Abstract
Throughout spermatogenesis, cellular cargoes including haploid spermatids are required to be transported across the seminiferous epithelium, either toward the microtubule (MT) plus (+) end near the basement membrane at stage V, or to the MT minus (−) end near the tubule lumen at stages VI to VIII of the epithelial cycle. Furthermore, preleptotene spermatocytes, differentiated from type B spermatogonia, are transported across the Sertoli cell blood-testis barrier (BTB) to enter the adluminal compartment. Few studies, however, have been conducted to explore the function of MT-dependent motor proteins to support spermatid transport during spermiogenesis. Herein, we examined the role of MT-dependent and microtubule plus (+) end–directed motor protein kinesin 15 (KIF15) in the testis. KIF15 displayed a stage-specific expression across the seminiferous epithelium, associated with MTs, and appeared as aggregates on the MT tracks that aligned perpendicular to the basement membrane and laid across the entire epithelium. KIF15 also tightly associated with apical ectoplasmic specialization, displaying strict stage-specific distribution, apparently to support spermatid transport across the epithelium. We used a loss-of-function approach by RNAi to examine the role of KIF15 in Sertoli cell epithelium in vitro to examine its role in cytoskeletal-dependent Sertoli cell function. It was noted that KIF15 knockdown by RNAi that reduced KIF15 expression by ~70% in Sertoli cells with an established functional tight junction barrier impeded the barrier function. This effect was mediated through remarkable changes in the cytoskeletal organization of MTs, but also actin-, vimentin-, and septin-based cytoskeletons, illustrating that KIF15 exerts its regulatory effects well beyond microtubules.
Keywords: testis, spermatogenesis, microtubule, cytoskeleton, microtubule-dependent motor protein, KIF15, Sertoli cells, blood-testis barrier, cargo transport, spermatid transport
In the mammalian testis, extensive intracellular protein trafficking takes place across the seminiferous epithelium to support cellular events pertinent to the epithelial cycle of spermatogenesis, including the timely transport of numerous cellular cargoes, such as endosomes, residual bodies, phagosomes, miRNAs, food vacuoles, and other biomolecules (1-4). The transport of these cargoes through the seminiferous epithelium is only possible with the involvement of F-actin–dependent (eg, myosin VIIa) (5) or microtubule (MT)-dependent (eg, dynein 1) (6) motor proteins. These motor proteins, in turn, work in concert with the Sertoli cell cytoskeletons that stretch across the entire seminiferous epithelium (3, 7-9). In brief, these 2 cytoskeletons serve as the tracks so that cargoes can be transported to be plus (+) or minus (−) ends of actin or MT filaments, both of which are polarized structures (3, 7). During the epithelial cycle, the most notable track-like structures are conferred by MTs, which are distinctively visible in stages I through XIV, that are aligned perpendicular to the basement membrane and laid across the entire seminiferous epithelium (10). Most of the F-actin cytoskeleton, however, is associated with the apical ectoplasmic specialization (ES) at the Sertoli-spermatid interface, and the basal ES at the Sertoli cell-cell interface at the blood-testis barrier (BTB), with the actin-based tracks notably detected at late stage VIII of the epithelial cycle, which are also aligned perpendicular to the basement membrane (7). MTs are polarized structures with the minus (−) slow-growing end oriented towards the adluminal compartment near the tubule lumen, whereas the plus (+) fast-growing end is oriented towards the basement membrane in the basal compartment at the tunica propria. A recent report has shown that dynein 1 is an MT-dependent minus (−) end motor protein that moves cargoes to the slow-growing minus (−) end of MTs near the tubule lumen in the seminiferous epithelium (6), known as retrograde sliding movement (11, 12). It was shown that dynein 1 was involved in the transport of developing spermatids and other organelles (eg, endosomes, food vacuoles) to the adluminal edge of the epithelium in the apical compartment, and also preleptotene spermatocytes across the BTB (6). When dynein 1 was knocked down by RNA interference (RNAi) and its expression was reduced by ~80%, the transport of elongated spermatids was grossly affected, since step-19 spermatids were trapped deep inside the seminiferous epithelium in stage IX to XII tubules failing to reach the adluminal edge of the epithelium when the release of spermatids had already occurred at stage VIII of the cycle (6). Furthermore, the organization of actin and microtubule cytoskeletons was also disrupted following dynein 1 knockdown (KD), since pertinent cellular events of intracellular protein trafficking that relied on dynein 1 motor protein to support cytoskeletal homeostasis were also compromised, as noted in biochemical assays that monitored the polymerization kinetics of both actin and microtubules (6). However, it remains to be examined how cellular cargoes (such as haploid spermatids, most notably elongating spermatids at stage V of the epithelial cycle, and residual bodies and phagosomes at stage VIII-IX of the cycle) are transported from the apical compartment, across the seminiferous epithelium, to the base of the epithelium near the basement membrane, which would require the MT-dependent fast-growing (+) end–dependent motor proteins, such as kinesins.
Kinesin is comprised of a group of structurally and functionally related motor proteins that use MT tracks in anterograde movement to transport cargoes toward the MT plus (+) ends, such as near the basement membrane in the seminiferous epithelium in the testis (13-15). In brief, kinesin transports cargoes away from the cell center, usually to cell peripheries. We sought herein to perform a detailed functional analysis on a selected kinesin motor protein, namely kinesin-15 (KIF15), which was shown to be highly expressed by Sertoli cells in adult rat testes in pilot experiments. Studies have shown that loss-of-function mutations in KIF15 lead to Braddock-Carey syndrome (16), characterized by congenital thrombocytopenia, microcephaly, and agenesis of the corpus callosum in humans. Braddock-Carey syndrome patients are typified by deletions of 21q22 and the RUNX1 gene and closely resemble patients with Down syndrome (17). Other studies have shown that KIF15 is a novel regulator of the endocytic trafficking of α2ß1-integrin (18), one of the most important collagen-binding receptors, also involved in pancreatic cancer proliferation (19), possibly through its role in regulating mitotic division. In HeLa cells, KIF15 is known to have redundant functions with kinesin-5 (20). As such KIF15 is a crucial motor protein in supporting multiple functions in the mammalian body. However, its function in the testis remains unexplored. Herein, we sought to examine the function of KIF15 in Sertoli cells, and its role in the homeostasis of microtubule-, actin-, vimentin-, and septin-based cytoskeletons in the testis.
Materials and Methods
Animals and Ethics Statement
Male Sprague-Dawley pups at 16 to 18 days of age in groups of 10 pups with a foster mother per group, and adult male Sprague-Dawley rats of 280 to 300 g body weight were purchased from Charles River Laboratories (Kingston, NY). Rats were housed at the Rockefeller University Comparative Bioscience Center (CBC) according to the applicable portions of the Animal Welfare Act and guidelines in the Department of Health and Human Services publication Guide for the Care and Use of Laboratory Animals. All animals were kept at the CBC in a temperature controlled environment at 20 ± 1 °C with a daily schedule of 12 hours of light and 12 hours of darkness. All rats had free access to water and standard rat chow. All experiments reported here involving the use of all animals were approved by the Rockefeller University Institutional Animal Care and Use Committee with Protocol Numbers 18-043H. All rats were euthanized by CO2 asphyxiation using a slow (20%-30%/min) displacement of chamber air from a compressed carbon dioxide tank via a gas regulator that regulated the airflow into an euthanasia chamber which was built and approved by the Rockefeller University Laboratory Safety and Environmental Health.
Antibodies
Antibodies used for different experiments reported herein were obtained commercially, unless otherwise specified, and had been characterized earlier in our laboratory, as listed in Table S1 (21), including their working dilutions for different applications, such as immunoblotting (IB) and immunofluorescence analysis (IF), and their Resource Identification Initiative (RRID) numbers. Table S1 is located in a digital research materials repository (21). These antibodies also cross-reacted with the corresponding proteins in rats as noted by the manufacturers. Antibodies from Proteintech Group (Rosemont, IL) included: KIF15 (22), SEPT7 (23), CAMSAP2 (24), MARK4 (25), and palladin (26). Antibodies from Abcam (Cambridge, MA) included: α-tubulin (27), β-tubulin (28), detyrosinated α-tubulin (29), acetylated α-tubulin (30), formin1 (31), MAP1a (32), and GAPDH (33). The antibody from BD Biosciences (San Jose, CA) was Eps8 (34). Antibodies from Santa Cruz Biotechnology (Dallas, TX) included: ß-actin (35), β1-integrin (36), Dync1h1 (37), EB1 (38), myosin VIIA (39), N-cadherin (40), N-WASP (41), vimentin (42), Goat IgG-HRP (43), and rabbit IgG-HRP (44). Antibodies from Thermo Fisher Scientific (Fair Lawn, NJ) included: β-catenin (mouse) (45), ß-catenin (rabbit) (46), JAM-A (47), N-cadherin (48), occludin (49), ZO-1 (50), mouse IgG-HRP (51), goat IgG-Alexa Fluor 488 (52), goat IgG-Alexa Fluor 555 (53), mouse IgG-Alexa Fluor 488 (54), mouse IgG-Alexa Fluor 555 (55), rabbit IgG-Alexa Fluor 488 (56), and rabbit IgG-Alexa Fluor 555 (57). Antibody from Sigma-Aldrich (Allentown, PA) included: tyrosinated α-tubulin (58) and Arp3 (59).
Isolation and Primary Sertoli Cell Cultures
Sertoli cells were isolated from 20-day-old rat testes for primary cultures as previously described (60). Sertoli cells from these testes were differentiated and ceased to divide mitotically when cultured in serum-free chemically defined F12/DMEM medium, and they were functionally and morphologically indifferent from Sertoli cells isolated from adult rat testes as reported earlier (61, 62). Freshly isolated Sertoli cells were plated on Matrigel (BD Biosciences, San Jose, CA)-coated culture dishes (either 6-, 12-, or 24-well dishes), coverslips (to be placed in 12-well dishes), and bicameral units (Millipore, Burlington, MA; to be placed in 24-well dishes). Cell density in culture dishes, coverslips, and bicameral units was at 0.3 to 0.4, 0.03 to 0.04, and 1.0 × 106 cells/cm2, respectively. These cell densities were selected based on pilot (or earlier) experiments from our laboratory (63, 64). As such, sufficient proteins (or mRNAs) were obtained from lysates for various experiments (eg, IB, tight junction [TJ]-barrier functional assay, biochemical assays) to investigate changes in phenotypes at the Sertoli cell-cell interface or cytoskeletal organization, changes in TJ-permeability barrier function, and actin or MT polymerization assays. In order to obtain highly purified Sertoli cells of ~98% purity, residual germ cells were lysed by a hypotonic treatment using 20mM Tris (pH 7.4 at 22 °C for 2 minutes) on day 2 after cell plating as described earlier (65). Cells were incubated in serum-free DMEM/F-12 (Sigma-Aldrich, St. Louis, MO) medium supplemented with growth factors and gentamicin in a humidified atmosphere of 95% air–5% CO2 (v/v) at 35 °C (60). For 6- and 12-well dishes to be used for biochemical assays or for IB (immunoblot analysis), each well contained 5 mL and 3 mL F12/DMEM medium, respectively. For IF (immunofluorescence microscopy analysis), 2 mL F12/DMEM were used per well in 12-well dishes with Sertoli cells cultured on a round coverslip in each well. For bicameral units placed in 24-well dishes, the apical and the basal chamber contained 0.5 mL F12/DMEM medium. All media were supplemented with growth factors: bovine insulin (10 µg/mL), human transferrin (5 µg/mL), EGF (2.5 ng/mL), bacitracin (5 µg/mL), and gentamicin (20 µg/mL). These Sertoli cell cultures were used for experiments on day 2 or 3 with an established functional TJ-permeability barrier, and ultrastructures of TJ, basal ES, gap junction, and desmosome that mimicked the Sertoli cell BTB in vivo were also detected by electron microscopy (66, 67). Sertoli cell cultures were >98% pure with negligible contamination of germ cells, Leydig cells, and/or peritubular myoid cells as confirmed by using corresponding primer pairs for specific cell markers by polymerase chain rection (PCR) (68).
KIF15 Knockdown (KD) by RNAi in Sertoli Cells Cultured In Vitro
KIF15 KD was performed by RNAi in Sertoli cell epithelium as noted in corresponding regimens in different experiments that assessed effects of KIF15 KD on Sertoli cell function. In brief, Sertoli cells cultured on day 2 (for IB, IF, and polymerization/spin-down assay) or day 3 (for transepithelial electrical resistance [TER], measurement to monitor TJ-barrier function) were transfected with specific KIF15 small interfering RNA (siRNA) duplexes vs non-targeting negative control (Ctrl RNAi) siRNA duplexes (Table 1). The siRNA duplexes were obtained from Dharmacon (Lafayette, CO). The siRNA duplexes were used at 100nM (for IB, IF, and polymerization/spin-down assay) with RNAiMAX (Life Technologies, Carlsbad, CA) as a transfection reagent. Transfection was done twice (or once, depending on the type of experiments, see regimen in a corresponding experiment), with each transfection lasting for 16 hour, cells were rinsed 3 times in-between with F12/DMEM to remove transfection reagents and cells were then allowed to recover for an 8-hour period based on the pilot experiment, which was necessary to obtain ~70% KIF15 expression knockdown. The Sertoli cells were then used for corresponding experiments as noted in different regimens outlined in different experiments. For cultures to be used for IF, cells were co-transfected with 1nM siGLO red transfection indicator (Dharmacon) to track successful transfection. In each experiment, replicates or triplicates of cultures were used for each treatment and control groups. Each experiment was derived from n = 3 independent experiments using different batches of Sertoli cells, excluding pilot experiments, which were used to establish the optimal experimental conditions. All samples within an experiment, including both treatment and control groups, were analyzed simultaneously, for IB, IF, or biochemical assays, to avoid intra-experimental variations.
Table 1.
siRNA Duplexes Used for RNAi Experiments
| Gene | Product | Catalog no. | Target sequence (5′ – 3′) |
|---|---|---|---|
| KIF15 | ON-TARGETplus Rat Kif15 (353302) siRNA-SMARTpool | L-097130-02 | GCGAGGAAGUUGAGCGAAC |
| GGACAAUGCCAGAUUAGAA | |||
| GCACAUUCAAAGAGAGUCA | |||
| CUUAAAGGCUCUUGAAACU | |||
| Non-Target | ON-TARGETplus Non-targeting Pool siRNA duplexes | D-001810–10 | UGGUUUACAUGUCGACUAA |
| UGGUUUACAUGUUGUGUGA | |||
| UGGUUUACAUGUUUUCUGA | |||
| UGGUUUACAUGUUUUCCUA |
Assessment of Sertoli Cell TJ-Permeability Barrier Function In Vitro
Sertoli cells cultured in vitro on Matrigel-coated bicameral units at 1.0 × 106 cells/cm2 were used to measure TER in ohms (Ω) across the cell epithelium to assess TJ-barrier function as described (60, 69). In brief, each bicameral unit was placed inside the well of a 24-well dish with 0.5 mL F12/DMEM each in the apical and the basal compartments. Transfection of Sertoli cells with KIF15 (vs control) siRNA duplexes was performed on day 3 and day 4 at 100nM for 16 hours twice (or transfected once for 24-hour), with an 8-hour interval in F12/DMEM for recovery, and Sertoli cell TJ-permeability was monitored daily by quantifying TER across the cell epithelium (60). In each experiment, each treatment vs control group had quadruple bicameral units. Each experiment was repeated with a total of n = 3 to 4 independent experiments using different batches of Sertoli cells, excluding pilot experiments.
BTB Integrity Assay In Vitro
Besides the physiological assay based on TER measurement, the Sertoli cell BTB integrity in vitro was assessed by using a membrane-impermeable biotin, EZ-LinkSulfo-NHS-LC-Biotin (Thermo Fisher Scientific, Waltham, MA) reagent. In brief, freshly isolated Sertoli cells were plated on Matrigel-coated round coverslips (placed in 12-well dishes) at a density of 0.03 to 0.04 × 106 cells/cm2 so that the cell-cell interface in the epithelium was distinctively visible by fluorescence microscopy. Thereafter, Sertoli cells were transfected with KIF15 RNAi vs non-targeting negative control (Ctrl RNAi) siRNA duplexes as described above. These cells were rinsed 3 times to remove siRNA duplexes and transfection medium. EZ-Link Sulfo-NHS-LC-Biotin, freshly diluted in 500 μL phosphate-buffered saline (PBS) (10mM sodium phosphate, 0.15M NaCl, pH 7.4 at 22 °C) at 0.1 mg/mL containing 0.1mM CaCl2/well was then added to the Sertoli cells in each culture well for 30 minutes to allow biotinylation. Thereafter, Sertoli cells were washed 3 times with PBS containing 100mM glycine (10mM sodium phosphate, 100mM glycine, 0.15M NaCl, pH 7.4 at 22° C) to quench and remove excess biotin reagent. Sertoli cells were then fixed in 4% paraformaldehyde (PFA) in PBS for 10 minutes, to be followed by an incubation with Alexa Fluor 488-streptavidin (Life Technologies, 1:250) for 30 minutes, and also co-stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma) to visualize cell nuclei. Samples were mounted in Prolong Gold Antifade reagent (Invitrogen, Life Technologies) for examination and image acquisition by fluorescence microscopy. Each experiment was performed with n = 3 independent assays, which yielded similar results.
Immunofluorescence Staining, F-Actin Staining, and Fluorescence Image Analysis
IF was performed using Sertoli cells cultured on coverslips or frozen cross-sections of testes at 7 μm in a cryostat at −22 °C. To visualize KIF15 or septin7 and other targeted proteins in testes, IF was fixed in modified Davidson’s fixative and paraffin embedded. For KIF15 and septin7 cell staining, Sertoli cells were cultured on coverslips for IF and fixed in ice-cold methanol for 5 minutes, and permeabilized in 0.1% Triton X-100 for 5 to 10 minutes. For other target proteins in Sertoli cells, either 4% PFA or ice-cold methanol was used for cell fixation. Paraffin sections were deparaffinized, rehydrated, and then subjected to antigen retrieval. Tissues or Sertoli cells were then blocked in 10% goat serum (volume/volume) or 5% bovine serum albumin (weight/volume) in PBS. Thereafter, samples were incubated with a specific primary and the corresponding secondary antibodies (Table S1) (21) and co-stained with DAPI to visualize cell nuclei. Slides were mounted in Prolong Gold Antifade reagent. For F-actin staining, frozen sections or Sertoli cells (fixed in 4% PFA) were incubated with phalloidin-conjugated Alexa Fluor 488 (Invitrogen). All images were examined using a Nikon Eclipse 90i Fluorescence Microscope system equipped with Nikon Ds-Qi1Mc or DsFi1 digital camera and images were acquired using Nikon NIS Elements AR 3.2 software package (Nikon, Tokyo, Japan). Image overlays were performed using Adobe Photoshop CS6 (Adobe, San Jose, CA). Fluorescence intensity was analyzed by ImageJ 1.45s (National Institutes of Health, Bethesda, MD) or Nikon NIS Elements AR (Version 3.2) software package. All samples, either Sertoli cells or testis cross-sections, in an experiment including both treatment and control groups were processed and analyzed in a single experimental session to avoid intra-experimental variations. Data shown herein were representative micrographs from a single experiment but each study had n = 3 independent experiments, which yielded similar results. For fluorescence intensity or distribution analysis in Sertoli cells, at least 200 cells were randomly selected and examined in both experimental and control groups with a total n = 3 experiments.
RNA Extraction and RT-PCR
Total RNA was isolated from rat testes, Sertoli cells, germ cells, and kidney using Trizol reagent (Life Technologies) (69). In brief, 2 µg total RNA from each sample was reverse transcribed by Moloney murine leukemia virus reverse transcriptase (M-MLV; Promega, Madison, WI) according to manufacturer’s instructions to obtain cDNAs, which served as templates for subsequent PCR using primer pairs specific to KIF6, KIF9, KIF15, KIF18A, KIF18B, KIF20A, KIF20B, and KIFC1, with S16 serving as the control (Table 2). PCR products were verified by direct DNA sequencing at Genewiz (South Plainfield, NJ) to confirm their identity. Each RT-PCR experiment was performed with n = 3 independent experiments using different batches of Sertoli cells or germ cells vs rat testes and kidneys (positive control) from n = 3 male rats.
Table 2.
Primers Used for RT-PCR
| Gene name | Accession number | Orientation | Nucleotide sequence | Nucleotide position | Product length (bp) | Annealing temperature (oC) |
|---|---|---|---|---|---|---|
| KIF6 | AY035403.1 | Sense | 5′-CGCCTACAGAAGGAGATTGC-3′ | 1085–1104 | 140 | 56 |
| Antisense | 5′-TCTGGATCTTGGTCCTCCAC-3′ | 1205–1224 | ||||
| KIF9 | NM_001192000.1 | Sense | 5′-ACCAGTTCAGGGTGGTTCTG-3′ | 1268–1287 | 102 | 54 |
| Antisense | 5′-CAGAAATGGCTGCAAAGTCA-3′ | 1350–1369 | ||||
| KIF15 | NM_181635.2 | Sense | 5′-GCATCGGTCGGACTGTATTT-3′ | 541–560 | 103 | 57 |
| Antisense | 5′-AGACCTGGTAGGCTTCAGCA-3′ | 624–643 | ||||
| KIF18A | NM_001137642.1 | Sense | 5′-AGCAAGAGCGTCCTCACATT-3′ | 1941–1960 | 172 | 56 |
| Antisense | 5′-CGGTAGTGGTTCAGGCAAAT-3′ | 2093–2112 | ||||
| KIF18B | NM_001039019.1 | Sense | 5′-TGCCAGGAAGAAAAGCAGTT-3′ | 433–452 | 174 | 57 |
| Antisense | 5′-GAGTAGCTGTTGGGCTGAGG-3′ | 587–606 | ||||
| KIF20A | NM_001108426.2 | Sense | 5′-GCAGGAAAACTTCGTCAAGC-3′ | 2299–2318 | 145 | 55 |
| Antisense | 5′-TCTGATACTGCTCGGCAATG-3′ | 2424–2443 | ||||
| KIF20B | NM_001107609.2 | Sense | 5′-GCATCAGAGTGTTGCCTTCA-3′ | 1002–1021 | 141 | 55 |
| Antisense | 5′-GCGAGATCACATAGCGACAA-3′ | 1123–1142 | ||||
| KIFC1 | NM_001005878.1 | Sense | 5′-CACAACCCTGGTCAAGTCCT-3′ | 132–151 | 109 | 57 |
| Antisense | 5′-TCGTGTTCGTTTCTTTGCAG-3′ | 221–240 | ||||
| S16 | NM_001169146.1 | Sense | 5′-TCCGCTGCAGTCCGTTCAAGTCTT-3′ | 87–110 | 385 | 60 |
| Antisense | 5′-GCCAAACTTCTTGGATTCGCAGCG-3′ | 448–471 |
Abbreviation: bp, base pairs.
MT Spin-Down Assay
MT spin-down assay was performed to estimate the relative levels of polymerized MTs vs free tubulins in Sertoli cell lysates as described (10, 70) using kits from Cytoskeleton (Catalog Number BK038, Cytoskeleton, Denver, CO) according to the manufacturer’s protocols. In brief, Sertoli cells were homogenized in 37 °C prewarmed lysis and MT stabilization buffer with a 25-gauge syringe needle. Lysates were precleared by centrifugation at 2000g for 5 minutes at 37 °C to remove cellular debris, followed by centrifugation at 100 000g at 37 °C for 30 minutes to separate polymerized tubulins/MTs (pellet) from tubulin monomers (supernatant). Supernatant was collected, and the pellet was resuspended in 250 µL of MilliQ water containing 2mM CaCl2. Cell lysates, pellet, and supernatant were then used for IB. Paclitaxel (20µM, also known as Taxol, an MT-stabilizing agent) vs CaCl2 (2mM, an MT depolymerization agent) was used in the Sertoli cell lysate to serve as the corresponding positive and negative controls, respectively. This assay assessed changes in the relative distribution of MTs/polymerized tubulins (pellet) vs free/nonpolymerized tubulin monomers supernatant, respectively, after KIF15 RNAi and compared to non-targeting negative control group.
Tubulin Polymerization Assay
Tubulin polymerization assay was performed to assess the ability of cell lysate from Sertoli cells following KIF15 RNAi vs the corresponding controls to polymerize tubulin oligomers (ie, α- and β-tubulins) in vitro according to manufacturer’s instructions (Cat No. BK-011-P, Cytoskeleton). In brief, each sample of 5 µL (containing ~10 to 20 µg total protein) cell lysates were incubated with 50 µL of tubulin reaction mix at 2 mg/mL tubulin and 15% glycerol in a Corning 96-well black flat-bottom polystyrene microplate (Corning, Lowell, MA), wherein polymerized α-/β-tubulin oligomers had high affinity to DAPI according to the manufacturer’s instructions. Fluorescence kinetics were monitored from the top to quantify DAPI-labeled MTs in a FilterMax F5 Multi-Mode Microplate Reader and the Multi-Mode Analysis Software 3.4 (Molecular Devices, Sunnyvale, CA) at 37 °C. Fluorimeter settings used for measurement were: kinetics, 100 cycles, 20-second interval; excitation wavelength, 360 nm; emission wavelength, 430nm; integration time, 0.25 ms. Tubulin polymerization rate was estimated by fluorescence intensity increase rate during the initial 10 minutes of the exponential phase, and obtained by linear regression analysis using Microsoft Excel 2016 (Microsoft, Redmond, WA).
Actin Polymerization Assay
To assess the ability of Sertoli cell lysates following KIF15 RNAi vs the corresponding controls to polymerize pyrene actin oligomers, an actin polymerization assay was performed according to manufacturer’s instructions (Cat No. BK003, Cytoskeleton). Polymerization and polymerization kinetics were monitored in a Corning 96-well black flat-bottom polystyrene microplate using a FilterMax F5 Multi-Mode Microplate Reader with measurements taken from the top. Data acquisition was performed using the Multi-Mode Analysis Software 3.4 (Molecular Devices, Sunnyvale, CA) at room temperature using the following fluorimeter settings: kinetics, 100 cycles, 20-second interval; excitation wavelength, 360 nm; emission wavelength, 430 nm; integration time, 0.25 ms. Phalloidin (1µM, an actin stabilizing agent) and urea (100mM, an actin depolymerization agent), as recommended by the manufacturer, were used as the corresponding positive and negative controls. Actin polymerization rate assessed by fluorescence intensity increase rate was obtained by linear regression analysis using Microsoft Excel 2016 (Microsoft, Redmond, WA).
Sertoli Cell Lysate Preparation, Protein Estimation, and Immunoblot Analysis
Lysates of Sertoli cells were used for protein estimation. IB to assess changes in the steady-state levels of different proteins using corresponding antibodies were performed as described (71, 72). Protein estimation was performed using Bio-Rad DC protein assay kits by spectrophotometry with a Bio-Rad Model 680 Plate Reader at 650 nm; bovine serum albumin was used as the standard for standard curve calibration against which unknowns were interpolated. Steady-state protein levels in immunoblots were assessed using in-house prepared chemiluminescence kits (73), and fluorescence signals were quantified using an Image Quant LAS 4000 Mini (GE Healthcare, Waukesha, WI) Imaging System with the Image Quant software package (Version 1.3). GAPDH, tubulin and/or β-actin served as protein loading controls. All samples within an experimental set were processed simultaneously to eliminate inter-experimental variations. Each sample had triplicate cultures for both treatment and control groups from n = 3 independent experiments using different batches of Sertoli cell cultures for in vitro studies. For in vivo studies, n = 4 rats were used for each time point.
Statistical Analysis
Data analyses were performed using with GraphPad Prism 6 software (GraphPad Software) using either Student t test (for 2-group comparisons), one-way analysis of variance (ANOVA) (for multi-group comparisons). Data presented are the mean ± SD of n = 3 to 5 independent experiments. P < 0.05 was considered statistically significant.
Results
Expression of KIFs and Stage-Specific Expression and Distribution of KIF15 in the Testis
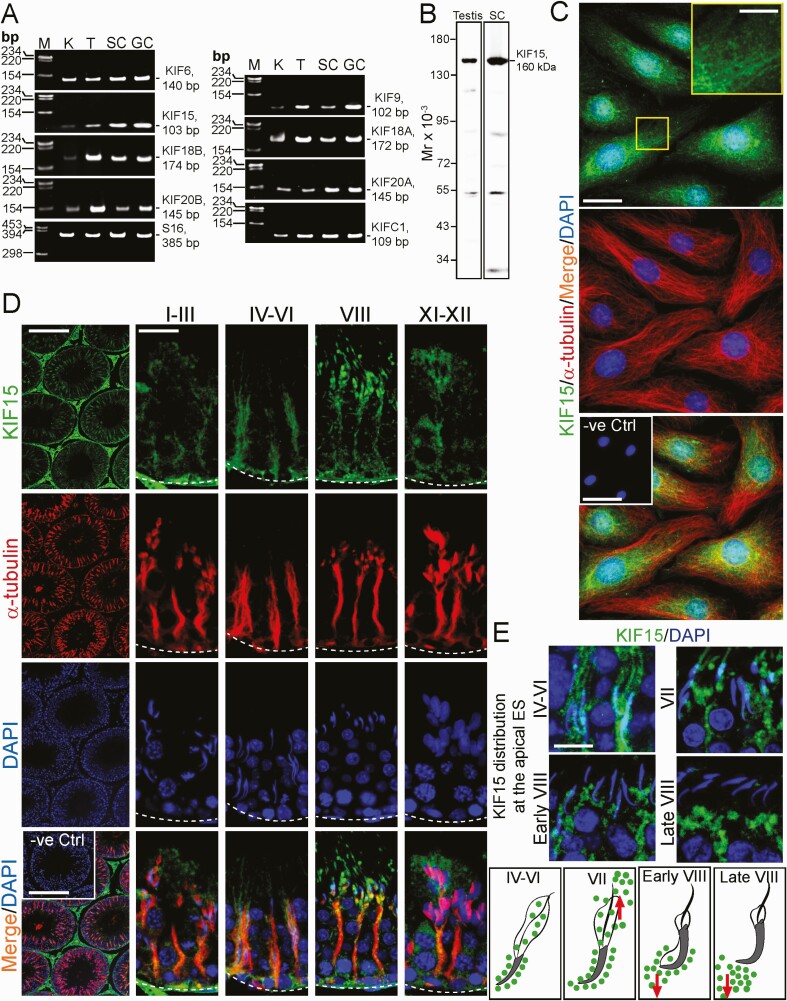
RT-PCR was performed used primer pairs specific to the corresponding KIFs with S16 (Table 2), which served as the PCR control. Sertoli cells, germ cells, and testes were found to express multiple KIFs as noted in Fig. 1A, illustrating that this family of microtubule plus (+) end–directed motor proteins is crucial to support spermatogenesis. The identity of the corresponding PCR products was confirmed by direct nucleotide sequencing in Genewiz (South Plainfield, NJ). Studies have shown that KIF15, similar to other KIF members, is a tetrameric proteins consisting of 2 light chains, and 2 heavy chains. Each heavy chain of KIF15 is about 160 kDa, comprising 1388 amino acid residues and the kinesin motor head that drives the kinesin-cargo complex toward the MT plus (+) end (12). Using an antibody specific to KIF15 (Table S1) (21), selected from 3 different vendors, a single prominent band of 160 kDa corresponding to the heavy chain of KIF15 was detected by SDS-PAGE and the band visualized by immunoblotting (IB) (Fig. 1B). This anti-KIF15 antibody was then used to localize KIF15 in Sertoli cells (Fig. 1C) and to examine the stage-specific expression and distribution across the seminiferous epithelium in cross-sections of adult rat testes (Fig. 1D). In Sertoli cells, KIF15 appeared as aggregates of fine grains that laid across the Sertoli cell cytosol, many of which laid on MTs and co-localized with α-tubulin (Fig. 1C). The negative control (-ve Ctrl) (see inset in bottom panel in Fig. 1C) in which the anti-KIF15 primary antibody (or anti-α-tubulin antibody) was substituted with normal rabbit IgG (or mouse IgG), yielded no notable staining (green or red fluorescence), illustrating the staining shown herein was specific to KIF15 (or α-tubulin) (Fig. 1C). KIF15 appeared as aggregates of fine grains (green fluorescence) that laid across the seminiferous epithelium along the MT-conferred tracks visualized by α-tubulin staining (red fluorescence), consistent with its role as the MT-dependent plus (+) end–directed motor protein (Fig. 1D). The staining noted in Fig. 1D was specific to KIF15, since the negative control, in which the primary antibody was substituted with the normal rabbit IgG, did not yielded any staining in cross-sections of testes (see inset of -ve Ctrl in the bottom panel). Figure 1E illustrates changes in the spatial expression of KIF15 at the apical ES when KIF15 was found to surround the entire spermatid heads in stages IV to VI and VII tubules. However, KIF15 immunoreactive substances were being pushed away from the spermatid heads during stage VII and early VIII of the epithelial cycle, either to the back or the front of spermatid heads (Fig. 1E). By stage VIII, KIF15 no longer associated with spermatid heads at the apical ES (Fig. 1E, see also schematic drawings that illustrate these changes in KIF15 distribution).
Figure 1.
Expression and distribution of KIF15 in Sertoli and germ cells and the seminiferous epithelium in rat testes. (A) RT-PCR using primer pairs specific to KIF15 and several other kinesins (Table 2) were used to assess the expression of these microtubule plus (+) end–directed motor proteins. Sertoli cells (SC), germ cells (GC), and testes (T) (as well as kidney [K], which served as a positive control) expressed all the kinesins examined in this experiment. DNA size markers (M) are shown with base pair (bp) units. KIF15 was elected for subsequent experiments because a monospecific antibody was available, see (B) and Table S1 (21). (B) A specific antibody against KIF15 was selected from multiple sources (Table S1) (21) which was used in our studies as noted in this immunoblot (IB) experiment. This is a representative experiment using lysates (40 µg protein per lane) from testes of a 60-day-old rat and SC (isolated from 20-day-old rat testes), yielding a prominent protein band of 160 kDa, corresponding to the heavy chain of KIF15. (C) In Sertoli cells, KIF15 (green fluorescence) appeared as dot-like aggregates (and were noted in enlarged yellow boxed area) that laid along the MT-based tracks conferred by α-tubulin (red fluorescence) with the negative control (-ve Ctrl, see inset in the third panel whereby the primary antibody was substituted by normal rabbit IgG). Scale bar, 40 µm, which apply to corresponding images; 15 µm in inset boxed in yellow; 160 µm in inset of -ve Ctrl. (D) Immunofluorescence analysis (IF) using cross-sections of testes and antibodies against KIF15 (green fluorescence) and α-tubulin (red fluorescence, to visualize microtubules [MTs]; α-tubulin together with ß-tubulin creates the α-/ß-tubulin oligomers which serve as the building blocks of MTs). In the seminiferous epithelium, KIF15 also appeared as dot-like structures and aggregates that laid along MT tracks. As such, KIF15 appeared as track-like structures that stretched across the seminiferous epithelium and aligned perpendicular to the basement membrane (annotated by dotted white line), co-localized with MTs, in all stages of the epithelial cycle with stages I-III, IV-VI, VIII, and IX-XII noted here. In negative control (-ve Ctrl), the primary antibody of KIF15 or α-tubulin was substituted with the corresponding IgG of the same animal species (ie, rabbit IgG for KIF15, and mouse IgG for α-tubulin), no red or green fluorescence was detected, illustrating staining for KIF15 and α-tubulin were specific. Scale bar, 350 µm and 80 µm, which apply to corresponding images in the same panel; and 350 µm in inset for -ve Ctrl. (E) Changes in the distribution of KIF15 at the apical ectoplasmic specialization (ES), surrounding the developing spermatids in stages IV-VI, VII, early and late VIII were shown, with the schematic drawings in the bottom panel. Red arrows illustrate the directional changes in KIF15 distribution during these epithelial stages. Scale bar, 40 µm, which applies to all other micrographs. All data shown here are representative results of an experiment, and n = 3 independent experiments (either testes from 3 rats or separate culture experiments) yielded similar findings.
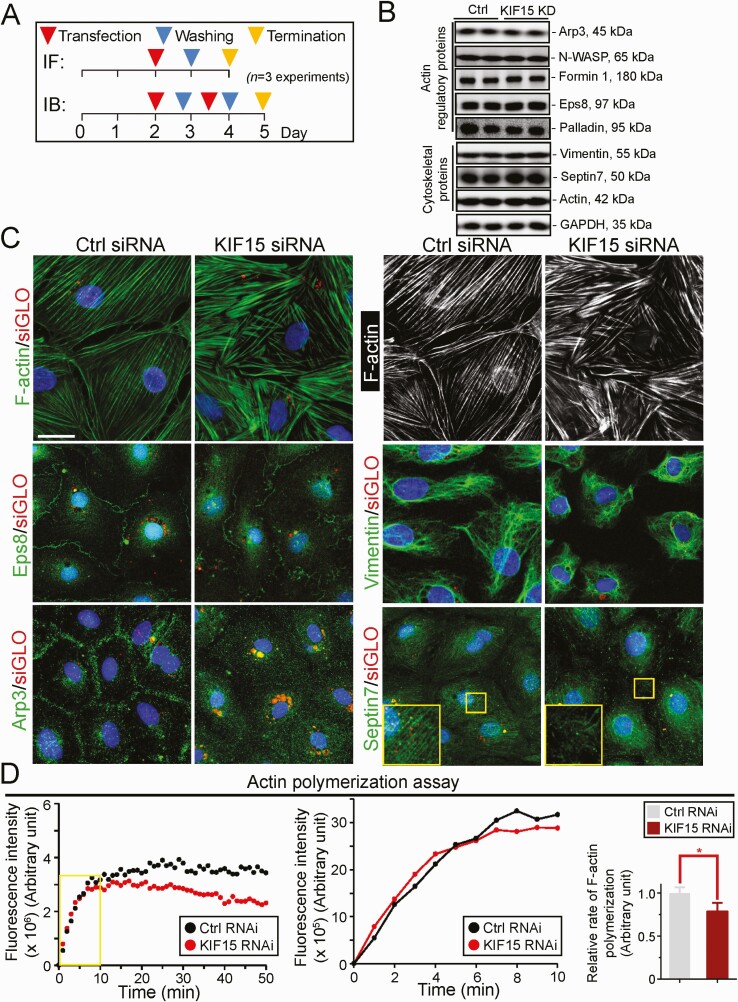
KIF15 knockdown (KD) in Sertoli Cells Perturbs Tight Junction (TJ)-Barrier Function
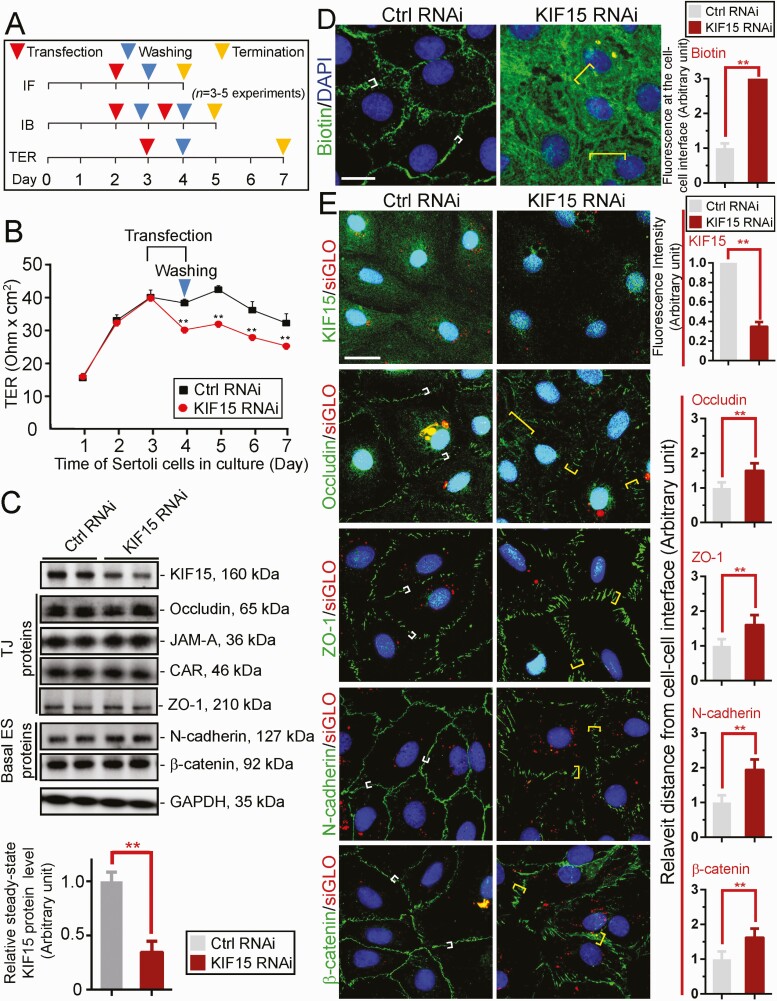
A loss-of-function study used to assess the functional significance of KIF15 in Sertoli cells was performed by RNAi using the regimen outlined in Fig. 2A with n = 3 independent experiments. Using specific KIF15 siRNA duplexes vs non-targeting control siRNA duplexes (Table 1), KD of KIF15 perturbed the Sertoli cell TJ-permeability barrier function (Fig. 2B). When the expression of KIF15 was downregulated by ~70% based on immunoblotting (IB) (Fig. 2C), the steady-state protein levels of several TJ and basal ES proteins were not affected, supporting the notion that there was no off-target effects of using the specific KIF15 siRNA duplexes for RNAi (Fig. 2C). The KIF15 KD-mediated disrupted TJ-barrier function, as noted in Fig. 2B, was further confirmed using a newly developed in vitro Sertoli cell BTB assay (Fig. 2D). In this assay, an intact Sertoli cell TJ-barrier was capable of blocking the entry of a small membrane-impermeable biotin (sulfo-NHS-LC-biotin, Mr 556.59) into the Sertoli cell cytosol but restricted to the Sertoli cell-cell interface (ie, cell cortical zone) (Fig. 2D, left panel). However, following KIF15 KD, biotin was capable of permeating into the cell cytosol when biotin distribution was visualized by Alexa Fluor 488-streptavidin (green fluorescence) since biotin has strong affinity to streptavidin (Fig. 2D, right panel). Findings from this study are semi-quantitatively shown in the bar graph in Fig. 2D (right panel) where the distribution of biotin at the cell-cell interface in controls (white brackets) is compared to the KIF15 KD cells (yellow brackets). The efficacy of KIF15 KD was also assessed by a considerable decline of KIF15 staining across Sertoli cell cytosol in KIF15 KD vs control cells (Fig. 2E, top panel). While KIF15 KD did not affect the relative steady-state levels of many BTB-associated proteins (Fig. 2C), its KD considerably perturbed the relative distribution of TJ (eg, occludin, ZO-1) and basal ES (eg, N-cadherin, ß-catenin) proteins (Fig. 2E).
Figure 2.
KIF 15 supports Sertoli cell blood-testis barrier (BTB) function through its effects to confer proper distribution of BTB-associated proteins at the Sertoli cell-cell interface. (A) Regimen used for n = 3 to 5 independent experiments reported here. (B) Sertoli TJ-permeability barrier was monitored by recording transepithelial electrical resistance (TER) across the cell epithelium cultured on bicameral units of n = 4 units from a representative experiment of n = 3 independent experiments which yielded similar results. ** P < 0.01 by Student’s t test compared to corresponding control group. (C) Representative immunoblotting (IB) data illustrating the relative protein levels of tight junction (TJ, occludin, ZO-1) and basal ectoplasmic specialization (ES), N-cadherin, ß-catenin proteins following KIF15 knockdown (KD) by RNAi, with GADPH serving as the protein loading control. Each lane contained 40 µg total protein. Bar graph shown in lower panel was composite data of IB such as those shown in the top panel, illustrating reduction of KIF15 expression by ~70%, with each bar representing a mean ± SD of n = 5 independent RNAi experiments. ** P < 0.01. (D) Results of an in vitro Sertoli cell BTB integrity assay wherein biotin (green fluorescence, EZ-link sulfo-NHS-LC-biotin, a membrane impermeable biotin, visualized by Alexa Fluor 488-streptavidin) (see “Materials and Methods”). In cell epithelium with an intact TJ-barrier, green fluorescence was confined to the cell-cell interface (white brackets), failing to enter the cell cytosol, but after KIF15 KD when the Sertoli BTB was perturbed, biotin entered freely into the cell cytosol (yellow brackets). Scale bar, 20 µm. Bar graph on the right panel is the composite data, with each bar representing a mean ± SD of n = 3 experiments (about 200 cells were randomly selected and scored for measurements). Biotin at the cell-cell interface in control (Ctrl RNAi, white brackets) was arbitrarily set at 1 for statistical comparison with KIF15 RNAi (yellow brackets) by Student’s t test. ** P < 0.01. (E) In the top panel, KIF15 (green fluorescence) was considerably diminished across Sertoli cell cytosol (as noted in the composite bar graph data on the right panel) following its KD. TJ proteins (occludin, ZO-1) and basal ES proteins (N-cadherin, ß-catenin) were localized tightly to the cell cortical zone at the cell-cell interface in controls (see white brackets). Following KIF15, these proteins no longer tightly localized to the cell-cell interface (see yellow brackets), but internalized, thereby impeding the TJ-barrier as noted in (B) and (D). Successful transfection was confirmed by siGLO. Bar graph on the right panel is the composite down wherein each bar represents a mean ± SD of n = 3 experiments shown on the left panel. Changes in protein distribution was semi-quantitatively analyzed by quantifying distribution of these proteins at the cell-cell interface (see white vs yellow brackets) with the white bracket arbitrarily set at 1 against which statistical comparison was performed. ** P < 0.01, by Student’s t test. Scale bar, 40 µm, which applies to all other micrograph in (E). All data shown in (D) and (E) are representative findings from an experiment with triplicate culture dishes, and a total of n = 3 independent experiments yielded similar results.
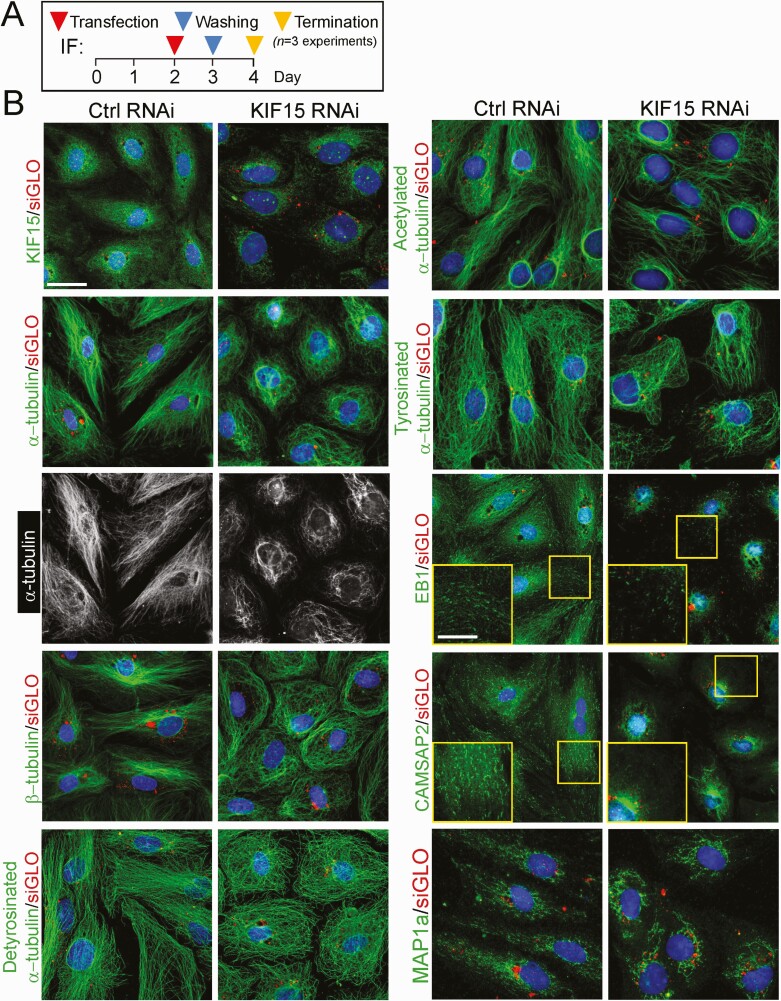
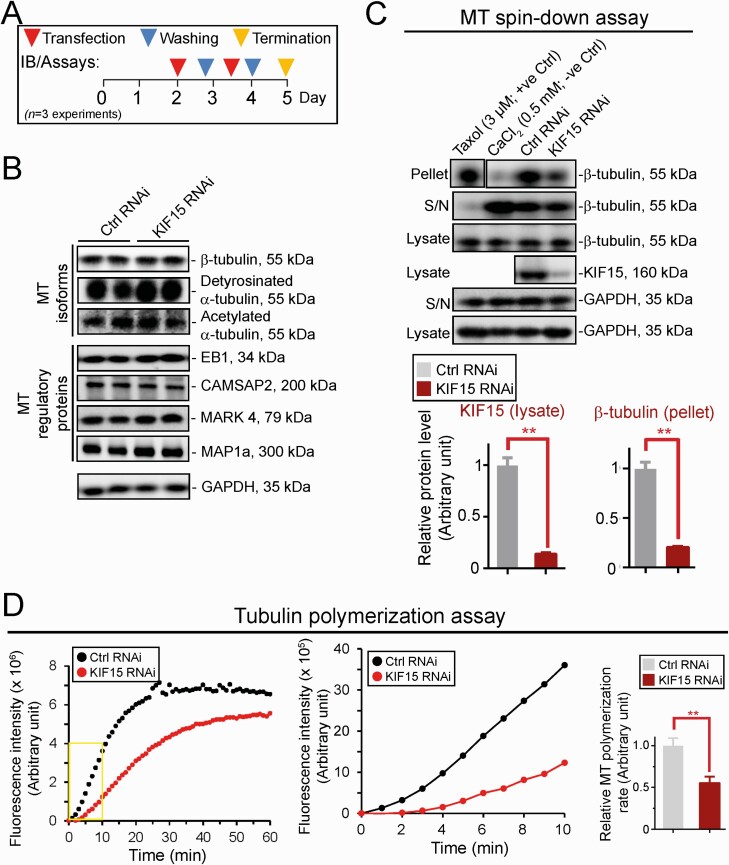
KIF15 KD in Sertoli Cells Perturbs Microtubule (MT) Cytoskeleton OrganizationWe next investigated the underlying mechanism(s) by which KIF15 KD perturbed the Sertoli cell TJ- barrier function noted in Fig. 2. Using the regimen in Fig. 3A, KIF15 KD effectively downregulated KIF15 expression across the Sertoli cell cytosol (Fig. 3B, left top panel). In control Sertoli cells transfected with non-targeting siRNA duplexes (Table 1), MTs (visualized by either α- or ß-tubulin staining which are the building blocks of MTs), appeared as linear filaments that stretched across the entire Sertoli cell cytosol (Fig. 3B; see also the black-and-white image for better clarity). We also examined the distribution of detyrosinated and acetylated α-tubulins (both are the stabilized forms of MTs (74, 75)), as well as tyrosinated α-tubulin (the dynamic form of MTs (74, 75), all of which were found to stretch across the entire Sertoli cell cytosol in control cells (Fig. 3B). Additionally, the +TIP (microtubule plus (+) end–tracking protein that binds to MT to confer stability) EB1 (end-binding protein 1) and also the −TIP (microtubule minus (−) end targeting protein that binds to MT to determine the length of MT protofilaments and confers stability) CAMSAP2 (calmodulin-regulated spectrin-associated protein 2), as well as the microtubule affinity protein 1a (MAP1a) that binds to MT to maintain its stability, all of which are also involved in stabilizing MTs to prevent MT shrinkage and catastrophe (3, 76-78), were all found to stretch across the Sertoli cell cytosol in control cells, similar to MTs when visualized by α- or ß-tubulin staining (Fig. 3B). In KIF15 silenced cells, MTs and the corresponding MT isoforms, as well as the MT regulatory proteins EB1, CAMSAP2, and MAP1a, no longer stretched across the cell cytosol to support MT function, but retracted from the cell peripheries (Fig. 3B). These changes thus impeded MT organization, perturbing the proper distribution of TJ and basal ES proteins to support the Sertoli TJ-permeability barrier function noted in Fig. 2B, 2D, and 2E. The findings noted in Fig. 3 were also supported by findings based on a biochemical study by monitoring the kinetics of MT polymerization as shown in Fig. 4. Figure 4A shows the regimen used for the experiments reported in Fig. 4B-4D. Based on immunoblot analysis, KIF15 KD by RNAi did not affect the steady-state protein levels of ß-tubulin or detyrosinated and acetylated α-tubulin, the isoforms known to stabilize MTs (75), nor the MT regulatory proteins EB1 (a +TIP), CAMSAP2 (a −TIP), MARK4, and MAP1a, all of which are involved in regulating MT dynamics to support spermatogenesis (3, 78) (Fig. 4B). However, using a MT spin-down assay that assessed the level of polymerized MTs in cell lysates, KIF15 KD considerably perturbed polymerized MTs found in Sertoli cell lysates based on this biochemical assay (Fig. 4C), as well as the polymerization kinetics (Fig. 4D). These findings thus support the notion that KIF15 supports spermatogenesis by maintaining MT homeostasis in Sertoli cell epithelium through its effects on MT plus (+) end cargo transport along the MT tracks across the seminiferous epithelium.
Figure 3.
KIF15 knockdown perturbs microtubule (MT) organization across Sertoli cell cytosol through changes in spatial expression of different MT isoforms and microtubule associated proteins (MAPs). (A) Regimen used for the experiments reported herein. (B) KIF15 knockdown was confirmed by considerable reduction on the expression of KIF15 (green fluorescence) across Sertoli cell cytosol as noted in the top 2 left panels. In control cells, α- and ß-tubulins, the building blocks of MTs, stretched across the Sertoli cell cytosol. The black-and-white images of the corresponding green fluorescence α-tubulin were also shown to confirm the considerable changes in MT organization after KIF15 KD. Detyrosinated α-tubulin and acetylated α-tubulin (both are known to confer MT stabilization (74), but also tyrosinated α-tubulin (known to confer MT dynamics (74)) all stretched across the entire Sertoli cell cytosol as linear protofilaments and noted in control Sertoli cells. However, KIF15 KD caused MTs and their corresponding isoforms to retract from cell cytosol and stay closer to Sertoli cell nuclei. Similarly, the +TIP (eg, EB1, known to stabilize MTs by binding to the microtubule plus (+) end (105)), −TIP (eg, CAMSAP2, known to determine MT length and stability by binding to the microtubule minus (−) end (106)), and structural MAPs (eg, MAP1a known to bind to MTs to maintain MT stability (77, 107)), were found to associate with the linear MT protofilaments that stretched across the entire Sertoli cell cytosol in control cells. However, following KIF15 KD, even though they remained associated with MTs, they no longer stretched across the cell cytosol but retracted from cell peripheries and moved closer to the cell nuclei. Scale bar, 40 µm, which applies to all other panels; 20 µm in insets.
Figure 4.
KIF15 knockdown perturbs MT polymerization and polymerization kinetics without affecting the steady-state protein levels of MT regulatory proteins and α-tubulin isoforms. (A) Regimen used for the experiment reported here, and from a total of n = 3 independent experiments using different batches of Sertoli cells. (B) KIF15 knockdown by RNAi did not affect the steady-state protein levels of several MT isoforms known to confer MT stabilization (eg, detyrosinated α-tubulin, acetylated α-tubulin (74)), but also several MAPs, including the +TIP (eg, EB1), −TIP (eg, CAMSAP2), MARK4 (a non-receptor Ser/Thr protein kinase, capable of phosphorylating structural MAPs such as MAP1a, causing their detachment from MTs and thereby destabilizing MTs and leading to MT catastrophe (77)), which are also known MT regulatory proteins. GAPDH served as a protein loading control. Immunoblotting (IB) data shown here are representative findings of an experiment from n = 3 independent experiments, which yielded similar results. No notable changes in steady-state protein levels were detected. (C) Representative findings of an MT spin-down assay which assessed changes in the ability of Sertoli cell lysates from control (Ctrl, transfected with non-targeting siRNA duplexes) vs KIF15 KD group to polymerize MTs detected in the pellet and compared to free tubulins in the supernatant (S/N). Composite data on the lower left panel illustrated the efficacy of KIF15 KD (see the fourth panel of IB data in upper panel). Bar graph on the lower right panel summarized findings of the polymerized MTs in the samples (see first panel of IB data in upper panel). Each bar represents a mean ± SD of n = 3 experiment with triplicate cultures in each experiment. GAPDH served as protein loading controls. ** P < 0.01 by Student’s t test. (D) Representative findings of a tubulin polymerization assay that assessed the ability of Sertoli cell lysates to polymerize α- and ß-tubulin oligomers in vitro after KIF15 vs control RNAi. Rate of tubulin polymerization was noted on the y-axis during the assay period of 60 minutes (left panel). Kinetics of tubulin polymerization were assessed during the initial 10 minutes (mid panel) (boxed in yellow on the left panel) during the initial exponential phase of polymerization. Composite data are shown on the right panel with each bar representing a mean ± SD of n = 3 independent experiments. Each experiment had triplicate culture dishes. ** P < 0.01 by Student’s t test.
KIF15 KD in Sertoli Cells Perturbs Actin Cytoskeleton Organization
We next examined if KIF15 KD also perturbed actin cytoskeleton using the regimen outlined in Fig. 5A. KIF15 KD was shown not to alter the steady-state levels of the proteins that modulate actin dynamics nor vimentin and septin7 (Fig. 5B). As noted in Fig. 5B, there were no changes in the expression of actin nucleation proteins Arp3 (which with N-WASP serves as an upstream activator of the Arp2/3 protein complex used to induce branched actin polymerization, thereby converting bundled actin filaments at the BTB/basal ES to a branched actin network) (79, 80) and formin 1 (known to induce linear actin polymerization) (81, 82) following KIF15 KD vs control groups. Also, the expression of actin barbed end capping and bundling protein Eps8 (83) and actin filament cross-linking protein palladin (84) were not affected (Fig. 5B). In control cells, F-actin stretched across the Sertoli cell cytosol and appeared mostly as linear filaments, supported by Eps8 and Arp3, which were expressed mostly at the Sertoli cell-cell interface (Fig. 5C) (the combined effects of these 2 actin regulators are known to confer actin plasticity to regulate spermatogenesis (85)). However, KIF15 KD caused extensive defragmentation and gross misalignment of actin filaments across Sertoli cell cytosol, since these fragments no longer aligned in an orderly manner across the cell cytosol as observed in control cells, possibly due to internalization of the 2 actin regulatory proteins Eps8 and Arp3 (Fig. 5C). Furthermore, the vimentin- and septin7-based cytoskeletons were also grossly affected as noted in Fig. 5C. Interestingly, KIF15 KD only mildly perturbed the polymerization kinetics of F-actin (Fig. 5D, middle and right panel), unlike its robust effects on MT polymerization dynamics shown in Fig. 4D. However, KIF15 KD perturbed the ability of Sertoli cell lysates to maintain long-term actin polymerization activity (Fig. 5D, left panel), possibly due to failure of transporting the much-needed newly synthesized cargoes (eg, Eps8 and Arp3) to the corresponding sites to support actin dynamics to sustain actin cytoskeletal organization, as noted in Fig. 5C.
Figure 5.
KIF15 knockdown perturbs actin, vimentin, and septin cytoskeletal organization. (A) Regimen used for the experiments reported here from n = 3 independent experiments. (B) IB illustrating the knockdown of KIF15 by RNAi did not interfere with the steady-state level of any actin regulatory proteins including Eps8, palladin and Arp3 except for their spatial expression as noted in (C), as well as F-actin, vimentin, and septin 7. (C) In control cells, F-actin and vimentin-based intermediate filament and septin7-based filaments stretched across the Sertoli cell cytosols as linear filaments (see also the black-and-white image of F-actin on the right panel). After KIF15 knockdown (KD), actin filaments were grossly truncated, resulting from defragmentation; whereas vimentin filaments retracted from cell peripheries and wrapped around the Sertoli cell nuclei, and the septin7-based filaments also appeared to be truncated. Defects noted in F-actin possibly due to disruptive spatial expression of Eps (an actin barbed end capping and bundling protein) and Arp3 (a branched actin polymerization protein) since both protein no longer expressed prominently at the Sertoli cell-cell interface to support Sertoli cell TJ-permeability barrier function following KIF15 KD, but notably internalized into the cell cytosol. Scale bar, 40 µm, which applies to other micrographs. (D) Results of a biochemical assay that monitored the rate (left panel) and kinetics (middle panel) of actin polymerization from n = 3 independent experiments, which yielded similar results. Bar graph on the right panel is the composite data to illustrate the kinetics of polymerization during the exponential phase of the polymerization assay (see middle panel) during the first 10 minutes (see boxed yellow box in the left panel). Each bar represents a mean ± SD of n = 3 experiments. There was a mild statistically significant change in actin polymerization kinetics (see right bar graph); however, KIF15 by RNAi impeded the ability of Sertoli cells to polymerize actin from 20 to 50 minutes as noted in the data shown on the left panel, compared with control cells treated with non-targeting siRNA duplexes. P < 0.05, by Student’s t test.
KIF15 is Involved in Maintaining MT Cytoskeletal Organization
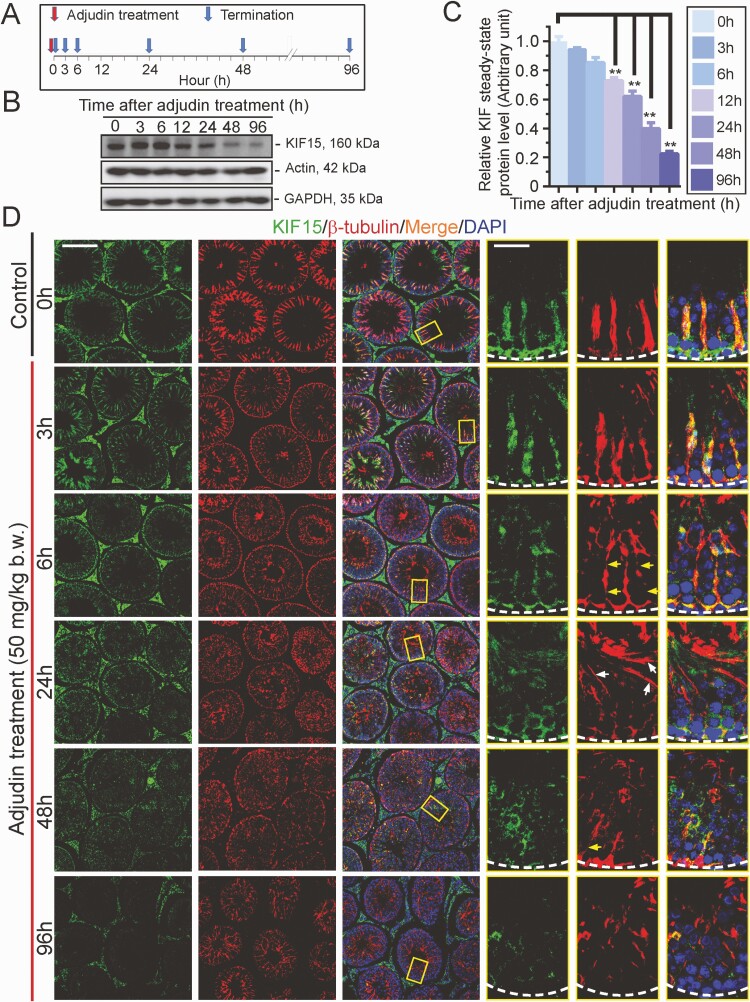
We further investigated the role of KIF15 in maintaining MT organization using an established animal model in which adult rats were treated with a single dose of adjudin (50 mg/kg body weight, oral gavage), which is known to induce germ cell exfoliation through its disruptive effects on actin and MT cytoskeletal organization (86). Using the regimen noted in Fig. 6A, adjudin induced a time-dependent downregulation of KIF15 (Fig. 6B, 6C). Using IF, KIF15 (green fluorescence) was found to stretch across the entire seminiferous epithelium in cross-sections of control testes (time 0) and laid perpendicular to the basement membrane (annotated by a dashed white line), co-localizing with MTs (red fluorescence, visualized by ß-tubulin staining) (Fig. 6D). However, KIF15 was grossly truncated, similar to MTs visualized by ß-tubulin, within 6 hours following adjudin treatment, and became grossly truncated and misaligned (eg, laying parallel to the basement membrane) by 24 hours (Fig. 6D). By 96 hours, KIF15 was virtually collapsed, laying close to the basement membrane of the tunica propria, displaying a pattern considerably more remarkable than MTs as noted in the low-magnification images in multiple tubules (Fig. 6D). These changes noted in this animal model thus support the notion that KIF15 is a crucial regulator of MT organization.
Figure 6.
Adjudin-induced defects in spermatogenesis across the seminiferous epithelium are associated with a declining and disruptive spatial expression of KIF15. Using an established model of studying the mechanism(s) by which developing spermatids anchor onto the seminiferous epithelium and their transport across the epithelium during spermiogenesis based on treatment of adult rats with a single dose (50 mg/kg body weight, oral gavage) (86, 108) (see “Materials and Methods”), we examined changes in KIF15 expression and its distribution across the epithelium following adjudin treatment. (A) Regimen used for the experiment reported herein with n = 4 rats per time point. (B) IB data from a representative experiment illustrating a time-dependent decline on KIF15 following adjudin treatment, wherein a downregulation of KIF15 protein level was notably detected by 12 hours from n = 3 independent experiments that yielded similar results. (C) Composite data (right panel) with each bar represents a mean ± SD of n = 3 experiments such as those shown in (B). P < 0.01, by ANOVA. Abbreviation: h, hour. (D) Immunofluorescence (IF) results in which KIF15 (green fluorescence) and MTs (red fluorescence, visualized by ß-tubulin staining, which together with α-tubulin creates the α-/ß-tubulin oligomeric building blocks of MTs), across the epithelium were visualized. In control testes, MTs appeared as track-like structures that stretched across the seminiferous epithelium and aligned perpendicular to the basement membrane (see dashed white lines in magnified images in insets) whereas the KIF15 motor protein that directed/carried cargoes to the microtubule (+) end appeared as dot-like structures that co-localized with the MTs (see yellow boxed insets and magnified in the three right panels). However, defects of KIF15 and MTs were notably recognized by as early as 6 hours (h) after adjudin treatment in which the MTs were grossly truncated (yellow arrows) and also misaligned when some tracks were laid parallel to the basement membrane (see insets by 24 h) (white arrows). Most importantly, a notably decline on the expression of KIF15 was noted, consistent with the IB data shown in (B). On the third column, yellow boxed areas were magnified and shown in the corresponding boxed insets on the last 3 right columns. Scale bar, 300 µm; 80 µm in inset, which apply to the corresponding low- and higher-magnification images. Results shown here are representative findings from an experiments from n = 3 independent experiments that yielded similar results.
KIF15 KD Impedes Septin Cytoskeletal Organization
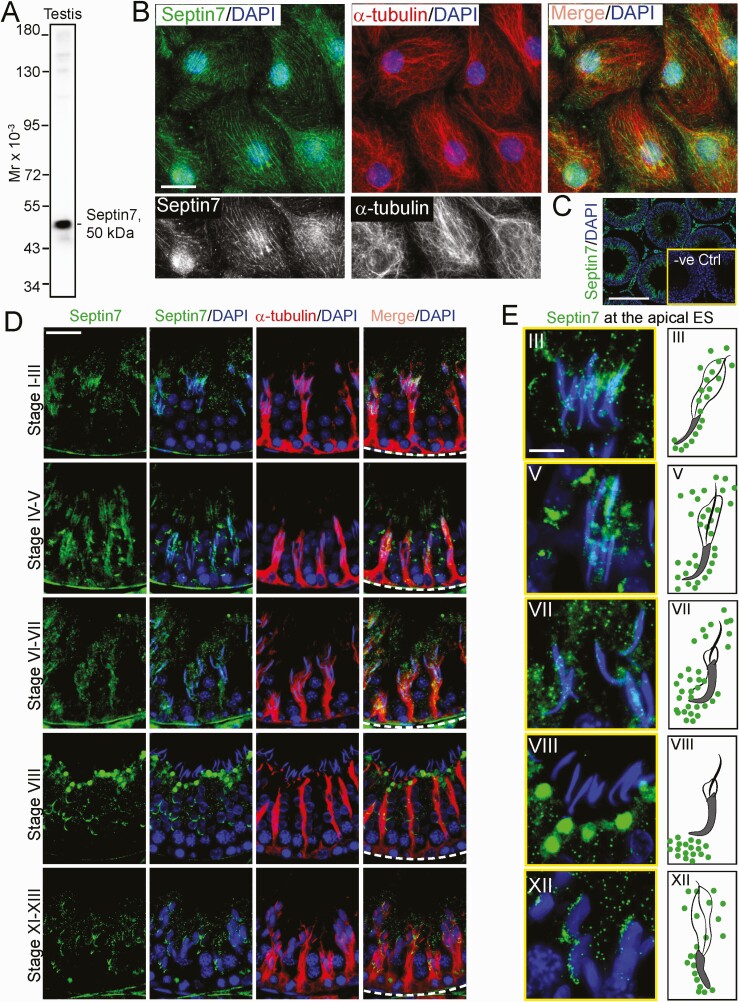
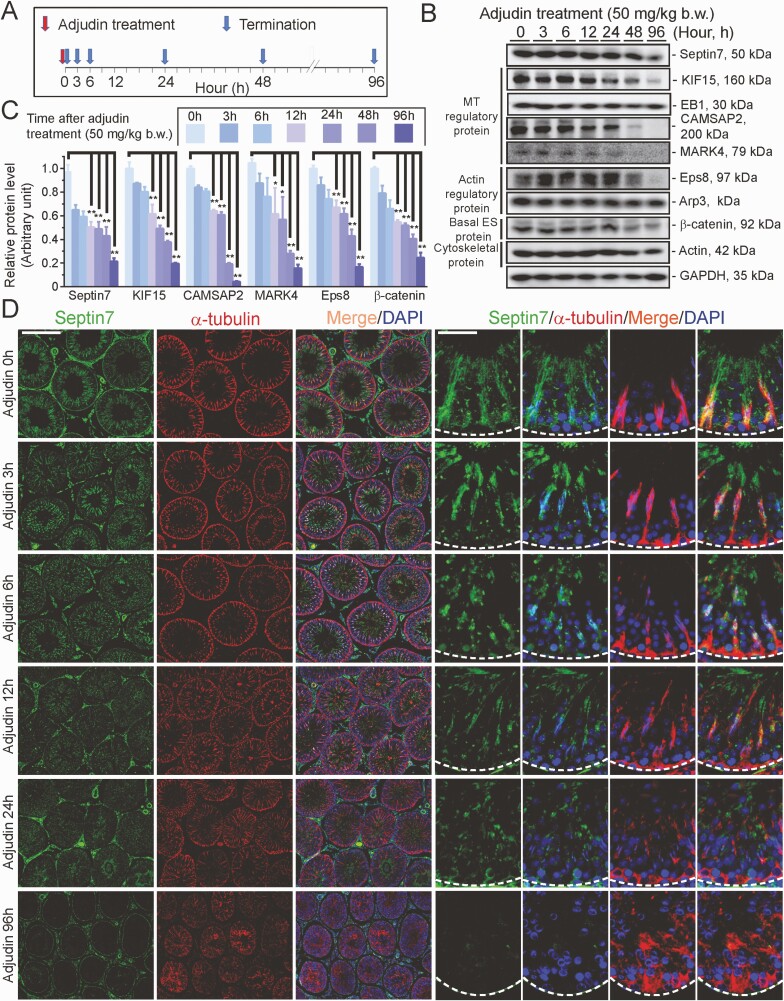
Since our findings, illustrated in Fig. 5B, 5C, show that KIF15 KD affected the organization of the septin cytoskeleton, we sought to understand the underlying mechanism for this observation. Few studies were found in the literature regarding the role of septin, the fourth cytoskeleton, in the testis, except that septin12 was shown to be involved in spermiogenesis due to its robust expression around the developing spermatid heads (87, 88). We selected septin7 in this investigation, as this is one of the few septins we had examined that had a spatial expression that shared similarities with MTs across the seminiferous epithelium (Fig. 7). We used an antibody specific to septin7 (Table S1; Fig. 7A) (21) to visualize septin7, which appeared as filaments that stretched across the Sertoli cell cytosol, and partially co-localized with MTs (Fig. 7B), supporting the notion that this cytoskeleton was localized adjacent to MTs to support spermatogenesis. The specificity of this antibody using cross-sections of testes was noted in Fig. 7C when the primary antibody (Table S1) (21) was substituted by normal rabbit IgG, which failed to generate any staining, showing that the staining shown in Fig. 7B-7E was specific to septin7. Septin7 (green fluorescence) was found to stretch across the entire seminiferous epithelium and laid perpendicular to the basement membrane (annotated by a dashed white line) but was also robustly expressed at the apical and basal ES, similar but not identical to MTs (red fluorescence) (Fig. 7D). The stage-specific changes in the distribution of septin7 at the apical ES that surrounded the spermatid heads in stages between III, V, VII, VIII, and XII are shown in Fig. 7E. It is noted in the schematic drawing on the right panel of Fig. 7E, which depicts changes in septin7 distribution surrounding the apical ES seen on the left panel in Fig. 7E. Septin7 initially distributed by surrounding the entire spermatid head at the apical ES in stages III and V tubules, which was being pushed away apically and basally, toward the tip and base of the spermatid head beginning in stage VII (Fig. 7E). As such, by stage VIII, no septin7 was found at the spermatid head until it reappeared again by stages XI to XII and localized primarily at the convex side of spermatid head (Fig. 7E). Using the regimen noted in Fig. 8A, we next used the adjudin model to examine any changes in septin7 expression in the testis by IB (Fig. 8B, 8C) and/or its organization across the seminiferous epithelium (Fig. 8D). Indeed, during adjudin-mediated germ cell exfoliation, a downregulation of septin7 expression was noted, but of also several proteins necessary to maintain the homeostasis of actin cytoskeleton (eg, Eps8, but not Arp3), and MT cytoskeleton (eg, CAMSAP2, MARK4, but not EB1) (Fig. 8B, 8C). Most notably, the spatial expression and distribution of septin7 was disrupted by as early as 6 hours following adjudin treatment, which became extensively disorganized as shown in Fig. 8D, and by 96 hours, the septin7-based cytoskeleton was virtually collapsed entirely, even worse than MTs, as illustrated in the low- and high-magnification images shown in Fig. 8D.
Figure 7.
Stage-specific distribution and expression of septin7 in adult rat testes. (A) Immunoblotting (IB) showing a single immunoreactive band of 50 kDa corresponding to the apparent molecular weight (Mr) of septin7 using an anti-septin7 antibody and lysates of testis (40 µg) from an adult rat, illustrating the specificity of this antibody selected from 3 different vendors (Table S1) (21). (B) Sertoli cells cultured in vitro for 3 days were used for visualization of septin cytoskeleton using an anti-septin7 antibody. Septin cytoskeleton (green fluorescence) appeared as linear filaments that stretched across the entire Sertoli cell cytosol, but notably distinguishable from MTs (visualized by α-tubulin) (red fluorescence) and these 2 cytoskeletons were only partially co-localized as illustrated in the merged image. The same image of septin7 and MT (visualized by α-tubulin) on the top panel were also shown partially in black-and-white in the lower panel illustrate the differences between these 2 cytoskeletons. Scale bar, 40 µm, which applies to other micrographs in this panel. (C) Representative immunofluorescence (IF) staining of septin7 (green fluorescence) in cross-section of an adult rat testis from n = 3 experiments which yielded similar results. Inset herein illustrated negative control (-ve Ctrl) using normal rabbit IgG substituted for the anti-septin7 antibody (Table S1) (21), yielding no detectable staining and thus illustrating that the green fluorescence of septin7 was authentic septin7 staining. Scale bar, 300 µm which also applies to the negative control (-ve Ctrl) image in inset. (D) Septin cytoskeleton visualized by septin7 (green fluorescence) staining appears as track-like structures that stretch across the entire seminiferous epithelium and aligned perpendicular to the basement membrane (see dashed white line on the last panel column), partially co-localized with MTs (red fluorescence, visualized by α-tubulin staining). However, unlike MTs, septin7 also localized prominently with late-stage elongating/elongated spermatids, most notably at the apical ectoplasmic specialization (ES) where septin7 was robustly expressed. Scale bar, 80 µm, which applies to other micrographs. (E) Interestingly, the spatial expression and distribution of septin7 at the apical ES site were notably changed during the epithelial cycle as noted in the left panel which were enlarged micrographs compared to (D) and shown herein. Trend of changes on the distribution of septin7 at the apical ES was further shown in the schematic drawings on the right panel, in which septin7 was found to be pushed away from the apical ES. In brief, septin7 was diffusing away from the back and the front of the spermatid head, beginning at stage VI-VII, and by stage VIII of the epithelial cycle, septin7 was no longer associated with spermatid heads at the apical ES until stage X-XII. Scale bar, 40µm, which applies to other micrographs.
Figure 8.
Adjudin-induced defects in spermatogenesis are associated with disruptive spatial expression of septin7 across the seminiferous epithelium. (A) Regimen used for the experiment reported here to investigate whether adjudin-induced germ cell exfoliation perturbed septin-based cytoskeletal organization. (B) Immunoblotting (IB) data from a representative experiment that illustrated a time-dependent downregulation on the expression of septin7 and several microtubule (MT) and actin regulatory proteins, including a BTB-associated basal ectoplasmic specialization (ES) protein ß-catenin, with ß-actin and GAPDH serving as the protein loading controls. (C) Composite IB data such as those shown in (B) with each bar representing a mean ± SD of n = 3 independent experiments; the analyzed proteins displayed a trend of downregulated expression. ** P < 0.01 by ANOVA. (D) Immunofluorescence using cross-sections of testes where septin cytoskeleton (green fluorescence, visualized by septin7 staining) and MTs (red fluorescence, visualized by α-tubulin staining) appeared as track-like structures that laid across the seminiferous epithelium and partially co-localized as noted in control testes at time 0 following adjudin treatment. Consistent with data shown in Fig. 7, septin cytoskeleton localized prominently with developing spermatid head at the site of apical ES. However, following adjudin treatment, the expression of septin cytoskeleton across the epithelium, similar to MTs, were grossly perturbed in which the filaments of septin and MT were truncated and randomly distributed without an organized pattern as noted in control testes. These changes thus failed to support germ cell adhesion that led to eventual germ cell exfoliation. Scale bar, 400 µm, which applies to micrographs in the first 3 columns of low-magnification images; and 80 µm, which applies to micrographs of the last 4 columns.
Discussion
KIF15 is a microtubule-dependent MT plus (+) end–directed motor protein that transports cargoes towards the fast-growing MT plus end (89, 90). Studies have shown MT-based tracks that stretch across the entire seminiferous epithelium are laid perpendicular to the basement membrane, whereby the polarized MT plus (+) ends are aligned in the adluminal and basal compartments, closest to the basement membrane (9, 91). KIF15 and its family members are likely involved in transporting maturing spermatids, in particular step 17 spermatids at stage V of the epithelial cycle, toward the basement membrane using MTs as tracks. KIF15 (and some of its family members) also move cargoes (e.g., residual bodies and phagosomes) from apical compartment near the tubule lumen to the base of the epithelium at stages VIII to IX of the epithelial cycle for their eventual degradation by the Sertoli cells. A functional KIF15, similar to other KIFs (there are ~45 kinesin proteins, derived from corresponding genes in vertebrates, which can be organized into at least 15 families) is a tetramer composed of 2 homodimeric heavy chains and 2 light chains (12, 92, 93). In humans, each KIF15 heavy chain is a polypeptide of 160 kDa composed of 1388 amino acid residues. The motor head of KIF15 locates at its N-terminal region which overlaps with the MT-binding region that interacts with the MT-based tracks, which is followed by the neck region and the coiled-coil domain, and the cargo binding tail, which in turn is supported by the light chain to confer cargo binding (12, 94). Even though there are more than 45 KIFs found in vertebrate cells and tissues, and several KIFs are expressed by Sertoli and germ cells in the rat testis as reported here, each KIF is likely uniquely required to support cell and tissue homeostasis. This notion is supported by findings reported herein whereby a loss-of-function blockade of KIF15 by RNAi cannot be superseded by other KIFs (eg, KIF6, KIF9, KIF18A, KIF18B, KIF20A, KIF20B, and KIFC1) which are also expressed by Sertoli and/or germ cells in the testis. For instance, a knockdown of KIF15 by ~70% in Sertoli cell epithelium, using an in vitro model that mimics the BTB in the testis in vivo with a functional TJ-permeability barrier, was found to perturb not just the MT organization, but also actin, vimentin-based intermediate filament, and septin cytoskeletons. This is likely because the loss of KIF15-mediated cargo transport failed to maintain intracellular protein trafficking, thereby perturbing the homeostasis of multiple cytoskeletons. For instance, studies have shown that dynamic organization and remodeling of microtubules are necessary to support cellular events including spermatogenesis, and these changes require active intracellular protein trafficking supported by motor proteins (75, 95). Indeed, this possibility is supported by 2 reports indicating that KIF15 is a novel regulator of endocytic trafficking of proteins (18, 96). For instance, KIF15 is involved in plasma membrane localization of the alternative clathrin adaptor Dab2 (Disabled-2, a protein involved in intracellular protein trafficking and signaling function (97)) necessary to support α2-integrin internalization (18). These findings also suggest that each member of the KIFs may be responsible to support transport of specific set(s)/type(s) of cargoes. Nonetheless, there are few reports in the literature that examine how motor proteins are involved in supporting spermatogenesis.
The observation that a disruption of KIF15 expression impeded actin, intermediate filament, and septin cytoskeletal organization is physiologically important, since this suggests that the organization of other cytoskeletons besides microtubules relies on the KIF15-mediated microtubule plus (+) end transport function. An earlier study using a yeast 2-hybrid screen has shown that KIF15 is an interacting partner of EspW (a regulator of actin filament organization (98)), which is involved in actin remodeling via a Rac1-dependent mechanism (99), suggesting the likely involvement of KIF15 in actin function. Interestingly, while a knockdown of KIF15 was found to impede the microtubule polymerization rate in Sertoli cells based on the use of a biochemical assay, it failed to impede actin polymerization rate. However, a considerable loss of KIF15 expression impeded the ability of Sertoli cells to polymerize actin filaments in Sertoli cells. Interestingly, KIF15 knockdown also perturbed the organization of septin-based cytoskeleton. In addition, this is the first report illustrating that septin7 is organized as track-like structures that partially co-localized with MTs, even though much of the septin7 is supporting apical and basal ES function. This finding is also consistent with earlier findings that septin12 is almost exclusively associated with developing apical ES to support spermiogenesis (87, 88). In this context, it is of interest to note that KIF15 has been shown to be involved in MT-mediated axonal outgrowth (90). A recent report has demonstrated that a MT-associated septin—septin9—suppresses kinesin-1/KIF5 but enhances kinesin-3/KIF1, and this differential effect of septin to mediate KIF function thus modulates partitioning of neuronal membrane proteins to affect MT-dependent membrane protein trafficking to support axon-dendrite membrane polarity necessary to maintain neuronal function (100). Since a KIF15 deletion mouse model of global KO is not available, neither a testis (or Sertoli cell)-specific KO genetic model, it remains to be explored if a loss of KIF15 function would impede male fertility. Findings report here thus support the physiological significance of KIF15 in maintaining MT cytoskeletal function, but also actin, intermediate filament, and septin cytoskeletal function to support spermatogenesis.
In this context, it is of interest to note that a knockdown of KIF15 was found to perturb vimentin-based intermediate filament organization in Sertoli cells, illustrating there is cytoskeletal crosstalk to support Sertoli cell function. While there is no intermediate filament-dependent motor protein(s) reported in the literature to support cargo transport as those found in F-actin (eg, myosin VIIa) and MTs (eg, dynein 1, KIF15) since intermediate filament cytoskeleton is not polarized structure, studies have demonstrated unequivocally that intermediate filament are also dynamic ultrastructures actively involved in cell migration, differentiation, cell migration, and stress response (101). More important, keratin-based intermediate filaments are crucial to support MT-dependent anterograde transport, ie, MT (+) end–directed transport mediated by KIF15; or retrograde transport, ie, MT (−) end–directed transport mediated by dynein 1 (101-103), illustrating the close functional relationship between intermediate filament and MT cytoskeletons. Nonetheless, elucidation of the precise role of KIF15 in supporting intermediate filament- and septin-mediated cell motility requires additional investigations. It is noted that a genetic model in mice based on global knockout was not found in the literature. Nonetheless, studies have shown that KIF15 promotes pancreatic cancer proliferation (19) and radioresistance of colorectal cancer (104) through the MEK-ERK signaling. For instance, KIF15 expression was considerably upregulated in human pancreatic cancer tissues and an elevated KIF15 expression correlated with poor prognosis and shorter patient survival times (19). In humans, loss-of-function mutations in KIF15 caused Braddock-Carey syndrome, characterized by microcephaly, congenital thrombocytopenia, and agenesis of the corpus callosum, and associated with intellectual disability (16, 17). In brief, its functional significance in male fertility has not been explored. Nonetheless, these findings suggest that other KIF members fail to supersede the loss-of-function due to mutation of the KIF15 gene in humans. This notion is also consistent with the findings noted in this report, since the transient loss-of-function of KIF15 in Sertoli cells by RNAi that led to defects in Sertoli cell function (eg, the TJ-permeability barrier function) also failed to be rescued by other KIF members that were expressed by these cells.
In summary, we have demonstrated that KIF15 is a crucial regulator of Sertoli cell function through its microtubule (+) end–directed cargo transport intrinsic activity, modulating cytoskeletal organization of MT, actin, intermediate filament, and septin.
Acknowledgments
Financial Support: This work was supported in part by grants from the Eunice Shriver National Institute of Child Health and Human Development (R01 HD056034 to C.Y.C.), the National Key Research and Development Program of China (2018YFC1003500 to S.F.), the National Natural Science Foundation of China (NSFC) (81971367 to L.L.; 81730042 to R.G.), the China Shenzhen Science Technology and Innovative Commission (SZSTI) (SZSTI-JCYJ20180508152336419 to C.K.C.W), and the Second Affiliated Hospital & Yuying Children’s Hospital of Wenzhou Medical University (to C.Y.C.).
Author Contributions: C.Y.C. conceived the study; C.Y.C., S.W., L.Lv, and L.L. designed research; S.W. and C.Y.C. performed research; L.Lv, L.W., B.M., J.L., X.S., R.G., C.K.C.W., F.S., and C.Y.C. contributed new reagents/analytic tools; S.W., L.Lv, and C.Y.C. performed data analysis and interpretation; S.W. and C.Y.C. prepared all figures; C.Y.C. and S.W. wrote the paper. All authors read and approved the final manuscript.
Glossary
Abbreviations
- BTB
blood-testis barrier
- CAMSAP2
calmodulin-regulated spectrin-associated protein 2
- DAPI
4′,6-diamidino-2-phenylindole
- EB1
end-binding protein 1
- ES
ectoplasmic specialization
- IB
immunoblotting
- IF
immunofluorescence microscopy analysis
- KD
knockdown
- KIF15
kinesin 15
- MAP1a
microtubule affinity protein 1a
- MT
microtubule
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- RNAi
RNA interference
- siRNA
small interfering RNA
- TER
transepithelial electrical resistance
- +TIP
microtubule plus (+) end tracking protein
- –TIP
microtubule minus (–) end targeting protein
- TJ
tight junction
Additional Information
Disclosures: The authors declare that there is no conflict of interest. The authors have nothing to declare.
Data Availability
Some data generated or analyzed during the study are included in this published report (eg, image files, gel-based immunoblot data, and composite data reported in Figs. 1-8, or in the data repositories listed in the References section, such as data deposited at https://figshare.com listed in (21) with doi:10.6084/m9.figshare.13488606.v1. Some or all datasets generated during and/or analyzed in the current reported here are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Xiao X, Wong EW, Lie PP, Mruk DD, Wong CK, Cheng CY. Cytokines, polarity proteins, and endosomal protein trafficking and signaling-the Sertoli cell blood-testis barrier system in vitro as a study model. Methods Enzymol. 2014;534:181-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 2: changes in spermatid organelles associated with development of spermatozoa. Microsc Res Tech. 2010;73(4):279-319. [DOI] [PubMed] [Google Scholar]
- 3. Wang L, Yan M, Wu S, et al. Microtubule cytoskeleton and spermatogenesis-lesson from studies of toxicant models. Toxicol Sci. 2020;177(2):305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clermont Y, Morales C, Hermo L. Endocytic activities of Sertoli cells in the rat. Ann N Y Acad Sci. 1987;513:1-15. [DOI] [PubMed] [Google Scholar]
- 5. Wen Q, Wu S, Lee WM, et al. Myosin VIIa supports spermatid/organelle transport and cell adhesion during spermatogenesis in the rat testis. Endocrinology. 2019;160(3):484-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wen Q, Tang EI, Lui WY, et al. Dynein 1 supports spermatid transport and spermiation during spermatogenesis in the rat testis. Am J Physiol Endocrinol Metab. 2018;315(5):E924-E948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang L, Yan M, Wu S, et al. Actin binding proteins, actin cytoskeleton and spermatogenesis—Lesson from toxicant models. Reprod Toxicol. 2020;96:76-89. [DOI] [PubMed] [Google Scholar]
- 8. Tang EI, Mruk DD, Cheng CY. Regulation of microtubule (MT)-based cytoskeleton in the seminiferous epithelium during spermatogenesis. Semin Cell Dev Biol. 2016;59:35-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Donnell L, O’Bryan MK. Microtubules and spermatogenesis. Semin Cell Dev Biol. 2014;30:45-54. [DOI] [PubMed] [Google Scholar]
- 10. Tang EI, Mok KW, Lee WM, Cheng CY. EB1 regulates tubulin and actin cytoskeletal networks at the sertoli cell blood-testis barrier in male rats: an in vitro study. Endocrinology. 2015;156(2):680-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roberts AJ, Kon T, Knight PJ, Sutoh K, Burgess SA. Functions and mechanics of dynein motor proteins. Nat Rev Mol Cell Biol. 2013;14(11):713-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wen Q, Tang EI, Xiao X, et al. Transport of germ cells across the seminiferous epithelium during spermatogenesis-the involvement of both actin- and microtubule-based cytoskeletons. Tissue Barriers. 2016;4(4):e1265042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miki H, Okada Y, Hirokawa N. Analysis of the kinesin superfamily: insights into structure and function. Trends Cell Biol. 2005;15(9):467-476. [DOI] [PubMed] [Google Scholar]
- 14. Webb S, Mukhopadhyay AG, Roberts AJ. Intraflagellar transport trains and motors: insights from structure. Semin Cell Dev Biol. 2020;107:82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fourriere L, Jimenez AJ, Perez F, Boncompain G. The role of microtubules in secretory protein transport. J Cell Sci. 2020;133(2):jcs237016. [DOI] [PubMed] [Google Scholar]
- 16. Sleiman PMA, March M, Nguyen K, et al. Loss-of-function mutations in KIF15 underlying a braddock-Carey genocopy. Hum Mutat. 2017;38(5):507-510. [DOI] [PubMed] [Google Scholar]
- 17. Braddock SR, South ST, Schiffman JD, Longhurst M, Rowe LR, Carey JC. Braddock-Carey syndrome: a 21q22 contiguous gene syndrome encompassing RUNX1. Am J Med Genet A. 2016;170(10):2580-2586. [DOI] [PubMed] [Google Scholar]
- 18. Eskova A, Knapp B, Matelska D, et al. An RNAi screen identifies KIF15 as a novel regulator of the endocytic trafficking of integrin. J Cell Sci. 2014;127(Pt 11):2433-2447. [DOI] [PubMed] [Google Scholar]
- 19. Wang J, Guo X, Xie C, Jiang J. KIF15 promotes pancreatic cancer proliferation via the MEK-ERK signalling pathway. Br J Cancer. 2017;117(2):245-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanenbaum ME, Macůrek L, Janssen A, Geers EF, Alvarez-Fernández M, Medema RH. Kif15 cooperates with eg5 to promote bipolar spindle assembly. Curr Biol. 2009;19(20):1703-1711. [DOI] [PubMed] [Google Scholar]
- 21. Cheng CY, Wu S. Supplemental Data. figshare. Posted December 24, 2020. 10.6084/m9.figshare.13488606.v1 [DOI]
- 22. RRID: AB_11182836,https://scicrunch.org/resolver/AB_11182836 [Google Scholar]
- 23. RRID: AB_2254298,https://scicrunch.org/resolver/AB_2254298 [Google Scholar]
- 24. RRID: AB_2068826,https://scicrunch.org/resolver/AB_2068826 [Google Scholar]
- 25. RRID: AB_2140610,https://scicrunch.org/resolver/AB_2140610 [Google Scholar]
- 26. RRID: AB_2158782,https://scicrunch.org/resolver/AB_2158782 [Google Scholar]
- 27. RRID: AB_2241126,https://scicrunch.org/resolver/AB_2241126 [Google Scholar]
- 28. RRID: AB_2210370,https://scicrunch.org/resolver/AB_2210370 [Google Scholar]
- 29. RRID: AB_869990,https://scicrunch.org/resolver/AB_869990 [Google Scholar]
- 30. RRID: AB_448182,https://scicrunch.org/resolver/AB_448182 [Google Scholar]
- 31. RRID: AB_2105244,https://scicrunch.org/resolver/AB_2105244 [Google Scholar]
- 32. RRID: AB_10903974,https://scicrunch.org/resolver/AB_10903974 [Google Scholar]
- 33. RRID: AB_2107448,https://scicrunch.org/resolver/AB_2107448 [Google Scholar]
- 34. RRID: AB_397544,https://scicrunch.org/resolver/AB_397544 [Google Scholar]
- 35. RRID: AB_630836, https://scicrunch.org/resolver/AB_630836 [Google Scholar]
- 36. RRID: AB_2130101, https://scicrunch.org/resolver/AB_2130101 [Google Scholar]
- 37. RRID: AB_2093483, https://scicrunch.org/resolver/AB_2093483 [Google Scholar]
- 38. RRID: AB_2141629, https://scicrunch.org/resolver/AB_2141629 [Google Scholar]
- 39. RRID: AB_2148626, https://scicrunch.org/resolver/AB_2148626 [Google Scholar]
- 40. RRID: AB_647794, https://scicrunch.org/resolver/AB_647794 [Google Scholar]
- 41. RRID:AB_2288632, https://scicrunch.org/resolver/AB_2288632 [Google Scholar]
- 42. RRID: AB_628437, https://scicrunch.org/resolver/AB_628437 [Google Scholar]
- 43. RRID:AB_634811, https://scicrunch.org/resolver/AB_634811 [Google Scholar]
- 44. RRID:AB_634837, https://scicrunch.org/resolver/AB_634837 [Google Scholar]
- 45. RRID: AB_138792, https://scicrunch.org/resolver/AB_138792 [Google Scholar]
- 46. RRID:AB_2533982, https://scicrunch.org/resolver/AB_2533982 [Google Scholar]
- 47. RRID: AB_2533241, https://scicrunch.org/resolver/AB_2533241 [Google Scholar]
- 48. RRID: AB_2313779, https://scicrunch.org/resolver/AB_2313779 [Google Scholar]
- 49. RRID: AB_2533977, https://scicrunch.org/resolver/AB_2533977 [Google Scholar]
- 50. RRID: AB_2533938, https://scicrunch.org/resolver/AB_2533938 [Google Scholar]
- 51. RRID:AB_228307, https://scicrunch.org/resolver/AB_228307 [Google Scholar]
- 52. RRID:AB_2534102, https://scicrunch.org/resolver/AB_2534102 [Google Scholar]
- 53. RRID:AB_2535853, https://scicrunch.org/resolver/AB_2535853 [Google Scholar]
- 54. RRID:AB_2534088, https://scicrunch.org/resolver/AB_2534088 [Google Scholar]
- 55. RRID:AB_141780, https://scicrunch.org/resolver/AB_141780 [Google Scholar]
- 56. RRID:AB_2576217, https://scicrunch.org/resolver/AB_2576217 [Google Scholar]
- 57. RRID:AB_2535850, https://scicrunch.org/resolver/AB_2535850 [Google Scholar]
- 58. RRID: AB_305328, https://scicrunch.org/resolver/AB_305328 [Google Scholar]
- 59. RRID:AB_476749, https://scicrunch.org/resolver/AB_476749 [Google Scholar]
- 60. Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol. 2011;763:237-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lui WY, Lee WM, Cheng CY. Transforming growth factor beta3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003;68(5):1597-1612. [DOI] [PubMed] [Google Scholar]
- 62. Li JC, Lee TW, Mruk TD, Cheng CY. Regulation of Sertoli cell myotubularin (rMTM) expression by germ cells in vitro. J Androl. 2001;22(2):266-277. [PubMed] [Google Scholar]
- 63. Li L, Mao B, Yan M, et al. Planar cell polarity protein Dishevelled 3 (Dvl3) regulates ectoplasmic specialization (ES) dynamics in the testis through changes in cytoskeletal organization. Cell Death Dis. 2019;10(3):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li L, Mao B, Wu S, et al. Endogenously produced LG3/4/5-peptide protects testes against toxicant-induced injury. Cell Death Dis. 2020;11(6):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Galdieri M, Ziparo E, Palombi F, Russo MA, Stefanini M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl. 1981;2:249-254. [Google Scholar]
- 66. Siu MK, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280(26):25029-25047. [DOI] [PubMed] [Google Scholar]
- 67. Li MW, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41(11):2302-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee NP, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25(2):200-215. [DOI] [PubMed] [Google Scholar]
- 69. Wen Q, Li N, Xiao X, et al. Actin nucleator Spire 1 is a regulator of ectoplasmic specialization in the testis. Cell Death Dis. 2018;9(2):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li N, Mruk DD, Tang EI, Lee WM, Wong CK, Cheng CY. Formin 1 regulates microtubule and F-actin organization to support spermatid transport during spermatogenesis in the rat testis. Endocrinology. 2016;157(7):2894-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu S, Yan M, Li L, et al. mTORC1/rpS6 and spermatogenic function in the testis-insights from the adjudin model. Reprod Toxicol. 2019;89:54-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Su W, Cheng CY. Cdc42 is involved in NC1 peptide-regulated BTB dynamics through actin and microtubule cytoskeletal reorganization. Faseb J. 2019;33(12):14461-14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mruk DD, Cheng CY. Enhanced chemiluminescence (ECL) for routine immunoblotting: an inexpensive alternative to commercially available kits. Spermatogenesis. 2011;1(2):121-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Janke C. The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206(4):461-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Janke C, Magiera MM. The tubulin code and its role in controlling microtubule properties and functions. Nat Rev Mol Cell Biol. 2020;21(6):307-326. [DOI] [PubMed] [Google Scholar]
- 76. Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol. 2015;16(12):711-726. [DOI] [PubMed] [Google Scholar]
- 77. Ramkumar A, Jong BY, Ori-McKenney KM. ReMAPping the microtubule landscape: how phosphorylation dictates the activities of microtubule-associated proteins. Dev Dyn. 2018;247(1):138-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mao BP, Ge R, Cheng CY. Role of microtubule +TIPs and -TIPs in spermatogenesis—insights from studies of toxicant models. Reprod Toxicol. 2020;91:43-52. [DOI] [PubMed] [Google Scholar]
- 79. Alekhina O, Burstein E, Billadeau DD. Cellular functions of WASP family proteins at a glance. J Cell Sci. 2017;130(14):2235-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Suetsugu S. Activation of nucleation promoting factors for directional actin filament elongation: allosteric regulation and multimerization on the membrane. Semin Cell Dev Biol. 2013;24(4):267-271. [DOI] [PubMed] [Google Scholar]
- 81. Romero S, Le Clainche C, Gautreau AM. Actin polymerization downstream of integrins: signaling pathways and mechanotransduction. Biochem J. 2020;477(1):1-21. [DOI] [PubMed] [Google Scholar]
- 82. Le S, Yu M, Bershadsky A, Yan J. Mechanical regulation of formin-dependent actin polymerization. Semin Cell Dev Biol. 2020;102:73-80. [DOI] [PubMed] [Google Scholar]
- 83. Lie PP, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. Faseb J. 2009;23(8):2555-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Qian X, Mruk DD, Wong EW, Lie PP, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in adult rat testes. Endocrinology. 2013;154(5):1907-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J. 2011;435(3):553-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cheng CY. Toxicants target cell junctions in the testis: insights from the indazole-carboxylic acid model. Spermatogenesis. 2014;4(2):e981485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lin YH, Kuo YC, Chiang HS, Kuo PL. The role of the septin family in spermiogenesis. Spermatogenesis. 2011;1(4):298-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lin YH, Lin YM, Wang YY, et al. The expression level of septin12 is critical for spermiogenesis. Am J Pathol. 2009;174(5):1857-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ohi R, Strothman C, Zanic M. Impact of the ‘tubulin economy’ on the formation and function of the microtubule cytoskeleton. Curr Opin Cell Biol. 2020;68:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Konjikusic MJ, Gray RS, Wallingford JB. The developmental biology of kinesins. Dev Biol. 2021;469:26-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tang EI, Mruk DD, Cheng CY. MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J Endocrinol. 2013;217(2):R13-R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Reddy AS, Day IS. Kinesins in the Arabidopsis genome: a comparative analysis among eukaryotes. BMC Genomics. 2001;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Drechsler H, McHugh T, Singleton MR, Carter NJ, McAinsh AD. The Kinesin-12 Kif15 is a processive track-switching tetramer. Elife. 2014;3:e01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yang JT, Laymon RA, Goldstein LS. A three-domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses. Cell. 1989;56(5):879-889. [DOI] [PubMed] [Google Scholar]
- 95. Hahn I, Voelzmann A, Liew YT, Costa-Gomes B, Prokop A. The model of local axon homeostasis—explaining the role and regulation of microtubule bundles in axon maintenance and pathology. Neural Dev. 2019;14(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kevenaar JT, Bianchi S, van Spronsen M, et al. Kinesin-binding protein controls microtubule dynamics and cargo trafficking by regulating kinesin motor activity. Curr Biol. 2016;26(7):849-861. [DOI] [PubMed] [Google Scholar]
- 97. Finkielstein CV, Capelluto DG. Disabled-2: a modular scaffold protein with multifaceted functions in signaling. Bioessays. 2016;38 Suppl 1:S45-S55. [DOI] [PubMed] [Google Scholar]
- 98. Tobe T, Beatson SA, Taniguchi H, et al. An extensive repertoire of type III secretion effectors in Escherichia coli O157 and the role of lambdoid phages in their dissemination. Proc Natl Acad Sci U S A. 2006;103(40):14941-14946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sandu P, Crepin VF, Drechsler H, McAinsh AD, Frankel G, Berger CN. The enterohemorrhagic Escherichia coli effector EspW triggers actin remodeling in a Rac1-dependent manner. Infect Immun. 2017;85(9):e00244-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Karasmanis EP, Phan CT, Angelis D, et al. Polarity of neuronal membrane traffic requires sorting of kinesin motor cargo during entry into dendrites by a microtubule-associated septin. Dev Cell. 2018;46(4):518-524. [DOI] [PubMed] [Google Scholar]
- 101. Leube RE, Moch M, Windoffer R. Intracellular motility of intermediate filaments. Cold Spring Harb Perspect Biol. 2017;9(6) :a021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Patteson AE, Vahabikashi A, Goldman RD, Janmey PA. Mechanical and non-mechanical functions of filamentous and non-filamentous vimentin. Bioessays. 2020;42(11):e2000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Seetharaman S, Etienne-Manneville S. Cytoskeletal crosstalk in cell migration. Trends Cell Biol. 2020;30(9):720-735. [DOI] [PubMed] [Google Scholar]
- 104. Ma Y, Zhan S, Lu H, et al. B7-H3 regulates KIF15-activated ERK1/2 pathway and contributes to radioresistance in colorectal cancer. Cell Death Dis. 2020;11(10):824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Akhmanova A, Steinmetz MO. Microtubule +TIPs at a glance. J Cell Sci. 2010;123(Pt 20):3415-3419. [DOI] [PubMed] [Google Scholar]
- 106. Akhmanova A, Steinmetz MO. Microtubule minus-end regulation at a glance. J Cell Sci. 2019;132(11):jcs227850. [DOI] [PubMed] [Google Scholar]
- 107. Bodakuntla S, Jijumon AS, Villablanca C, Gonzalez-Billault C, Janke C. Microtubule-associated proteins: structuring the cytoskeleton. Trends Cell Biol. 2019;29(10):804-819. [DOI] [PubMed] [Google Scholar]
- 108. Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60(2):146-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some data generated or analyzed during the study are included in this published report (eg, image files, gel-based immunoblot data, and composite data reported in Figs. 1-8, or in the data repositories listed in the References section, such as data deposited at https://figshare.com listed in (21) with doi:10.6084/m9.figshare.13488606.v1. Some or all datasets generated during and/or analyzed in the current reported here are not publicly available but are available from the corresponding author on reasonable request.