Abstract
Background
Dexanabinol is a synthetic analogue of tetrahydrocannabinol identified as a potential anti-cancer therapeutic by e-Therapeutics PLC. Dexanabinol was selected for further investigation based on its preclinical tumoricidal activity. This phase I dose-escalation trial examined the safety, drug penetration into the central nervous system (CNS), preliminary antitumor activity, and recommended phase II dose.
Methods
Dexanabinol formulated in cremophor/ethanol was administered once weekly via 3-hour intravenous infusion to patients with brain cancer.
Results
A total of 26 patients were dosed once weekly at 2, 4, 8, 16, 24, 28, and 36 mg/kg. Two patients at 36 mg/kg were nonevaluable for dose level confirmation, having withdrawn early for reasons unrelated to study treatment. A recommended phase II dose of dexanabinol was established at 28 mg/kg due to related, reversible adverse events at higher dose levels that required medications for symptomatic relief. The most common drug-related toxicities were the depressed level of consciousness and lightheadedness, diarrhea, itching, fatigue, chest discomfort, and tingling in the mouth. Systemic exposure to dexanabinol (AUC0-t and Cmax) increased from 2 to 36 mg/kg, with dose nonproportionality apparent at the highest dose; dexanabinol was present in appreciable levels in the cerebrospinal fluid (CSF), which implies the possibility of exposure of intracranial tumors to drug. Five of 24 efficacy-evaluable patients (21%) experienced stable disease with a median duration of 2 cycles (28-day cycle) as the best response.
Conclusions
Dexanabinol administered weekly by intravenous infusion was safe and well-tolerated up to 28 mg/kg in brain cancer patients, but has limited antitumor activity in patients with brain cancer.
Keywords: brain cancer, cerebrospinal fluid drug exposure, dexanabinol, NFκB, tetrahydrocannabinol
Key Points.
Weekly intravenous infusion of dexanabinol demonstrated safety.
Dexanabinol was detectable in cerebrospinal fluid at approximately 0.5% of systemic concentrations.
Importance of the Study.
Protection against apoptosis is a key hallmark of cancer, and the ability to selectively induce apoptosis in malignant cells versus normal cells is an attractive strategy for the treatment of brain cancer. This clinical trial evaluated the candidate agent dexanabinol that was identified through network pharmacology for its ability to disrupt survival mechanisms in cancer cells and cross the blood–brain barrier and demonstrated a broad range of tumoricidal activity along with multivalent anti-apoptotic and anti-inflammatory properties in preclinical studies. Results of this study demonstrated the safety and tolerability of dexanabinol in patients with brain cancer. Although single-agent activity was insufficient to warrant further investigation in larger trials, the study incorporated an assessment of dexanabinol drug levels in cerebrospinal fluid that could address a central question in neuro-oncology regarding CNS drug penetration.
Brain metastases are the most common intracranial neoplasm, occurring in 6–14% of cancer patients affecting between 100 000 and 240 000 Americans annually, and are a significant cause of morbidity and mortality.1–3 Among adults, lung cancer accounts for approximately half of these cases. Other metastatic disease to the brain includes breast cancer (~15% of cases), melanoma (~10%), renal cancer, colorectal cancer, lymphoma, and tumors of unknown primary. The incidence of brain metastases has been increasing for a number of reasons, including longer survival of patients with the metastatic primary disease from more effective systemic therapy and enhanced detection. Current treatment modalities include surgery, stereotactic radiosurgery (SRS), whole brain radiation (WBRT), and chemotherapy. For metastases that reoccur, there is no FDA approved treatment besides radiation therapy. Based on various prognostic factors, median survival of patients with brain metastases ranges from 3.4 to 14.8 months.
Primary malignant gliomas, glioblastoma (GBM) in particular, represent the second most common malignant brain cancer. Standard of care radiochemotherapy results in a median survival of 14 months4 and the addition of a tumor-treating fields (TTFields) device increase median survival to approximately 21 months.5 Despite advances in treatment for newly diagnosed glioma patients, essentially all patients will experience disease recurrence. For patients with recurrent disease, conventional chemotherapy is generally ineffective with response rates <20%. Like metastatic cancers to the brain, there is a high frequency of diffuse and leptomeningeal metastases from primary gliomas. Genome-wide studies have confirmed that GBM is a heterogeneous group of diseases that can be sub-classified by shared genetic aberrations.6,7 With dismal prognoses and few effective treatments, clearly new therapies are critically needed for brain cancer patients.
Protection from apoptosis is a key survival factor for cancer cells. Many cancer types are characterised by elevated constitutive NFκB activity, which can provide a survival mechanism for malignant cells via a predominantly anti-apoptotic function.8 Up-regulation of NFĸB activity also plays a key role in the evolution of chemoresistance, with exposure to prior therapy tending to select for NFĸB up-regulation.9,10
Dexanabinol is a synthetic analogue of tetrahydrocannabinol with limited affinity to cannabinoid receptors 1 and 2 and reduced psychotropic activity in comparison to other cannabinoids. It was initially developed as a neuroprotective agent due to its anti-inflammatory activity and its ability to noncompetitively inhibit the N-methyl-D-aspartate (NMDA) receptor,11,12 and has previously undergone clinical trials for traumatic brain injury and patients undergoing coronary artery bypass surgery.13–15 Dexanabinol was subsequently found to exhibit cytotoxicity against tumors in vitro and in vivo. Dexanabinol was further selected for investigation as a potential antitumor agent based on its ability to inhibit NFκB nuclear translocation and the phosphorylation and degradation of the inhibitor of NFκB (IκB), resulting in a reduction in NFκB transcriptional activity and the accumulation of the mRNA of its target genes: TNFα and interleukin 6 (IL-6).16 Dexanabinol can directly block the action of TNFα at the posttranscriptional level as well,17 and reduce the secretion of Prostaglandin E2 (PGE2) by regulating the gene expression of the enzyme cyclooxygenase-2 (COX-2).18 COX-2 expression is up-regulated in tissue samples from a wide variety of solid tumours19 and its inhibition in tumor and stromal cells can lead to anti-proliferative and pro-apoptotic actions within the tumour.20
Taken together, targeted induction of apoptosis in cancer cells versus normal cells provides an attractive strategy for the treatment of brain cancer, a pernicious disease with debilitating neurological side effects, and poor prognosis. Here, we report the results of a phase I dose-escalation trial of dexanabinol administered via 3-hour weekly intravenous infusion in adult patients with brain cancer.
Methods
Patient Eligibility
Eligible patients were ≥18 years old with a histologically confirmed diagnosis of brain cancer (eg, glioblastoma, anaplastic astrocytoma, anaplastic oligodendroglioma, anaplastic mixed oligoastrocytoma, low grade gliomas, brain metastases, meningiomas, or leptomeningeal metastases) having failed prior standard therapy or, in the case of meningioma, had no other standard therapy option. Patients had Karnofsky performance status (KPS) scores ≥60%, a life expectancy of ≥8 weeks, and adequate bone marrow (absolute neutrophil count ≥1.5 × 109/L, platelet count 100 × 109/L, hemoglobin ≥9.0 g/dL, white blood cell count ≥3.0 × 109/L), renal (serum creatinine ≤1.5 × institution's upper limit of normal [ULN], estimated glomerular filtration rate >50 mL/min), liver (AST/SGOT and ALT/SGPT ≤2.5 × ULN, total bilirubin ≤1.5 × ULN, alkaline phosphatase ≤2.5 × ULN unless considered tumor-related), and coagulation (INR ≤1.4, PT/aPTT ≤1.2 × ULN) functions. Women of child-bearing potential and men with partners of child-bearing potential agreed to use adequate contraception while on the study. Patients were required to have recovered from acute toxic effects of prior therapy and not to have received investigational agents within 28 days of study entry; cytotoxic therapy within 28 days (42 days if nitrosourea, 23 days if temozolomide, 21 days if procarbazine, and 14 days if vincristine); noncytotoxic agents within 7 days, or surgery within 4 weeks. Patients were excluded if they had a history of allergic reactions to medicines containing polyoxyethylated castor oil uncontrolled with premedications; a severe or uncontrolled concurrent medical disorder; impaired cardiac function; were on enzyme-inducing anti-epileptic drugs; or were pregnant or nursing females.
Approval by the UC San Diego Human Research Protections Program (#111827) was obtained and the study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines. Patients provided written informed consent. The clinical trial was registered on ClinicalTrials.gov as NCT 01654497.
Treatment Regimen and Dose Escalation
Dexanabinol formulated in cremophor/ethanol and diluted into saline was administered intravenously over 3 h once weekly during each cycle of 28 days. Planned dose levels were 2, 4, 8, 16, 24, 28, 36, 40, and 44 mg/kg. Intra-patient dose escalation was not allowed. Cohort expansion occurred in the 36 mg/kg dose level.
Definition of DLT and MTD
Dose escalation followed a standard 3 + 3 design and was also guided by an assessment of all grade toxicities and trends in adverse events seen in subsequent dosing cycles. Cohort expansion to 6 patients was required if one dose-limiting toxicity (DLT) was reported, and dose escalation would stop if 2 DLTs were observed in those 6 patients. DLT was defined as any possible drug-related, clinically relevant, grade 3 or 4 nonhematologic toxicity (except alopecia; unpremedicated nausea/vomiting; or nausea, vomiting, or diarrhea lasting ≤24 h with standard prophylaxis and/or treatment), grade 4 diarrhea and vomiting of any duration, grade 3 febrile neutropenia, grade 4 febrile neutropenia of any duration, grade 4 neutropenia lasting >5 days, grade 4 thrombocytopenia or thrombocytopenia with clinically significant bleeding, grade 4 anemia of any duration, any clinically significant toxicity that precludes administration of the next scheduled dose beyond 7 days, or dose reduction for any reason. The maximum tolerated dose (MTD) was defined as the highest dose tested in which fewer than 33% of patients experienced DLT attributable to the study drug. The recommended phase II dose was defined as a dose equal to or below the MTD and would take into account any cumulative toxicity and review of the study data as well as data from the concurrent study of dexanabinol in patients with solid tumor (ETS2101-001: NCT01489826).
Safety
Safety evaluations of hematology and chemistry were conducted weekly in cycles 1 and 2 and every 2 weeks thereafter. Physical examinations were performed on days 1 and 8 in cycles 1 and 2, then days 1 and 15 thereafter. Vital signs were collected weekly and ECG was performed predose and within 1 h postdose. Performance status assessment, neurological exam, and urinalysis occurred monthly. Toxicities were assessed using NCI CTCAE version 4.03.
Pharmacokinetic Analysis
Pharmacokinetic (PK) data were obtained to guide the optimal dose of dexanabinol. Blood samples (5 mL) were collected before the dexanabinol dose and at 0.5, 1, 2, and 4 h after the end of infusion on day 1 of Cycle 1, within 30 min after the end of infusion on day 8 of Cycle 1, and within 30 min after the end of infusion on day 1 of Cycle 2. Cerebrospinal fluid (CSF) samples (3 mL) were also collected via lumbar puncture within 30 min after the end of infusions on day 8 of Cycle 1 and day 1 of Cycle 2.
Dexanabinol plasma and CSF concentrations were determined using a validated method of high pressure liquid chromatography with tandem mass spectrometry at POOL Laboratories (Canterbury, Kent, United Kingdom). Noncompartmental analysis with a serial sampling design was used to calculate the key PK parameters using PKSolver 2.0 (China Pharmaceutical University).
Tumor Response
Tumor response was assessed by MRI after every 2 treatment cycles, or earlier if clinically indicated, according to the Response Assessment in Neuro-Oncology (RANO) criteria.
Results
Patient Characteristics
Between May 2012 and May 2015, 34 brain cancer patients were enrolled onto the study at UC San Diego (Table 1). 26 patients received at least one dose of dexanabinol (8 were excluded: 5 noneligible, 3 patient choice). Patients were administered dexanabinol once weekly in sequential dose cohorts at 2 mg/kg (n = 3), 4 mg/kg (n = 3), 8 mg/kg (n = 3), 16 mg/kg (n = 3), 24 mg/kg (n = 3), 28 mg/kg (n = 3) and 36 mg/kg (n = 8). Dose cohort expansion occurred at the 36 mg/kg level; 2 patients were replaced at this dose level due to noncompletion of at least 3 doses, giving 6 efficacy-evaluable patients at this dose.
Table 1.
Baseline Characteristics of All Treated Patients (N = 26)
| Characteristic | No. of Patients | % |
|---|---|---|
| Age, years | ||
| Median | 54.5 | |
| Range | 25–79 | |
| Gender | ||
| Female | 6 | 23.1 |
| Male | 20 | 76.9 |
| Racial origin | ||
| American Indian or Alaskan Native | 1 | 3.8 |
| Asian | 2 | 7.7 |
| Black or African American | 1 | 3.8 |
| White | 21 | 80.8 |
| More than one | 1 | 3.8 |
| Pathology | ||
| Anaplastic astrocytoma | 6 | 23.1 |
| Anaplastic meningioma | 1 | 3.9 |
| Anaplastic oligodendroglioma | 2 | 7.7 |
| Brain metastases | 2 | 7.7 |
| Glioblastoma | 12 | 46.2 |
| Meningioma | 3 | 11.5 |
| Karnofsky performance status | ||
| 90 | 5 | 19.2 |
| 80 | 5 | 19.2 |
| 70 | 15 | 57.7 |
| 60 | 1 | 3.8 |
| No. of prior regimens | ||
| Median | 2 | |
| Range | 1–6 |
Safety and Tolerability
No DLTs occurred. Toxicities were generally mild. The most common adverse events attributed to dexanabinol were hypokalemia, depressed level of consciousness, diarrhea, fatigue, lightheadedness, pruritus, alanine aminotransferase increased, and hypophosphatemia. All but one patient experienced one or more adverse events during the trial. Grade 3 treatment-related adverse events included depressed level of consciousness (n = 2), hypocalcemia (n = 1), and hypophosphatemia (n = 1). No Grade 4 or 5 adverse events occurred. Table 2 summarizes the number of patients with treatment-related toxicities by dose level and CTCAE grade.
Table 2.
Number of Patients with Treatment-related AEs, by Dose Level of Dexanabinol
| CTCAE Grade | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 mg/kg (n = 3) |
4 mg/kg (n = 3) |
8 mg/kg (n = 3) |
16 mg/kg (n = 3) |
24 mg/kg (n = 3) |
28 mg/kg (n = 3) |
36 mg/kg (n = 8) |
Total Patients (n = 26) |
||||||||||||||||
| Adverse event | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | No. | % |
| Cardiac | |||||||||||||||||||||||
| Chest pain | 1 | 1 | 3.8 | ||||||||||||||||||||
| Prolonged QTc | 1 | 1 | 3.8 | ||||||||||||||||||||
| PTT prolonged, intermittent | 1 | 1 | 3.8 | ||||||||||||||||||||
| GI disorders | |||||||||||||||||||||||
| Diarrhea | 1 | 1 | 2 | 4 | 15.4 | ||||||||||||||||||
| GERD | 1 | 1 | 3.8 | ||||||||||||||||||||
| Oral dysesthesia | 1 | 1 | 3.8 | ||||||||||||||||||||
| Vomiting | 2 | 2 | 7.7 | ||||||||||||||||||||
| General disorders and administration site conditions | |||||||||||||||||||||||
| Fatigue | 1 | 2 | 1 | ||||||||||||||||||||
| Infusion-related reaction | 1 | 1 | 3.8 | ||||||||||||||||||||
| Infections and infestations | |||||||||||||||||||||||
| Thrush | 1 | 1 | 3.8 | ||||||||||||||||||||
| Investigations | |||||||||||||||||||||||
| ALT increased | 3 | 1 | 4 | 15.4 | |||||||||||||||||||
| Creatinine increased | 1 | 1 | 3.8 | ||||||||||||||||||||
| PTT prolonged, intermittent | 1 | 1 | 3.8 | ||||||||||||||||||||
| Metabolic and nutritional disorders | |||||||||||||||||||||||
| ALT increased | 3 | 1 | 4 | 15.4 | |||||||||||||||||||
| Creatinine increased | 1 | 1 | 3.8 | ||||||||||||||||||||
| Creatinine increased, intermittent | 1 | 1 | 3.8 | ||||||||||||||||||||
| Hypoalbuminemia | 1 | 1 | 3.8 | ||||||||||||||||||||
| Hypocalcemia | 1 | 1 | 3.8 | ||||||||||||||||||||
| Hypokalemia | 1 | 1 | 2 | 1 | 5 | 19.2 | |||||||||||||||||
| Hypokalemia, intermittent | 1 | 1 | 3.8 | ||||||||||||||||||||
| Hypoglycemia, intermittent | 1 | 1 | 3.8 | ||||||||||||||||||||
| Hypomagnesemia | 1 | 1 | 3.8 | ||||||||||||||||||||
| Hyponatremia | 1 | 1 | 3.8 | ||||||||||||||||||||
| Hypophosphatemia | 1 | 2 | 1 | 4 | 15.4 | ||||||||||||||||||
| Musculoskeletal and connective tissue disorders | |||||||||||||||||||||||
| Steroid myopathy | 1 | 1 | 3.8 | ||||||||||||||||||||
| Nervous system disorders | |||||||||||||||||||||||
| Depressed level of consciousness | 3 | 2 | 5 | 19.2 | |||||||||||||||||||
| Dizziness | 1 | 1 | 3.8 | ||||||||||||||||||||
| Gait disturbance | 1 | 1 | 3.8 | ||||||||||||||||||||
| Headache | 2 | 2 | 7.7 | ||||||||||||||||||||
| Lightheadedness | 2 | 1 | 3 | 11.5 | |||||||||||||||||||
| Psychiatric disorders | |||||||||||||||||||||||
| Confusion | 1 | 1 | 3.8 | ||||||||||||||||||||
| Renal and urinary disorders | |||||||||||||||||||||||
| Hematuria | 1 | 1 | 3.8 | ||||||||||||||||||||
| Urinary tract infection | 1 | 1 | 3.8 | ||||||||||||||||||||
| Skin and subcutaneous tissue disorders | |||||||||||||||||||||||
| Pruritus | 2 | 2 | 7.7 | ||||||||||||||||||||
Abbreviations: AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events.
Most adverse events occurred during the first cycle of investigational treatment but could also be observed up through the fourth cycle. Depressed level of consciousness was observed in 5 patients at the highest dose level of 36 mg/kg and considered to be drug-related. Although all patients recovered during the same study visit following treatment with naloxone, it was decided this dose level should not be further explored. Mild lightheadedness and fatigue occurred at lower doses and did not require any intervention.
In reviewing data from all cohorts of the current study plus those of another active study in parallel (ETS2101-001: NCT01489826), depressed level of consciousness, pruritus, and diarrhea were clear drug-related adverse events that were reversible but required medications for symptomatic relief as needed at higher dose levels, particularly at 36 mg/kg dose level. The recommended phase II dose was, therefore, defined as 28 mg/kg.
Pharmacokinetics of Dexanabinol
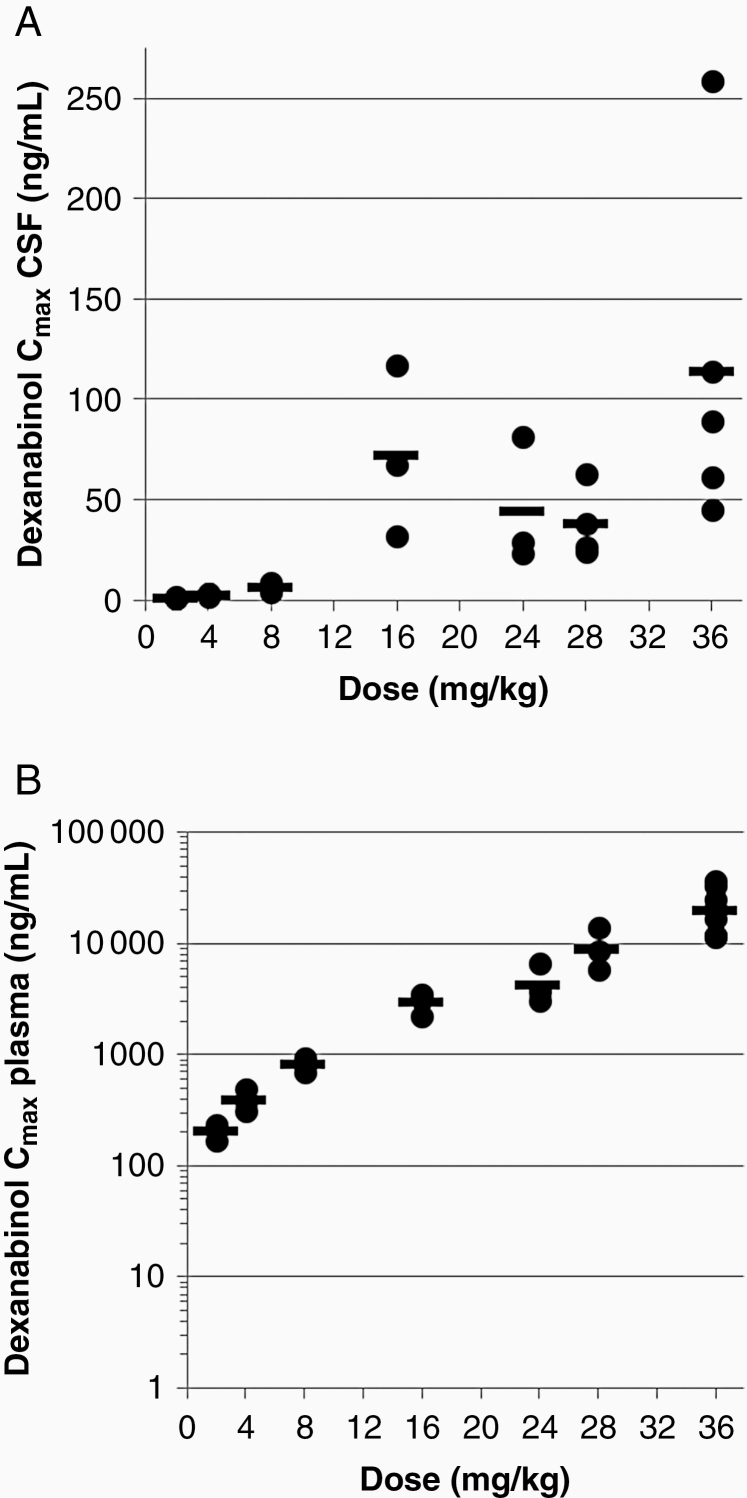
Following a 3-hour intravenous infusion of dexanabinol, the geometric mean maximum concentrations of dexanabinol (Cmax) of 204 to 20, 100 ng/mL in plasma and of 1.1 to 114 ng/mL in CSF were reached (tmax) at 0.5 h post end of infusion (Table 3; Figure 1). Systemic exposure to dexanabinol (AUC0-4h and Cmax) increased greater than dose proportionately. There was no excessive accumulation of dexanabinol in plasma following weekly doses, consistent with the short half-life relative to the dosing interval. Dexanabinol concentrations in CSF were approximately 0.5% of systemic concentrations.
Table 3.
Key Mean Pharmacokinetic Parameters of Dexanabinol
| Dose (mg/kg) |
Dose Fold Increase | Plasma |
t
1/2 (h)b |
CSF | ||||
|---|---|---|---|---|---|---|---|---|
|
C
max (ng/mL)a |
C max Fold Increase |
t
max (h)b |
AUC0-4h (ng.h/mL)a |
AUC0-4h Fold Increase |
C
max (ng/mL)c |
|||
| 2 | 1.0 | 204 (15.3) | 1.0 | 0.5 | 475 (24.1) | 1.0 | 2.2 | 1.1 (30.3) |
| 4 | 2.0 | 387 (23.1) | 1.9 | 1.0 | 881 (11.6) | 1.9 | 1.7 | 2.5 (34.2) |
| 8 | 2.0 | 813 (14.0) | 2.1 | 0.5 | 1890 (19.6) | 2.1 | 2.3 | 6.3 (41.5) |
| 16 | 2.0 | 2885 (22.6) | 3.5 | 0.5 | 5750 (29.3) | 3.0 | 1.8 | 72 (58.9) |
| 24 | 1.5 | 4226 (45.1) | 1.5 | 0.5 | 10 800 (18.3) | 1.9 | 3.4 | 44 (71.6) |
| 28 | 1.2 | 7018 (26.8) | 1.7 | 0.5 | 15 400 (17.1) | 1.4 | 1.7 | 38 (47.8) |
| 36 | 1.3 | 20 139 (45.5) | 2.9 | 0.5 | 38 300 (35.5) | 2.5 | 2.0 | 114 (74.9) |
Abbreviations: AUC, area under the concentration-time curve for 0 to 4 h after end of infusion; Cmax, maximum measured concentration; CV, coefficient of variation; tmax, time from end of infusion to maximum concentration.
aGeometric means (CV%) reported.
bMedian.
cArithmetic means (CV%) reported.
Figure 1.
Maximum concentration (Cmax) of the dexanabinol achieved in CSF (A) and plasma (B) by dose cohort.
Antitumor Activity
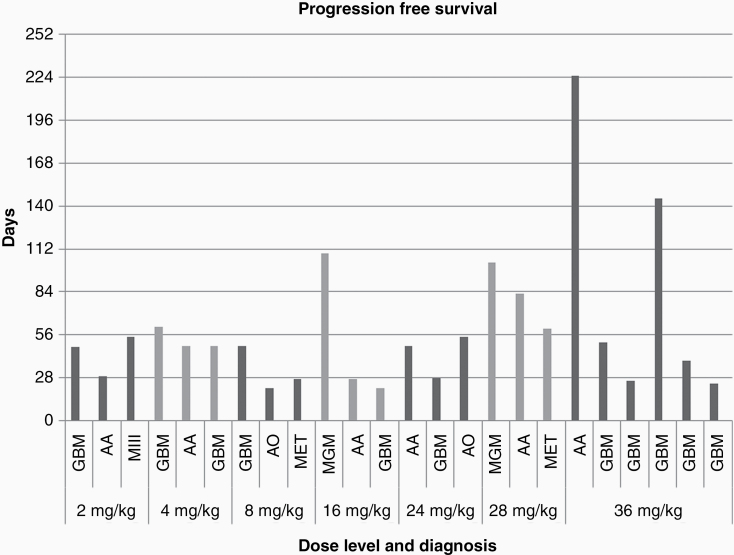
24 patients were evaluated for response. Five patients exhibited stable disease (21%) at doses of 16 mg/kg or above, with 4 patients having stable disease for greater than 4 cycles before disease progression (Table 4). These patients included those with meningioma (n = 2), anaplastic astrocytoma (n = 2), and glioblastoma (n = 1). Nineteen patients exhibited progressive disease only and no complete or partial responses were observed. Overall, median progression free survival (PFS) was 49 days (95% CI: 48–79 days). The PFS data are summarized according to the different cancer types (Figure 2).
Table 4.
Number of Cycles, PFS and Best Response by Dose Level (N = 24)
| Dose (mg/kg) |
No. Cycles | PFS (Days) | Best Response | No. Evaluable | |||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | CR | PR | SD | PD | ||
| 2 | 1.75 | 1–2 | 48 | 29–55 | 0 | 0 | 0 | 3 | 3 |
| 4 | 1.75 | 1.75–2.25 | 60 | 49–61 | 0 | 0 | 0 | 3 | 3 |
| 8 | 1 | 1–1.75 | 27 | 21–49 | 0 | 0 | 0 | 3 | 3 |
| 16 | 1 | 1–4 | 27 | 21–109 | 0 | 0 | 1 | 2 | 3 |
| 24 | 1.75 | 1–2 | 49 | 28–55 | 0 | 0 | 0 | 3 | 3 |
| 28 | 3 | 2.25–4 | 79 | 60–103 | 0 | 0 | 2 | 1 | 3 |
| 36 | 1.75 | 1–8 | 51 | 24–225 | 0 | 0 | 2 | 4 | 6 |
Abbreviations: CR, complete response; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
Figure 2.
Progression free survival (days) by dose level and diagnosis. Abbreviations: GBM, glioblastoma; AA, anaplastic astrocytoma; MIII, anaplastic meningioma; AO, anaplastic oligodendroglioma; MET, metastasis.
Discussion
Dexanabinol is generally well tolerated, the most common adverse events related to dexanabinol being hypokalemia, depressed level of consciousness, diarrhea, fatigue, lightheadedness, pruritus, increased alanine aminotransferase, and hypophosphatemia. Five out of 8 patients treated at the highest dose level (dexanabinol 36 mg/kg) experienced the depressed level of consciousness. The 2 patients who experienced grade 3 depressed level of consciousness received a higher total dose of dexanabinol based on their body weight, which may have contributed to the severity of the events. Although the events of depressed level of consciousness did not meet protocol-defined dose-limiting toxicity, the high incidence in a small number of patients was concerning, Thus, the recommended phase II dose of dexanabinol was established at 28 mg/kg due to related, reversible adverse events of depressed level of consciousness, pruritus, and diarrhea at higher dose levels that required medications for symptomatic relief, and emergent adverse events in clinical study ETS2101-001.
Dexanabinol was present at appreciable levels in the CSF, albeit at a small fraction of systemic levels. With CSF drug levels serving as a surrogate for potential intracranial tumor drug exposure, it is unlikely that intratumoral drug levels reached therapeutic concentrations. Considering that dexanabinol is very highly bound to plasma proteins, it would have been of interest to further examine the residence time of dexanabinol in CSF; unfortunately, more than half of the patients discontinued study treatment prior to cycle 2 and did not provide additional CSF samples for analyses. A linear pharmacokinetic profile was observed in CSF up to 8 mg/kg dosing and up to 24 mg/kg in blood. The nonlinearity in CSF at higher doses may be related to tumor burden and the degree of enhancement/leakiness of the blood–brain barrier in the tumors, particularly considering the various tumor histologies included in the study.
No objective tumor responses occurred. While the primary purpose of the study was to determine safety, preliminary signs of antitumor activity could have guided the selection of specific tumor histologies for further evaluation. However, the few cases of the stable disease could not necessarily be attributed to the efficacy of dexanabinol since they generally occurred in patients with lower grade histologies.
In conclusion, the first phase I trial of dexanabinol for adults with brain cancer established the safety and tolerability of dexanabinol in this patient population and that dexanabinol crosses the blood–brain barrier. However, due to the lack of antitumor activity observed and its discontinued development by the manufacturer, additional investigation is not forthcoming.
Acknowledgments
We acknowledge the patients that participated in this study. We thank Minya Pu, Emily Pittman, and Karen Messer from UC San Diego Moores Cancer Center for help with statistical analysis. Funding and study drug was provided by e-Therapeutics.
Presented in part at the: 20th Annual Society for Neuro-Oncology Annual Scientific Meeting and Education Day, San Antonio, TX, November 19–22, 2015.
Funding
This investigator-initiated study was supported by research funding and study drug from e-Therapeutics PLC.
Conflict of interest statement. The authors declare no competing interests.
Authorship Statement. Conception and design: S.K., T.M.J. Collection and assembly of data: T.M.J., D.P., L.R., A.N., B.B., S.K. Data analysis and interpretation: T.M.J., D.P., S.K. Manuscript writing: T.M.J., S.K. Final approval of manuscript: All authors.
References
- 1. Lowery FJ, Yu D. Brain metastasis: unique challenges and open opportunities. Biochim Biophys Acta Rev Cancer. 2017;1867(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Norden AD, Wen PY, Kesari S. Brain metastases. Curr Opin Neurol. 2005;18(6):654–661. [DOI] [PubMed] [Google Scholar]
- 3. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5–14. [DOI] [PubMed] [Google Scholar]
- 4. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 5. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897): 1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Network CGAR. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008; 455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bassères DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25(51):6817–6830. [DOI] [PubMed] [Google Scholar]
- 9. Patel NM, Nozaki S, Shortle NH, et al. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-kappaB is enhanced by IkappaBalpha super-repressor and parthenolide. Oncogene. 2000;19(36):4159–4169. [DOI] [PubMed] [Google Scholar]
- 10. Arlt A, Vorndamm J, Breitenbroich M, et al. Inhibition of NF-kappaB sensitizes human pancreatic carcinoma cells to apoptosis induced by etoposide (VP16) or doxorubicin. Oncogene. 2001;20(7):859–868. [DOI] [PubMed] [Google Scholar]
- 11. Eshhar N, Striem S, Kohen R, Tirosh O, Biegon A. Neuroprotective and antioxidant activities of HU-211, a novel NMDA receptor antagonist. Eur J Pharmacol. 1995;283(1-3):19–29. [DOI] [PubMed] [Google Scholar]
- 12. Biegon A, Joseph AB. Development of HU-211 as a neuroprotectant for ischemic brain damage. Neurol Res. 1995;17(4):275–280. [DOI] [PubMed] [Google Scholar]
- 13. Brewster ME, Pop E, Foltz RL, et al. Clinical pharmacokinetics of escalating i.v. doses of dexanabinol (HU-211), a neuroprotectant agent, in normal volunteers. Int J Clin Pharmacol Ther. 1997;35(9):361–365. [PubMed] [Google Scholar]
- 14. Knoller N, Levi L, Shoshan I, et al. Dexanabinol (HU-211) in the treatment of severe closed head injury: a randomized, placebo-controlled, phase II clinical trial. Crit Care Med. 2002;30(3):548–554. [DOI] [PubMed] [Google Scholar]
- 15. Maas AI, Murray G, Henney H III, et al. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006;5(1):38–45. [DOI] [PubMed] [Google Scholar]
- 16. Jüttler E, Potrovita I, Tarabin V, et al. The cannabinoid dexanabinol is an inhibitor of the nuclear factor-kappa B (NF-kappa B). Neuropharmacology. 2004;47(4):580–592. [DOI] [PubMed] [Google Scholar]
- 17. Shohami E, Gallily R, Mechoulam R, Bass R, Ben-Hur T. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-alpha inhibitor and an effective neuroprotectant. J Neuroimmunol. 1997;72(2):169–177. [DOI] [PubMed] [Google Scholar]
- 18. Garzon A, Avraham A, Fink G. . Dexanabinol and Dexanabinol Analogs Regulate Inflammation Related Genes. No. 10/942,504 in States U (ed), 2005. [Google Scholar]
- 19. Koki AT, Masferrer JL. Celecoxib: a specific COX-2 inhibitor with anticancer properties. Cancer Control. 2002;9(2 Suppl):28–35. [DOI] [PubMed] [Google Scholar]
- 20. Mazhar D, Gillmore R, Waxman J. COX and cancer. QJM. 2005;98(10):711–718. [DOI] [PubMed] [Google Scholar]