Abstract
Background:
The focus of caries management has shifted to the early detection of caries and noninvasive methods of management of incipient lesions with novel remineralizing agents.
Aim:
The aim of this study is to evaluate and compare the remineralization potential of a novel laboratory synthesized strontium-doped nanohydroxyapatite (SrnHAp) paste to a commercially available regular dentifrice.
Materials and Methods:
Sixty enamel specimens (4 mm × 4 mm × 1 mm) were divided into two groups based on the type of dentifrice applied: Group I – regular toothpaste and Group II – SrnHAp paste. Calcium/phosphorous ratio of all sound specimens was evaluated using Scanning Electron Microscopy-Energy Dispersive X-ray analysis. Samples in both groups were subjected to demineralization, and the calcium/phosphorous ratio was analyzed. The samples were then subjected to remineralization using the specific agents in each group, and the mean calcium–phosphorus ratio was assessed. Cytotoxic evaluation of both pastes was done by direct microscopic observation and MTT assay.
Statistical Analysis:
Comparison of mean calcium and phosphorous values of sound enamel, demineralized, and remineralized specimen in Groups I and II was done using the one-way ANOVA and Tukeys post hoc test. Intergroup comparison after remineralization was done using the Student's t-test.
Results and Conclusion:
Group II showed higher remineralization potential than Group I and was statistically significant. Cytotoxicity of novel paste was less compared to the regular toothpaste. SrnHAp showed better remineralization than regular toothpaste and can be considered for enamel repair in incipient carious lesions.
Keywords: Cytotoxic evaluation, remineralization, scanning electron microscopy-energy dispersive X-ray, strontium-doped nanohydroxyapatite
INTRODUCTION
Dental caries is one of the significant public health problems and we continue to search for ways to reduce the risk of caries in patients. The focus of caries management has shifted to the early detection of caries and noninvasive methods of management of incipient lesions with novel remineralizing agents.[1]
White-spot lesions are the earliest phase of the caries process and are reversible. The mechanical and crystallographic studies on white-spot lesions revealed around 10% loss in mineral content, making the area softer and prone to enamel caries.[2] Noncavitated lesions as well as caries extending up to the dentinoenamel junction can be arrested. This is possible if the cariogenic challenges of the specific microenvironment are sufficiently controlled or/and if therapeutic agents are applied for tissue healing.[3]
Recently, nanoparticles of hydroxyapatite (nHAp) with the same chemical composition as of tooth enamel Ca10(PO4)6(OH)2 has been used for remineralization. Nanohydroxyapatite (nHAp) shows higher Ca2+ ion release rates and superior functional properties due to uniform grain size.[4] Fluorides which are considered as a gold standard for remineralization cause hypermineralization of the surface layers and fail to strengthen the teeth from within, while nHAp induces mineralization from within the teeth along with the natural therapy of saliva.[5]
However, the main drawbacks of nHAp are lack of strength, brittleness, high degree of crystallinity, and low solubility at neutral pH.[6] Many researchers have tried replacing calcium (Ca2+) ion with strontium ion (Sr2+) in varied ratios to increase the acid reactivity of apatite, improvement in solubility, and increased fluoride release successfully.[7,8,9,10] Hence, strontium-doped nHAp (SrnHAp) may be beneficial for inducing enamel repair and remineralization.
Evaluation of remineralization will require the measurement of even small changes in a tooth's mineral content. Scanning electron microscopy (SEM) along with energy dispersive X-ray analysis (EDX) is a microanalytical technique that is employed to quantitatively estimate the amounts of mineral in a given tooth sample.[11] SEM helps to assess the topographic changes of the enamel surface.
The present study evaluates and compares the remineralization potential of a novel laboratory synthesized paste-containing SrnHAp to a commercially available regular dentifrice. Toxicology assessment was also done to correlate its possibility for future application.
MATERIALS AND METHODS
Preparation of enamel specimens
Sixty intact premolars, extracted for orthodontic reasons were cleaned and used for the study after approval from the Institutional Ethical Committee.
Sixty enamel specimens of 4 mm × 4 mm × 1 mm size were prepared from the buccal surfaces of the teeth by sectioning. The samples were divided into two groups of thirty specimens each. Group I (control group) in which the samples was treated with commercially available regular toothpaste and Group II treated with the novel SrnHAp paste.
All sound enamel samples were subject to SEM to assess the surface topography and EDX analysis to evaluate the mean calcium and phosphorus values.
Preparation of strontium-doped nanohydroxyapatite paste
SrnHAp was prepared by co-precipitation method which is described as follows.[12] 50 mL of the solution containing calcium nitrate tetrahydrate (0.57M) and strontium nitrate (0.18M) in 1:1 ratio was prepared. After stirring the solution for 15 min, 50 mL of aqueous solution of ammonium dihydrogen orthophosphate (0.45M) was added drop wise. The pH was maintained at ≥10 by adding ammonia at a temperature of 80°C. The suspension obtained was washed with distilled water, centrifuged at 5500 rpm for 30 min and lyophilized. The fine powder of 25 mol% SrnHAp thus obtained was used for the preparation of the paste.
100 g of the novel paste was prepared. The paste was constituted in the following proportion using 25 mol % SrnHAp 50% by weight (wt), sorbitol, and glycerine as humectant 30% by wt, sodium lauryl sulphate as detergent 2% by wt, sodium alginate as binder 3% by wt, and de-ionized water 15% by wt.[13,14] [Table 1]. All the dry ingredients were finely powdered in a mortar and pestle and mixed with glycerine and deionized water until a paste like consistency was obtained. It was then assimilated into a tube labeled novel SrnHAp paste and used for the experiment.
Table 1.
Table of ingredients (strontium-doped nanohydroxyapatite paste)
| Ingredients (/100 g) | Quantity (w/w) | Role |
|---|---|---|
| 25 mol% SrnHAp | 50 | Remineralising agent and abrasive |
| Sorbitol | 14 | Humectant |
| Glycerine | 16 | |
| Sodium lauryl sulphate | 2 | Detergent |
| Sodium alginate | 3 | Binder |
| Deionized water | 15 | Vehicle |
SrnHAp: Strontium-doped nanohydroxyapatite
Demineralization of the samples
The samples in both the groups were subjected to demineralization with freshly prepared McInne's demineralizing solution (l ml of 36% hydrochloric acid, 1 ml of 30% hydrogen peroxide, and 0.2 ml of anesthetic ether in the ratio of 5:5:1).[15] The demineralizing solution was applied to the surface of enamel samples using a cotton applicator for 5 min. It was then washed under running tap water, damped dry with absorbent paper, and then stored in artificial saliva for 24 h to prevent dehydration. After 24 h interval, the demineralization cycle was repeated in the same manner as described above. All the demineralized specimen were washed in running water, damped dry, and subjected to SEM-EDX analysis. The mean calcium and phosphorus content of demineralized specimen was recorded.
Remineralization of the samples
The demineralized specimens were subjected to remineralization with, regular dentifrice (Colgate Strong Teeth Anticavity Toothpaste) in Group I and the laboratory synthesized SrnHAp paste in Group II. All the samples were brushed using a motorized toothbrush for 3 min twice daily (12 h interval), rinsed with running tap water, and stored in artificial saliva for 28 consecutive days. The samples were then subjected to SEM-EDX analysis to evaluate the mean calcium and phosphorus content after remineralization.
Cytotoxic evaluation of the novel laboratory synthesized strontium-doped nanohydroxyapatite dentifrice
Cells seeding in 96 well plates
The L929 (Fibroblast) cell line was cultured in 25 cm2 tissue culture flask with Dulbecco's modified Eagles medium (DMEM) supplemented with 10% fetal bovine serum, L-glutamine, sodium bicarbonate (Merck, Germany), and antibiotic solution containing: Penicillin (100U/ml), streptomycin (100 μg/ml), and amphoteracin B (2.5 μg/ml). Cultured cell lines were kept at 37°C in a humidified 5% CO2 incubator (NBS Eppendorf, Germany). The viability of cells was evaluated by direct observation of cells by inverted-phase contrast microscope and followed by MTT assay method.
Two-day-old confluent monolayer of cells was trypsinized, and the cells were suspended in 10% growth medium. 100 μl cell suspension (5 × 103 cells/well) was seeded in 96 well-tissue culture plate and incubated at 37°C in a humidified 5% CO2 incubator. 1 mg of the sample was weighed and dissolved in 1 mL DMEM using a cyclomixer.
After 24 h, the growth medium was removed and freshly prepared with each of the sample pastes in 5% DMEM, five times serially diluted by two fold dilution (100 μg, 50 μg, 25 μg, 12.5 μg, and 6.25 μg in 500 μl of 5% DMEM) and each concentration of 100 μl were added in triplicates to the respective wells and incubated at 37°C. Nontreated control cells were also maintained.
Cytotoxicity assay by direct microscopic observation
The entire plate was observed after 24 h in an inverted phase-contrast tissue culture microscope, and images were recorded. Changes in the morphology of the cells, including rounding or shrinking of cells, granulation, and vacuolization in the cytoplasm if present, were considered as the indicators of cytotoxicity.
Cytotoxicity Assay by MTT Method
Fifteen mg of MTT (Sigma, M-5655) was reconstituted in 3 ml phosphate-buffered saline and sterilized by filter sterilization.
After 24 h incubation period, the sample content in wells was removed, and 30 μl of reconstituted MTT solution was added to all test and cell control wells, the plate was gently shaken well, then incubated at 37°C for 4 h. After the incubation period, the supernatant was removed and 100 μl of MTT solubilization solution (dimethyl sulphoxide) was added. The wells were mixed gently to solubilize the formazan crystals. The absorbance values were measured by using microplate reader at a wavelength of 540 nm.
Statistical analysis
The comparison of mean calcium and phosphorous values of sound enamel, demineralized and remineralized specimen in Groups I and II was done using the one-way ANOVA and Tukeys post hoc test. Intergroup comparison of Group I and Group II after remineralization was done using the Student's t-test.
RESULTS
Comparative analysis
The mean calcium and phosphorus values of sound enamel specimen, demineralized as well as remineralized specimen in Group I (regular toothpaste) are given in Tables 1 and 2. Following remineralization with the regular toothpaste, the mean calcium and phosphorus values obtained were lesser than that of sound enamel specimens and were statistically significant (P = 0.000) [Table 2 and Graph 1].
Table 2.
Group I - mean calcium phosphorus values (scanning electron microscopy-energy dispersive X-ray analysis)
| Group I | Mean calcium | Mean phosphorous |
|---|---|---|
| Baseline (B) | 65.32±0.53 | 21.05±0.76 |
| Demineralized (D) | 55.15±0.47 | 15.77±0.57 |
| Remineralized (R) | 55.14±0.47 | 15.77±0.57 |
| P | 0.000 | 0.000 |
| Post hoc test | B > D = R | B > D = R |
Graph 1.
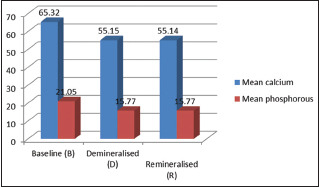
Group I - mean calcium phosphorus values
The mean calcium and phosphorus values of sound enamel specimen, demineralized as well as remineralized specimen in Group II are given in Table 3. Mean calcium and phosphorus values after remineralization with SrnHAp paste were higher than the demineralized samples and were statistically significant (P = 0.000) [Table 3 and Graph 2].
Table 3.
Group II - mean calcium phosphorus values (scanning electron microscopy-energy dispersive X-ray analysis)
| Group II | Mean calcium | Mean phosphorous |
|---|---|---|
| Baseline (B) | 65.25±0.59 | 20.76±0.81 |
| Demineralized (D) | 55.03±0.59 | 15.59±0.48 |
| Remineralized (R) | 65.4±0.86 | 20.5±0.76 |
| P | 0.000 | 0.000 |
| Post hoc test | B = R > D | B = R > D |
Graph 2.
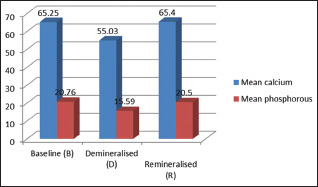
Group II - mean calcium phosphorus values (scanning electron microscopy-energy dispersive X-ray analysis)
SrnHAp paste showed higher calcium and phosphorus values (65.48 ± 0.53 and 21.05 ± 0.76, respectively) after remineralization than the regular dentifrice (55.24 ± 0.49 and 15.76 ± 0.54, respectively) and was statistically significant (P < 0.001) [Table 4 and Graph 3].
Table 4.
Intergroup comparison of mean calcium phosphorus values after remineralization
| Group | Mean calcium | Mean phosphorous |
|---|---|---|
| Group I (regular toothpaste) | 55.24±0.49 | 15.76±0.54 |
| Group II (SrnHAp paste) | 65.48±0.53 | 21.05±0.76 |
| P | 0.001 | 0.001 |
SrnHAp: Strontium-doped nanohydroxyapatite
Graph 3.
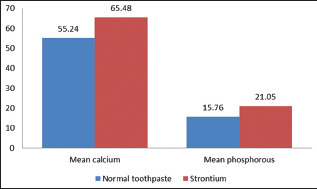
Intergroup comparison of mean calcium phosphorus values after remineralization
SEM images of the sound enamel specimens in Group I and II showed smooth surfaces [Figure 1]. After demineralization, specimens showed an uneven and irregular surface with porosities [Figure 2]. Following remineralization, Group I specimens showed open pores with few mineral crystals [Figure 3]. Group II showed smoother surface with more mineral deposition [Figure 4].
Figure 1.
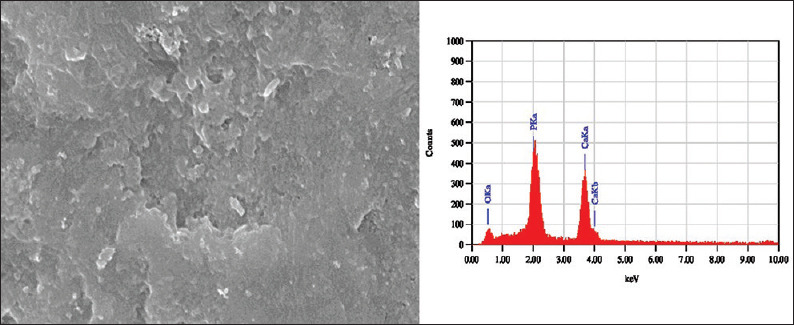
Scanning electron microscopy-energy dispersive X-ray images of sound enamel specimen
Figure 2.
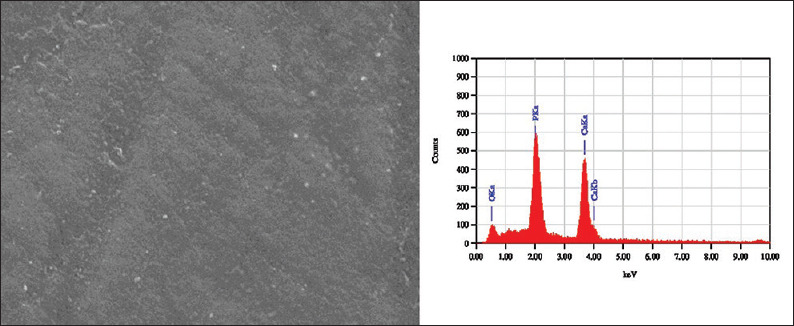
Scanning electron microscopy-energy dispersive X-ray images of demineralized enamel sample
Figure 3.
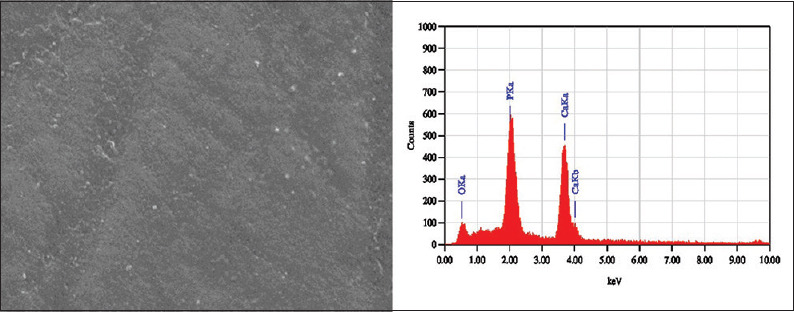
Scanning electron microscopy-energy dispersive X-ray image of Group I specimen treated with regular toothpaste
Figure 4.
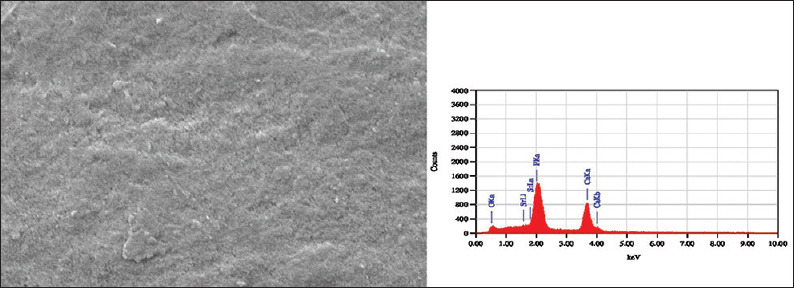
Scanning electron microscopy-energy dispersive X-ray image of Group II specimen treated with strontium-doped nanohydroxyapatite paste. Energy dispersive X-ray images show strontium peaks in Group II specimens evidently showing its presence
Toxicology assessment
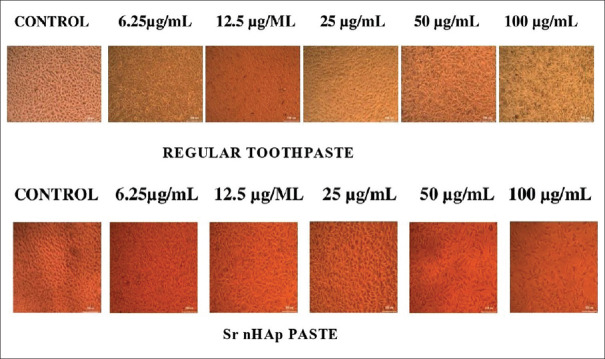
Percentage viability assessment of cells treated with both regular and SrnHAp pastes was done at different dilutions by comparing the optical density of the samples with that of the untreated controls. At all dilutions, the percentage viability of cells was greater with SrnHAp paste compared to the regular toothpaste [Table 5 and Figure 5].
Table 5.
Percentage viability assessment of cells treated with both regular and strontium-doped nanohydroxyapatite pastes done at different dilutions
| Sample concentration (µg/mL) | OD value I | OD value II | OD value III | Average OD | Percentage viability |
|---|---|---|---|---|---|
| Control (untreated cells) | 0.5535 | 0.6474 | 0.5508 | 0.5839 | 100.00 |
| Sample code: Regular paste | |||||
| 6.25 | 0.5415 | 0.5436 | 0.5442 | 0.5431 | 93.01 |
| 12.5 | 0.5258 | 0.5289 | 0.5273 | 0.5273 | 90.31 |
| 25 | 0.4621 | 0.4537 | 0.4604 | 0.4587 | 78.56 |
| 50 | 0.4408 | 0.4315 | 0.4326 | 0.4350 | 74.49 |
| 100 | 0.4281 | 0.4257 | 0.4174 | 0.4237 | 72.57 |
| Sample concentration (µg/mL) | OD value I | OD value II | OD value III | Average OD | Percentage viability |
| Control (untreated cells) | 0.6124 | 0.6307 | 0.6202 | 0.6211 | 100.00 |
| Sample code: SrnHAp paste | |||||
| 6.25 | 0.603 | 0.5962 | 0.6132 | 0.6041 | 97.27 |
| 12.5 | 0.5791 | 0.5845 | 0.5507 | 0.5714 | 92.00 |
| 25 | 0.4892 | 0.5026 | 0.5142 | 0.5020 | 80.82 |
| 50 | 0.4522 | 0.4909 | 0.4977 | 0.4803 | 77.33 |
| 100 | 0.4421 | 0.4804 | 0.4752 | 0.4659 | 75.01 |
Cytotoxic evaluation: The percentage of growth inhibition was calculated using the formula: 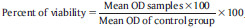 . OD: Optical density, SrnHAp: Strontium doped nanohydroxyapatite
. OD: Optical density, SrnHAp: Strontium doped nanohydroxyapatite
Figure 5.
Cell viability assay (MTT) with Group I and II specimens- Note the increased cell growth with Group II (strontium-doped nanohydroxyapatite) specimens in all the concentrations and reduced cell growth with Group I suggesting improved biological nature of strontium doped nanohydroxyapatite in comparison to regular toothpaste. Control depicts untreated cells
LC50 Value (ED50PLUS is a pharmacological analysis tool in the form of a Microsoft Excel worksheeet, which allows you to create and analyse dose-response curves. it works on Windows95/98/Me/NT/2000 operating system.)
Regular Paste – 193.921 μg/mL
SrnHAp Paste – 202.62 μg/mL.
The LC50 values for the SrnHAp paste are higher than the regular toothpaste, which shows it is less toxic than regular tooth paste.
DISCUSSION
Many remineralization agents have been developed to control demineralization and promote remineralization of incipient carious lesions. These include fluorides, calcium supplements as well as hydroxyapatite (HA) itself. Limitations of nHAp include large crystal size, questionable biocompatibility, lack of strength, brittleness, the high degree of crystallinity and low solubility at neutral pH requiring an acidic pH to dissolve.[6] Moreover, the strength of nHAp was not enough to provide adequate protection for the enamel surface leaving it rough as before and further amenable to plaque accumulation and acid attack from microbial.[12] In a study by Krishnan et al., strontium was doped in different concentrations to nHAp to overcome this problem.[12] It is reported that the incorporation of strontium (Sr) for Calcium (Ca) in HA allows the formation of a pure but nonstoichiometric HA with low (Sr + Ca)/P ratio. This partial replacement of Ca2+ ions by Sr2+ ions in HAp matrix is responsible for the increase in solubility and increases the acid reactivity.[16] It is found that Sr-Hap (above 10 mol% substitution) is a more soluble material and its increased bioactivity, due to Sr2+ release, makes it more desirable in vivo.
Krishnan et al. also stated that the increase in crystallinity and reduced particle size of 25% Sr-nHAp favors diffusion through small incipient carious lesions and white-spot areas making it the material of choice for enamel repair.[12] However, the strontium-doped HA used was in a solution form, and its application on tooth surface could be difficult. Hence, a thicker material in paste form incorporating the SrnHAp in its formulation will be beneficial because of its better retention on the tooth surface and easy application.
The present study evaluated the remineralization potential and cytotoxicity of a novel SrnHAp incorporated paste.
The caries demineralization process was simulated by using McInnes solution on the enamel specimen. Demineralization with McInnes bleaching solution shows a significant reduction in microhardness of enamel.[15] All the samples were kept in artificial saliva to simulate the oral environment.
Mean calcium and phosphorus values were determined using EDX analysis. EDX is considered as the “gold standard” for the determination of mineral loss or gain in experimentally induced initial carious lesions. It provides a precise quantitative measurement of the mineral content.[17,18] It is a micro-analytical technique used along with SEM SEM analysis is the structural part, and EDX analysis is the elemental part at an ultrastructural level.[19] The topographic changes of the enamel layer that is in sound enamel, demineralized, and remineralized specimen were assessed with SEM. After remineralization with the novel SrnHAp paste, the calcium and phosphorus values were almost similar to that of natural teeth. The SrnHAp paste showed a higher remineralization potential compared to the regular toothpaste and was statistically significant. The SEM images of Group II specimen treated with SrnHAp paste showed a smoother surface with almost complete obliteration of surface pores compared to Group I, which showed an open porous structure. SrnHAp paste appears to have better remineralization potential than the regular dentifrice, and this can be attributed to the reduced particle size, which helps to penetrate the pores and reduced crystal size which increases the solubility.
Similar results were observed in a study by Krishnan et al. in which SrnHAp in solution was found to be superior to casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) cream and nHAp toothpaste for repair of demineralized enamel surface.[6] The study concluded that the presence of strontium increased dissolving capacity of the material and improved retention on the tooth surface making it a better choice than CPP-ACP and novamin for remineralization or repair of enamel.
Evaluation of cytotoxicity of the novel toothpaste was done by direct microscopic observation and MTT assay. The percentage viability assessment of the cells with the SrnHAp paste, exhibited nontoxic nature of the material. Uninterrupted cellular growth was seen with the paste when added at different dilutions. The fibroblastic growth promotion was much more prominent in SrnHAp paste compared to the regular dentifrice [Figure 5]. LC50 values were higher for the SrnHAp paste. The novel paste showed improved cell viability and reduced cytotoxicity compared to the regular dentifrice as evidenced through MTT assay and LC50 values.
The improved remineralization potential and reduced cytotoxicity make this novel SrnHAp paste a suitable agent for remineralization of incipient caries and white-spot lesions.
However, further evaluation with different concentrations of SrnHAp and assessment of other characteristics is required before the application of the paste clinically.
CONCLUSION
Within the limitations of this study , it can be concluded that SrnHAp paste showed better remineralization potential and favorable surface changes in enamel compared to the regular dentifrice. The cytotoxic evaluation showed the nontoxic nature of the paste. The novel SrnHAp paste can be considered for enamel repair and remineralization in incipient caries and white-spot lesions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rao A, Malhotra N. The role of remineralizing agents in dentistry: A review. Compend Contin Educ Dent. 2011;32:26–33. [PubMed] [Google Scholar]
- 2.Livas C, Kuijpers-Jagtman AM, Bronkhorst E, Derks A, Katsaros C. Quantification of white spot lesions around orthodontic brackets with image analysis. Angle Orthod. 2008;78:585–90. doi: 10.2319/0003-3219(2008)078[0585:QOWSLA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Burke FJ. From extension for prevention to prevention of extension: Minimal intervention dentistry. Dent Update. 2003;30:492–8, 500, 502. doi: 10.12968/denu.2003.30.9.492. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Pan H, Tao J, Xu X, Mao C, Gu X, et al. Repair of enamel by using hydroxyapatite nanoparticles as the building blocks. J Mater Chem. 2008;18:4079–84. [Google Scholar]
- 5.Kim MY, Kwon HK, Choi CH, Kim BI. Combined effects of nano-hydroxyapatite and NaF on remineralization of early caries lesion. Key Eng Mater. 2007;1347:330–2. [Google Scholar]
- 6.Nan K, Wu T, Chen J, Jiang S, Huang Y, Pei G. Strontium doped hydroxyapatite film formed by micro-arc oxidation. Mater Sci Eng C. 2009;29:1554–8. [Google Scholar]
- 7.O'Donnell MD, Fredholm Y, de Rouffignac A, Hill RG. Structural analysis of a series of strontium-substituted apatites. Acta Biomater. 2008;4:1455–64. doi: 10.1016/j.actbio.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Featherstone JD, Shields CP, Khademazad B, Oldershaw MD. Acid reactivity of carbonated apatites with strontium and fluoride substitutions. J Dent Res. 1983;62:1049–53. doi: 10.1177/00220345830620100801. [DOI] [PubMed] [Google Scholar]
- 9.Thuy TT, Nakagaki H, Kato K, Hung PA, Inukai J, Tsuboi S, et al. Effect of strontium in combination with fluoride on enamel remineralization in vitro. Arch Oral Biol. 2008;53:1017–22. doi: 10.1016/j.archoralbio.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Shahid S, Hassan U, Billington RW, Hill RG, Anderson P. Glass ionomer cements: Effect of strontium substitution on esthetics, radiopacity and fluoride release. Dent Mater. 2014;30:308–13. doi: 10.1016/j.dental.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Hedge MN, Shetty S, Pardal D. Remineralization of enamel subsurface lesion using casein phosphopeptide-amorphous calcium phosphate. J Conserv Dent. 2007;10:19–25. [Google Scholar]
- 12.Krishnan V, Bhatia A, Varma H. Development, characterization and comparison of two strontium doped nano hydroxyapatite molecules for enamel repair/regeneration: An in vitro study. J Dent Mater. 2016;32:646–59. doi: 10.1016/j.dental.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Peter S. Essentials of Preventive and Community Dentistry. 3rd ed. New Delhi: Arya (Medi) Publishing House; 2006. p. 311. [Google Scholar]
- 14.Moharamzadeh K. In: Biocompatibilty of oral care products. In: Biocompatibility of Dental Biomaterials. Sheldon R, editor. Publisher location: UK: Elsevier Woodhead Publishing Series in Biomaterials; 2017. pp. 113–29. [Google Scholar]
- 15.Darshan HE, Shashikiran ND. The effect of Mcinnes solution on enamel and the effect of Tooth mousse on bleached enamel: An in vitro study. J Conserv Dent. 2008;11:86–91. doi: 10.4103/0972-0707.44058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang S, Gao S, Cheng L, Yu H. Combined effects of nano-hydroxyapatite and Galla chinensis on remineralisation of initial enamel lesion in vitro. J Dent. 2010;38:811–9. doi: 10.1016/j.jdent.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira GM, Ritter AV, Heymann HO, Swift E, Jr, Donovan T, Brock G, et al. Remineralization effect of CPP-ACP and fluoride for white spot lesions in vitro. J Dent. 2014;42:1592–602. doi: 10.1016/j.jdent.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acharya AB, Surve SM, Thakur SL. A clinical study of the effect of calcium sodium phosphosilicate on dentin hypersensitivity. J Clin Exp Dent. 2013;5:e18–22. doi: 10.4317/jced.50955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hegde MN, Moany A. Remineralization of enamel subsurface lesions with casein phosphopeptide-amorphous calcium phosphate: A quantitative energy dispersive X-ray analysis using scanning electron microscopy: An in vitro study. J Conserv Dent. 2012;15:61–7. doi: 10.4103/0972-0707.92609. [DOI] [PMC free article] [PubMed] [Google Scholar]