology. 2015;136(2):367–75. [DOI] [PubMed] [Google Scholar]
- 69.Dyer AA, Lau CH, Smith TL, Smith BM, Gupta RS. Pediatric emergency department visits and hospitalizations due to food-induced anaphylaxis in Illinois. Ann Allergy Asthma Immunol. 2015;115(1):56–62. [DOI] [PubMed] [Google Scholar]
- 70.Bohlke K, Davis RL, DeStefano F, Marcy SM, Braun MM, Thompson RS. Epidemiology of anaphylaxis among children and adolescents enrolled in a health maintenance organization. Journal of Allergy and Clinical Immunology. 2004;113(3):536–42. [DOI] [PubMed] [Google Scholar]
- 71.Lieberman J, Sublett J, Ali Y, Haselkorn T, Damle V, Chidambaram A, et al. INCREASED INCIDENCE AND PREVALENCE OF PEANUT ALLERGY IN CHILDREN AND ADOLESCENTS IN THE UNITED STATES. Annals of Allergy, Asthma & Immunology. 2018;121(5, Supplement):S13. [Google Scholar]
- 72.FAIR Health I Food Allergy in the United States: Recent Trends and Costs 2017.
- 73.Motosue MS, Bellolio MF, Van Houten HK, Shah ND, Campbell RL. National trends in emergency department visits and hospitalizations for food-induced anaphylaxis in US children. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2018;29(5):538–44. [DOI] [PubMed] [Google Scholar]
- 74.Gupta RS, Springston EE, Smith B, Warrier MR, Pongracic J, Holl JL. Geographic variability of childhood food allergy in the United States. Clinical pediatrics. 2012;51(9):856–61. [DOI] [PubMed] [Google Scholar]
- 75.Botha M, Basera W, Facey-Thomas HE, Gaunt B, Gray CL, Ramjith J, et al. Rural and urban food allergy prevalence from the South African Food Allergy (SAFFA) study. The Journal of allergy and clinical immunology. 2019;143(2):662–8.e2. [DOI] [PubMed] [Google Scholar]
- 76.Tang ML, Mullins RJ. Food allergy: is prevalence increasing? Internal medicine journal. 2017;47(3):256–61. [DOI] [PubMed] [Google Scholar]
- 77.Mahesh PA, Wong GW, Ogorodova L, Potts J, Leung TF, Fedorova O, et al. Prevalence of food sensitization and probable food allergy among adults in India: the EuroPrevall INCO study. Allergy. 2016;71(7):1010–9. [DOI] [PubMed] [Google Scholar]
- 78.Tham EH, Loo EXL, Zhu Y, Shek LPC. Effects of Migration on Allergic Diseases. International archives of allergy and immunology. 2019;178(2):128–40. [DOI] [PubMed] [Google Scholar]
- 79.Koplin JJ, Peters RL, Ponsonby AL, Gurrin LC, Hill D, Tang ML, et al. Increased risk of peanut allergy in infants of Asian-born parents compared to those of Australian-born parents. Allergy. 2014;69(12):1639–47. [DOI] [PubMed] [Google Scholar]
- 80.Panjari M, Koplin JJ, Dharmage SC, Peters RL, Gurrin LC, Sawyer SM, et al. Nut allergy prevalence and differences between Asian-born children and Australian-born children of Asian descent: a state-wide survey of children at primary school entry in Victoria, Australia. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2016;46(4):602–9. [DOI] [PubMed] [Google Scholar]
- 81.National Research Council (US) Panel on Race E, and Health in Later Life. 7. Immigrant Health: Selectivity and Acculturation. . Anderson NBBR, Cohen B, editor. Washington (DC): National Academies Press; 2004. [Google Scholar]
- 82.Allen KJ, Koplin JJ. What can urban/rural differences in food allergy prevalence tell us about the drivers of food allergy? Journal of Allergy and Clinical Immunology. 2019;143(2):554–6. [DOI] [PubMed] [Google Scholar]
- 83.Allen CW, Bidarkar MS, vanNunen SA, Campbell DE. Factors impacting parental burden in food-allergic children. Journal of Paediatrics and Child Health. 2015;51(7):696–8. [DOI] [PubMed] [Google Scholar]
- 84.Wassenberg J, Cochard M-M, DunnGalvin A, Ballabeni P, Flokstra-de Blok BMJ, Newman CJ, et al. Parent perceived quality of life is age-dependent in children with food allergy. Pediatric Allergy and Immunology. 2012;23(5):412–9. [DOI] [PubMed] [Google Scholar]
- 85.Howe L, Franxman T, Teich E, Greenhawt M. What affects quality of life among caregivers of food-allergic children? Annals of Allergy, Asthma & Immunology. 2014;113(1):69–74.e2. [DOI] [PubMed] [Google Scholar]
- 86.Ward CE, Greenhawt MJ. Treatment of allergic reactions and quality of life among caregivers of food-allergic children. Annals of Allergy, Asthma & Immunology. 2015;114(4):312–8.e2. [DOI] [PubMed] [Google Scholar]
- 87.Warren CM, Gupta RS, Sohn M-W, Oh EH, Lai N, Garfield CF, et al. Differences in empowerment and quality of life among parents of children with food allergy. Annals of Allergy, Asthma & Immunology. 2015;114(2):117–25.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chow C, Pincus DB, Comer JS. Pediatric Food Allergies and Psychosocial Functioning: Examining the Potential Moderating Roles of Maternal Distress and Overprotection. Journal of Pediatric Psychology. 2015;40(10):1065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Warren CM, Otto AK, Walkner MM, Gupta RS. Quality of Life Among Food Allergic Patients and Their Caregivers. Current allergy and asthma reports. 2016;16(5):38. [DOI] [PubMed] [Google Scholar]
- 90.Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA pediatrics. 2013;167(11):1026–31. [DOI] [PubMed] [Google Scholar]
- 91.Bilaver LA, Kester KM, Smith BM, Gupta RS. Socioeconomic Disparities in the Economic Impact of Childhood Food Allergy. Pediatrics. 2016;137(5). [DOI] [PubMed] [Google Scholar]
- 92.Bilaver LA, Chadha AS, Doshi P, O’Dwyer L, Gupta RS. Economic burden of food allergy: A systematic review. Ann Allergy Asthma Immunol. 2019;122(4):373–80.e1. [DOI] [PubMed] [Google Scholar]


